Abstract
Muscle-in-vein conduits are used alternatively to nerve grafts for bridging nerve defects. The purpose of this study was to examine short- and long-term regeneration results after digital nerve reconstruction with muscle-in-vein conduits. Static and moving two-point discriminations and Semmes-Weinstein Monofilaments were used to evaluate sensory recovery 6–12 months and 14–35 months after repair of digital nerves with muscle-in-vein in 7 cases. Both follow-ups were performed after clinical signs of progressing regeneration disappeared. In 4 of 7 cases, a further recovery of both two-point discriminations and in another case of only the static two-point discrimination of 1–3 mm could be found between the short-term and long-term follow-up examination. Moreover, a late recovery of both two-point discriminations was demonstrated in another case. Four of 7 cases showed a sensory improvement by one Semmes-Weinstein Monofilaments. This pilot study suggests that sensory recovery still takes place even when clinical signs of progressing regeneration disappear.
Keywords: peripheral nerve, regeneration, muscle-in-vein conduits, digital nerves, sensory recovery, Semmes-Weinstein, two-point discrimination, outcome, short-term, long-term
Introduction
Injuries of peripheral nerves are common and often accompanied with extended tissue loss. If no primary nerve coaptation is possible, the nerve gap has to be bridged to avoid tension on the nerve sutures, which would lead to poor clinical results (Battiston et al., 2005). For this purpose, several options are available like conventional nerve autografting (Millesi, 1992; Paprottka et al., 2013) and several artificial conduits consisting of collagen, polyglycolic acid, caprolactone, etc. (Griffin et al., 2013). Artificial conduits have the advantage not to cause donor site morbidity, but produce additional material costs, may cause foreign body reactions and should not be used for nerve gaps larger than 2–3 cm (Griffin et al., 2013; Paprottka et al., 2013).
A more recent method for bridging nerve gaps are the so-called muscle-in-vein conduits (MVC), first described by Brunelli et al. (1993). While veins form a barrier against dispersion of outgrowing axons and inhibit ingrowth of scar tissue into the conduit (Brunelli et al., 1993; Battiston et al., 2000), muscle tissue provides an optimal environment for the ingrowth of regenerating axons into newly formed bands of proliferating Schwann cells along the basal laminae of the muscle fibers. Additionally, the interposition of muscle tissue prevents the collapse of the veins and therefore enables bridging of larger defects, up to 6 cm reported in the literature (Battiston et al., 2005; Manoli et al., 2014). Regenerating axons can correctly orientate within muscle-in-vein conduits due to the accumulation of neurotrophic factors from the distal stump, generating a concentration gradient, which enables growing axons to reach their proper target (Lundborg et al., 1981, 1982, 1994; Tos et al., 2000; Fornaro et al., 2001). An important advantage of this technique compared to nerve autografts is that no loss of sensation occurs at the donor site (Battiston et al., 2000) and that the graft can be harvested directly at the lesion area since MVCs are available almost in every region of the body (Marcoccio and Vigasio, 2010). Functional results after reconstruction with MVCs were shown to be comparable the ones after reconstruction with nerve grafts (Manoli et al., 2014) whereas the number of axons after nerve repair with MVCs was shown to be even superior to nerve grafts after bridging defects up to 2 cm in the rat model (Brunelli et al., 1993).
Depending on the level of injury, completion of nerve regeneration of the digits is often expected after about one year in most cases. This is especially the case after direct nerve coaptation, while regeneration after bridging defects with MVCs or autografts is expected to last longer. After this time clinical signs of progressing regeneration do not mostly exist any more. However, it is known that histomorphological changes may occur up to 2 years after functional regeneration took place in rodents (Mackinnon et al., 1991). In addition to this, further regeneration in terms of outcome improvement has been observed in the empirical clinical practice. The aim of this study was to evaluate the short-term (6-12 months) and long-term (more than 14 months) regeneration results after digital nerve repair with MVCs.
Subjects and Methods
The retrospective study was approved by the ethics committee of the University of Tuebingen (117/2012BO2).
