Summary
Amyotrophic lateral sclerosis (ALS) is a motor neuron disease characterized by serious muscle atrophy and weakness. The purpose of this study was to find prognostic factors in patients with mild ALS using application forms for the Specified Disease Treatment Research Program in Japan. We classified ALS as mild, moderate and severe. The subjects consisted of 363 patients with mild ALS who underwent needle electromyography at registration and were followed for more than one year. Time to progression to severe ALS and time to deterioration of activities of daily living such as speech dysfunction, upper limb dysfunction, and walking disability were used as outcomes. Cox proportional hazards model analysis was performed to identify prognostic factors. Of the patients with initially mild ALS, 38.3% (139/363) had progressed severe ALS at the last follow-up. In multivariate analysis of time to progression to severe ALS, bulbar onset (hazard ratio [95% confidence interval]: 1.68 [1.13–2.49], p = 0.010), tongue atrophy (1.69 [1.14–2.51], p = 0.009), dyspnea (1.57 [1.02–2.41], p = 0.042) and active denervation findings (ADFs) of the cervical-upper limb area (1.81 [1.25–2.63], p = 0.002) emerged as prognostic factors. Furthermore ADFs in the trunk area were prognostic factors for upper limb dysfunction and walking disability (1.72 [1.05–2.81], p = 0.031, and 1.97 [1.09–3.59], p = 0.026). In conclusion ADFs of the cervical-upper limb area and trunk area were prognostic factors in ALS patients.
Keywords: Amyotrophic lateral sclerosis, prognostic factors, needle electromyography, denervation findings, bulbar onset
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a motor neuron disease characterized by serious muscle atrophy and weakness. ALS progresses rapidly with a median survival time from symptom onset of 2–4 years (1), and effective treatment has not been established because of the unknown etiology. Adult onset and rapid progression of limb muscle weakness, muscle atrophy, fasciculation, or exaggerated deep tendon reflex lead suspicion of ALS. The diagnosis of ALS is difficult and it is important that the detection of upper or lower motor neuron disorders at each site of the brainstem, cervical, thoracic and lumbosacral spinal cord by taking medical history and physical observation carefully. A variety of prognostic factors for ALS have been reported (2), and onset age is a strong prognostic factor in ALS, with decreasing survival time correlating with increasing age of onset (3–5). ALS is classified as bulbar onset type, which start with dysarthria, dysphagia, or dyspnea, and extremity onset type, which with muscle weakness or atrophy in an arm or leg. Bulbar onset is associated with a worse prognosis than extremity onset (3,4).
In Japan, the Specified Disease Treatment Research Program provides a public subsidy for medical expenses for incurable diseases. Patients in each prefecture are required to submit an application form for this program. These forms allow clinical information to be obtained for incurable disease on a national basis, which is useful for studies on epidemiology and pathogenesis, and for evaluation of treatment and drugs (6). However, greater emphasis has been placed on administrative applications, while few systematic analyses have been performed for research use. ALS is designated as a specified disease of the program. In this study, we analyzed prognostic factors for progression of ALS using application forms.
2. Methods
2.1. Patients
The subjects were patients who registered from 2004 to 2005 in the Japanese Specific Disease Treatment Research Program. Data from application forms for patients who registered with a diagnosis of ALS were submitted to an advisory board on intractable diseases from 47 administrative divisions in Japan. This board included neurologists. These data were evaluated using the diagnostic criteria for ALS defined by the Committee on Intractable Degenerative CNS Diseases of the Ministry of Health and Welfare of Japan (2002), which are based on the diagnostic criteria of the ALS Committee of the World Foundation of Neurology (2000) (7). Patients who received certification then updated their information annually from September to November in future years.
2.2. Protocol approval and patient consent
This study conforms to the ethical guidelines for epidemiological research issued by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare. The ethics committee of the National Institute of Public Health approved this study (NIPH-IBRA No.10021; 10 June, 2010). All data were provided by the Ministry of Health, Labor and Welfare (Notification of Health Service bureau, MHLW; No.0708-1; 8 Jul. 2010). All patients gave consent to utilization of their clinical data in research studies.
