Abstract
Monocarboxylate transporters (MCTs), which carry monocarboxylates such as lactate across biological membranes, have been associated with cerebral ischemia/reperfusion process. In this study, we studied the effect of ischemic preconditioning (IPC) on MCT4 immunoreactivity after 5 minutes of transient cerebral ischemia in the gerbil. Animals were randomly designated to four groups (sham-operated group, ischemia only group, IPC + sham-operated group and IPC + ischemia group). A serious loss of neuron was found in the stratum pyramidale of the hippocampal CA1 region (CA1), not CA2/3, of the ischemia-only group at 5 days post-ischemia; however, in the IPC + ischemia groups, neurons in the stratum pyramidale of the CA1 were well protected. Weak MCT4 immunoreactivity was found in the stratum pyramidale of the CA1 in the sham-operated group. MCT4 immunoreactivity in the stratum pyramidale began to decrease at 2 days post-ischemia and was hardly detected at 5 days post-ischemia; at this time point, MCT4 immunoreactivity was newly expressed in astrocytes. In the IPC + sham-operated group, MCT4 immunoreactivity in the stratum pyramidale of the CA1 was increased compared with the sham-operated group, and, in the IPC + ischemia group, MCT4 immunoreactivity was also increased in the stratum pyramidale compared with the ischemia only group. Briefly, present findings show that IPC apparently protected CA1 pyramidal neurons and increased or maintained MCT4 expression in the stratum pyramidale of the CA1 after transient cerebral ischemia. Our findings suggest that MCT4 appears to play a significant role in the neuroprotective mechanism of IPC in the gerbil with transient cerebral ischemia.
Keywords: nerve regeneration, monocarboxylate transporters, ischemic preconditioning, ischemia/reperfusion injury, hippocampus, CA1 pyramidal neurons, neural regeneration
Introduction
Ischemic stroke, which is usually a consequence of blood flow reduction below a critical threshold because of the occlusion of a major brain artery, may cause irreversible brain damage (Kirino and Sano, 1984). An important feature of cerebral ischemic injury is the vulnerability of specific neuronal populations; especially, pyramidal neurons in the stratum pyramidale (SP) of the hippocampal CA1 region of the Mongolian gerbil do not die immediately but rather survive over several days and the death is referred to as “delayed neuronal death” (Kirino, 1982). It has been known that the delayed neuronal death is related to multiple mechanisms including changes of energy metabolism, oxidative stress, neuroinflammation, and neurotoxicity (Won et al., 1999, 2001; Chan, 2001; Lee et al., 2011b). One of the mechanisms is that ischemic brain displays the fast reduction in ATP supplies by increasing the rate of anaerobic glycolysis, leading to lactate accumulation, which links glycolysis and oxidative metabolism (Brooks, 2007), may participate in the imbalance of energy metabolism during ischemia/reperfusion. However, effects of lactate on neuronal damage/death induced by cerebral ischemia are still controversial (Schurr et al., 2001; Schurr, 2002).
Ischemic preconditioning (IPC) protects the brain against sub-lethal short-term ischemia, leading to increased tolerance of the brain to lethal ischemia/reperfusion injury (Kirino et al., 1991; Liu et al., 1992; Nishi et al., 1993; Stagliano et al., 1999; Gidday, 2006; Lehotsky et al., 2009). IPC induces expression of diverse families of genes involved in cytoprotection, which, in turn, encode proteins that can enhance tolerance to cerebral ischemia (Gidday, 2006). Nevertheless, the mechanisms underlying these tolerance are rather complex and have not been fully understood yet (Kardesoglu et al., 2011).
On the other hand, lactate, the end product of glycolysis from hypoxic condition, plays an important role in cellular metabolism (Schurr, 2006). Lactate is catalyzed by monocarboxylate transporters (MCTs), and one of the essentials of their roles is their rapid transport across the plasma membrane (Halestrap and Price, 1999). Although 14 members belonging to the MCT family have been identified up to now, only three different MCTs (MCT1, MCT2 and MCT4) have been shown to be produced in the brain (Pierre and Pellerin, 2005). These three MCTs have been found in neurons and astrocytes (Hanu et al., 2000; Debernardi et al., 2003; Gao et al., 2014), and are stimulated during excitotoxic injuries or after ischemic conditions (Schurr et al., 1999, 2001; Cater et al., 2001; Ros et al., 2001). To the best of our knowledge, few studies regarding cellular changes in MCT4 expression after transient cerebral ischemia have been investigated (Tseng et al., 2003). In addition, little is known regarding the expression pattern of MCT4 and phenotypes of cells expressing MCT4 in the brain of IPC-mediated animals after transient cerebral ischemia.
Therefore, this study was performed to examine temporal changes and cellular expression of MCT4 in the hippocampus following transient global cerebral ischemia using the gerbil. The gerbil model has been well used as an animal model of transient global cerebral ischemia (Hu et al., 2007; Min et al., 2012; Radenovic et al., 2014). We, furthermore, examined the effects of IPC on MCT4 expression in the hippocampus following transient global cerebral ischemia in the gerbil.
Materials and Methods
Experimental animals
The male Mongolian gerbils (Meriones unguiculatus) were obtained from the Experimental Animal Center of Kangwon National University (Chuncheon, South Korea). Gerbils were used at 6 months of age, weighing 65–75 g. The gerbils were housed in a conventional state under adequate temperature and humidity (23°C, 60%) control with a 12 hour light/dark cycle. The procedures for animal handling and care adhered to guidelines that are in compliance with the current international laws and policies (Guide for the Care and Use of Laboratory Animals, The National Academies Press, 8th Ed., 2011), and they were approved by the Institutional Animal Care and Use Committee (IACUC, Kangwon University). All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in this study.
