Abstract
Endothelial progenitor cells (EPCs) improve survival and reduce organ failure in cecal ligation and puncture-induced sepsis; however, expanded EPCs may represent an even better approach for vascular repair. To date, no study has compared the effects of non-expanded EPCs (EPC-NEXP) with those of expanded EPCs (EPC-EXP) and mesenchymal stromal cells of human (MSC-HUMAN) and mouse (MSC-MICE) origin in experimental sepsis. One day after cecal ligation and puncture sepsis induction, BALB/c mice were randomized to receive saline, EPC-EXP, EPC-NEXP, MSC-HUMAN or MSC-MICE (1 × 105) intravenously. EPC-EXP, EPC-NEXP, MSC-HUMAN, and MSC-MICE displayed differences in phenotypic characterization. On days 1 and 3, cecal ligation and puncture mice showed decreased survival rate, and increased elastance, diffuse alveolar damage, and levels of interleukin (IL)-1β, IL-6, IL-10, tumor necrosis factor-α, vascular endothelial growth factor, and platelet-derived growth factor in lung tissue. EPC-EXP and MSC-HUMAN had reduced elastance, diffuse alveolar damage, and platelet-derived growth factor compared to no-cell treatment. Tumor necrosis factor-α levels decreased in the EPC-EXP, MSC-HUMAN, and MSC-MICE groups. IL-1β levels decreased in the EPC-EXP group, while IL-10 decreased in the MSC-MICE. IL-6 levels decreased both in the EPC-EXP and MSC-MICE groups. Vascular endothelial growth factor levels were reduced regardless of therapy. In conclusion, EPC-EXP and MSC-HUMAN yielded better lung function and reduced histologic damage in septic mice.
Electronic supplementary material
The online version of this article (doi:10.1186/s13287-015-0226-7) contains supplementary material, which is available to authorized users.
Keywords: Sepsis, Inflammation, Growth factors, Cell therapies
Findings
Background
Mesenchymal stem cell (MSC) treatment has been widely used in many experimental models including sepsis [1–6]. MSC mechanisms of action might include: a decrease in alveolar–capillary barrier permeability [4, 6, 7]; enhanced alveolar fluid clearance [8, 9]; a shift in macrophage profile from pro-inflammatory to anti-inflammatory [10]; improved bacterial clearance [11, 12]; and mitochondrial transfer [13].
Sepsis leads to several immunological events that alter endothelial function in the macrocirculation and microcirculation [14]. Endothelial barrier dysfunction and microvascular leak contribute critically to the pathogenesis of organ failure in sepsis and of sepsis-related complications such as acute respiratory distress syndrome (ARDS) [15]. Therefore, reconstitution of the endothelial layer might be initiated via migration and proliferation of surrounding mature endothelial cells (ECs). Since differentiated ECs have a low proliferative potential, their capability to substitute damaged endothelium is restricted. Studies have observed that endothelial progenitor cells (EPCs) are increasingly mobilized during sepsis and that their migration is associated with clinical outcome [16, 17]. EPCs are precursor cells that can differentiate into mature ECs and create new blood vessels [18]. Generally, EPCs can be identified on the basis of their expression of CD133, CD34, KDR, and/or VE-cadherin cellular markers [19]. A recent study reported that EPCs improve survival and reduce organ failure in experimental sepsis [20]. Nevertheless, expanded EPCs represent an even better approach for vascular repair in myocardial ischemia [20, 21]. To date, no study has compared the effects of non-expanded EPCs (EPC-NEXP), expanded EPCs (EPC-EXP), and MSCs of human (MSC-HUMAN) and mouse (MSC-MICE) origin in experimental sepsis.
Within this context, we hypothesized that human umbilical cord blood-derived EPCs would be non-inferior or superior to MSCs at improving survival, lung function, and histology in experimental sepsis. The aim of the present study was to compare the efficacy of expanded and non-expanded human EPCs and human or murine MSC therapy in treating lung injury in a murine model of sepsis.
Methods
This study was approved by the Ethics Committee of the Health Sciences Centre, Federal University of Rio de Janeiro (CEUA-019). All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences, USA.