2.3. Definition of variables
Analysis factors such as sex, onset age, initial symptom at onset, clinical sings at registration, and findings of needle EMG at registration were recorded. The initial symptoms at onset were determined from an interview with the patient or an introductory letter, and included the presence or absence of dysarthria, dysphagia, dyspnea, neck muscle weakness, upper limb muscle weakness, and lower limb muscle weakness. Also, patients were classified into extremity onset type: only muscle weakness at onset, and bulbar onset type that started with dysarthria, dysphagia or dyspnea at onset. Clinical signs at registration were also evaluated by neurologists, and included tongue atrophy, dysarthria, dysphagia, dyspnea, muscle strength, and muscle atrophy. Muscle strength was manually tested and scored using the Medical Research Council (MRC) 6-point scale (range: 0–5) (8) in 11 muscle groups: neck flexor muscles; shoulder abductor muscles (right and left); elbow flexor muscles (right and left); wrist flexor muscles (right and left); hip flexor muscles (right and left), and ankle flexor muscles (right and left). A MRC score ≤ 3 was defined as indicating muscle weakness. The presence of muscle atrophy were observed in 10 muscle groups: neck muscle group, upper limb (right and left), girdle muscles (right and left), paraspinal muscles, pelvicrural muscle (right and left), and lower extremities (right and left). Needle electromyography (EMG), a method of puncture of muscles with needle electrodes to record action potentials caused by natural shrinkage and voluntary contraction, was performed in the cranial, cervical-upper limb, trunk, and lumbar-lower limb areas. The presence of active denervation findings (ADFs), defined as fibrillation potentials (Fib-Ps) or positive sharp waves (PSWs); and chronic denervation findings (CDFs), defined as enlarged action potentials and decreased interference patterns, was also evaluated (9–11). ADFs are found when innervation of muscles is lost (12), and these findings reflect neurodegeneration before appearance of clinical signs such as muscle weakness and muscle atrophy in ALS (9,13). CDFs were found when reinnervation occurred following denervation.
Severity of ALS was classified into 5 grades (Table 1) by evaluation of neurological signs at staging and assessment of activities of daily living (ADL) on the modified Rankin Scale (mRS) by the Research Committee of CNS Degenerative Diseases, Ministry of Health, Labour and Welfare. The mRS is a marker of severity of ALS (14). In this study, mild ALS was defined as not requiring daily assistance (grades 1 and 2), moderate ALS as requiring daily assistance (grades 3 and 4), and severe ALS as requiring life support such as tubal feeding, gastrostoma, positive pressure ventilation, tracheotomy, and an artificial ventilator (grade 5, Table 1).
Table 1. Classification of ALS severity by the Research Committee for CNS Degenerative Diseases, Ministry of Health, Labour and Welfare.
| Severity | Definition |
|---|---|
| Mild | |
| Grade 1 | Movement disturbance of one extremity or anarthria by bulbar paralysis. No limitation in activities of daily living (ADL). |
| Grade 2 | Apparent movement disturbance in one or two muscle regions in 6 body segments: each limb, trunk, tongue, face, palatal, and pharyngeal region. Slight limitation, but can live an independent life by oneself. |
| Moderate | |
| Grade 3 | Muscle weakness at more than 3 positions of the above 6 body segments. Cannot do social activities (housework, job) and has mild limitation requiring assistance in ADL. |
| Grade 4 | Inability of any one of breathing, swallowing, or keeping a sitting position. Requires total assistance for ADL. |
| Severe | |
| Grade 5 | Bedridden, requiring life support including tracheotomy, parenteral nutrition, and an artificial respirator |
ALS, amyotrophic lateral sclerosis. The ALS severity classification (grades 1 to 5) is based on evaluation of neurological signs at staging and social life using the modified Rankin scale (mRS). Mild ALS (grades 1 and 2) is defined as not requiring daily assistance. Moderate ALS (grades 3 and 4) is defined as requiring daily assistance. Severe ALS (grade 5) is defined as requiring life support.
2.4. Outcomes
Four outcomes were evaluated: time for progression to severe ALS (grade 5, Table 1) as a main outcome and deterioration of ADLs based on loss of speech function, loss of upper limb function, and loss of walking ability as sub outcomes. ADLs were classified into 5 grades referring the Japanese version of the ALSFRS-R (15), as validated by Ohashi et al. (16). The time at which each ADLs deterioration was defined as follows: loss of speech function occurred when the patient lost useful speech; loss of upper limb function occurred when the patient became unable to grip a pen; loss of walking ability occurred when the patient had no purposeful leg movement, respectively.
2.5. Statistical analysis
Cox proportional hazards regression analyses were performed for time from registration to progression within 3 years. The hazard ratio (HR) and corresponding 95% confidence interval (CI) and p-value were estimated by Wald test. In univariate analysis, candidate prognostic factors were identified at p < 0.05. In multivariate analysis, prognostic factors were selected from these candidate factors using backward selection at p < 0.05. To construct a prognostic classification, regression tree analysis for each outcome was performed using prognostic factors as dependent variables. For validation of the prognostic classification (tree structure), regression tree analysis with 1000 bootstrap samples was performed, and the reliability of the crude tree structure was investigated (17). The stratified progression-free rates were estimated using the Kaplan-Meier method to show the prognostic classification, and a log-rank test was used for comparison between the stratified groups.
For validation of the severity classification (mild, moderate, and severe), we explored associations with other severity-related measures using a Pearson chi-squared test complemented by Haberman's residual analysis (18). To explore the reliability of the severity classification, we analyzed data at the first visit because the number of censors at the last visit was more than that at the first visit. The significance level was p = 0.05. All analyses were performed using R ver. 3.1.1. (R Foundation, Austria).
