Abstract
Ototoxic drugs can be used to produce a loss of cochlear hair cells to create animal models of deafness. However, to the best of our knowledge, there is no report on the establishment of a rat deafness model through the combined application of aminoglycosides and loop diuretics. The aim of this study was to use single or combined administration of furosemide and kanamycin sulfate to establish rat models of deafness. The rats received intravenous injections of different doses of furosemide and/or intramuscular injections of kanamycin sulfate. The auditory brainstem response was measured to determine the hearing threshold after drug application. Immunocytochemistry and confocal microscopy were performed to evaluate inner ear morphology. In the group receiving combined administration of furosemide and kanamycin, the auditory brainstem response threshold showed significant elevation 3 days after administration, higher than that produced by furosemide or kanamycin alone. The hair cells showed varying degrees of injury, from the apical turn to the basal turn of the cochlea and from the outer hair cells to the inner hair cells. The spiral ganglion cells maintained a normal morphology during the first week after the hair cells completely disappeared, and then gradually degenerated. After 2 months, the majority of spiral ganglion cells disappeared, but a few remained. These findings demonstrate that the combined administration of furosemide and kanamycin has a synergistic ototoxic effect, and that these drugs can produce hair cell loss and hearing loss in rats. These findings suggest that even in patients with severe deafness, electronic cochlear implants may partially restore hearing.
Keywords: nerve regeneration, sensorineural deafness, kanamycin, furosemide, ototoxic drug, spiral ganglion cells, hair cell, neural regeneration
Introduction
Animal models of deafness are an important tool for studying the pathogenesis of deafness and for evaluating therapeutic strategies for restoring hearing. Deafness can be achieved through noise exposure (Yang et al., 2012), ototoxic drug administration (Ding et al., 2010) and gene mutation (Yang et al., 2009a). Drug administration is more frequently used than the other two approaches because it can result in the complete elimination of auditory hair cells. To date, numerous methods utilizing ototoxic drugs have been used to induce deafness, such as local application of carboplatin and cisplatin, neomycin infiltration through the round window membrane (He et al., 2009; Zhou et al., 2009), and intramuscular injection of kanamycin, gentamicin and amikacin (Staecker et al., 2007). Nevertheless, these methods may have severe side effects. For example, the local administration of ototoxic drugs may cause infection and mechanical damage of the inner ear, and the systemic administration of aminoglycosides may cause severe kidney dysfunction and death. Therefore, safer and more effective drug administration methods need to be explored. Fortunately, the combined application of aminoglycosides and loop diuretics, such as kanamycin and furosemide, has been demonstrated to cause hair cell injury in a relatively rapid and safe manner (West et al., 1973; Xu et al., 1993; Liberman et al., 2002; Nourski et al., 2004). This inspired us to explore a novel method for producing animal models of deafness. In this study, we examined the effects of kanamycin and furosemide on the rat cochlea.
To the best of our knowledge, this is the first report on the establishment of a rat deafness model through the combined application of aminoglycosides and loop diuretics.
Materials and Methods
Drug administration
Ninety healthy 4-week-old Sprague-Dawley rats, weighing 100–110 g, were randomly assigned into six groups, with 15 animals in each group. These rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.45 mL/100 g). Furosemide was obtained from Tianjin Jin Yao Amino Acid Co., Ltd., Tianjin, China (batch No. 0606191). Kanamycin sulfate (product No. K4000) was obtained from Sigma (St. Louis, MO, USA). Furosemide solution for intravenous injection was freshly prepared with saline before use. The left jugular vein was exposed and injected with furosemide, whereas kanamycin sulfate was injected intramuscularly into the thigh as described previously (West et al., 1973). The control group did not receive any treatment. The Furosemide 200 mg/kg and Kanamycin 1,000 mg/kg groups were injected with furosemide and kanamycin alone at doses of 200 mg/kg and 1,000 mg/kg, respectively. The Furosemide 100 mg/kg + Kanamycin 500 mg/kg, Furosemide 100 mg/kg + Kanamycin 1,000 mg/kg and Furosemide 200 mg/kg + Kanamycin 100 mg/kg groups were administered both drugs at the indicated doses. All rats exhibited normal behavior and eating after drug administration, and no signs of abnormal vestibular function were observed. The care and use of animals were approved by the Institutional Animal Care and Use Committee of Chinese PLA General Hospital, China.
