Abstract
Peach (Prunus persica, Rosaceae) is an extremely popular tree fruit worldwide, with an annual production near 20 million tons. Peach is widely thought to have origins in China, but its evolutionary history is largely unknown. The oldest evidence for the peach has been Chinese archaeological records dating to 8000–7000 BP. Here, we report eight fossil peach endocarps from late Pliocene strata of Kunming City, Yunnan, southwestern China. The fossils are identical to modern peach endocarps, including size comparable to smaller modern varieties, a single seed, a deep dorsal groove, and presence of deep pits and furrows. These fossils show that China has been a critical region for peach evolution since long before human presence, much less agriculture. Peaches evolved their modern morphology under natural selection, presumably involving large, frugivorous mammals such as primates. Much later, peach size and variety increased through domestication and breeding.
Peach (Prunus persica, Rosaceae) is a universally known tree fruit with an annual production near 20 million tons1; it is also a genetic model organism2. China has a long history of peach cultivation known from both historical and archaeological evidence3,4. The word peach (“ ”) has long appeared in Chinese literature, e.g., the books Shi-Jing (1,100–600 BC) and Shi-Ji (1st century BC)3. Despite the significant fossil record of Rosaceae and the genus Prunus5,6,7,8,9,10,11,12,13,14,15, the origins of the peach and its unique features remain unknown. No wild population has been confirmed16, and the long history of trade and complex genomics of peach cultivars present considerable obstacles17. Recently, we found eight fossil peach endocarps, in the late Pliocene Ciying Formation in Kunming, Yunnan, southwestern China (Fig. 1), whose morphological characters are identical to modern peaches. This discovery of the oldest fossil peaches provides important evidence for the origins and evolution of the modern fruit.
”) has long appeared in Chinese literature, e.g., the books Shi-Jing (1,100–600 BC) and Shi-Ji (1st century BC)3. Despite the significant fossil record of Rosaceae and the genus Prunus5,6,7,8,9,10,11,12,13,14,15, the origins of the peach and its unique features remain unknown. No wild population has been confirmed16, and the long history of trade and complex genomics of peach cultivars present considerable obstacles17. Recently, we found eight fossil peach endocarps, in the late Pliocene Ciying Formation in Kunming, Yunnan, southwestern China (Fig. 1), whose morphological characters are identical to modern peaches. This discovery of the oldest fossil peaches provides important evidence for the origins and evolution of the modern fruit.
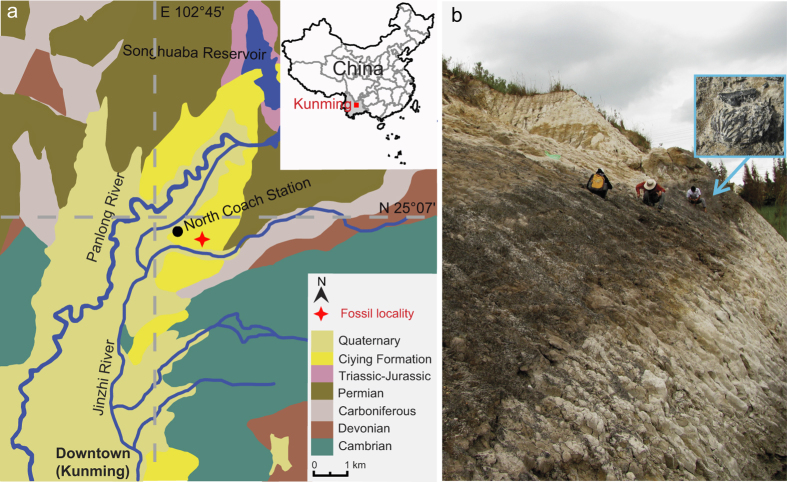
Figure 1. Fossil locality in Kunming, Yunnan Province, southwestern China.
(a) Geologic map, modified from Bureau of Geology and Mineral Resources of Yunnan Province, 199030 with the software Adobe Illustrator CS4. (b) Stratigraphic section, arrow showing the fossil-bearing layer; inset shows fossil peach endocarp in situ.