3. Results
3.1. Patients
From 2004 to 2005, application forms were submitted by 2,359 patients with ALS, of whom 985 submitted updated application forms for more than one year. All patients fulfilled the diagnostic criteria, as judged by an advisory board. The initial analysis included 959 patients with sporadic ALS, after exclusion of 26 patients with a family history of ALS. Of these 959 patients, 363 had ALS of mild severity and had undergone needle EMG at registration. The characteristics of these patients are shown in Table 2. The patients comprised 218 men and 145 women, and had a median age at disease onset of 62.0 years (range: 18–87 years) and a mean follow-up period of 1.52 ± 0.72 years. The numbers of patients with loss of speech function, loss of upper limb function, and loss of walking ability were 14/363 (3.9%), 6/362 (1.7%), and 0/362 (0%), respectively.
Table 2. Baseline characteristic of ALS patients.
| Variable (n = 363) | Number of patients | % |
|---|---|---|
| Gender: male | 145/363 | 39.9 |
| Age at onset (years old) | ||
| ≤ 40 | 11/363 | 3.0 |
| 41–64 | 220/363 | 60.6 |
| ≥ 65 | 132/363 | 36.4 |
| Onset type : Bulbar onset | 222/360 | 61.7 |
| Tongue atrophy at registration: presence | 172/361 | 47.6 |
| Dysarthria at registration: presence | 193/363 | 53.2 |
| Dysphagia at registration: presence | 145/361 | 40.2 |
| Dyspnea at registration: presence | 50/357 | 14.0 |
| Neck flexors strength at registration: with muscle weakness | 47/358 | 13.1 |
| Shoulder abductors strength at registration: with muscle weakness | 107/360 | 29.7 |
| Elbow flexors strength at registration: with muscle weakness | 94/359 | 26.2 |
| Wrist extensors strength at registration: with muscle weakness | 77/359 | 21.4 |
| Hip flexors strength at registration: with muscle weakness | 39/359 | 10.9 |
| Ankle extensors at registration: with muscle weakness | 63/352 | 17.9 |
| Active denervation findings at registration: presence | ||
| Cranial area | 96/363 | 26.4 |
| Cervical-upper limb area | 226/363 | 62.3 |
| Trunk area | 42/363 | 11.6 |
| Lumbar-lower limb area | 161/363 | 44.4 |
| Chronic denervation findings at registration: presence | ||
| Cranial area | 154/363 | 42.4 |
| Cervical-upper limb area | 294/363 | 81.0 |
| Trunk area | 73/363 | 20.1 |
| Lumbar-lower limb area | 254/363 | 70.0 |
ALS: amyotrophic lateral sclerosis.
3.2. Severity classification
The classification of mild, moderate and severe ALS was validated based on significant associations found with measures related to progression of ALS, including levels of speech function (p < 0.001), upper limb function (p < 0.001), and walking ability (p < 0.001), and the number of areas with muscle weakness (p < 0.001) and muscle atrophy (p < 0.001) (Table 3).
Table 3. Associations between severity classification and other severity-related measures.
| Variable | Category |
p value | ||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| Speech function | ||||
| 1 | 59* | 53* | 11 | < 0.001 |
| 2 | 43* | 33 | 17 | |
| 3 | 13 | 9 | 9 | |
| 4 | 7 | 12 | 12 | |
| 5 | 11 | 16 | 58* | |
| Upper limb function (handwriting) | < 0.001 | |||
| 1 | 59* | 11 | 9 | |
| 2 | 56* | 26 | 28 | |
| 3 | 8 | 23* | 12 | |
| 4 | 8 | 23* | 12 | |
| 5 | 2 | 34* | 41* | |
| Walking ability | < 0.001 | |||
| 1 | 54* | 15 | 11 | |
| 2 | 50* | 22 | 25 | |
| 3 | 28 | 39* | 17 | |
| 4 | 1 | 37* | 18 | |
| 5 | 0 | 11 | 34* | |
| Number of areas with muscle weakness | < 0.001 | |||
| ≤ 1 | 71* | 13 | 15 | |
| 2–5 | 41* | 29 | 17 | |
| 6–8 | 12 | 25 | 23 | |
| 9–11 | 5 | 43* | 43* | < 0.001 |
| Number of areas with muscle atrophy | ||||
| ≤ 1 | 28* | 1 | 7 | |
| 2–4 | 50* | 23 | 14 | |
| 5–7 | 31 | 39* | 20 | |
| 8–10 | 24 | 61* | 65* | |
ALS: amyotrophic lateral sclerosis. The significance of the association between severity and each index of ALS progression was assessed using a Pearson chi-squared test of independence. Residual analysis was also performed for identifying the categories responsible for a significant chi-square statistic.
indicates a significant large number (p < 0.05).
3.3. Time to progression
Of the patients with initially mild ALS, 38.3% (139/363) had progressed severe ALS at the last follow-up. The rate of patients with loss of speech function, loss of upper limb function, and loss of walking ability at the last follow-up were 31.1% (113/363), 30.6% (111/363), and 22.0% (80/363), respectively.