Auditory brainstem response (ABR) measurements
ABR measurements were performed as described previously (Liberman et al., 2002). Briefly, the rats were anesthetized by intramuscular injection of xylazine (0.1 mg/kg) and ketamine (30 mg/kg). The recording electrodes were inserted at the vertex and pinna. ABRs were evoked with clicks and/or 5-ms tone pips (0.5 ms rise/fall with 2 ms duration) at frequencies of 4, 8, 16 and 32 kHz. The signal was amplified, filtered, and averaged using the Smart EP system (Intelligent Hearing System, Miami, FL, USA). The sound level was raised in 20 and/or 5-dB steps. At each level, 1,024 responses were averaged. Both ears were measured.
Immunocytochemistry and scanning electron microscopy (SEM)
The cochleae were perfused with 4.0% formaldehyde and dissected in 0.01 M PBS. The bony wall and the spiral ligament were carefully removed, and the organ of Corti was separated from the modiolus and stored in PBS. The dissected corti were treated with 0.2 % Triton X-100/PBS. Goat serum (10%) was used to block nonspecific binding. The tissue was then incubated with chicken anti-200 kDa Neurofilament Heavy polyclonal (diluted 1:200; Abcam, Cambridge, MA, USA) for 48 hours on a shaker at 4°C. The samples were washed with PBS, followed by incubation for 1 hour at 37°C with rabbit anti-chicken secondary antibody (Alexa Fluor 488; Invitrogen, Grand Island, NY, USA). To stain nuclei, tissues were incubated with the DNA-specific Hoechst dye (10 mg/mL; Polysciences) for 1 hour at room temperature, as described previously (Yang et al., 2009b). The samples were mounted on glass slides with antifade solution (Prolong Antifade Kit, Molecular Probes) and examined using a confocal scanning system (LSM 510 META, Zeiss, Oberkochen, Germany).
For SEM, the cochleae were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) containing 2 mM CaCl2, washed in PBS, and post-fixed for 15 minutes with 1% OsO4 in the same buffer. The tissues were dehydrated in an ethanol series, critical-point dried using CO2, sputter-coated with gold, and then examined using a Hitachi S-3700N scanning electron microscope (Hitachi, Tokyo, Japan).
Hematoxylin-eosin (HE) staining
The rats from each group were deeply anesthetized with urethane (1.5 g/kg, intraperitoneally), followed by transcardial perfusion with physiological saline and 4% paraformaldehyde fixative. After the cochlea was removed from the temporal bone, it was perfused again with 4% paraformaldehyde fixative in 10 mM PBS. Cochleae were decalcified with 10% ethylenediamine tetraacetic acid overnight at 4°C in a refrigerator. All cochleae were rinsed in PBS and washed for 30 minutes with rotation in 20% sucrose at room temperature and maintained overnight at 4°C in a 20% sucrose solution. Cochleae were placed in the cryomold under a dissecting microscope and filled halfway with OCT. The cochleae were placed in the OCT and oriented by aligning an imaginary plane through the modiolus parallel to the bottom of the embedding mold. The mold was then immediately placed in a dry ice/ethanol bath. Eight-micron sections were cut with a Leica Cryomicrotome 2800 (Leica Microsystems, Heidelberg, Germany). Sections were mounted on Fisherbrand Superfrost plus slides. HE staining was performed as previously described (Llewellyn, 2009).
Spiral ganglion cell, hair cell, inner hair cell and outer hair cell counts
The number of spiral ganglion neurons was determined as described previously (Murillo-Cuesta, 2010). Briefly, neurons were counted in all 3 turns on the same side of the modiolus. In each section, all neurons containing a nucleus with a soma size larger than 20 μm were counted. We counted the total number of bundles from three cochlear locations (each 1-mm in length) from six normal (control group) cochleae as reference. Six cochleae from the Furosemide 200 mg/kg, Kanamycin 1,000 mg/kg, Furosemide 100 mg/kg + Kanamycin 500 mg/kg, Furosemide 100 mg/kg + Kanamycin 1,000 mg/kg, and Furosemide 200 mg/kg + Kanamycin 100 mg/kg groups were also examined. The three sites were 14.5–15.5, 10.5–11.5 and 4.0–5.0 mm from the basal end of the basilar membrane. The status of the bundles was determined by visual inspection of the bundles under a scanning electron microscope (Hitachi S-3700N).
Statistical analysis
All data are presented as the mean ± SD and were analyzed using the statistical software STATA7.0 (StataCorp LP, College Station, TX, USA). Student's t-test was used, and a P value < 0.05 was considered significant.