Patients
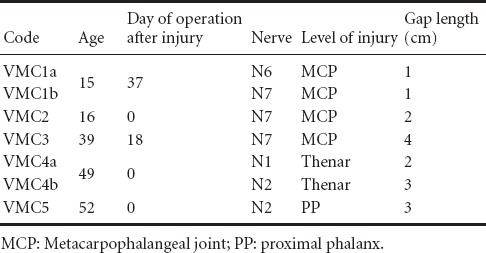
Five patients with seven isolated nerve injuries from the level of the middle hand to the level of the proximal phalanx on the palmar side of the digits were included in the study after reconstruction with MVCs. Four reconstructions were performed primarily and three secondarily up to 37 days after injury. The length of MVCs varied between 1–4 cm. Combined injuries of blood vessels, nerves, tendons and bones have been excluded from the study. All participants understood the background of the study clearly and gave their written consent before proceeding with the follow-up examination. Patient's age ranged from 15 to 52 years. Other pathologies able to influence digital sensitivity, i.e., nerve compression syndromes, were excluded through clinical examination. The demographic data of patients including age, time of reconstruction, localization and level of injury as well as graft length are summarized in Table 1.
Table 1.
Patients’ demographic data
Operation
All surgical procedures were carried out with the aid of an operating microscope. MVCs were constructed by harvesting a subcutaneous vein from the palmar side of the forearm, which was slightly wider than the injured nerve. Using the same incision, a thin muscle strip was excised mostly from the flexor digitorum superficialis or the flexor carpi radialis muscles and pulled into the vein. The muscle-in-vein conduit was then interposed between the two nerve stumps using sutures with 10-0 nylon (Brunelli et al., 1993; Manoli et al., 2014).
Follow-up examinations
All patients were examined twice, after clinical signs of progressing regeneration in terms of subjective improvement and on-going distal advancement of Hoffmann-Tinel's sign in line of the anatomical course of the injured nerve, disappeared. The short-term follow up examination (FU1) was performed during the first 6–12 months after surgery. The second long-term follow up examination was performed at least 14 and up to 35 months after surgery (FU2). The minimum time between FU1 and FU2 was 7 months (Tables 2, 3). Both times sensory recovery was evaluated using the static and moving two-point discrimination (2PD) tests as well as the Semmes-Weinstein monofilament test (Figure 1).
Table 2.
Results of static and moving 2PD
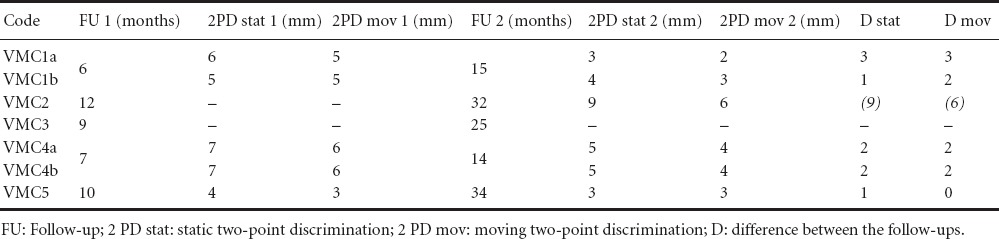
Table 3.
Results of SWM-test
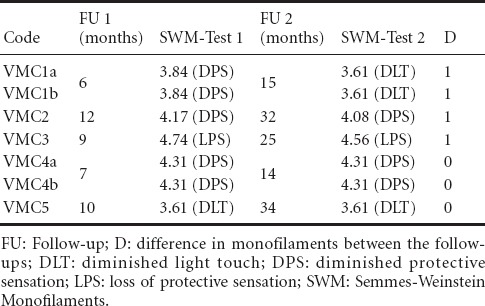
Figure 1.
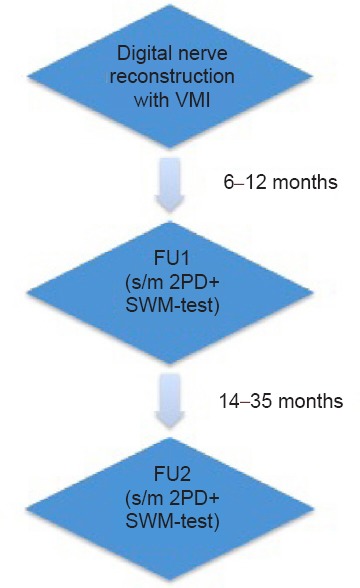
Flowchart of follow-up examination.