The results of univariate regression analysis of the time to progression to severe ALS are shown in Table 4. In this analysis, the candidate prognostic factors were bulbar onset (HR: 2.28 [95% CI: 1.63–3.19], p < 0.001), tongue atrophy at registration (2.26 [1.60–3.19], p < 0.001), dysarthria at registration (2.23 [1.56–3.18], p < 0.001), dysphagia at registration (2.25 [1.61–3.15], p < 0.001), dyspnea at registration (2.00 [1.33–3.00], p = 0.001), ADFs of the cervical-upper limb area at registration (1.59 [1.10–2.29], p = 0.013), and CDFs of the cervical-upper limb area at registration (1.41 [1.01— 1.96], p = 0.044). The results of univariate regression analysis of the times to loss of speech function, loss of upper limb function and loss of walking ability are also shown in Table 4.
Table 4. Univariate Cox regression analyses for times to loss of speech function, loss of walking ability, and loss of upper limb function.
| Variable | Progression to severe |
Loss of speech function |
Loss of upper limb function |
Loss of walking ability |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Gender (men) | 1.01 | 0.72 – 1.43 | 0.936 | 0.66 | 0.46 – 0.96 | 0.031 | 1.36 | 0.91 – 2.02 | 0.131 | 1.40 | 0.86 – 2.25 | 0.175 |
| Age at onset | 1.01 | 1.00 – 1.03 | 0.167 | 1.03 | 1.01 –1.05 | 0.003 | 0.99 | 0.97 – 1.00 | 0.103 | 0.99 | 0.97 – 1.01 | 0.428 |
| Bulbar onset | 2.28 | 1.63 – 3.19 | < 0.001 | 5.69 | 3.78 – 8.54 | < 0.001 | 0.55 | 0.36 – 0.84 | 0.005 | 1.17 | 0.75 – 1.84 | 0.492 |
| Tongue atrophy at registration | 2.26 | 1.60 – 3.19 | < 0.001 | 2.58 | 1.74 – 3.83 | < 0.001 | 0.72 | 0.49 – 1.06 | 0.092 | 0.94 | 0.61 – 1.47 | 0.793 |
| Dysarthria at registration | 2.23 | 1.56 – 3.18 | < 0.001 | 5.10 | 3.17 – 8.19 | < 0.001 | 0.69 | 0.47 – 1.00 | 0.048 | 0.94 | 0.61 – 1.46 | 0.781 |
| Dysphagia at registration | 2.25 | 1.61 – 3.15 | < 0.001 | 4.49 | 3.01 – 6.68 | < 0.001 | 0.63 | 0.42 – 0.95 | 0.026 | 1.06 | 0.68 – 1.67 | 0.795 |
| Dyspnea at registration | 2.00 | 1.33 – 3.00 | 0.001 | 1.92 | 1.21 – 3.04 | 0.006 | 0.88 | 0.51 – 1.52 | 0.637 | 1.39 | 0.79 – 2.43 | 0.258 |
| Neck flexors strength at registration | 1.36 | 0.86 – 2.14 | 0.187 | 0.94 | 0.54 – 1.64 | 0.825 | 1.15 | 0.68 – 1.96 | 0.604 | 1.20 | 0.65 – 2.21 | 0.570 |
| Shoulder abductors strength at registration | 1.06 | 0.74 – 1.51 | 0.760 | 0.56 | 0.36 – 0.87 | 0.010 | 2.54 | 1.75 – 3.68 | < 0.001 | 1.05 | 0.66 – 1.68 | 0.824 |
| Elbow flexors strength at registration | 0.87 | 0.59 – 1.28 | 0.475 | 0.47 | 0.29 – 0.77 | 0.003 | 2.20 | 1.50 – 3.21 | < 0.001 | 0.85 | 0.52 – 1.41 | 0.538 |
| Wrist extensors strength at registration | 0.99 | 0.66 – 1.51 | 0.973 | 0.59 | 0.35 – 1.00 | 0.050 | 3.01 | 2.03 – 4.48 | < 0.001 | 1.23 | 0.72 – 2.08 | 0.451 |
| Hip flexors strength at registration | 0.80 | 0.44 – 1.45 | 0.462 | 0.54 | 0.25 – 1.16 | 0.113 | 0.85 | 0.44 – 1.62 | 0.614 | 2.00 | 1.10 – 3.63 | 0.023 |