Results
Ototoxic effects of furosemide and kanamycin on ABR measurements
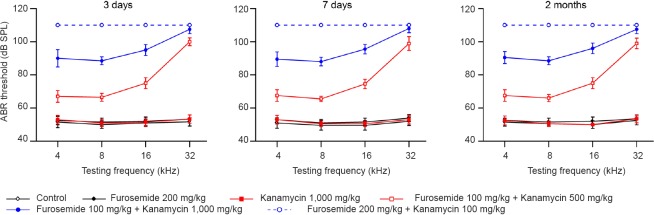
ABR measurements were performed at three time points (3 days, 1 week and 2 months) after drug administration to test whether the combined administration of furosemide and kanamycin is able to induce stable long-term hearing loss. Figure 1 shows the average ABR thresholds for each group measured at four frequencies (4, 8, 16 and 32 kHz). Compared with the control group at each time point, the ABR thresholds of the groups receiving kanamycin or furosemide alone (Furosemide 200 mg/kg and Kanamycin 1,000 mg/kg groups) did not show significant changes at any of the four frequencies (Figure 1; P > 0.05). In contrast, in the groups receiving both drugs (Furosemide 100 mg/kg + Kanamycin 500 mg/kg, Furosemide 100 mg/kg + Kanamycin 1,000 mg/kg and Furosemide 200 mg/kg + Kanamycin 100 mg/kg groups), the ABR thresholds were significantly elevated compared with the control group or the Furosemide 200 mg/kg group at each of the four different frequencies (P < 0.01). A high dose of kanamycin caused more severe hearing impairment, shown by a greater increase in ABR threshold in the Furosemide 100 mg/kg + Kanamycin 1,000 mg/kg group than in the Furosemide 200 mg/kg + Kanamycin 100 mg/kg group. Surprisingly, the ABR thresholds were too high to be detected even when the largest stimulus intensity (110 dB SPL) was given. At 3, 7 and 30 days, the ABR threshold values among the different groups were compared, and no significant differences were observed.
Figure 1.
Auditory brainstem response (ABR) thresholds of rats measured at 3 and 7 days and 2 months after injections of furosemide and/or kanamycin sulfate.
In the groups receiving drugs (Furosemide 100 mg/kg + Kanamycin 500 mg/kg, Furosemide 100 mg/kg + Kanamycin 1,000 mg/kg, Furosemide 200 mg/kg + Kanamycin 100 mg/kg), the ABR thresholds were significantly increased compared with the control group or with the Furosemide 200 mg/kg group at all tested frequencies (P < 0.01). The data are expressed as the mean ± SD of 15 rats in each group and were analyzed using Student's t-test.
Ototoxic effects of furosemide and kanamycin on auditory hair cells
To examine the ototoxic effects of furosemide and kanamycin on auditory hair cells, we performed SEM in the basilar membrane at 1 week after drug administration. In the groups treated with furosemide or kanamycin alone (Furosemide 200 mg/kg group and Kanamycin 1,000 mg/kg group), the stereocilia of the inner hair cells and outer hair cells on the cuticular plate exhibited a normal shape and organization (Figure 2), as seen in the control group (Figure 2). In the Furosemide 100 mg/kg + Kanamycin 500 mg/kg group, however, the stereocilia of the outer hair cells were completely depleted in the basal turn while remaining intact in the apical and middle turns (Figure 2). The stereocilia of the inner hair cells remained intact in the whole extent of the basilar membrane. Similar observations were made in the Furosemide 100 mg/kg + Kanamycin 1,000 mg/kg group, where the stereocilia of the outer hair cells were completely missing in the basilar membrane, while the stereocilia of the inner hair cells remained intact in the apical turn and were sporadically present in the middle turn, but were completely missing in the basal turn. In comparison, a thorough depletion of the stereocilia of both inner hair cells and outer hair cells were observed throughout the entire basilar membrane in the Furosemide 200 mg/kg + Kanamycin 100 mg/kg group. Notable scarring of the basilar membrane was also observed.
Figure 2.