Induction of transient forebrain ischemia and IPC
As previously described (Park et al., 2014), the animals were divided into four groups: sham-operated, ischemia only, IPC + sham-operated and IPC + ischemia groups. In the sham-operated group, gerbil bilateral common carotid arteries were exposed and no ischemia was given. In the ischemia only group, 5 minutes of transient forebrain ischemia was performed. In the IPC + sham-operated group, 2 minute sublethal ischemia was performed prior to sham-operation. In the IPC + ischemia group, animals were subjected to 2 minute sublethal ischemia 1 day prior to 5 minutes of transient ischemia. The IPC procedure has been proven to be very effective in protecting neurons against ischemic damage in this ischemic model (Nakamura et al., 2006). Animals in the ischemia only and IPC + ischemia groups were sacrificed at 1, 2 and 5 days (n = 14 per time point), because pyramidal neurons of the hippocampal CA1 region began to die 4 days after ischemia/reperfusion (Kirino, 1982).
Transient cerebral ischemia was performed according to our previous method (Lee et al., 2011b). In brief, the experimental animals were anesthetized with 2.5% isoflurane and the bilateral common carotid arteries were gently exposed via a ventral midline incision under an operating microscope. Ischemia/reperfusion was induced with non-traumatic aneurysm clips (Yasargil, Aesculap, Tuttlingen, Germany). The complete interruption of blood flow was confirmed by observing the central artery in retinae with an ophthalmoscope (HEINE K180®, Heine Optotechnik, Herrsching, Germany). After 2 minutes or 5 minutes of occlusion, the aneurysm clips were removed from the common carotid arteries. The body temperature under free-regulating or normothermic (37 ± 0.5°C) conditions was monitored with a rectal temperature probe (TR-100; Fine Science Tools, Foster City, CA, USA) and maintained using a thermometric blanket before, during and after the surgery until the animals completely recovered from anesthesia. Thereafter, animals were kept in the thermal incubator (Mirae Medical Inc, Seoul, South Korea) to control humidity and temperature (60%, 23°C) of animals until the animals were euthanized.
Tissue preparation for histological observation
As previously reported (Lee et al., 2015), gerbils were anesthetized with pentobarbital sodium on days 1, 2 and 5 after ischemia/reperfusion (n = 7 per time point per group), and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by fixative solution (4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4). The brains were harvested and post-fixed in the same fixative for 6 hours. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter, theses tissues were embedded in tissue-freezing medium and serially sectioned into 30 μm coronal sections on a cryostat (Leica, Wetzlar, Germany).
Cresyl violet (CV) and fluoro-Jade B (F-J B) histofluorescence staining
To find the neuronal death/damage induced by ischemia/reperfusion, we performed CV and F-J B histofluorescence staining as previously described (Lee et al., 2014). In brief, the sections were stained with cresyl violet acetate solution (1.0% (w/v), Sigma-Aldrich, St. Louis, MO, USA), dehydrated in a graded ethanol series, cleared in xylene, and coverslipped. The stained sections were mounted with Canada balsam (Kanto chemical, Tokyo, Japan). For F-J B histofluorescence, the sections were immersed in a F-J B (0.0004%, Histochem, Jefferson, AR, USA) staining solution. After washing, the sections were investigated using an epifluorescent microscope (Carl Zeiss, Göttingen, Germany) with blue excitation light (450–490 nm) and a barrier filter. With this tool, neurons that undergo degeneration brightly fluoresce in comparison to the background (Schmued and Hopkins, 2000).
Neuronal nuclei (NeuN) and MCT4 immunohistochemistry
The immunohistochemical staining was performed according to a previously reported method (Lee et al., 2014). The brain sections were blocked with normal goat serum (10% in 0.05 M PBS), followed by staining with mouse anti-NeuN (a neuron-specific soluble nuclear antigen) (diluted 1:1,000, Chemicon International, Temecula, CA, USA) and diluted rabbit anti-MCT4 antibodies (diluted 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. The sections were then incubated with anti-mouse and rabbit antibodies (diluted 1:200; Vector Laboratories Inc., Burlingame, CA, USA) for 1 hour at room temperature and developed using Vectastain ABC kit (Vector Laboratories Inc.). They were visualized with the chromogen 3,3′-diaminibenzidine (50 mg in 100 mL of 0.1 M Tris-HCl buffer) and 0.01% hydrogen peroxidase. The negative control was performed with pre-immune serum instead of primary antibody for building up the specificity of the immunostaining. The immunoreactivity of negative control was not detected in any structures.
The anatomical landmarks corresponding to anteroposterior from −1.4 to −2.2 mm of gerbil brain atlas was used to select brain sections. The number of NeuN-immunoreactive and F-J B-positive cells was counted in a 200 × 200 μm2 area, selected approximately at the center of the CA1 in the stratum pyramidale. Cell number of each animal per group was obtained by averaging the total cell numbers. Twenty sections per animal were selected to quantitatively analyze MCT4 immunoreactivity. Cellular immunoreactivity of MCT4 was graded in the hippocampal CA1 and CA3 regions. Digital images of the hippocampal CA1 region (strata oriens, pyramidale and radiatum in the hippocampus proper) were captured with an AxioM1 light microscope (Carl Zeiss) equipped with a digital camera (Axiocam, Carl Zeiss) connected to a PC monitor. Semi-quantification of the intensity of MCT4-immuoreactive structures was evaluated with a digital image analysis software (MetaMorph 4.01, Universal Imaging Corp.). The level of immunoreactivity was scaled as −, ±, + or ++, representing no staining (gray scale value: ≥ 200), weakly positive (gray scale value: 150–199), moderate (gray scale value: 100–149), or strong (gray scale value: ≤ 99), respectively (Lee et al., 2011a).
