Over the past decade, nine separate gene therapy clinical trials for advanced Parkinson's disease (PD) have been launched and completed, involving the dosing of nearly 12-dozen PD volunteers who incurred significant risks to hopefully reduce symptoms and gain a better life. Each study attempted to directly or indirectly correct or compensate for the dysfunction and death of dopamine neurons (Bartus et al., 2014) that originate in the substantia nigra (located in the brain stem), projecting their axon terminals to the putamen (centrally located within each cerebrum). All told, incalculable hours were spent planning and executing these clinical trials, collectively costing hundreds of millions of dollars. After all is said and done–what has been gained?
It would be understandable if one answered that question by pointing out that treatments for PD have not improved following these efforts, for advanced patients are left with the same choices–and the same disappointing prognosis–they faced a decade ago. However, as with any nascent and highly complicated endeavor, especially one also applying highly innovative and unproven technology, initial progress is often better measured by how much new information has been gained to guide further efforts, rather than how closely the ultimate goals may have been met.
One of the major impediments to gaining more information from central nervous system (CNS) gene therapy clinical trials is the lack of concrete information regarding what is actually happening inside the human brain following administration of a gene therapy vector. It is for this reason that autopsy cases from patients enrolled in these trials are proving to be so invaluable, providing information posthumously that would not otherwise be obtainable. To date, only four subjects from all those enrolled in the 9 PD gene therapy trials have died and donated their brains for study (Bartus et al., 2011, 2015); all four cases had been enrolled in the same neurotrophic factor gene therapy program (AAV2-NRTN), which accounts for nearly 60% of all the gene therapy PD subjects dosed, to date. Based on the relatively common inclusion/exclusion criteria employed by these studies, all four of these autopsy cases were reasonably typical of the other subjects enrolled in any of the PD gene therapy trials (for more information, see Bartus et al., 2015). While all were diagnosed as idiosyncratic PD at time of enrollment, the diagnosis of one subject was later changed to multiple system atrophy-Parkinson's (confirmed at autopsy); the histological responses to AAV2-NRTN in this subject, however, were indistinguishable from those of the three PD subjects. When one considers the relatively small number of gene therapy autopsy cases available, it is somewhat remarkable how much novel information can be extracted, especially when complemented by a larger pool of control (untreated) specimens from PD brain banks. For this reason, all stakeholders in the gene therapy field are indebted to the patients and their families who donate the brains of patient volunteers for further study after active participation in the trial has ended.
Excellent safety record for PD gene therapy trials: One of the more remarkable transformations in gene therapy over the past decade is the recognition that viral vectors can be delivered safely using stereotactic surgical procedures to the brains of patients suffering a serious, chronic neurodegenerative diseases. Throughout the 1990s and through the first decade of the new millennium, the safety of gene therapy–especially for CNS indications–remained the number one concern. Though that concern was a key obstacle for regulatory agencies, investors and many other stakeholders, it has since been effectively eradicated, due largely (but not solely) to the safety results achieved in the nine PD gene therapy trials. While the vast majority of the safety data involves clinical observations showing minimal treatment-related adverse events, data from brain autopsies (albeit small numbers) offer uniquely corroborating physical support, showing the lack of any histological abnormalities, inflammatory reactions or any other neuropathology in the target organ.
Long-term, accurately targeted, bioactive protein expression: Arguably, some of the more important data autopsy tissue has provided is the demonstration that long-term expression of biologically active protein can be selectively achieved in the targeted brain sites following a single viral vector injection. These observations in human brain are not entirely surprising, given that investigators had already shown long-term expression in several animal studies (e.g., Bankiewicz et al., 2006), while positron emission tomography scans had also provided surrogate evidence of long-term expression in PD subjects (Mittermeyer et al., 2012). Nonetheless, the immunocytochemical analysis from human PD brains provides proof-positive that gene therapy is able to perform as designed and may therefore represent an enabling technology for long-term delivery of many biologics to human brain, spinal cord and eyes.
As shown in Figure 1 (Section I, panels C and E), delivery of the gene for the neurotrophic factor, neurturin (NRTN) produced protein expression 4+ years, post-dosing. Evidence that the protein remains biologically active is provided by the enhanced tyrosine hydroxylase (TH) within the region of NRTN expression (Figure 1, Section 1, panels D and F), showing partial recovery of the dopamine phenotype of these degenerating neurons following neurotrophic factor delivery.
Figure 1.