Isolation and expansion of EPCs
Mononuclear cells were isolated from human umbilical cord blood. EPCs (CD133+) were selected using CD133-coupled magnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany), following the manufacturer’s instructions. After isolation, CD133+ cells were expanded as described elsewhere [22]. Immunophenotypic analysis was performed by staining 5 × 105 isolated and expanded EPCs. EPCs were analyzed after isolation, whereas expanded cells were analyzed after 30 days of culture. The cells were incubated with various conjugated monoclonal antibodies against the following human antigens: CD133; CD34; CD45; CD14; CD31; CD105; and von Willebrand factor. Quantitative analyses were performed using a FACSCalibur flow cytometer and FlowJo software (Flowjo, Ashland, OR, USA) [22].
Isolation and expansion of human MSCs
Bone marrow-derived human MSCs were isolated and expanded as described elsewhere [23].
Immunophenotypic analysis was performed by staining 5 × 105 expanded human MSCs. The cells were incubated with conjugated monoclonal antibodies against the following human antigens: CD45; CD14; CD 90; CD73; CD166; CD105; HLA-DR; CD34; CD29; and CD19. Quantitative analyses were performed using a FACSCalibur flow cytometer and FlowJo software (Flowjo) [22].
Isolation and expansion of mouse MSCs
MSCs from mice were isolated from 8-week-old BALB/c mouse femur and tibia bone marrow stromal cells as described elsewhere [2]. Immunophenotypic analysis was performed by staining 1 × 105 expanded mouse MSCs. The cells were incubated with conjugated monoclonal antibodies against the following antigens: CD19; CD34; CD45; CD29; and Sca1+. Quantitative analyses were performed using a FACSCalibur flow cytometer and FlowJo software (Flowjo).
Experimental protocol
BALB/c mice (8–10 weeks old, n = 169) were used. Sepsis was induced by cecal ligation and puncture (CLP) on day 0 [3]. Briefly, animals were anesthetized with sevoflurane and a midline laparotomy was performed. The cecum was carefully isolated and a 3–0 cotton ligature was placed below the ileocecal valve to prevent bowel obstruction. Finally, the cecum was punctured twice with an 18-gauge needle. In the sham group, an abdominal incision was made, but there was no cecal ligation or perforation. Both layers of the abdominal cavity were closed, followed by fluid resuscitation (sterile saline) subcutaneously. Sham and CLP animals received tramadol (0.05 mg/kg body weight, subcutaneously) for postoperative analgesia, repeated every 8 hours. After this step, animals were returned to their cages, where they received water and food ad libitum.
On day 1, the mortality rate in the CLP group was 34 %. The surviving animals were randomized to be euthanized for evaluation of lung mechanics, histology, and inflammatory mediators in lung tissue, or treated with the following therapies: saline (0.05 ml), EPC-EXP, EPC-NEXP, MSC-HUMAN, or MSC-MICE (1 × 105 in 0.05 ml saline, intravenously), after which animals were analyzed on day 3. EPC-NEXP were extracted and injected, whereas EPC-EXP, MSC-HUMAN and MSC-MICE were used at the third passage. To evaluate the cell viability, cells were subjected to trypan blue exclusion assay. For trypan blue staining, cell suspension was mixed with 0.4 % trypan blue solution at a 1:1 ratio. After 1–2 minutes incubation at room temperature, the mixture was loaded onto one chamber of a Neubauer hemocytometer and squares of the chamber were observed under a light microscope. The viable/live (clear) and non-viable/dead (blue) cells were evaluated. The number of viable cells was calculated using the formula: (number of live cells counted/total number of cells counted) × 100. From the number of viable cells, we calculated the exact concentration to obtain 1 × 105 cells in 0.05 ml.
Lungs mechanics, histological data and mediators in lung tissue homogenate (n = 8 for each experimental group) were measured, as described in the following sections.
Lung mechanics
On days 1 and 3 after induction of sepsis, mice were sedated (diazepam 1 mg, intraperitoneally), anesthetized (thiopental sodium 20 mg/kg, intraperitoneally), tracheotomized, paralyzed (vecuronium bromide 0.005 mg/kg, intravenously), and mechanically ventilated. The anterior chest wall was surgically removed and a positive end-expiratory pressure of 2 cmH2O was applied. After a 10-minute ventilation period, lung static elastance (Est,L) was measured by the end-inflation occlusion method [24].