Double immunofluorescence staining
To examine the cell type containing MCT4 immunoreactivity, the sections were processed by double immunofluorescence staining at 5 days after the ischemic surgery. Double immunofluorescence staining was performed using rabbit anti-MCT4 antibody (diluted 1:100, Santa Cruz Biotechnology)/goat anti-ionized calcium-binding adapter molecule 1 (Iba-1) (diluted 1:100, Santa Cruz Biotechnology) for microglia or mouse anti-glial fibrillary acidic protein (GFAP) (diluted 1:200, Chemicon International) for astrocytes. The sections were incubated in the mixture of antisera overnight at room temperature. After three PBS washes for 10 minutes each, they were then incubated in a mixture of both Cy3-conjugated goat anti-rabbit IgG (diluted 1:200; Jackson ImmunoResearch, West Grove, PA, USA), FITC-conjugated donkey anti-mouse IgG (diluted 1:200; Jackson ImmunoResearch), and FITC-conjugated donkey anti-goat IgG (diluted 1:200; Jackson ImmunoResearch) for 2 hours at room temperature. The confocal microscope (LSM510 META NLO, Carl Zeiss, Germany) was used for detecting the immunoreactions. The pre-immune serum instead of primary antibody was used for negative control in order to establish the specificity of the immunostaining.
Western blot analysis
To acquire the scientific data for changes in protein level of MCT4 in the IPC-induced hippocampal CA1 region after transient cerebral ischemia, animals (n = 7 per time point) were sacrificed at designated times (sham, 1, 2 and 5 days) after ischemia/reperfusion and used for western blot analysis.
As previously reported (Lee et al., 2014), the brain was transversely cut into transverse slices of 400 μm thicknesses on a vibratome (Leica), and the hippocampal CA1 region was dissected with a surgical blade under stereoscopic microscope. The tissues were homogenized in 50 mM PBS (pH 7.4) containing 0.1 mM ethylene glycol bis (2-aminoethyl Ether)-N,N,N′N′ tetraacetic acid (EGTA) (pH 8.0), 0.2% Nonidet P-40, 10 mM ethylendiamine tetraacetic acid (EDTA) (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mM dithiothreitol (DTT). After centrifugation, the protein level was determined in the supernatants using a Micro BCA protein assay kit with bovine serum albumin as the standard (Pierce Chemical, Rockford, IL, USA). Aliquots containing 20 μg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM dithiothreitol, 6% sodium dodecyl sulfate, 0.3% bromophenol blue, and 30% glycerol. Then, each aliquot was loaded onto a 12.5% polyacryamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Crop, East Hills, NY, USA). To reduce background staining, the membranes were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 minutes and then with rabbit anti-MCT4 antibody (diluted 1:1,500, Santa Cruz Biotechnology), peroxidase-conjugated goat anti-rabbit IgG (Sigma) and an ECL kit (Pierce Chemical). Antibodies against β-actin was used for loading controls (Abcam Incorporated, Cambridge, MA, USA).
According to our previous method (Lee et al., 2011a), the result of western blot analysis was scanned and the quantification of the analysis was done using Scion Image software (Scion Corp., Frederick, MD, USA) which was used to count relative optical density (ROD): a ratio of the ROD was calibrated as %, with sham-operated group designated as 100%.
Statistical analysis
All data are presented as the mean ± SEM. Differences of the means among the groups were statistically analyzed by Student's t-test or one-way analysis of variance (ANOVA) and post-hoc Bonferroni multiple comparisons were performed to elucidate ischemia-related differences among the groups. A level of P < 0.05 was considered statistically significant.
Results
CV-positive (CV+) cells
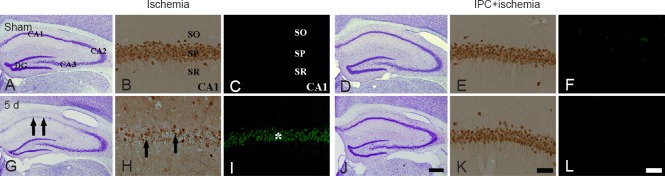
In the sham-operated group, CV+ cells were easily observed in all the subregions of the gerbil hippocampus (Figure 1A). In the ischemia only group, the morphology and number of CV+ cells were not changed until 2 days after ischemia/reperfusion (data not shown). Meanwhile, the number of CV+ cells was significantly decreased in the stratum pyramidale of the CA1 region, not CA2/3 regions, at 5 days after ischemia/reperfusion (Figure 1G). In the IPC + sham-operated group, pyramidal neurons in the CA1-3 regions were well stained with CV, and the distribution pattern of CV+ cells was similar to that in the sham-operated group (Figure 1D). In the IPC + ischemia group, CV+ pyramidal cells in the CA1 region were well protected 5 days after transient ischemia compared with those in the ischemia only group (Figure 1J).
Figure 1.
Cresyl violet (CV) staining (the 1st and 4th columns), neuronal nuclei (NeuN) immunohistochemistry (the 2nd and 5th columns) and fluoro-Jade B (F-J B) histofluorescence staining (the 3rd and 6th columns) in the hippocampus of the ischemia only (left three columns) and ischemic preconditioning + ischemia (right three columns) groups after sham operation (A – F) and at 5 days (d; G – L) after transient cerebral ischemia.