| Ankle extensors at registration | 0.58 | 0.34 – 0.99 | 0.047 | 0.60 | 0.33 – 1.09 | 0.10 | 0.66 | 0.37 – 1.18 | 0.163 | 1.58 | 0.92 – 2.70 | 0.097 |
| Active denervation findings at registration | ||||||||||||
| Cranial area | 1.45 | 1.02 – 2.06 | 0.039 | 2.07 | 1.43 – 3.02 | < 0.001 | 1.02 | 0.67 – 1.55 | 0.925 | 1.00 | 0.61 – 1.62 | 0.990 |
| Cervical-upper limb area | 1.59 | 1.10 – 2.29 | 0.013 | 0.95 | 0.65 – 1.38 | 0.777 | 1.90 | 1.24 – 2.92 | 0.003 | 1.07 | 0.68 – 1.70 | 0.772 |
| Trunk area | 1.12 | 0.68 – 1.87 | 0.652 | 0.80 | 0.43 – 1.49 | 0.479 | 1.96 | 1.20 – 3.18 | 0.007 | 1.94 | 1.09 – 3.45 | 0.025 |
| Lumbar-lower limb area | 0.96 | 0.69 – 1.34 | 0.809 | 0.69 | 0.47 – 1.01 | 0.061 | 1.30 | 0.90 – 1.90 | 0.162 | 1.47 | 0.95 – 2.28 | 0.086 |
| Chronic denervation findings at registration | ||||||||||||
| Cranial area | 1.41 | 1.01 – 1.96 | 0.044 | 1.99 | 1.37 – 2.89 | < 0.001 | 0.76 | 0.51 – 1.12 | 0.162 | 0.96 | 0.61 – 1.50 | 0.848 |
| Cervical-upper limb area | 1.59 | 0.98 – 2.58 | 0.060 | 1.05 | 0.65 – 1.70 | 0.852 | 1.45 | 0.85 – 2.46 | 0.169 | 1.24 | 0.68 – 2.24 | 0.484 |
| Trunk area | 1.14 | 0.76 – 1.71 | 0.537 | 0.88 | 0.54 – 1.43 | 0.603 | 1.00 | 0.63 – 1.60 | 0.987 | 1.06 | 0.61 – 1.84 | 0.826 |
| Lumbar-lower limb area | 1.01 | 0.70 – 1.44 | 0.969 | 0.64 | 0.44 – 0.93 | 0.019 | 0.93 | 0.62 – 1.39 | 0.722 | 1.31 | 0.80 – 2.15 | 0.278 |
HR: Hazard Ratio; CI: Confidence Interval; MRC: Medical Research Council; EMG: electromyography. The p-value and 95%CI were calculated using a Wald test. Variable (reference); Gender (women), Age at onset (1), onset type (extremity onset)), tongue atrophy at registration (absence), dysarthria at registration (absence), dysphagia at registration (absence), dyspnea at registration (absence), muscle strength at registration (no muscle weakness: MRC score ≤ 3), EMG finding at registration (absence). In an area tested on the right and left, the lower muscle strength was used for analysis.
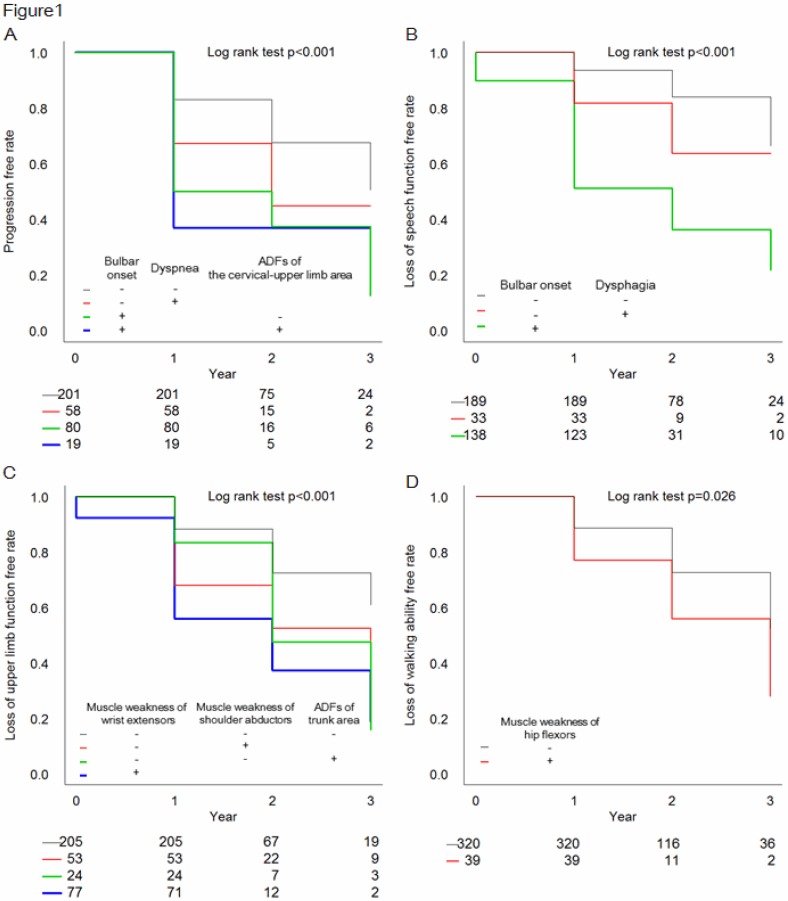
The results of multivariate regression analysis of the time to progression to severe ALS are shown in Table 5. Bulbar onset (1.68 [1.13–2.49], p = 0.010), tongue atrophy at registration (1.69 [1.14–2.51], p = 0.009), dyspnea at registration (1.57 [1.02–2.41], p = 0.042), and ADFs of the cervical-upper limb area at registration (1.81 [1.25–2.63], p = 0.002) emerged as prognostic factors for time for progression to severe ALS. The results of regression tree analysis are shown in Figure 1A as the stratified progression-free rate. ADFs of the cervical-upper limb area were found to be significant in progression to severe ALS. The results of multivariate analysis and regression tree analysis for the times to the three sub outcomes are shown in Table 5 and Figure 1B–D. These results indicated that ADFs of the trunk area were prognostic factors for upper limb dysfunction and walking disability.