Lung histology
The left lung was fixed in 4 % buffered formaldehyde solution, paraffin-embedded, cut into slices (4 μm thick), and stained with hematoxylin and eosin. Diffuse alveolar damage (DAD) was quantified using a weighted scoring system. In brief, values from 0 to 4 were used to represent the severity of edema, inflammation and atelectasis, with 0 standing for no effect and 4 for maximum severity. In addition, the extent of each score characteristic per field of view was graded on a scale of 0 to 4, with 0 standing for no visible damage and 4 for complete involvement. Scores were calculated as the product of severity and extent of each feature, and ranged from 0 to 16. Finally, the overall DAD score was calculated as the sum of single score characteristics, yielding values from 0 to 48 [25].
Protein expression of inflammatory mediators and growth factors
Protein expression of interleukin (IL)-1β, IL-6, IL-10, tumor necrosis factor (TNF)-α, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) was measured in the lung tissue of EPC-NEXP, EPC-EXP, MSC-HUMAN, and MSC-MICE animals using commercially available enzyme-linked immunosorbent assay kits, in accordance with manufacturer instructions.
Statistical analysis
Functional variables were tested with one-way analysis of variance followed by Tukey’s post-hoc test (Prism for Mac, Version 5.0a, GraphPad Software). The Kruskal-Wallis test followed by Dunn’s post-hoc test was used to compare DAD scores and molecular biology data. Survival rates were compared by log-rank test. Data are expressed as mean ± standard deviation or as median and interquartile range as appropriate. Significance was accepted at P < 0.05.
Results
Phenotypic characterization
The staining patterns of cells are provided in Table 1.
Table 1.
Phenotypic characterization
| EPC-NEXP | EPC-EXP | MSC-HUMAN | MSC-MICE | |
|---|---|---|---|---|
| CD45 | 3.52 % | 1.54 % | 0.25 % | 0.32 % |
| CD34 | 68.6 % | 22.5 % | 0.17 % | 0.24 % |
| CD14 | – | 0.48 % | 1.79 % | – |
| CD133 | 94.9 % | 23.0 % | – | – |
| CD105 | – | 99.6 % | 82.0 % | – |
| CD73 | – | – | 84.4 % | – |
| CD29 | – | 96.8 % | 86.2 % | 99.1 % |
| CD90 | – | – | 92.6 % | – |
| CD166 | – | 87.9 % | 63.4 % | – |
| CD19 | – | – | 0.17 % | 0.71 % |
| CD31 | – | 82.6 % | – | – |
| HLA-DR | – | – | 0.98 % | – |
| CD146 | – | 95.1 | – | – |
| Sca-1 | – | – | – | 88.6 % |
| vWF | – | 97.5 % | – | – |
EPC-CD Cluster of differentiation, EXP expanded endothelial progenitor cell, EPC-NEXP non-expanded endothelial progenitor cell, HLA Human leukocyte antigen, MSC-HUMAN Mesenchymal stem cell of human origin, MSC-MICE Mesenchymal stem cell of mouse origin, Sca-1 Stem cell antigen-1, vWF Von Willebrand factor
Survival rate
The survival rate of untreated animals (CLP) was 66 % on day 1 and 59 % on day 3 (out of 100 % on day 0). On day 3, the survival percentage did not differ among untreated CLP animals, and the MSC-MICE, MSC-HUMAN, EPC-NEXP, and EPC-EXP groups (89, 96, 82, 76, and 100 % respectively). These percentages were calculated from the CLP animals that had survived through day 1 (See Additional file 1).
Expanded EPCs and human MSCs ameliorated lung mechanics
Est,L was significantly increased in CLP mice at days 1 and 3 compared to sham-operated animals (P < 0.01). Est,L was reduced significantly in the EPC-EXP and MSC-HUMAN groups compared to CLP. The EPC-NEXP and MSC-MICE groups showed no significant difference from CLP. MSC-HUMAN animals exhibited lower Est,L compared to the EPC-NEXP and MSC-MICE groups (Fig. 1).