In the ischemia only group, a few CV+ neurons (arrows), NeuN+ neurons (arrows) and many F-J B+ cells (asterisk) were found in the SP at 5 d after transient ischemia. However, the distribution patterns of CV+ and NeuN+ neurons and F-J B+ cells in the SP were similar to those in the sham-operated (sham) group. SO: Stratum oriens; SP: stratum pyramidale; SR: stratum radiatum. Scale bars: 800 μm (A, D, G, J) and 50 μm (B, C, E, F, H, I, K, L).
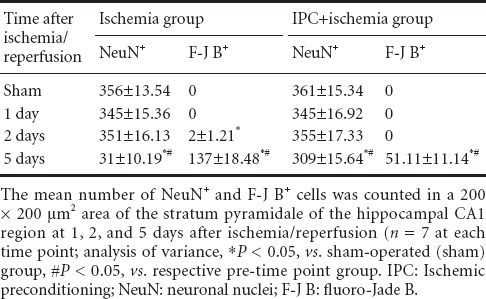
NeuN+ and F-J B+ neurons
IPC-mediated neuroprotection of CA1 pyramidal neurons was assessed with anti-NeuN immunohistochemistry and F-J B histofluorescence staining (Figure 1). In the sham-operated group, NeuN+ cells were detected in pyramidal neurons of the CA1 region (Table 1, Figure 1B), and no F-J B+ cells were found (Table 1, Figure 1C). In the ischemia only group, however, at 5 days after ischemia/reperfusion, the number of NeuN+ cells was dramatically decreased in the stratum pyramidale of the CA1 region (Table 1, Figure 1H), and a large number of F-J B+ cells were observed in the stratum pyramidale (Table 1, Figure 1I). In the IPC+ sham-operated group, intact NeuN immunoreactivity was easily detected in the CA1 pyramidal neurons (Table 1, Figure 1E), whereas, no CA1 pyramidal neurons were stained with F-J B (Table 1, Figure 1F). In the IPC + ischemia group at 5 days post-ischemia, based on distribution patterns of NeuN+ and F-J B+ cells in the stratum pyramidale, CA1 pyramidal neurons were well protected compared with those in the ischemia only group (Table 1, Figure 1K and L).
Table 1.
Changes in the number of pyramidal neurons of the hippocampal CA1 region of the ischemia only and IPC + ischemia groups
Change in MCT4 immunoreactivity
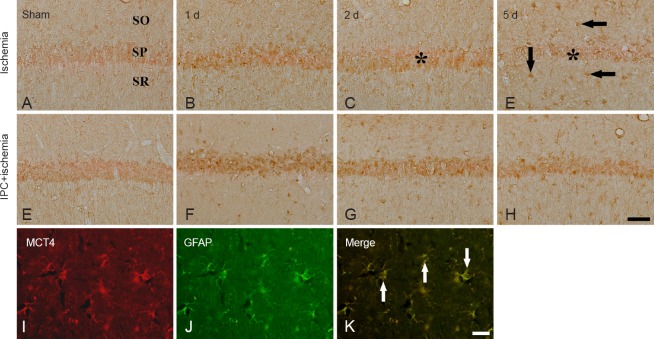
CA1 region: MCT4 immunoreactivity in the sham-operated group was weakly detected in the stratum pyramidale of the CA1 region (Table 2, Figure 2A). One day after ischemia/reperfusion, MCT4 immunoreactivity in the stratum pyramidale was slightly increased compared with that in the sham-operated group (Table 2, Figure 2B), and 2 days after ischemia/reperfusion, MCT4 immunoreactivity in the stratum pyramidale was decreased (Table 2, Figure 2C). Five days after ischemia/reperfusion, MCT4 immunoreactivity in the stratum pyramidale was rarely detected; at this time point, moderate MCT4 immunoreactivity was newly expressed in cells in the stratum oriens and radiatum (Table 2, Figure 2D). In the IPC + sham-operated group, MCT4 immunoreactivity in the stratum pyramidale region was slightly increased compared with that in the sham-operated group (Table 2, Figure 2E). In the IPC + ischemia groups, MCT4 immunoreactivity in the stratum pyramidale was strong at 2 days post-ischemia, and the immunoreactivity was maintained until 5 days after ischemia/reperfusion (Table 2, Figure 2F–H). Cells in the stratum oriens and radiatum showed moderate MCT4 immunoreactivity from 1 day after ischemia/reperfusion (Table 2, Figure 2F–H).
Table 2.
Semi-quantitative analysis of the immunoreactivity of monocarboxylate transporter 4 in the hippocampal CA1 and CA3 regions in the ischemia only and ischemic preconditioning + ischemia groups
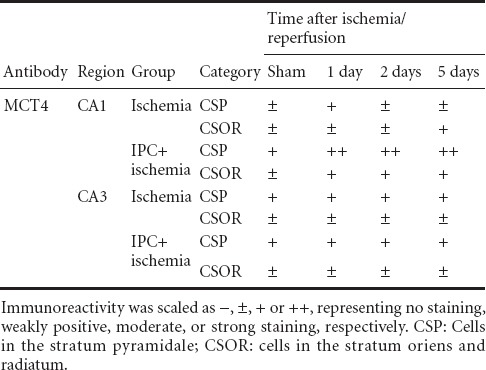
Figure 2.
Immunohistochemistry of monocarboxylate transporter 4 (MCT4) in the CA1 region of the ischemia only (upper panel), and ischemic preconditioning + ischemia groups (middle panel) after sham operation (A, E), and 1 day (B, F), 2 days (C, G) and 5 days (D, H) after ischemia/reperfusion.