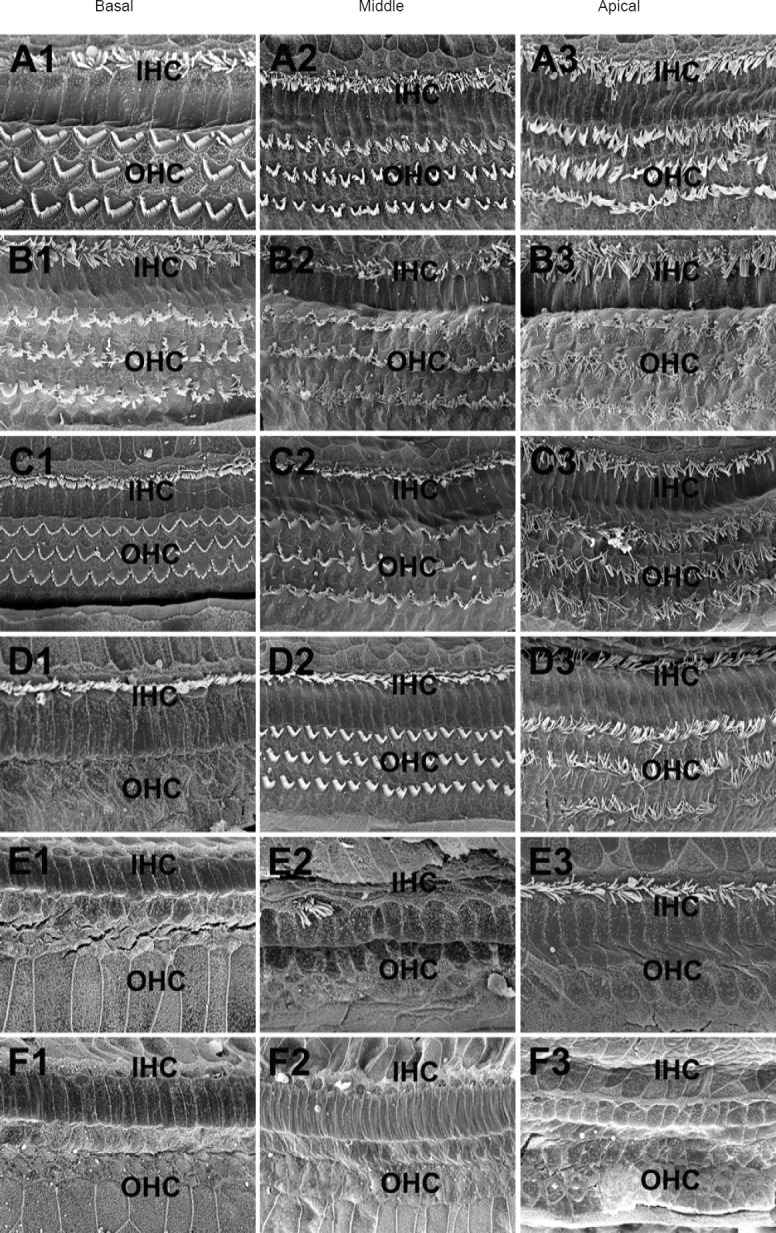
Scanning electron microscopy to evaluate the cochlear toxicity of injections of furosemide and/or kanamycin sulfate in rats 1 week after drug administration.
A1–A3, B1–B3 and C1–C3 show the basal, middle and apical turns of the rat cochlea in the control, Furosemide 200 mg/kg and Kanamycin 1,000 mg/kg group, respectively. One row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs) were observed. D1–D3 show the basal, middle and apical turns in the Furosemide 100 mg/kg + Kanamycin 500 mg/kg group. In the apical and middle turns, one row of IHCs and three rows of OHCs could be observed. The IHCs were intact in the basal turn, while the OHCs were completely missing. E1–E3 show the basal, middle and apical turns in the Furosemide 100 mg/kg + Kanamycin 100 mg/kg group. In the apical turn, the IHCs were intact, while the OHCs were completely missing. In the middle turn, the IHCs were extensively missing, while the OHCs were missing completely. In the basal turn, both IHCs and OHCs were completely missing. F1–F3 show the basal, middle and apical turns in the Furosemide 200 mg/kg + Kanamycin 100 mg/kg group. The IHCs and OHCs in the entire cochlea were missing. Scale bars: 10 μm.
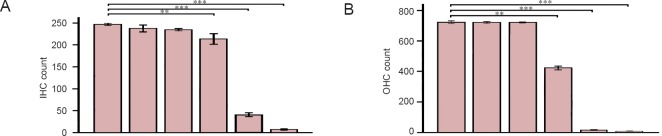
To quantify bundle damage caused by furosemide and kanamycin sulfate, the total number of bundles of inner hair cells and outer hair cells was counted (Figure 3A, B). As shown in Figure 3, combined injection of furosemide and kanamycin sulfate caused a significant reduction in the number of bundles of inner and outer hair cells in the Furosemide 100 mg/kg + Kanamycin 500 mg/kg, Furosemide 100 mg/kg + Kanamycin 1,000 mg/kg and Furosemide 200 mg/kg + Ka- namycin 100 mg/kg groups compared with control group (P < 0.01 for both inner and outer hair cells, Student's t-test). However, single administration of furosemide or kanamycin sulfate caused less damage, and no significant difference between control group and the Furosemide 200 mg/kg group or Kanamycin 1,000 mg/kg group was observed (P > 0.05).
Figure 3.
Stereocilia bundle counts in the normal (without injection) and injected cochleae at 1 week after drug administration.