Examination of static and moving 2PD (Dellon, 1978) was carried out with a two-point discriminator (Touch-Test®, North Coast Medical Inc., U.S.A.). Testing intervals of 1 mm ranging from 1–15 mm could be assessed. Both points of the discriminator were applied at the level of the distal interphalangeal joint and were slowly moved distally to the fingertip. The lowest possible pressure was applied, so that the patient could appreciate the stimulus and respond without hesitation. Testing began with 8 mm and stopped at 2 mm. The results had to be verified three times to be valid (Manoli et al., 2014).
Homecraft Rolyan® Semmes-Weinstein monofilaments (SWM) were used to assess pressure perception on the palmar side of the hand. The set consisted of 20 monofilaments whereas each monofilament was labeled with the logarithm to base 10 of the pressure force it produces onto the skin. In order to obtain objective results each monofilament was vertically pressed onto the skin until it slightly bended holding it for 1–2 seconds. The examination began always with the 2.83 monofilament followed by the next thicker monofilament until the tested person stated a perception with closed eyes. The perception measured with the thinner possible monofilament had to be verified three times to be valid (Manoli et al., 2014).
Results
The exact time points of the follow-up examinations (FU1 and FU2) are summarized with the results of 2PD and Semmes-Weinstein-Monofilament test in Tables 2 and 3, respectively.
Static and moving 2PD
In 4 of 7 cases, a further recovery of both 2PDs and in one case of only the static 2PD of 1–3 mm could be found between the short-term and long-term follow-up examination (Table 2). Moreover, a late recovery of the 2PD was demonstrated in another case (VMC2). In this case, no measurable static or moving 2PD could be detected in the FU1 after 12 months, while at 20 months a static 2PD of 9 mm and a moving 2PD of 6 mm could be measured. Only one case (VMC3) did not show any measurable static or moving 2PD after both 9 and 25 months. The best improvement was found in two patients with four nerve injuries (VMC1 and VMC4) that had a short FU1 after 6 and 7 months, respectively, and a FU2 already after 14 and 15 months, respectively. Here a maximal improvement of 3 mm for both the static and moving 2PD could be detected for VMC1a. VMC4a–b showed an equal improvement of 2 mm in FU2, while VMC1b showed an improvement of 1 and 2 mm for static and moving 2PDs, respectively. VMC5 examined after 10 and 34 months showed a slight improvement of 1 mm for the static 2PD and no improvement for the moving 2PD.
Semmes-Weinstein-Monofilament test (SWM-test)
Regarding the short-term and long-term follow up examination results, 4 of 7 cases showed further sensory recovery of one level according to the SWM-test (Table 3). One patient with two nerve injuries (VMC1a-b) had an improvement that yielded a gain in sensitivity from “diminished protective sensation” (DPS) to “diminished light touch” (DLT), while the other two cases with an improvement of one level stayed on the same category of sensation according to the SWM-test (DPS and “loss of protective sensation” (LPS), respectively). The 4 cases that demonstrated an improvement, involved 3 younger patients of this series with 15–39 years of age, while the other 3 cases without any improvement according to the SWM-test involved 2 patients with 49 and 52 years of age. This age distribution was not concordant to the results of the static and moving 2PD.
Discussion
It is important for both surgeon and patient to know when the endpoint of sensory recovery of digital nerves has been reached. This information cannot only provide the patient with a realistic prognosis, but it is also important for planning other necessary surgical procedures in the injured hand. Previous clinical studies examining parameters of peripheral nerve regeneration were dealing mostly with more proximal injuries of mixed nerves as the median and the ulnar nerve (Rosen and Lundborg, 2001; Ruijs et al., 2005). These studies reported significant improvements in peripheral nerve regeneration up to 5 years after nerve repair. However, the recovery curve of the oligofascicular, sensory digital nerves cannot be directly compared to one of the more proximally located and essentially thicker polyfascicular, mixed nerves of humans or the mixed sciatic nerve of rodents. In the rat model for instance, it is known that the number of axons distal to a repair zone increases dramatically up to 3 months, plateaus between 6 to 9 months and returns to about normal levels after 2 years (Mackinnon et al., 1991).