Table 5. Multivariate Cox regression analyses for time to progression to severe ALS, tongue atrophy and dysarthria.
| Variable | Progression to severe |
Loss of speech function |
Loss of upper limb function |
Loss of walking ability |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Gender (men) | ||||||||||||
| Age at onset | ||||||||||||
| Bulbar onset | 1.68 | 1.13 – 2.49 | 0.010* | 3.81 | 2.24 – 6.48 | < 0.001* | ||||||
| Tongue atrophy at registration | 1.69 | 1.14 – 2.51 | 0.009 | |||||||||
| Dysarthria at registration | ||||||||||||
| Dysphagia at registration | 1.87 | 1.11 – 3.14 | 0.018* | |||||||||
| Dyspnea at registration | 1.57 | 1.02 – 2.41 | 0.042* | |||||||||
| Neck flexors strength at registration | ||||||||||||
| Shoulder abductors strength at registration | 1.91 | 1.25 – 2.91 | 0.003* | |||||||||
| Elbow flexors strength at registration | ||||||||||||
| Wrist extensors strength at registration | 2.12 | 1.35 – 3.32 | 0.001* | |||||||||
| Hip flexors strength at registration | 1.88 | 1.05 – 3.55 | 0.033* | |||||||||
| Ankle extensors at registration | ||||||||||||
| Active denervation findings at registration | ||||||||||||
| Cranial area | 1.81 | 1.25 – 2.63 | 0.002* | |||||||||
| Cervical-upper limb area | 1.72 | 1.05 – 2.81 | 0.031* | 1.97 | 1.09 – 3.59 | 0.026 | ||||||
| Trunk area | ||||||||||||
| Lumbar-lower limb area | ||||||||||||
| Chronic denervation findings at registration | ||||||||||||
| Cranial area | ||||||||||||
| Cervical-upper limb area | ||||||||||||
| Trunk area | ||||||||||||
| Lumbar-lower limb area | ||||||||||||
HR: Hazard Ratio; CI: Confidence Interval; MRC: Medical Research Council; EMG: electromyography. The p-value and 95%CI were calculated using a Wald test. Variable (reference); Gender (women), Age at onset (1), Onset type (extremity onset), tongue atrophy at registration (absence), dysarthria at registration (absence), dysphagia at registration (absence), dyspnea at registration (absence), muscle strength at registration (no muscle weakness: MRC score ≤ 3), EMG finding at registration (absence). In an area tested on the right and left, the lower muscle strength was used for analysis. Variables marked with * were chosen as results of regression tree analysis.
Figure 1.
Stratified Kaplan-Meier curves for four outcomes based on regression tree analysis. Curves are shown for progression to severe ALS (A), loss of speech (B), loss of upper limb function (C), and loss of walking ability (D). The curves were compared by log-rank test. For progression to severe ALS, patients were classified into 5 groups based on onset type (+: bulbar onset, −: extremity onset), presence or absence of respiratory problems at registration, and ADFs of the cervical-upper limb area at registration (A). For loss of speech, patients were classified into 3 groups based on onset type and presence or absence of dysphagia at registration (B). For loss of upper limb function, patients were classified into 4 groups based on presence or absence of weakness of wrist extensors at registration, of shoulder abductors at registration, and ADFs of the trunk area at registration (C). For loss of walking ability, patients were classified into 2 stratified groups based on presence or absence of weakness of hip flexors at registration (D). The stratified Kaplan-Meier curve for each outcome was well-distinguished by log-rank test (A: p < 0.001, B: p < 0.001, C: p < 0.001, D: p = 0.026).
4. Discussion
In this study we tried to examine prognostic factors for progression ALS by interannual analysis of application forms, which provided important medical data on a national scale, focusing on patients with initial mild ALS who did not require daily assistance. The main outcome in our study: progression to severe ALS, is a stage at which a patient requires a ventilator and parenteral nutrition for life support. Thus an observation period of 3 years should be sufficient to analyze prognostic factors for ALS considering median survival time of 2–4 years (1).
Our results indicated that bulbar onset, tongue atrophy, dyspnea, and ADFs of the cervical-upper limb area were prognostic factors for progression from mild to severe ALS (Table 5). Many studies reported bulbar onset and tongue atrophy were important prognostic factors (19), however a few studies reported the possibility of ADFs as prognostic factors of ALS (20).