(A, B) The numbers of inner hair cell (A) and outer hair cell (B) bundles. The data are presented as the mean ± standard deviation (SD) of six cochleae in each group. Status of the bundles was determined by visual inspection of the bundles under a scanning electron microscope. Hair bundles with no clear signs of trunction, fusion or folding were included in the bundle count. Student's t-test was used for statistical analysis. **P < 0.05, ***P < 0.01. The colored boxes on the each histogram from left to right indicate the six groups, i.e., control, Furosemide 200 mg/kg, Kanamycin 1,000 mg/kg, Furosemide 100 mg/kg + Kanamycin 500 mg/kg, Furosemide 100 mg/kg + Kanamycin 1,000 mg/kg and Furosemide 200 mg/kg + Kanamycin 100 mg/kg, respectively.
Change in the number of spiral ganglion cells after the loss of hair cells
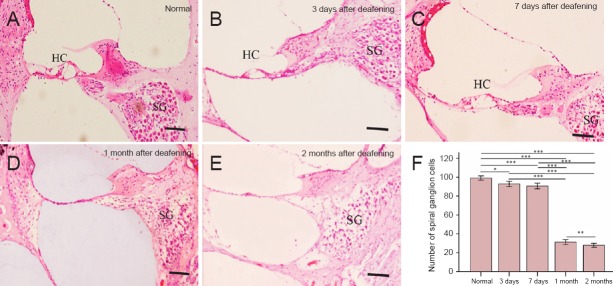
Spiral ganglion cells in the Furosemide 200 mg/kg + Kanamycin 100 mg/kg group were counted at different time points after drug administration. As shown in Figure 5, 3 days after drug administration, hair cells had nearly completely disappeared, but the framework of the organ of Corti appeared intact. A few hair cells remained, and supporting cells and spiral ganglion cells appeared mostly unaffected (Figure 4). The appearance of spiral ganglion cells was similar 7 days after drug administration in this group. However, 1 month after drug administration, hair cells had completely disappeared, and the framework of the organ of Corti had collapsed. Some basal cells were observed, but most of the spiral ganglion cells were lost. Two months after drug administration, spiral ganglion cell damage was more severe compared with the other time points. There were no significant differences in the number of spiral ganglion cells between the Furosemide 200 mg/kg + Kanamycin 100 mg/kg group (92.9 ± 0.5 at 3 days, 90.7 ± 0.8 at 7 days) and the control group (99.1 ± 0.7, P > 0.05; Figure 4). However, 1 and 2 months after drug administration, the number of spiral ganglion cells was significantly lower in the Furosemide 200 mg/kg + Kanamycin 100 mg/kg group (31.2 ± 0.9, 28.0 ± 0.9) than in the control group (99.1 ± 0.7, P < 0.01; Figure 4).
Figure 5.
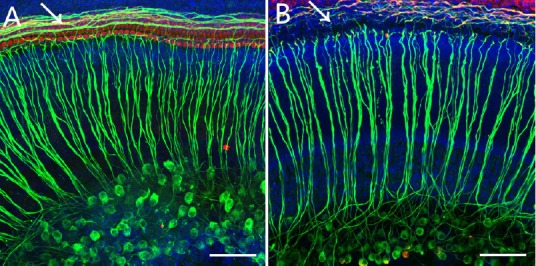
Immunofluorescence staining of the whole cochlear basilar membrane preparation in the control group (A) and the Furosemide 200 mg/kg + Kanamycin 100 mg/kg group (B) 7 days after drug administration.
The number of auditory nerve fibers in the Furosemide 200 mg/kg + Kanamycin 100 mg/kg group was reduced compared with the control group. In the control group, a complete row of IHCs and three rows of OHCs were visible. The arrows point to the contacts between HCs and nerve fibers. After injury, the nerve fibers were damaged. Green fluorescence indicates the nerve fibers and SG cells stained with neurofilament-specific antibody, and the blue fluorescence indicates Hoechststained cell nuclei. Scale bars: 20 μm. IHC: Inner hair cell; OHC: outer hair cell; HC: hair cell; SG: spiral ganglion.
Figure 4.
Hematoxylin-eosin staining of frozen sections of the cochlea (A–E) and spiral ganglion (SG) cell counts (F) in untreated rats and in rats given injections of furosemide and kanamycin sulfate (Furosemide 200 mg/kg + Kanamycin 100 mg/kg group) at 3 and 7 days and 1 and 2 months after drug administration.
The morphology of cochleae in the control group (A) and in the Furosemide 200 mg/kg + Kanamycin 100 mg/kg group (B–E). Hair cells (HCs) had almost fully disappeared, but SG cells were intact at 3 and 7 days after drug administration. HCs were completely absent, and most SG cells were lost at 1 and 2 months after drug administration. Scale bars: 20 μm. (F) The SG cell count in the control and Furosemide 200 mg/kg + Kanamycin 100 mg/kg groups at 3 and 7 days and 1 and 2 months after drug administration. The data are expressed as the mean ± SD (n = 6). ***P < 0.001, **P < 0.01, *P < 0.05.