MCT4 immunoreactivity in the stratum pyramidale (SP, asterisk) was significantly increased at 2 days post-ischemia. Five days after ischemia/reperfusion, MCT4 immunoreactivity in the SP (asterisk) was decreased; at this time point, cells in the stratum oriens (SO) and radiatum (SR) show MCT4 immunoreactivity. In the ischemic preconditioning + ischemia groups, MCT4 immunoreactivity in the SP was higher than that in the ischemia only group. Double immunofluorescence staining (lower panel) for MCT4 (red, I) and glial fibrillary acidic protein (GFAP, green, J) and merged images (K) in the CA1 region of the ischemia only group at 5 days post-ischemia. MCT4+ cells were co-stained with GFAP+ astrocytes (arrows). Scale bar: 50 μm (A–H), 10 μm (I–K).
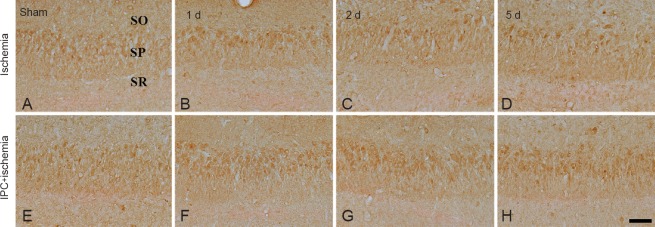
CA3 region: Strong MCT4 immunoreactivity was detected in neurons of the stratum pyramidale in the CA3 region of the sham-operated group (Table 2, Figure 3A). MCT4 immunoreactivity in neurons of the stratum pyramidale was not changed until 5 days after ischemia/reperfusion in the ischemia only group (Table 2, Figure 3B–D). In the IPC + sham-operated group, MCT4 immunoreactivity in the CA3 region was similar to that in the sham-operated group (Table 2, Figure 3E). In the IPC + ischemia groups, the pattern of MCT4 immunoreactivity did not change until 5 days after ischemia/reperfusion (Table 2, Figure 3F–H).
Figure 3.
Immunohistochemistry for MCT4 in the CA3 region of the ischemia only (upper panel) and IPC + ischemia groups (lower panel) after sham operation (A, E), and at 1 day (B, F), 2 days (C, G) and 5 days (D, H) after ischemia/reperfusion.
MCT4 immunoreactivity of the ischemia only and ischemic preconditioning + ischemia groups was not significantly changed compared with that in the sham-operated group. SP: Stratum pyramidale; SO: stratum oriens; SR: stratum radiatum; MCT4: monocarboxylate transporter 4; IPC: ischemic preconditioning. Scale bar: 50 μm.
Expression of MCT4 in astrocytes
To elucidate the type of MCT4+ cells that were found in the stratum oriens and radiatum of the CA1 region at 5 days post-ischemia (Figure 2D), we conducted double immunofluorescence staining using MCT4/Iba-1 and MCT4/GFAP antibodies. We found that MCT4+ cells were co-localized with GFAP+ astrocytes, not Iba-1+ microglia (data not shown), in the ischemic CA1 region of the ischemia only group (Figure 2I–K).
IPC-mediated effects on MCT4 levels
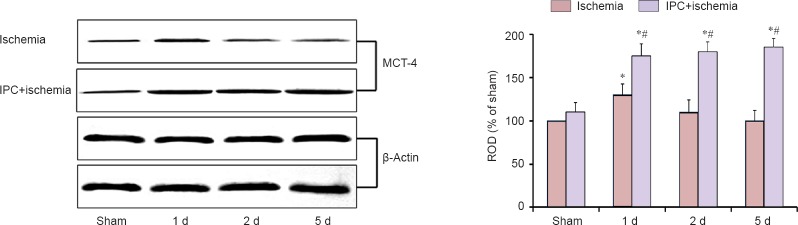
Western blot analysis showed that the pattern of changes in MCT4 protein levels in the CA1 region after ischemia/reperfusion was similar to that observed in the immunohistochemistry (Figure 4). In the ischemia only group, MCT4 protein level was significantly increased 1 day after ischemia/reperfusion, and the level was low at 5 days post-ischemia (Figure 4). In the IPC + sham-operated group, MCT4 protein level was a little higher than that in the sham-operated group; however, MCT4 level in the IPC + ischemia group was significantly increased 1 day after ischemia/reperfusion and maintained with time (Figure 4).
Figure 4.
Western blot analysis for MCT4 protein in the hippocampal CA1 region derived from the ischemia only and IPC + ischemia groups.
Relative optical density (ROD) as % values of immunoblot band was also represented (n = 7 at each time point; analysis of variance; *P < 0.05, vs. sham-operated (sham) group; #P < 0.05, vs. ischemia group). The bars indicate the mean ± SEM. MCT4: Monocarboxylate transporter 4; IPC: ischemic preconditioning.
Discussion
IPC, which protects neuron from ischemic insults, is capable of inducing neuronal tolerance to a subsequent longer or lethal period of ischemia in animal models (Kirino et al., 1991; Liu et al., 1992; Nishi et al., 1993; Stagliano et al., 1999; Gidday, 2006; Lehotsky et al., 2009). IPC was firstly demonstrated by Kitagawa et al. (1991) in a gerbil model of global cerebral ischemia. Nakamura et al. (2006) determined the preconditioning timing and period and suggested that at least 1 day interval between sublethal 2 minute ischemia and lethal 5 minute ischemia was necessary for protecting the pyramidal cells of the CA1 region of the gerbil. In the present study, IPC protected against transient ischemic injury in the hippocampal CA1 region after 5 minutes of transient cerebral ischemia in gerbils. It has been well known that, 5 days after transient ischemia, CA1 pyramidal neurons show the typical feature of neuronal death. The numbers CV+ and NeuN+ neurons significantly reduced and many F-J B+ neurons were observed. However, the number of viable neurons in the hippocampal CA1 was significantly increased after IPC. To the best of our knowledge, a better understanding of the mechanism underlying IPC-mediated neuroprotection help to improve the strategies for ischemic injury, because IPC frequently provides strong tolerance against ischemic insults in the brain.