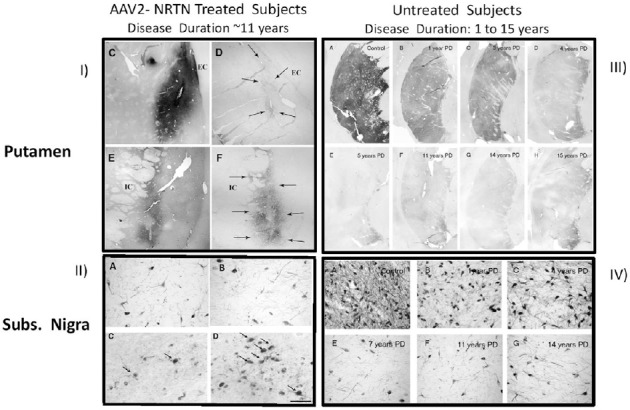
Immunohistochemical data from Parkinson's disease (PD) autopsy cases.
I) Upper lefthand section: C and E show NRTN-staining in the targeted putamen of two brains from subjects previously given AAV2-NRTN; D and F show tyrosine staining in same area (adapted from Bartus et al., 2011, 2015). II) Lower left-hand section: Dopamine neuron cell bodies in the substantia nigra compacta from 4 AAV2-NRTN cases. Note a complete lack of any NRTN-positive neurons in the 1.5 and 3months, post-dosing autopsy cases panels A and B. While an increase in the number of NRTN-positive neurons was seen following 4 years of continuous NRTN expression (arrows in panels C and D), this still only represented < 5% of all remaining dopamine neurons (adapted from Bartus et al., 2015). III) Upper right-hand section: Tyrosine hydroxylase staining in the putamen of an age-matched control and a series of PD cases at varying times, post-diagnosis (i.e., B–H: 1–15 years), reflecting stark loss of dopamine activity in the terminals of the degenerating dopamine neurons soon after initial diagnosis (From Kordower et al., 2013). IV) Lower right-hand section: Tyrosine hydroxylase staining in the substantia nigra dopamine neuronal cell bodies of an age-matched control and a series of PD cases at varying times, post-diagnosis (i.e., D–G: 1–14 years), reflecting early loss of substantial numbers dopamine neurons with a few years after initial diagnosis (from Kordower et al., 2013).
Dopamine neurons are already severely degenerated in the PD subjects enrolled in gene therapy trials: Perhaps the most striking (and possibly surprising), contribution of autopsy tissue from PD gene therapy trials is the revelation for how much neurodegeneration has already occurred in the dopamine neurons at the time patients are enrolled. Figure 1 (Section 1, panels D and F) shows virtually no TH staining is seen outside the region of NRTN-transgene expression in these autopsy cases (panels C and E). Scores of histology sections evaluated throughout the putamen of all four cases showed similar paucity of TH. In other words, except for areas where NRTN expression from gene therapy restored some modest signal, no evidence for this key dopamine phenotypic marker remained in the putamen of any of the 4 patients. While the number of cases is small, based on similar inclusion/exclusion criteria, these patients likely are representative of the majority of others enrolled in all the trials. Reinforcing that latter point, the paucity of TH staining in the AAV2-NRTN cases is consistent with data from a broad survey of autopsied brains from PD brain banks (Kordower et al., 2013). This analysis showed that a marked decline in TH immunohistochemical staining occurs in the putamen very soon after diagnosis (Figure 1, Section III, panels A–H) with the lack of TH appearing very similar to that seen in the AAV-NRTN cases. Importantly, the brain bank samples found virtually no TH signal remaining in the putamen after 5-years, post-diagnosis. Because patients with ≤ 5 years post-diagnosis are typically excluded from gene therapy protocols, these two autopsy studies offer mutually corroborating evidence for the advanced stage of degeneration of all subjects enrolled in past trials.
Similarly severe degeneration in the cell bodies of these same dopamine neurons (which originate in the substantia nigra and project to the putamen) is also revealed in an analysis of the autopsy tissue from gene therapy PD patients. Figure 1 (Section II panels A and B) shows that very few dopamine neuronal cell bodies remain in the patients enrolled into the AAV-NRTN trial and that those neurons remaining are dysfunctional, showing little evidence for the ability to transport NRTN-protein expressed in the terminal area (putamen) to the substantial nigra, even after four years of continuous NRTN expression (Figure 1, Section II, panels C and D). Studies in animal models of PD, with similar NRTN expression, have consistently shown several areas of dense NRTN-positive neurons in the substantia nigra, due to the topographic distribution of dopamine neurons in the substantia nigra and their axonal projections to the putamen. The lack of a more robust NRTN signal in the autopsy cases provides compelling evidence that axonal transport deficits in the remaining neurons are far more severe than what has been modeled in PD animal studies.