Synergistic toxicity of furosemide and kanamycin on neurofilaments and spiral ganglion cells
Immunofluorescence staining revealed that, in the control group at 7 days, neurofilaments (in green) and inner hair cells and outer hair cells (in red) on the basilar membrane of the cochlea were well organized (Figure 5A). In contrast, in the Furosemide 200 mg/kg + Kanamycin 100 mg/kg group, the hair cells were completed disorganized and the neurofilaments were ruptured 7 days after drug administration (Figure 5B). In the same focal plane, there was no clear nuclear damage in the spiral ganglion cells.
Discussion
Sprague-Dawley rats were used in this study because they are more suitable than other rodent species for deafness model establishment. First, rats cost less to breed than guinea pigs. Second, rats are more amenable to cochlear surgeries because they have a larger body size than mice. Loop diuretics, such as furosemide and ethacrynic acid, have been shown to cause edema and cystic degeneration of the cochlear stria vascularis, a decrease in the magnitude of the cochlear action potential, inhibition of K+-Na+-ATPase, and induction of edema of the outer hair cells in the organ of Corti (Henly and Rybak, 1995). The ototoxicity of aminoglycoside antibiotics, such as kanamycin, is primarily towards cochlear hair cells, and results in irreversible hearing loss (Wästerström and Bredberg, 1986). In animal experiments, a single injection of a conventional dose of a loop diuretic, such as furosemide, may cause reversible hearing loss due to the transient disruption of the microcirculation of the cochlear stria vascularis. In comparison, a single injection of a conventional dose of an aminoglycoside antibiotic, such as kanamycin, often does not cause inner ear dysfunction or pathologic changes. Only after multiple injections, massive necrosis of hair cells may occur because of the accumulation of high concentrations of kanamycin in the perilymph fluid and delayed excretion (Alam et al., 1998; Murillo-Cuesta et al., 2010). In this study, we found that a single injection of kanamycin or furosemide did not cause an elevation in ABR threshold or hair cell loss in rats. We speculate that this apparent lack of effect may be due to the time points after drug administration that ABR thresholds were measured. Because the effects of loop diuretics on the cochlear stria vascularis are temporary, hearing may have been restored to normal 3 days after drug administration (when the first ABR test was performed). Other studies have shown that administration of low-dose antibiotics does not cause significant changes in the cochlear sensory epithelium (Murillo-Cuesta et al., 2010).
Previous studies have demonstrated that loop diuretics and aminoglycosides may mutually reinforce ototoxicity (Brummett et al., 1979). Yamane et al. (1988) reported that furosemide could promote the entrance of kanamycin into the inner ear lymph, enhancing the ototoxicity of kanamycin. Our study also shows that furosemide and kanamycin have a synergistic ototoxic effect. Furosemide damages the blood-labyrinth barrier, causing a change in permeability, which results in increased entrance of kanamycin into the inner ear. Furosemide may also damage the excretory function of the cochlear stria vascularis, causing slower excretion, resulting in a greater accumulation of kanamycin in the inner ear. When furosemide was administered at a low dose of 100 mg/kg, the elevation in ABR threshold was positively correlated with the dose of kanamycin (within a certain range). The combined injection of furosemide (100 mg/kg) and kanamycin (500 mg/kg) caused a slight increase in the ABR threshold. Injection of kanamycin at a high dose (1,000 mg/kg) caused a significant increase in ABR threshold. When a high dose of furosemide was given (200 mg/kg), a very small dose of kanamycin (100 mg/kg) was sufficient to increase ABR threshold to > 110 dB SPL at each frequency. The SEM results are consistent with the ABR thresholds. When a low dose of furosemide (100 mg/kg) was given, the loss of cochlear hair cells was positively correlated with the dose of kanamycin within a certain range. When a high dose of furosemide was given (200 mg/kg), a very small dose of kanamycin (100 mg/kg) was sufficient to cause the loss of all inner and outer hair cells in the cochlea. We speculate that while the low dose of furosemide (100 mg/kg) caused only mild damage to the stria vascularis, the blood-labyrinth barrier was partially and temporarily disrupted, allowing kanamycin to slowly accumulate in the lymph. Therefore, a large dose of kanamycin (1,000 mg/kg) can result in severe injury to cochlear hair cells. When a large dose of furosemide (200 mg/kg) was administered, it caused even greater damage to the stria vascularis, likely disrupting the blood-labyrinth barrier for an extended period of time, allowing kanamycin to enter the lymph rapidly. Russell et al. (1979) concluded that differences in ototoxicity in different animals may be attributable to differences in the clearance rates of kanamycin and ethacrynic acid, and that the toxic effects of combined application primarily depends on the dose of ethacrynic acid. Our study also showed that when the dose of ethacrynic acid is low, even a high dose of kanamycin could only achieve 53.3% hair cell loss. When the dose of ethacrynic acid was high, low dose kanamycin caused complete hair cell loss.