Order—Rosales Bercht. & J. Presl
Family—Rosaceae Juss.
Genus—Prunus L.
Subgenus—Amygdalus L.
Species—Prunus kunmingensis T. Su, P. Wilf et Z.K. Zhou sp. nov.
Holotype—KUN PC2015001 (Fig. 2a) (designated here).
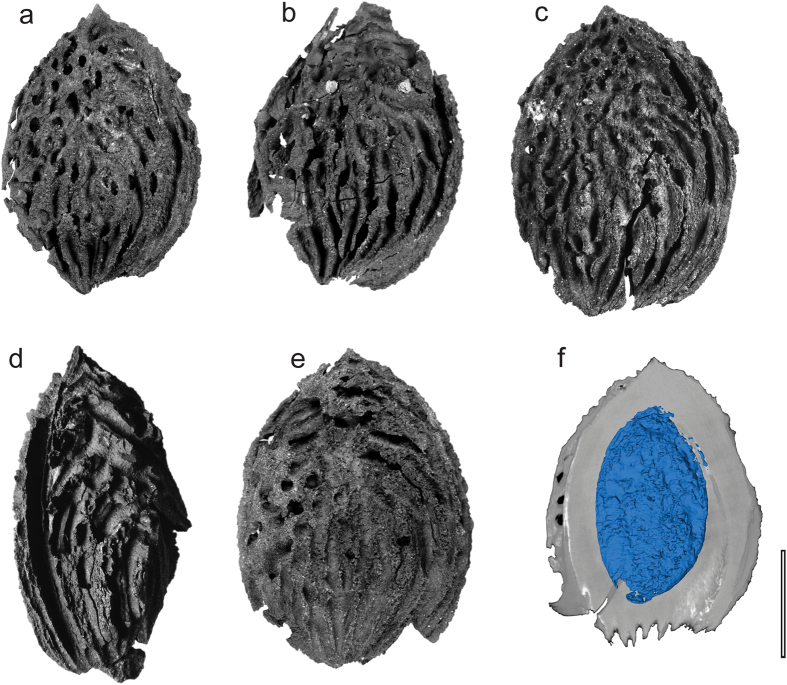
Figure 2. Prunus kunmingensis.
(a–e) KUN PC2015001-KUN PC2015005. (f) CT scan showing longitudinal section and seed (KUN PC2015001). Scale bar = 1 cm. See the three dimensional reconstruction in Animation S1.
Paratypes—KUN PC2015002 (Fig. 2b), KUN PC2015003 (Fig. 2c), KUN PC2015004 (Fig. 2d), KUN PC2015005 (Fig. 2e), KUN PC2015006, KUN PC2015007, KUN PC2015008.
Locality—The late Pliocene Ciying Formation, North Terminal Bus Station, Kunming, central Yunnan Province, southwestern China (Fig. 1).
Repository—The Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (KUN).
Etymology—The specific epithet "kunmingensis" refers to the discovery location in Kunming.
Description—Stony endocarps (Fig. 2) elliptical, flattened in lateral view (presumably compressed), base obtuse, apex apiculate, length 2.6–3.0 cm, width 1.8–2.3 cm, length:width ratio 1.3–1.6:1, thickness 0.8–1.2 cm. Endocarp exterior surface with both furrows and pits. Single deep groove of vascular bundle canal on dorsal side, extending from base to apex. Ridge on ventral side. Transverse furrows (Fig. 2a) one or two, following edges of both dorsal and ventral sides. Longitudinal furrows (Fig. 3a) seven to ten, radiating apically from the base over less than half the endocarp length. Deep pits (Fig. 2) mainly situated near the apex. Endocarp interior surface (Fig. 3c) smooth, with linear striations; internal sclerids (Fig. 3e) apparently diagenetically altered. Seed (Fig. 2f) single, flattened, elliptical, base round, apex acute, length ~1.9 cm, width ~1.0 cm, replaced by iron compounds.