Progression to severe ALS is associated with decreased swallowing function and respiratory function. Swallowing is controlled by muscles that are innervated mainly by the pons and medulla oblongata such as the glossopharyngeal nerve, vagal nerve and hypoglossal nerve (21,22), and bulbar onset and tongue atrophy are associated with loss of this function. Breathing is controlled by complex relationships among many muscles, of which the diaphragm and the anterior, middle and posterior scalene muscles, which function in intake, are innervated by C3–C4, C4–C7, C2–C7 and C5–C8, respectively. Muscles innervated by the brachial plexus (C5–T1) are tested in needle EMG of the cervical- upper limb area. Then, ADFs in this area might reflect neurodegeneration of muscles involved in respiratory functions. ADFs are said to show neurodegeneration from before appearance of clinical signs such as muscle weakness and muscle atrophy in ALS (10,14). Therefore we analyzed the prognosis of patients without neck flexors muscle weakness nor shoulder abductors muscle weakness at registration and found that the number of patients with ADFs of the cervical-upper limb area who progressed to severe ALS within 3 years were significant large (χ2 = 4.00, p = 0.045).
Figure 1A suggested that patients with both bulbar onset and ADFs of the cervical-upper limb area had poor prognosis. Further analysis found that of patients with extremity onset, the number of patients with ADFs of the cervical-upper limb area who progressed to severe ALS within 3 years were significant large (χ2 = 3.89, p = 0.049). This suggested that ADFs of the cervical-upper limb area were also important for predicting prognosis ALS in patients with extremity onset.
Furthermore, ADFs in the trunk area were prognostic factors for upper limb dysfunction and walking disability (Table 5). In needle EMG of the trunk area, muscles of the thoracic spinal cord are tested, including the paraspinal muscles and abdominal rectus muscle (23,24). Degeneration of motor neurons may spread contiguously throughout the three-dimensional anatomy of connected and neighboring neurons in ALS (25,26), and this may explain upper limb dysfunction resulting from proximal progression of denervation of the trunk area and walking disability due to distal progression.
In this study, CDFs were not prominent as risk factors. CDFs were found when reinnervation occurred following denervation, but occasionally did not occur, especially in extremely in cases with fast progression. Furthermore, the Awaji criteria (2008) indicate that detection of fasciculation potential (FP), which was not investigated in this study, in muscle with chronic findings carries the same significance as active findings such as Fib-Ps and PSWs (10,27). Previous study said that FP is a specific finding in ALS, and occurs inconsistently in the initial stage of ALS before appearance of Fib-Ps and PSWs (28). We performed multivariate regression analysis and identified the detection of either ADFs or CDFs of the cervical-upper area as prognostic factors for progression to severe ALS (3.00 [1.51–5.94], p = 0.002). Interpretation of these associations with CDFs requires further studies.
There are other limitations in this study, including the short follow-up period and ambiguity of the time to outcomes. However, of the patients with initially mild ALS, 38.3% had severe ALS at the last follow-up. Thus, due to the large number of events, our prognostic analysis has certain reliability.
ALS causes a lethal respiratory failure but, recently many patients introduce an artificial respiratory management. However, much previous reports on prognostic factors were set an outcome as time to death, which could not predict the degree of ALS progress. In this study we identified needle EMG findings as prognostic factors, which closely associated with the pathology of ALS, based on nationwide data of ALS patients. The needle EMG is an invasive diagnostic procedure, however, our findings suggest that this experiment is useful for not only accurate diagnosis ALS (29,30) but also prediction ALS prognosis or progression.
Acknowledgements
This study was supported by Health Labour Science Research Grants from the Japanese Ministry of Health, Labour and Welfare, and Research on Measures against Intractable Disease(Notification of Health Service bureau, MHLW; No.0708-1; 8 Jul. 2010) and the National Institute of Public Health approved this study (NIPH-IBRA No.10021; 10 June, 2010).
References
- 1. Ringel SP, Murphy JR, Alderson MK, et al. The natural history of amyotrophic lateral sclerosis. Neurology. 1993; 43:1316–1322. [DOI] [PubMed] [Google Scholar]
- 2. Chiò A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, Traynor BG; Eurals Consortium. Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler. 2009; 10:310–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. del Aguila MA, Longstreth WT, McGuire V, Koepsell TD, van Belle G. Prognosis in amyotrophic lateral sclerosis: A population-based study. Neurology. 2003; 60:813–819. [DOI] [PubMed] [Google Scholar]
- 4. Magnus T, Beck M, Giess R, Puls I, Naumann M, Toyka KV. Disease progression in amyotrophic lateral sclerosis: Predictors of survival. Muscle Nerve. 2002; 25:709–714. [DOI] [PubMed] [Google Scholar]