Deafness caused by combined application of furosemide and kanamycin in rats was bilaterally symmetric and hearing loss occurred 3 days after drug administration. Pathology studies on the cochlea of rats with deafness found that cochlear hair cell damage always gradually expands from the cochlear basal turn to the apical turn, and the damage to the outer hair cells always precedes that of inner hair cells. Outer hair cells may have a greater capacity to accumulate kanamycin, and hair cells at the basal turn may have a greater ability than hair cells at the apical turn. We speculate that hair cells at different sites may have different drug uptake capacities related to the distribution of drug transporter on the cell membrane (Ding et al., 2007). Hair cells at the basal turn also have more stereocilia and transduction channels. Studies have shown that after intramuscular injection of kanamycin, degeneration of supporting cells occurs significantly later than that of hair cells, and that the degeneration of spiral ganglion cells and nerve fibers occurs much later. Therefore, it is thought that damage to inner ear sensory cells is the root cause of kanamycin-induced hearing impairment (Alam et al., 1998). Our results are consistent with these previous studies. However, we found that the combined use of furosemide and kanamycin not only causes damage to the inner ear sensory epithelium (Figure 2, 5), it also results in injury to auditory nerve fibers.
Nourski et al. (2004) found that ethacrynic acid and kanamycin produced hair cell injury without causing significant inhibition of the auditory nerve response. The discrepancy between their findings and ours may be due to differences in the time points of observation. The time points of observation were within 10 hours after drug administration in the Nourski study, while our observations were carried out between 3 days and 2 months after drug administration. It is possible that the auditory nerve fibers were not damaged within 10 hours after drug administration, and that the damage may occur slowly. Furosemide is a loop diuretic, and studies on ototoxicity (Rybak et al., 1992) have shown that furosemide can cause pathologic changes in the border cells of the stria vascularis, and the degree of edema in border cells is a major indicator of ototoxicity caused by a loop diuretic. The stria vascularis plays an important role in maintaining the inner ear microenvironment, which is necessary for the normal functioning and viability of hair cells (Patuzzi, 2011).
The degeneration of spiral ganglion cells after cochlear trauma is secondary to the loss of inner hair cells (Sugawara et al., 2005). It is thought that the loss of stimulation from inner hair cells plays a key role in the degenerative process.
In summary, we established a rat model of deafness through the single administration of furosemide or kanamycin as well as by combined administration. We found that only combined administration of kanamycin and furosemide resulted in severe hearing impairment. Furthermore, injury to the cochlear outer hair cells was more severe than injury to the inner hair cells. The majority of supporting cells were undamaged. Spiral ganglion cell damage was not apparent acutely after drug administration, but damage to the auditory nerve fibers was noticeable. No hair cell regeneration was observed. When a high dose of furosemide was used, even a low dose of kanamycin caused severe damage to the cochlea. A single injection of furosemide or kanamycin resulted in little or no cochlear damage.
Footnotes
Funding: This study was supported by grants from the National Program on Key Basic Research Project of China (973 Program), No. 2011CBA01000, 2012CB967900.
Conflicts of interest: None declared.