Once again, data from the survey of autopsied brains from PD brain banks (Kordower et al., 2013) offer independent corroboration for the significant loss of substantia nigra dopamine neurons in this population of PD patients. The survey shows that between 1 to 4 years after diagnosis, a marked loss of dopamine neurons rapidly occurs (Figure 1, Section IV, panels A–G). After 4 to 5 years, the loss reaches an asymptote, leaving a small, residual number of neurons that survive for years. Collectively, these results predict that the majority of PD subjects enrolled into gene therapy trials likely show similarly substantial loss of dopamine neurons (e.g., Figure 1, Section II, panels A and B) and lack of TH activity in the terminals of the putamen.
Preliminary clinical results confirm predictions from autopsy tissue: earlier intervention may be essential: Can past clinical data be used to empirically support the hypothesis (based on the autopsy tissue) that the stage of degeneration in patients enrolled in gene therapy trials may be too far advanced to be responsive to treatment? To further test this idea, we performed exploratory analyses using scores on the primary endpoint (Unified Parkinson's Disease Rating Scale; UPDRS, motor-off) from two separate double-blind, controlled phase 2 PD AAV2-NRTN data bases, as described below.
The first analysis used the data base from a recent phase2b trail that failed to show a significant effect on the primary endpoint (UPDRS, motor-off) (Olanow et al., 2015). For the exploratory test, we segregated the patients’ UPDRS scores and plotted them based on the number of years each patient had previously been diagnosed. We asked the question, is the duration of disease related to any improvement on the primary endpoint? As shown in Figure 2A, subjects diagnosed ≤ 5 years earlier from dosing showed evidence suggesting possible benefit from AAV2-NRTN (though this exploratory analysis is not sufficiently powered to conduct analytic statistics). Importantly, subjects dosed 10+ years earlier showed no evidence of any benefit (Olanow et al., 2015). A paired t-test (not corrected for multiple comparisons, nor presented for analytic purposes but merely to add perspective) suggests a possible difference in change from baseline between treated patients diagnosed ≤ 5 years versus 10+ years. Both outcomes are entirely consistent with predictions based on the autopsy results.
Figure 2.
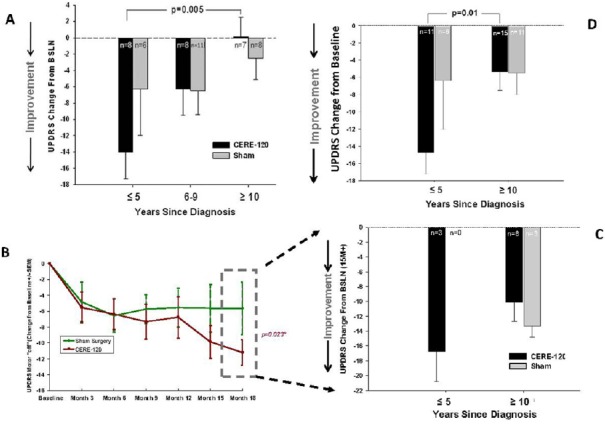
Changes from baseline in Unified Parkinson's Disease Rating Scale (UPDRS), Part 3 (motor) in the practically-defined off state.
(A) Data from recent phase 2b AAV2-NRTN controlled trial, showing strong trend toward more improvement in earlier-diagnosed subgroup of patients (i.e., ≤ 5 years, post-diagnosis), relative to those diagnosed further back in time. Note especially little change in baseline from subjects diagnosed 10+ years in either treatment or control group, with more apparent improvement in earlier-diagnosed subjects compared to later diagnosed subjects (from Olanow et al., 2015). The P value is post-hoc, not corrected for possible multiple comparisons and should not be taken literally; it is not intended for analytic purposes but provided to add perspective. (B) Data from initial Phase 2a AAV-NRTN controlled trial, wherein secondary analysis of subset of subjects who remained blinded beyond 12 months, showed significant effect on primary endpoint (UPDRS, motor off); dashed box portion of graph (from Marks et al., 2010). Those data were then used to compile graph and analysis shown in panel C. (C) Change from baseline in UPDRS for all (3 only) subjects ≤ 5 years post-diagnosis and all subjects 10+ years post-diagnosis, showing that none of the treatment effect seen in panel B was contributed by the subjects with 10+ years, post-diagnosis. (D) Data from panel A and panel C pooled to provide more statistically reliable perspective of possible impact of years, post-diagnosis in ability to improve PD clinical symptoms.