Due to this background knowledge and lacking exact information about the long-term recovery curve after digital reconstruction with MVCs, the current pilot study was performed to examine if any objective improvements in sensation after clinical signs of regeneration like progression of Hoffmann-Tinel's sign disappear, should be expected. Although, the study may have some limitations such as the small number of patients with 7 cases included in total making a statistical analysis difficult, the diversity of defect lengths and patients’ age as well as the variable time points of follow-up examinations, interesting observations have been made. Both methods used to assess sensory recovery demonstrated an improvement in the second follow-up examination compared to the first one in most of the cases. Therefore, sensory recovery may still take place even when obvious clinical signs of progressing regeneration disappear. For the clinical practice, this would be not only important for an accurate patient information concerning the recovery expectation after digital nerve reconstruction with MVCs but also for the rehabilitation, which could include a longer therapy of resensitization. Further studies with higher number of patients examining the regeneration curve over time after reconstruction of digital nerves with muscle-in-vein-conduits compared to primary nerve coaptation would be very useful.
Another interesting aspect of the current study is the discussion about the quality characteristics of 2PD and SWMs. Our results demonstrated a rather better improvement in the 2PD ability than pressure perception measured by SWMs. While the 2PD test measures the spatial discrimination, the SWM-test measures the pressure force onto the skin. Static two-point discrimination evaluates the density of the slowly adapting fiber system and is related to the ability to use the hand for fine motor tasks. Moving 2PD evaluates the density of the quickly adapting fiber system. Hand function requiring moving touch, like buttoning, can be assessed by this test. Moving 2PD should return earlier, about 2 to 6 months before static 2PD does. Spatial discrimination is a method to assess tactile gnosis, which is a more differentiate kind of perception than pressure. Therefore, it could indeed recover over a longer time period. However, the 2PD test is not considered to be objective and reliable enough, since it is not possible to perfectly standardize the pressure being applied onto the skin, especially when either one or two points are being used (Bell-Krotoski et al., 1993). In a recent study of our department, a relatively strong correlation between the static and moving 2PD could be found, while both static and moving 2PD were weakly correlated to the SWM-test (Manoli et al., 2014). Wong et al. (2006) found an even poorer correlation between moving 2PD and the SWM-test after repair of the median nerve. Though results of SWM-test are considered to be more reliable and reproducible than the ones of 2PD, we believe that both methods should be used to assess sensory recovery, because they deliver different kinds of information. Despite its drawbacks concerning reproducibility, 2PD may be a more sensitive method to assess progression of regeneration in its late phase.
The age factor has been a subject of disagreement in previous studies dealing with peripheral nerve regeneration. While some supported the thesis that patients’ age influences the regeneration results (Rosen and Lundborg, 2001; Meek et al., 2005), other observed that only patients younger than 10 years have a better regeneration potential (Steinberg and Koman, 1991). In a previous study of our department, no correlation between the patients’ age (range 11–72 years) and regeneration results could be observed (Manoli et al., 2014). In the current study, the two eldest patients were the ones that demonstrated no improvement in the SMW-test. Since this test measures pressure force onto the skin, it can be also influenced by an age relevant skin induration, as it could be observed in these two male patients.
Conclusion
This pilot study suggests that sensory recovery still takes place when no clinical signs of progressing regeneration exist any more. Moreover, it is recommended to use both 2PD and SWM to evaluate sensory recovery, since the two methods examine different kinds of perception during different phases of the recovery curve.
References
- 1.Battiston B, Tos P, Cushway TR, Geuna S. Nerve repair by means of vein filled with muscle grafts I. Clinical results. Microsurgery. 2000;20:32–36. doi: 10.1002/(sici)1098-2752(2000)20:1<32::aid-micr6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 2.Battiston B, Geuna S, Ferrero M, Tos P. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005;25:258–267. doi: 10.1002/micr.20127. [DOI] [PubMed] [Google Scholar]