Fig. 1.
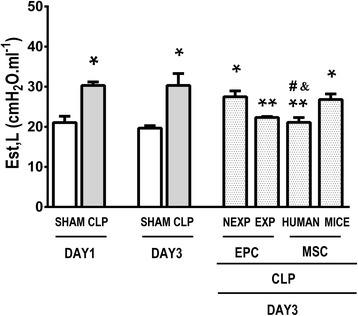
Static lung elastance on days 1 and 3. Mice were subjected to cecal ligation and puncture (CLP). A sham-operated group was used as a control CLP. At day 1, some animals were euthanized after establishment of ARDS in order to evaluate static lung elastance (Est,L), while other animals were randomized to receive saline or non-expanded endothelial progenitor cells (EPC-NEXP), expanded endothelial progenitor cells (EPC-EXP), human mesenchymal stem cells (MSC-HUMAN), or mouse mesenchymal stem cells (MSC-MICE), intravenously. Values expressed as mean ± standard deviation of eight animals in each group. *P < 0.05, versus respective sham group. **P < 0.05, versus CLP group at day 3. # P < 0.05, versus EPC-NEXP group. &P < 0.05, versus MSC-MICE group
Expanded EPCs reduced the DAD score
Histological evaluation revealed greater edema, neutrophil infiltration, atelectasis, and total DAD score in CLP compared to sham animals at days 1 and 3. The total DAD score was reduced after EPC-EXP and MSC-HUMAN therapies compared to CLP; however, animals in the MSC-HUMAN group had higher total DAD scores than sham-operated animals. Edema was significantly decreased in the EPC-EXP, MSC-HUMAN and MSC-MICE groups compared to CLP. Inflammation and atelectasis were significantly reduced in EPC-EXP compared to CLP (Fig. 2). EPC-EXP led to reduced edema and inflammation compared to EPC-NEXP. EPC-EXP yielded decreased atelectasis compared to MSC-MICE.
Fig. 2.
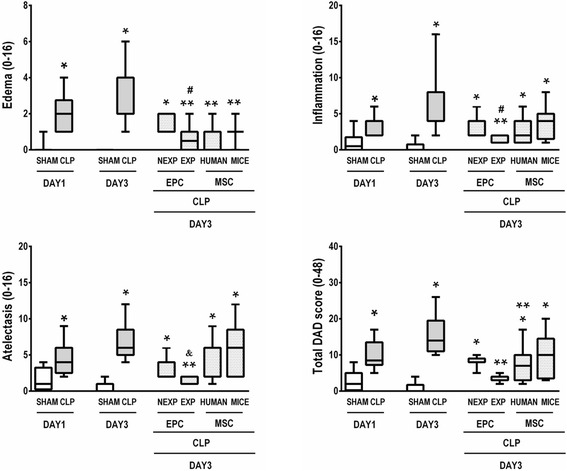
Diffuse alveolar damage in animals with lung injury induced by sepsis. Cecal ligation and puncture (CLP) animals were randomized to receive saline or non-expanded endothelial progenitor cells (EPC-NEXP), expanded endothelial progenitor cells (EPC-EXP), human mesenchymal stem cells (MSC-HUMAN), or mouse mesenchymal stem cells (MSC-MICE), intravenously. Values expressed as a box-and-whiskers plot of eight animals in each group. *P < 0.05, versus respective sham group. **P < 0.05, versus CLP group at day 3. # P < 0.05, versus EPC-NEXP group. &P < 0.05, versus MSC-MICE group. DAD Diffuse alveolar damage
Effects of different cell therapies on inflammatory mediators and growth factors in lung tissue
Levels of inflammatory mediators and growth factors in lung tissue were higher in CLP compared to sham animals at days 1 and 3 (Fig. 3). TNF-α levels were decreased after cell therapies in the EPC-EXP, MSC-HUMAN, and MSC-MICE groups. IL-1β levels were decreased only by expanded EPC therapy, while IL-10 was decreased only by mouse MSC therapy. IL-6 level was decreased both after expanded EPC and after mouse MSC therapies. VEGF levels were decreased by all cell therapies. PDGF was decreased by expanded EPC and human MSC treatments (Fig. 3).