As a further test of the hypothesis, we next selected the data from an earlier PD Phase 2a AAV2-NRTN trial, which had shown that subjects who remained blinded longer than 12mo (the evaluation period for the primary endpoint) performed significantly better than controls on the primary measure (UPDRS, motor-off) (Figure 2B; Marks et al., 2010; Bartus et al., 2013). In this case, we asked a corollary question: how much of the benefit observed in these patients was contributed by scores from subjects with ≤ 5 years, post-diagnosis, versus scores from patients with 10+ years, post-diagnosis? As shown in Figure 2C, patients whose disease had progressed for 10+ years contributed nothing to the significant effect previously reported. While only 3 subjects in that trial were diagnosed ≤ 5 years earlier, all showed very robust responses to treatment, clearly contributing to the difference in groups observed. Because none of the earlier diagnoses patients happened to be randomized to the sham group, no control comparison is possible. Nonetheless, the results of this exploratory analysis are once again consistent with predictions of the autopsy data. In other words, too many subjects enrolled in gene therapy trials may be too far advanced to show significant benefit from existing treatments.
As a final exercise, the data from the two separate, randomized, double-blind phase 2 trials were pooled in an effort to increase the numbers of subjects and therefore provide a more statistically reliable estimate of the impact disease duration on the ability to improve clinical symptoms. While clearly exploratory in nature, and again not sufficiently powered, these data nonetheless suggest that duration of disease is a variable that should be given far more consideration in future protocol design. It may be worrisome that according to public disclosures regarding inclusion/exclusion criteria (e.g., Clinical Trials.gov), the ongoing gene therapy and protein infusion studies for PD continue to specifically exclude subjects diagnosed ≤ 5 years earlier (i.e., the subjects more likely to benefit from treatment), while also continuing to include subjects diagnosed 10 or even many more years earlier (i.e., those least likely to benefit). Indeed, the preliminary evidence for PD presented in this perspective is consistent with an emerging theme involving a wide variety of neurodegenerative diseases, ranging from Alzheimer's to monogenic childhood diseases like Batten disease, Angelman syndrome and Rett syndrome.
The author acknowledges with sincere appreciation the collaborative contributions and past input from several key colleagues, including Tiffany Baumann and Chris Herzog who provided substantial support and assistance with clinical/regulatory activities and preclinical activities, respectively. Also, the collaborative work performed, and insight shared regarding analysis of autopsy tissue by Jeffrey Kordower is greatly appreciated, as are past suggestions and advice from Eugene Johnson, Warren Olanow and Anthony Lang.
References
- 1.Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, Cunningham J. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Bartus RT, Weinbert MS, Samulski RJ. Parkinson's disease gene therapy: Success by design meets failure by efficacy. Mol Ther. 2014;22:487–497. doi: 10.1038/mt.2013.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartus RT, Baumann TL, Brown L, Kruegel BR, Ostrove JM, Herzog CD. Advancing neurotrophic factors as treatments for age-related neurodegenerative diseases: developing and demonstrating “clinical proof-of-concept” for AAV-neurturin (CERE-120) in Parkinson's disease. Neurobiol Aging. 2013;34:35–61. doi: 10.1016/j.neurobiolaging.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Johnson E, Olanow CW, Mufson EJ, Kordower JH. Bioactivity of AAV2-NeurturinNRTN gene therapy (CERE-120) in Parkinson's disease: A comparison of post-mortem and nonhuman primate brain tissue. Mov Disord. 2011;26:27–36. doi: 10.1002/mds.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartus RT, Kordower JH, Johnson EM, Jr, Brown L, Kruegel BR, Chu Y, Baumann TL, Lang AE, Olanow CW, Herzog CD. Post-mortem assessment of the short and long-term effects of the trophic factor neurturin in patients with α-synucleinopathies. Neurobiol Dis. 2015;78:162–171. doi: 10.1016/j.nbd.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA, et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neuro. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 8.Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, Kaplan PL, Forsayeth J, Aminoff MJ, Bankiewicz KS. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease. Hum Gene Ther. 2012;23:377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren Olanow C, Bartus RT, Baumann TL, Factor S, Boulis N, Stacy M, Turner DA, Marks W, Larson P, Starr PA, Jankovic J, Simpson R, Watts R, Guthrie B, Poston K, Henderson JM, Stern M, Baltuch G, Goetz CG, Herzog C, et al. Gene delivery of neurturin to putamen and substania nigra in Parkinson disease: A double-blind, randomized controlled trial. Ann Neurol. 2015;78:248–257. doi: 10.1002/ana.24436. [DOI] [PubMed] [Google Scholar]