- 3.Bell-Krotoski J, Weinstein S, Weinstein C. Testing sensibility, including touch-pressure, two-point discrimination, point localization, and vibration. J Hand Ther. 1993;6:114–123. doi: 10.1016/s0894-1130(12)80292-4. [DOI] [PubMed] [Google Scholar]
- 4.Brunelli GA, Battiston B, Vigasio A, Brunelli G, Marocolo D. Bridging nerve defects with combined skeletal muscle and vein conduits. Microsurgery. 1993;14:247–251. doi: 10.1002/micr.1920140407. [DOI] [PubMed] [Google Scholar]
- 5.Dellon AL. 1978 The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg Am. 3:474–481. doi: 10.1016/s0363-5023(78)80143-9. [DOI] [PubMed] [Google Scholar]
- 6.Fornaro M, Tos P, Geuna S, Giacobini-Robecchi MG, Battiston B. Confocal imaging of Schwann-cell migration along muscle-vein combined grafts used to bridge nerve defects in the rat. Microsurgery. 2001;21:153–155. doi: 10.1002/micr.1029. [DOI] [PubMed] [Google Scholar]
- 7.Griffin JW, Hogan MV, Chhabra AB, Deal DN. Peripheral nerve repair and reconstruction. J Bone Joint Surg Am. 2013;95:2144–2151. doi: 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- 8.Lundborg G, Dahlin L, Danielsen N, Zhao Q. Trophism, tropism, and specificity in nerve regeneration. J Reconstr Microsurg. 1994;10:345–354. doi: 10.1055/s-2007-1006604. [DOI] [PubMed] [Google Scholar]
- 9.Lundborg G, Dahlin LB, Danielsen NP, Hansson HA, Larsson K. Reorganization and orientation of regenerating nerve fibres perineurium and epineurium in preformed mesothelial tubes - an experimental study on the sciatic nerve of rats. J Neurosci Res. 1981;6:265–281. doi: 10.1002/jnr.490060302. [DOI] [PubMed] [Google Scholar]
- 10.Lundborg G, Dahlin LB, Danielsen N, Gelberman RH, Longo FM, Powell HC, Varon S. Nerve regeneration in silicone chambers: influence of gap length and of distal stump components. Exp Neurol. 1982;76:361–375. doi: 10.1016/0014-4886(82)90215-1. [DOI] [PubMed] [Google Scholar]
- 11.Mackinnon SE, Dellon AL, O’Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve. 1991;14:1116–1122. doi: 10.1002/mus.880141113. [DOI] [PubMed] [Google Scholar]
- 12.Manoli T, Schulz L, Stahl S, Jaminet P, Schaller HE. Evaluation of sensory recovery after reconstruction of digital nerves of the hand using muscle-in-vein conduits in comparison to nerve suture or nerve autografting. Microsurgery. 2014;34:608–615. doi: 10.1002/micr.22302. [DOI] [PubMed] [Google Scholar]
- 13.Marcoccio I, Vigasio A. Muscle-in-vein nerve guide for secondary reconstruction in digital nerve lesions. J Hand Surg. 2010;35:1418–1426. doi: 10.1016/j.jhsa.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Meek MF, Coert JH, Robinson PH. Poor results after nerve grafting in the upper extremity: Quo vadis? Microsurgery. 2005;25:396–402. doi: 10.1002/micr.20137. [DOI] [PubMed] [Google Scholar]
- 15.Millesi H. Munich: Urband & Schwarzenberg; 1992. Chirurgie der peripheren Nerven. [Google Scholar]
- 16.Paprottka FJ, Wolf P, Harder Y, Kern Y, Paprottka PM, Machens HG, Lohmeyer JA. Sensory recovery outcome after digital nerve repair in relation to different reconstructive techniques: meta-analysis and systematic review. Plast Surg Int. 2013;2013:704589. doi: 10.1155/2013/704589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen B, Lundborg G. The long term recovery curve in adults after median or ulnar nerve repair: a reference interval. J Hand Surg. 2001;26:196–200. doi: 10.1054/jhsb.2001.0567. [DOI] [PubMed] [Google Scholar]
- 18.Ruijs AC, Jaquet JB, Kalmijn S, Giele H, Hovius SE. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg. 2005;116:484–494. doi: 10.1097/01.prs.0000172896.86594.07. discussion 495-486. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg D, Koman L. Factors affecting the results of peripheral nerve repair. In: Gelberman RH, editor. Operative Nerve Repair and Reconstruction. Philadelphia: Lippincott Williams and Wilkins; 1991. pp. 349–364. [Google Scholar]
- 20.Tos P, Battiston B, Geuna S, Giacobini-Robecchi MG, Hill MA, Lanzetta M, Owen ER. Tissue specificity in rat peripheral nerve regeneration through combined skeletal muscle and vein conduit grafts. Microsurgery. 2000;20:65–71. doi: 10.1002/(sici)1098-2752(2000)20:2<65::aid-micr4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Wong KH, Coert JH, Robinson PH, Meek MF. Comparison of assessment tools to score recovery of function after repair of traumatic lesions of the median nerve. Scand J Plast Reconstr Surg Hand Surg. 2006;40:219–224. doi: 10.1080/02844310600652878. [DOI] [PubMed] [Google Scholar]