Fig. 3.
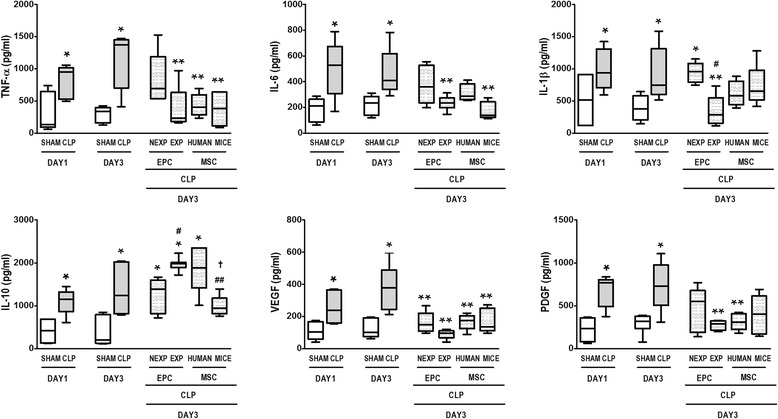
Lung inflammation on days 1 and 3. Lung tissue protein expressions of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-10, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF). Cecal ligation and puncture (CLP) animals were randomized to receive saline or non-expanded endothelial progenitor cells (EPC-NEXP), expanded endothelial progenitor cells (EPC-EXP), human mesenchymal stem cells (MSC-HUMAN), or mouse mesenchymal stem cells (MSC-MICE), intravenously. Values expressed as a box-and-whiskers plot of eight animals in each group. *P < 0.05, versus respective sham group. **P < 0.05, versus CLP group at day 3. # P < 0.05, versus EPC-NEXP group. ## P < 0.05, versus EPC-EXP group. † P < 0.05, versus MSC-HUMAN group
Discussion
In the present study we observed that exogenously administered expanded human cord blood-derived CD133+ cells (EPC-EXP) and MSC-HUMAN were effective in improving lung morpho-function compared to CLP mice treated with saline.
Disruption of the vascular barrier is a critical step in the development of multiple organ failure in sepsis [26]. Several studies have demonstrated the role of circulating EPCs in sepsis [16, 27]. Some demonstrated that septic patients have increased numbers of circulating EPCs as compared with control subjects [16, 17]; however, another study indicated that patients with sepsis have significantly reduced numbers of circulating EPCs [27]. A recent experimental study demonstrated that mice subjected to CLP-induced sepsis had reduced circulating EPC counts at 24 hours, and that exogenous EPC administration improved survival [20].
The protective effect of expanded EPCs observed in this study is consistent with the beneficial effects of MSCs in sepsis [3, 20]. Activated MSCs could reprogram macrophages, resulting in reduced TNF-α and IL-6 but increased IL-10 production [4], which is in accordance with our results with expanded EPC administration. The EPC-EXP group experienced greater improvement of lung function and reduction of lung inflammation, whereas MSC-MICE animals exhibited reduced lung inflammation. Human MSCs also led to lung function recovery, while reducing levels only of TNF-α. One interesting finding was that IL-1β expression decreased only after EPC-EXP administration, which could explain the better overall results achieved with this therapy. IL-1β mediates inflammatory and proliferative effects in many experimental models of lung injury, including sepsis, ventilator-induced lung injury, and bleomycin [28–30]. Increased levels of IL-1β are found in the bronchoalveolar lavage fluid and serum of patients with ARDS [31, 32].
The endothelium plays an important role in sepsis, and the clinical outcome of septic patients is largely dependent on their ability to reconstitute damaged endothelium. Angiogenic factors, including VEGF signaling pathways, have recently been receiving great attention in critically ill patients, including those with sepsis [33], because of their pivotal roles in both angiogenesis and microvascular permeability. In our study, we observed a decrease in VEGF expression levels regardless of the cell therapy administered. Additionally, VEGF plays an important role in mobilizing EPCs under pathologic conditions such as cancer and sepsis [34]. While the decrease in circulating EPCs observed after anti-VEGF treatment is beneficial in cancer, it may not be so in sepsis, which may explain why expanded EPCs were effective in CLP-induced sepsis in the present study.