An increasing body of evidence shows that lactate protected neurons under excitotoxic or ischemic condition (Schurr et al., 1999, 2001; Cater et al., 2001; Ros et al., 2001). When perfused in combination with glutamate, lactate protected against glutamate excitotoxicity and reduced lesion size (Ros et al., 2001). In addition, lactate concentrations increased during hypoxia and ischemia (Lampl et al., 1990; Huguet et al., 1998). It was reported that the uptake of lactate into brain cells was performed via MCTs (Hanu et al., 2000; Simpson et al., 2007) and that MCTs displayed the recovery of neuronal function after insults such as ischemia or hypoxia (Schurr et al., 1997; Schurr and Rigor, 1998). Schurr et al. (2001) demonstrated that the blockade of MCT exacerbated neuronal damage in a rat model of transient cerebral ischemia (Schurr et al., 2001).
Neurons could easily intake lactate through its membrane MCTs and then convert it into pyruvate, along with three carboxylic acid cycles of aerobic metabolism (Kitano et al., 2003). Moreover, there has been evidence regarding modification of MCT expression in cerebral ischemic situations. For examples, a strong MCT1 immunoreactivity was detected in hippocampal neurons of a rat model of transient global ischemia (Tseng et al., 2003). In addition, a significant increase in MCT4 was found within and around the infarct zone of permanent focal ischemia at 120 hours after ischemia in the rat (Zhang et al., 2005), and interestingly, they suggested that these changes of MCT4 in the infarct zone could be associated with penetrating neutrophils and microglia/macrophages. Moreover, Ullah et al. (2006) reported that MCT4 expression was up-regulated by hypoxia via a hypoxia inducible factor-1α-dependent mechanism. In this study, weak MCT4 immunoreactivity was found in the CA1 pyramidal neurons in the sham-operated group, and MCT4 immunoreactivity was reduced from 2 days post-ischemia and hardly detected at 5 days post-ischemia. We, in addition, found that MCT4 immunoreactivity in the CA1 pyramidal neurons was increased in the IPC + sham-operated group compared with that in the sham-operated group and that in the IPC + ischemia group, MCT4 immunoreactivity was increased compared with the ischemia only group. There is evidence that neurons in vitro and in vivo primarily expressed MCT2 (Hanu et al., 2000; Debernardi et al., 2003). This finding is supported by a previous report (Rosafio and Pellerin, 2014) that changing MCT4 expression yielded deleterious consequences on cell survival after hypoxic exposure, but neither MCT1 nor MCT2 level appeared to be regulated by hypoxia. Therefore, these indicate that the elevation of MCT4 immunoreactivity in the ischemic CA1 pyramidal neurons is related to neuroprotection against transient global ischemic injury.
It has been reported that MCTs are found in astrocytes in the cerebral cortex, cerebellum, striatum and hippocampus (Rafiki et al., 2003; Pellerin et al., 2005; Pierre and Pellerin, 2005). However, the precise identity of MCT4 expression in hippocampal astrocytes has not been investigated in the animal model of transient global cerebral ischemia.
In the present study, we found that MCT4 immunoreactivity was newly expressed at 5 days post-ischemia in astrocytes when the CA1 pyramidal neurons underwent cell death; however, IPC dramatically attenuated MCT4 expression in astrocytes after ischemia/reperfusion. Astrocytes are major cell types that serve many essential physiological functions in the central nerve system. These maintain blood-brain barrier and clear extracellular potassium that accumulates with neuronal activity and metabolize excitatory amino acids (Sofroniew and Vinters, 2010). Astrocytes also store energy in the form of glycogen that can be metabolized to lactate for export to neurons for additional fuel during ischemia (Swanson and Choi, 1993; Folbergrova et al., 1996). Thus, it is possible that MCT4 activation in reactive astrocytes may alter their functions in ischemic injury and that, in pathological conditions including ischemic injury, the capacity of astrocytes up-regulating MCT4 expression could be protective for neighboring neurons. Now, we can suggest that additional studies with cell-type specific MCT4 knockout animal models would help to reveal roles of MCT4 in altering functions of reactive astrocytes following in vivo ischemia.
In brief, we show that MCT4 immunoreactivity was changed in pyramidal neurons and newly expressed in astrocytes in the ischemic CA1 region and that IPC protected pyramidal neurons from ischemic damage and elevated or maintained the expression of MCT4 in astrocytes in ischemic CA1 region. We, therefore, suggest that MCT4 may be a key factor in protecting neurons from ischemia/reperfusion insults.
Acknowledgments
We would like to thank Mr. Sung Uk Lee (Department of Physiology, College of Medicine, and Institute of Neurodegeneration and Neuroregeneration, Hallym University, Chuncheon, South Korea) for his technical help in this study.
Footnotes
Funding: This work was supported by a Priority Research Centers Program grant (NRF-2009-0093812) through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning, and by 2014 Research Grant from Kangwon National University.
Conflicts of interest: None declared.