Copyedited by Barry P, Raye W, Li CH, Song LP, Zhao M
References
- 1.Alam SA, Ikeda K, Kawase T, Kikuchi T, Katori Y, Watanabe K, Takasaka T. Acute effects of combined administration of kanamycin and furosemide on the stria vascularis studied by distortion product otoacoustic emission and transmission electron microscopy. Tohoku J Exp Med. 1998;186:79–86. doi: 10.1620/tjem.186.79. [DOI] [PubMed] [Google Scholar]
- 2.Brummett RE, Brown RT, Himes DL. Quantitative relationships of the ototoxic interaction of kanamycin and ethacrynic acid. Arch Otolaryngol. 1979;105:240–246. doi: 10.1001/archotol.1979.00790170010003. [DOI] [PubMed] [Google Scholar]
- 3.Ding D, Jiang H, Salvi RJ. Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear Res. 2010;259:16–23. doi: 10.1016/j.heares.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding D, Jiang H, Wang P, Salvi R. Cell death after co-administration of cisplatin and ethacrynic acid. Hear Res. 2007;226:129–139. doi: 10.1016/j.heares.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 5.He J, Yin S, Wang J, Ding D, Jiang H. Effectiveness of different approaches for establishing cisplatin-induced cochlear lesions in mice. Acta Otolaryngol. 2009;129:1359–1367. doi: 10.3109/00016480902856604. [DOI] [PubMed] [Google Scholar]
- 6.Henley CM, Rybak LP. Ototoxicity in developing mammals. Brain Res Brain Res Rev. 1995;20:68–90. doi: 10.1016/0165-0173(94)00006-b. [DOI] [PubMed] [Google Scholar]
- 7.Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- 8.Llewellyn BD. Nuclear staining with alum hematoxylin. Biotech Histochem. 2009;84:159–177. doi: 10.1080/10520290903052899. [DOI] [PubMed] [Google Scholar]
- 9.Murillo-Cuesta S, Contreras J, Cediel R, Varela-Nieto I. Comparison of different aminoglycoside antibiotic treatments to refine ototoxicity studies in adult mice. Lab Anim. 2010;44:124–131. doi: 10.1258/la.2009.009046. [DOI] [PubMed] [Google Scholar]
- 10.Nourski KV, Miller CA, Hu N, Abbas PJ. Co-administration of kanamycin and ethacrynic acid as a deafening method for acute animal experiments. Hear Res. 2004;187:131–133. doi: 10.1016/s0378-5955(03)00336-8. [DOI] [PubMed] [Google Scholar]
- 11.Patuzzi R. Ion flow in stria vascularis and the production and regulation of cochlear endolymph and the endolymphatic potential. Hear Res. 2011;277:4–19. doi: 10.1016/j.heares.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Russell NJ, Fox KE, Brummett RE. Ototoxic effects of the interaction between kanamycin and ethacrynic acid. Cochlear ultrastructure correlated with cochlear potentials and kanamycin levels. Acta Otolaryngol. 1979;88:369–381. doi: 10.3109/00016487909137181. [DOI] [PubMed] [Google Scholar]
- 13.Rybak LP, Whitworth C, Weberg A, Scott V. Effects of organic acids on the edema of the stria vascularis induced by furosemide. Hear Res. 1992;59:75–84. doi: 10.1016/0378-5955(92)90104-u. [DOI] [PubMed] [Google Scholar]
- 14.Staecker H, Praetorius M, Baker K, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28:223–231. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- 15.Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–147. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wästerström SA, Bredberg G. Ototoxicity of kanamycin in albino and pigmented guinea pigs. II. A scanning electron microscopic study. Am J Otol. 1986;7:19–24. [PubMed] [Google Scholar]
- 17.West BA, Brummett RE, Himes DL. Interaction of kanamycin and ethacrynic acid. Severe cochlear damage in guinea pigs. Arch Otolaryngol. 1973;98:32–37. doi: 10.1001/archotol.1973.00780020036009. [DOI] [PubMed] [Google Scholar]
- 18.Xu SA, Shepherd RK, Chen Y, Clark GM. Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear Res. 1993;70:205–215. doi: 10.1016/0378-5955(93)90159-x. [DOI] [PubMed] [Google Scholar]
- 19.Yamane H, Nakai Y, Konishi K. Furosemide-induced alteration of drug pathway to cochlea. Acta Otolaryngol Suppl. 1998;447:28–35. doi: 10.3109/00016488809102854. [DOI] [PubMed] [Google Scholar]
- 20.Yang SM, Chen W, Guo WW, Jia S, Sun JH, Liu HZ, Young WY, He DZ. Regeneration of stereocilia of hair cells by forced Atoh1 expression in the adult mammalian cochlea. PLoS One. 2012;7:e46355. doi: 10.1371/journal.pone.0046355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang SM, Hou ZH, Yang G, Zhang JS, Hu YY, Sun JH, Guo WW, He Dz, Han DY, Young WY, Yang X. Chondrocyte-specific Smad4 gene conditional knockout results in hearing loss and inner ear malformation in mice. Dev Dyn. 2009a;238:1897–1908. doi: 10.1002/dvdy.22014. [DOI] [PubMed] [Google Scholar]
- 22.Yang SM, Guo WW, Hu YY, Sun YX, Hou ZH, Sun JH, Wang X, He DZ, Zhai SQ, Young WY, Han DY, Yang X. Smad 5 haploinsufficiency leads to hair cell and hearing loss. Dev Neurobiol. 2009b;69:153–161. doi: 10.1002/dneu.20692. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Ding D, Kraus KS, Yu D, Salvi RJ. Functional and structural changes in the chinchilla cochlea and vestibular system following round window application of carboplatin. Audiol Med. 2009;7:189–199. doi: 10.3109/16513860903335795. [DOI] [PMC free article] [PubMed] [Google Scholar]