- 5. Chiò A, Mora G, Leone M, Mazzini L, Cocito D, Giordana MT, Bottacchi E, Mutani R; Piemonte and Valle d'Aosta Register for ALS (PARALS). Early symptom progression rate is related to ALS outcome: A prospective population-based study. Neurology. 2002; 59:99–103. [DOI] [PubMed] [Google Scholar]
- 6. Kimura E, Kobayashi S, Kanatani Y, Ishihara K, Mimori T, Takahashi R, Chiba T, Yoshihara H. Developing an electronic health record for intractable diseases in Japan. Stud Health Technol Inform. 2011; 169:255–259. [PubMed] [Google Scholar]
- 7. Brooks BR, Miller RG, Swash M, Munsat TL; El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000; 1:293–299. [DOI] [PubMed] [Google Scholar]
- 8. Medical Research Council. Aids to the examination of the peripheral nervous system, Memorandum no. 45, Her Majesty's Stationery Office, London, 1981. [Google Scholar]
- 9. de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, Mills K, Mitsumoto H, Nodera H, Shefner J, Swash M. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008; 119:497–503. [DOI] [PubMed] [Google Scholar]
- 10. Mills KR. The basics of electromyography. J Neurol Neurosurg Psychiatry. 2005; 76 (Suppl 2):ii32–ii35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson E. Needle electromyography. Muscle Nerve. 2009; 40:666; author reply 666–667. [DOI] [PubMed] [Google Scholar]
- 12. Lewis P Rowland, Timoth A Pedley. Merritt's Neurology Twelfth edition. Lippincott Williams & Wilkins, Philadelphia, USA, 2010; pp. 83–92. [Google Scholar]
- 13. Blijham PJ, Schelhaas HJ, Ter Laak HJ, van Engelen BGM, Zwarts MJ. Early diagnosis of ALS: The search for signs of denervation in clinically normal muscles. J Neurol Sci. 2007; 263:154–157. [DOI] [PubMed] [Google Scholar]
- 14. Tetsuka S, Morita M, Ikeguchi K, Nakano I. Creatinine/cystatin C ratio as a surrogate marker of residual muscle mass in amyotrophic lateral sclerosis. Neurol Clin Neurosci. 2013; 1:32–37. [Google Scholar]
- 15. Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999; 169:13–21. [DOI] [PubMed] [Google Scholar]
- 16. Ohashi Y, Tashiro K, Itoyama Y, Nakano I, Sobue G, Nakamura S, Sumino S, Yanagisawa N. Study of functional rating scale for amyotrophic lateral sclerosis: Revised ALSFRS (ALSFRS-R) Japanese version. No To Shinkei. 2001; 53:346–355. (in Japanese) [PubMed] [Google Scholar]
- 17. Zhou B, Nakatani E, Teramukai S, Nagai Y, Fukushima M; Alzheimer's Disease Neuroimaging Initiative. Risk classification in mild cognitive impairment patients for developing Alzheimer's disease. J Alzheimers Dis. 2012; 30:367–375. [DOI] [PubMed] [Google Scholar]
- 18. Haberman SJ. The analysis of residuals in cross-classified tables. Biometrics. 1973; 29:205–220. [Google Scholar]
- 19. Weikamp JG, Schelhaas HJ, Hendriks JC, de Swart BJ, Geurts AC. Prognostic value of decreased tongue strength on survival time in patients with amyotrophic lateral sclerosis. J Neurol. 2012; 259:2360–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Carvalho M, Scotto M, Lopes A, Swash M. Clinical and neurophysiological evaluation of progression in amyotrophic lateral sclerosis. Muscle Nerve. 2003; 28:630–633. [DOI] [PubMed] [Google Scholar]
- 21. Luchesi KF, Kitamura S, Mourão LF. Higher risk of complications in odynophagia- associated dysphagia in amyotrophic lateral sclerosis. Arq Neuropsiquiatr. 2014; 72:203–207. [DOI] [PubMed] [Google Scholar]
- 22. Aydogdu I, Tanriverdi Z, Ertekin C. Dysfunction of bulbar central pattern generator in ALS patients with dysphagia during sequential deglutition. Clin Neurophysiol. 2011; 122:1219–1228. [DOI] [PubMed] [Google Scholar]
- 23. Makki AA, Benatar M. The electromyographic diagnosis of amyotrophic lateral sclerosis: Does the evidence support the El Escorial criteria? Muscle Nerve. 2007; 35:614–619. [DOI] [PubMed] [Google Scholar]
- 24. de Carvalho MA, Pinto S, Swash M. Paraspinal and limb motor neuron involvement within homologous spinal segments in ALS. Clin Neurophysiol. 2008; 119:1607–1613. [DOI] [PubMed] [Google Scholar]
- 25. Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: Deconstructing motor neuron degeneration. Neurology. 2009; 73:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanouchi T, Ohkubo T, Yokota T. Can regional spreading of amyotrophic lateral sclerosis motor symptoms be explained by prion-like propagation? J Neurol Neurosurg Psychiatry. 2012; 83:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nodera H, Izumi Y, Kaji R. New diagnostic criteria of ALS (Awaji criteria). Brain Nerve. 2007; 59:1023–1029. (in Japanese) [PubMed] [Google Scholar]
- 28. Bokuda K, Shimizu T. Fasciculation potentials in ALS-Significance and relationship with clinical features. Rinsho Shinkeigaku. 2014; 54:1083–1085. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 29. Swash M. Shortening the time to diagnosis in ALS: The role of electrodiagnostic studies. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000; 1(Suppl 1):S67–S72. [PubMed] [Google Scholar]
- 30. Daube JR. Electrodiagnostic studies in amyotrophic lateral sclerosis and other motor neuron disorders. Muscle Nerve. 2000; 23:1488–1520. [DOI] [PubMed] [Google Scholar]