Several studies have shown that PDGF can accelerate tissue repair and wound healing in acute injury and in some forms of chronic injury, such as radiation-induced chronic non-healing wounds [35, 36]. Nevertheless, it is unclear whether PDGF has beneficial effects in acute critical conditions such as sepsis. Our results demonstrated that administration of expanded EPCs decreased PDGF expression levels. In a rodent model of traumatic hemorrhagic shock, administration of exogenous PDGF improved animal survival and increased tissue blood flow and mitochondrial function in vital organs [37]. However, our results demonstrated that EPC-EXP reduced PDGF expression levels, which may be explained by the different experimental models used in the aforementioned study by Liu et al. [37], in accordance with previous work published by our group with cell therapy and endotoxemia [1].
A recent study evaluated the efficacy of expanded and non-expanded EPCs in modulating myocardial function [22]. The authors observed that both expanded and non-expanded EPCs improved cardiac function. However, in our model of sepsis, in contrast to the morphofunctional benefits of EPC-EXP, EPC-NEXP did not improve lung function or histology. The reasons whereby expanded EPCs led to better lung morphofunction and reduced inflammation as compared with non-expanded EPCs remain to be elucidated, but may be associated with differences in the immunophenotype of these cells. EPC-NEXP exhibited immunophenotypic markers associated with immature cells able to differentiate into both hematopoietic and endothelial cells, depending on stimuli [20]. In contrast, EPC-EXP were already committed to the endothelial lineage and able to promote rapid neovascularization in ischemic areas immediately after infusion, thus improving the environment with nutrients and engraftment of stem cells that may act both on immunomodulation and repair of damaged tissues [20, 21]. Since these cells were studied during a short period, we may hypothesize that the mechanisms of action of EPC-EXP could be attributable to an immunomodulatory effect rather than to engraftment.
Conclusions
In septic mice, expanded cord blood-derived EPCs and human MSCs were associated with specific improvement in lung function and histology, while the other cellular types analyzed, MSC-MICE and EPC-NEXP, were not so effective. Further studies are needed to better understand the therapeutic potential of EPC-EXP, especially as a novel therapy for sepsis.
Acknowledgments
The authors would like to express their gratitude to Mr. Andre Benedito da Silva and Mrs. Ana Lucia Neves da Silva for their help with technical assistance during the experiments and to Mrs. Moira Elizabeth Schottler and Mr. Filippe Vasconcellos for their assistance in editing the manuscript. This study was supported by the Brazilian Council for Scientific and Technological Development (CNPq), the Rio de Janeiro State Research Foundation (FAPERJ), the Department of Science and Technology (DECIT)/Brazilian Ministry of Health, German Academic Exchange Service (DAAD), and the Coordination for the Improvement of Higher Education Personnel (CAPES).
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CLP
Cecal ligation and puncture
- DAD
Diffuse alveolar damage
- EC
Endothelial cell
- EPC
Endothelial progenitor cell
- EPC-EXP
expanded endothelial progenitor cell
- EPC-NEXP
non-expanded endothelial progenitor cell
- Est,L
Lung static elastance
- IL
Interleukin
- MSC
Mesenchymal stem cell
- MSC-HUMAN
Mesenchymal stem cell of human origin
- MSC-MICE
Mesenchymal stem cell of mouse origin
- PDGF
Platelet-derived growth factor
- TNF
Tumor necrosis factor
- VEGF
Vascular endothelial growth factor
Additional file
Survival rate in untreated CLP animals and those treated with cells. Survival rate at days 1 and 3 are related to day 0; values in parentheses are survival rate related to day 1. (DOCX 12 kb)
Footnotes
Andreas Güldner, Tatiana Maron-Gutierrez, Soraia Carvalho Abreu and Debora Gonçalves Xisto contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AG, TMG, SCA, DGX, MGdA, and PRMR conceived and designed the experiments. ACS and PRdSB performed isolation and culture of expanded EPCs, non-expanded EPCs, and human MSCs. AG, TMG, SCA, DGX, and PRdSB performed the experiments. AG, TMG, SCA, DGX, PRdSB, and JDS analyzed the data. AG, TMG, SCA, DGX, ACS, PRdSB, JDS, PB, MGdA, and PRMR wrote this short report. All authors approved the final version of the manuscript.