Copyedited by Jackson C, Li HF, Liu ZF, Song LP, Liu WJ, Zhao M
References
- 1.Brooks GA. Lactate: link between glycolytic and oxidative metabolism. Sports Med. 2007;37:341–343. doi: 10.2165/00007256-200737040-00017. [DOI] [PubMed] [Google Scholar]
- 2.Cater HL, Benham CD, Sundstrom LE. Neuroprotective role of monocarboxylate transport during glucose deprivation in slice cultures of rat hippocampus. J Physiol. 2001;531:459–466. doi: 10.1111/j.1469-7793.2001.0459i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res. 2003;73:141–155. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- 5.Folbergrova J, Katsura KI, Siesjo BK. Glycogen accumulated in the brain following insults is not degraded during a subsequent period of ischemia. J Neurol Sci. 1996;137:7–13. doi: 10.1016/0022-510x(96)82226-x. [DOI] [PubMed] [Google Scholar]
- 6.Gao C, Wang C, Liu B, Wu H, Yang Q, Jin J, Li H, Dong S, Gao G, Zhang H. Intermittent hypoxia preconditioning-induced epileptic tolerance by upregulation of monocarboxylate transporter 4 expression in rat hippocampal astrocytes. Neurochem Res. 2014;39:2160–2169. doi: 10.1007/s11064-014-1411-2. [DOI] [PubMed] [Google Scholar]
- 7.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 8.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343 Pt. 1999;2:281–299. [PMC free article] [PubMed] [Google Scholar]
- 9.Hanu R, McKenna M, O’Neill A, Resneck WG, Bloch RJ. Monocarboxylic acid transporters, MCT1 and MCT2 in cortical astrocytes in vitro and in vivo. Am J Physiol Cell Physiol. 2000;278:C921–930. doi: 10.1152/ajpcell.2000.278.5.C921. [DOI] [PubMed] [Google Scholar]
- 10.Hu Z, Zeng L, Xie L, Lu W, Zhang J, Li T, Wang X. Morphological alteration of Golgi apparatus and subcellular compartmentalization of TGF-beta1 in Golgi apparatus in gerbils following transient forebrain ischemia. Neurochem Res. 2007;32:1927–1931. doi: 10.1007/s11064-007-9382-1. [DOI] [PubMed] [Google Scholar]
- 11.Huguet F, Guerraoui A, Barrier L, Guilloteau D, Tallineau C, Chalon S. Changes in excitatory amino acid levels and tissue energy metabolites of neonate rat brain after hypoxia and hypoxia-ischemia. Neurosci Lett. 1998;240:102–106. doi: 10.1016/s0304-3940(97)00907-5. [DOI] [PubMed] [Google Scholar]
- 12.Kardesoglu E, Isilak Z, Uz O, Yiginer O. Ischemic conditioning: a current concept in reducing reperfusion injury. Chin Med J (Engl) 2011;124:480. [PubMed] [Google Scholar]
- 13.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 14.Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984;62:201–208. doi: 10.1007/BF00691853. [DOI] [PubMed] [Google Scholar]
- 15.Kirino T, Tsujita Y, Tamura A. Induced tolerance to ischemia in gerbil hippocampal neurons. J Cereb Blood Flow Metab. 1991;11:299–307. doi: 10.1038/jcbfm.1991.62. [DOI] [PubMed] [Google Scholar]
- 16.Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, Ueda H, Handa N, Kimura K, Kamada T. ‘Ischemic tolerance’ phenomenon detected in various brain regions. Brain Res. 1991;561:203–211. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- 17.Kitano T, Nisimaru N, Shibata E, Iwasaka H, Noguchi T, Yokoi I. Monocarboxylates and glucose utilization as energy substrates in rat brain slices under selective glial poisoning--a 31P NMR study. Mol Cell Biochem. 2003;244:77–81. [PubMed] [Google Scholar]
- 18.Lampl Y, Paniri Y, Eshel Y, Sarova-Pinhas I. Cerebrospinal fluid lactate dehydrogenase levels in early stroke and transient ischemic attacks. Stroke. 1990;21:854–857. doi: 10.1161/01.str.21.6.854. [DOI] [PubMed] [Google Scholar]
- 19.Lee CH, Park JH, Choi JH, Yoo KY, Ryu PD, Won MH. Heat shock protein 90 and its cochaperone, p23, are markedly increased in the aged gerbil hippocampus. Exp Gerontol. 2011a;46:768–772. doi: 10.1016/j.exger.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Lee CH, Park JH, Yoo KY, Choi JH, Hwang IK, Ryu PD, Kim DH, Kwon YG, Kim YM, Won MH. Pre- and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Exp Neurol. 2011b;229:450–459. doi: 10.1016/j.expneurol.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Lee JC, Kim IH, Cho GS, Park JH, Ahn JH, Yan BC, Kwon HM, Kim YM, Cheon SH, Cho JH, Lee HY, Won MH, Seo JY. Ischemic preconditioning-induced neuroprotection against transient cerebral ischemic damage via attenuating ubiquitin aggregation. J Neurol Sci. 2014;336:74–82. doi: 10.1016/j.jns.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Lee JC, Kim IH, Park JH, Ahn JH, Cho JH, Cho GS, Tae HJ, Chen BH, Yan BC, Yoo KY, Choi JH, Lee CH, Hwang IK, Kwon YG, Kim YM, Won MH. Ischemic preconditioning protects hippocampal pyramidal neurons from transient ischemic injury via the attenuation of oxidative damage through upregulating heme oxygenase-1. Free Radic Biol Med. 2015;79:78–90. doi: 10.1016/j.freeradbiomed.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Lehotsky J, Burda J, Danielisova V, Gottlieb M, Kaplan P, Saniova B. Ischemic tolerance: the mechanisms of neuroprotective strategy. Anat Rec (Hoboken) 2009;292:2002–2012. doi: 10.1002/ar.20970. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Kato H, Nakata N, Kogure K. Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sublethal ischemia. Brain Res. 1992;586:121–124. doi: 10.1016/0006-8993(92)91380-w. [DOI] [PubMed] [Google Scholar]