Authors’ information
AG, MD/PhD, Researcher with expertise in sepsis and acute respiratory distress syndrome.
TMG, PhD, Researcher with expertise in stem cells and experimental acute respiratory distress syndrome.
SCA, PhD, Researcher with expertise in stem cells and regenerative medicine.
DGX, PhD, Researcher with expertise in stem cells and regenerative medicine.
ACS, PhD, Researcher with expertise in stem cells and regenerative medicine.
PRdSB, RRT, Researcher with expertise in respiratory physiology.
JDS, PhD, Researcher with expertise in stem cells and experimental acute respiratory distress syndrome.
PB, MD/PhD, Professor, Researcher with expertise in stem cells and regenerative medicine.
MGdA, MD/PhD, Professor, Researcher with expertise in experimental acute respiratory distress syndrome, mechanical ventilation.
PRMR, MD/PhD, Professor, Researcher with expertise in experimental sepsis, stem cells, regenerative medicine, acute respiratory distress syndrome, and mechanical ventilation.
Contributor Information
Andreas Güldner, Email: Andreas.Gueldner@uniklinikum-dresden.de.
Tatiana Maron-Gutierrez, Email: tati.maron@gmail.com.
Soraia Carvalho Abreu, Email: soraiafisio@gmail.com.
Debora Gonçalves Xisto, Email: deboraxisto@gmail.com.
Alexandra Cristina Senegaglia, Email: alexandra.senegaglia@pucpr.br.
Patty Rose da Silva Barcelos, Email: patty.barcelos@gmail.com.
Johnatas Dutra Silva, Email: johnatasdutra@gmail.com.
Paulo Brofman, Email: paulo.brofman@pucpr.br.
Marcelo Gama de Abreu, Email: mgabreu@uniklinikum-dresden.de.
Patricia Rieken Macedo Rocco, Phone: +55 21 3938-6530, Email: prmrocco@biof.ufrj.br.
References
- 1.Araujo IM, Abreu SC, Maron-Gutierrez T, Cruz F, Fujisaki L, Carreira H, Jr, et al. Bone marrow-derived mononuclear cell therapy in experimental pulmonary and extrapulmonary acute lung injury. Crit Care Med. 2010;38(8):1733–41. doi: 10.1097/CCM.0b013e3181e796d2. [DOI] [PubMed] [Google Scholar]
- 2.Maron-Gutierrez T, Silva JD, Asensi KD, Bakker-Abreu I, Shan Y, Diaz BL, et al. Effects of mesenchymal stem cell therapy on the time course of pulmonary remodeling depend on the etiology of lung injury in mice. Crit Care Med. 2013;41(11):e319–33. doi: 10.1097/CCM.0b013e31828a663e. [DOI] [PubMed] [Google Scholar]
- 3.Maron-Gutierrez T, Silva JD, Cruz FF, Alegria S, Xisto DG, Assis EF, et al. Insult-dependent effect of bone marrow cell therapy on inflammatory response in a murine model of extrapulmonary acute respiratory distress syndrome. Stem Cell Res Ther. 2013;4(5):123. doi: 10.1186/scrt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107(12):5652–7. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ornellas DS, Maron-Gutierrez T, Ornellas FM, Cruz FF, Oliveira GP, Lucas IH, et al. Early and late effects of bone marrow-derived mononuclear cell therapy on lung and distal organs in experimental sepsis. Respir Physiol Neurobiol. 2011;178(2):304–14. doi: 10.1016/j.resp.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047–57. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 7.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4(9):e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187(7):751–60. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106(38):16357–62. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302(10):L1003–13. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67(6):533–9. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–65. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck GC, Rafat N, Yard B, Hanusch C. The role of endothelial progenitor cells in sepsis. Anaesthesist. 2007;56(5):423–8. doi: 10.1007/s00101-007-1183-z. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med. 2011;3(88):88ps25. doi: 10.1126/scitranslmed.3002011. [DOI] [PubMed] [Google Scholar]
- 16.Patschan SA, Patschan D, Temme J, Korsten P, Wessels JT, Koziolek M, et al. Endothelial progenitor cells (EPC) in sepsis with acute renal dysfunction (ARD) Crit Care. 2011;15(2):R94. doi: 10.1186/cc10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becchi C, Pillozzi S, Fabbri LP, Al Malyan M, Cacciapuoti C, Della Bella C, et al. The increase of endothelial progenitor cells in the peripheral blood: a new parameter for detecting onset and severity of sepsis. Int J Immunopathol Pharmacol. 2008;21(3):697–705. doi: 10.1177/039463200802100324. [DOI] [PubMed] [Google Scholar]
- 18.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 19.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–8. [PubMed] [Google Scholar]
- 20.Fan H, Goodwin AJ, Chang E, Zingarelli B, Borg K, Guan S, et al. Endothelial progenitor cells and a stromal cell-derived factor-1alpha analogue synergistically improve survival in sepsis. Am J Respir Crit Care Med. 2014;189(12):1509–19. doi: 10.1164/rccm.201312-2163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103(5):634–7. doi: 10.1161/01.CIR.103.5.634. [DOI] [PubMed] [Google Scholar]
- 22.Senegaglia AC, Barboza LA, Dallagiovanna B, Aita CA, Hansen P, Rebelatto CL, et al. Are purified or expanded cord blood-derived CD133+ cells better at improving cardiac function? Exp Biol Med. 2010;235(1):119–29. doi: 10.1258/ebm.2009.009194. [DOI] [PubMed] [Google Scholar]
- 23.Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, Barchiki F, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2008;233(7):901–13. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 24.Bates JH, Decramer M, Chartrand D, Zin WA, Boddener A, Milic-Emili J. Volume-time profile during relaxed expiration in the normal dog. J Appl Physiol. 1985;59(3):732–7. doi: 10.1152/jappl.1985.59.3.732. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira GP, Silva JD, Marques PS, Goncalves-de-Albuquerque CF, Santos HL, Vascocellos AP, et al. The effects of dasatinib in experimental acute respiratory distress syndrome depend on dose and etiology. Cell Physiol Biochem. 2015;36(4):1644–58. doi: 10.1159/000430325. [DOI] [PubMed] [Google Scholar]
- 26.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31(9):2316–23. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 27.Cribbs SK, Sutcliffe DJ, Taylor WR, Rojas M, Easley KA, Tang L, et al. Circulating endothelial progenitor cells inversely associate with organ dysfunction in sepsis. Intensive Care Med. 2012;38(3):429–36. doi: 10.1007/s00134-012-2480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank JA, Pittet JF, Wray C, Matthay MA. Protection from experimental ventilator-induced acute lung injury by IL-1 receptor blockade. Thorax. 2008;63(2):147–53. doi: 10.1136/thx.2007.079608. [DOI] [PubMed] [Google Scholar]
- 29.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117(12):3786–99. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107(12):1529–36. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154(3 Pt 1):602–11. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 32.Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med. 1996;153(6 Pt 1):1850–6. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 33.Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, et al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med. 2006;203(6):1447–58. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang RY, Liu YY, Qu HP, Tang YQ. The angiogenic factors and their soluble receptors in sepsis: friend, foe, or both? Crit Care. 2013;17(4):446. doi: 10.1186/cc12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Judith R, Nithya M, Rose C, Mandal AB. Application of a PDGF-containing novel gel for cutaneous wound healing. Life Sci. 2010;87(1–2):1–8. doi: 10.1016/j.lfs.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Yan G, Sun H, Wang F, Wang J, Wang F, Zou Z, et al. Topical application of hPDGF-A-modified porcine BMSC and keratinocytes loaded on acellular HAM promotes the healing of combined radiation-wound skin injury in minipigs. Int J Radiat Biol. 2011;87(6):591–600. doi: 10.3109/09553002.2011.570854. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Zhang J, Zhu Y, Xiao X, Peng X, Yang G, et al. Beneficial effects of platelet-derived growth factor on hemorrhagic shock in rats and the underlying mechanisms. Am J Physiol Heart Circ Physiol. 2014;307(9):H1277–87. doi: 10.1152/ajpheart.00006.2014. [DOI] [PubMed] [Google Scholar]