- 25.Min D, Mao X, Wu K, Cao Y, Guo F, Zhu S, Xie N, Wang L, Chen T, Shaw C, Cai J. Donepezil attenuates hippocampal neuronal damage and cognitive deficits after global cerebral ischemia in gerbils. Neurosci Lett. 2012;510:29–33. doi: 10.1016/j.neulet.2011.12.064. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura H, Katsumata T, Nishiyama Y, Otori T, Katsura K, Katayama Y. Effect of ischemic preconditioning on cerebral blood flow after subsequent lethal ischemia in gerbils. Life Sci. 2006;78:1713–1719. doi: 10.1016/j.lfs.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Nishi S, Taki W, Uemura Y, Higashi T, Kikuchi H, Kudoh H, Satoh M, Nagata K. Ischemic tolerance due to the induction of HSP70 in a rat ischemic recirculation model. Brain Res. 1993;615:281–288. doi: 10.1016/0006-8993(93)90039-p. [DOI] [PubMed] [Google Scholar]
- 28.Park YS, Cho JH, Kim IH, Cho GS, Park JH, Ahn JH, Chen BH, Shin BN, Shin MC, Tae HJ, Cho YS, Lee YL, Kim YM, Won MH, Lee JC. Effects of ischemic preconditioning on VEGF and pFlk-1 immunoreactivities in the gerbil ischemic hippocampus after transient cerebral ischemia. J Neurol Sci. 2014;347:179–187. doi: 10.1016/j.jns.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 29.Pellerin L, Bergersen LH, Halestrap AP, Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res. 2005;79:55–64. doi: 10.1002/jnr.20307. [DOI] [PubMed] [Google Scholar]
- 30.Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- 31.Radenovic L, Selakovic V, Olivan S, Calvo AC, Rando A, Janac B, Osta R. Neuroprotective efficiency of tetanus toxin C fragment in model of global cerebral ischemia in Mongolian gerbils. Brain Res Bull. 2014;101:37–44. doi: 10.1016/j.brainresbull.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Rafiki A, Boulland JL, Halestrap AP, Ottersen OP, Bergersen L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. 2003;122:677–688. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 33.Ros J, Pecinska N, Alessandri B, Landolt H, Fillenz M. Lactate reduces glutamate-induced neurotoxicity in rat cortex. J Neurosci Res. 2001;66:790–794. doi: 10.1002/jnr.10043. [DOI] [PubMed] [Google Scholar]
- 34.Rosafio K, Pellerin L. Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultured mouse cortical astrocytes via a hypoxia-inducible factor-1alpha-mediated transcriptional regulation. Glia. 2014;62:477–490. doi: 10.1002/glia.22618. [DOI] [PubMed] [Google Scholar]
- 35.Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 36.Schurr A. Lactate, glucose and energy metabolism in the ischemic brain (Review) Int J Mol Med. 2002;10:131–136. [PubMed] [Google Scholar]
- 37.Schurr A. Lactate: the ultimate cerebral oxidative energy substrate. J Cereb Blood Flow Metab. 2006;26:142–152. doi: 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- 38.Schurr A, Rigor BM. Brain anaerobic lactate production: a suicide note or a survival kit? Dev Neurosci. 1998;20:348–357. doi: 10.1159/000017330. [DOI] [PubMed] [Google Scholar]
- 39.Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation. J Neurochem. 1997;69:423–426. doi: 10.1046/j.1471-4159.1997.69010423.x. [DOI] [PubMed] [Google Scholar]
- 40.Schurr A, Miller JJ, Payne RS, Rigor BM. An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J Neurosci. 1999;19:34–39. doi: 10.1523/JNEUROSCI.19-01-00034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schurr A, Payne RS, Miller JJ, Tseng MT, Rigor BM. Blockade of lactate transport exacerbates delayed neuronal damage in a rat model of cerebral ischemia. Brain Res. 2001;895:268–272. doi: 10.1016/s0006-8993(01)02082-0. [DOI] [PubMed] [Google Scholar]
- 42.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagliano NE, Perez-Pinzon MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 1999;19:757–761. doi: 10.1097/00004647-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Swanson RA, Choi DW. Glial glycogen stores affect neuronal survival during glucose deprivation in vitro. J Cereb Blood Flow Metab. 1993;13:162–169. doi: 10.1038/jcbfm.1993.19. [DOI] [PubMed] [Google Scholar]
- 46.Tseng MT, Chan SA, Schurr A. Ischemia-induced changes in monocarboxylate transporter 1 reactive cells in rat hippocampus. Neurol Res. 2003;25:83–86. doi: 10.1179/016164103101200978. [DOI] [PubMed] [Google Scholar]
- 47.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 48.Won MH, Kang T, Park S, Jeon G, Kim Y, Seo JH, Choi E, Chung M, Cho SS. The alterations of N-Methyl-D-aspartate receptor expressions and oxidative DNA damage in the CA1 area at the early time after ischemia-reperfusion insult. Neurosci Lett. 2001;301:139–142. doi: 10.1016/s0304-3940(01)01625-1. [DOI] [PubMed] [Google Scholar]
- 49.Won MH, Kang TC, Jeon GS, Lee JC, Kim DY, Choi EM, Lee KH, Choi CD, Chung MH, Cho SS. Immunohistochemical detection of oxidative DNA damage induced by ischemia-reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999;836:70–78. doi: 10.1016/s0006-8993(99)01611-x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang F, Vannucci SJ, Philp NJ, Simpson IA. Monocarboxylate transporter expression in the spontaneous hypertensive rat: effect of stroke. J Neurosci Res. 2005;79:139–145. doi: 10.1002/jnr.20312. [DOI] [PubMed] [Google Scholar]