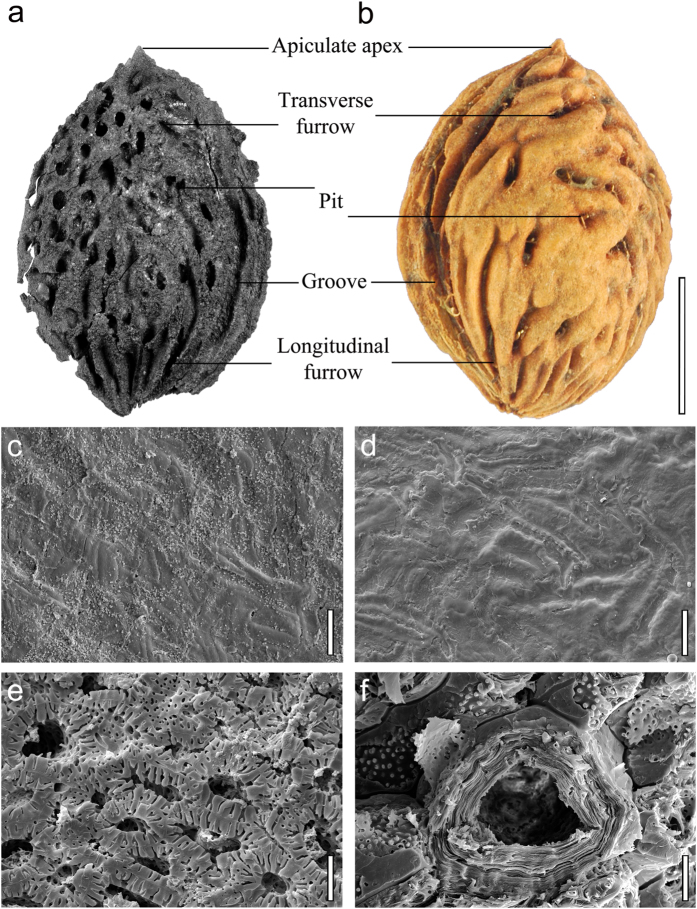
Figure 3.
Morphological comparison of endocarps between Prunus kunmingensis (a,c,e) and modern peach (b,d,f). (a,b) Gross morphology. (c,d) Endocarp interior surface with linear striations. (e,f) Diagenetically altered fossil sclereids (e) and modern sclereids (f) along a transverse section of the endocarp. Scale bars: a–b = 1 cm; c–d = 30 μm; e–f = 15 μm.
Discussion
Several characters of the fossils, including the large, single-seeded endocarp, elliptic shape, and the deep vascular bundle canal along the edge of the dorsal side, unambiguously assign them to the genus Prunus8. Although molecular phylogenies are revising infrageneric relationships18, Rehder’s19 widely-used classification of Prunus recognizes five subgenera: Amygdalus, Cerasus, Laurocerasus, Padus, and Prunus. Of these, subgenus Amygdalus is consistent with the fossils in having the largest endocarp size (mean lengths and widths usually more than 1.5 cm16,20) and because it is the only subgenus with species that have both furrows and pits on the endocarps (Figs 2 and 3a).
Subgenus Amygdalus has two sections, Amygdalus and Persica21. Endocarp shape and size are similar in both sections, but deep furrows are usually absent in section Amygdalus21. Many additional features of the fossils show their close affinity to the living peach, Prunus persica, as seen in our full character matrix for 36 modern Prunus species, plus the fossils, that shows identical scores for the fossils and modern peaches (Fig. 4; Supplementary Table 1). The most distinctive features of peaches that are seen in the fossil endocarps, in combination, are the large size, apiculate apex, presence of both pits and furrows on the exterior surface, and typical linear striations on the interior endocarp surface (Fig. 3). In sum, the well-preserved fossil endocarps show no differences from the living peach (Supplementary Figure 1) and could be assigned to the extant species. However, other organs of the ancient plant are not yet known, and we instead propose the new species name, Prunus kunmingensis, to provide an unambiguous epithet for the fossils in the absence of a whole-plant reconstruction.
Figure 4. Nonmetric Multidimentional Scaling analysis of 36 Prunus species based on 12 morphological characters of endocarps.
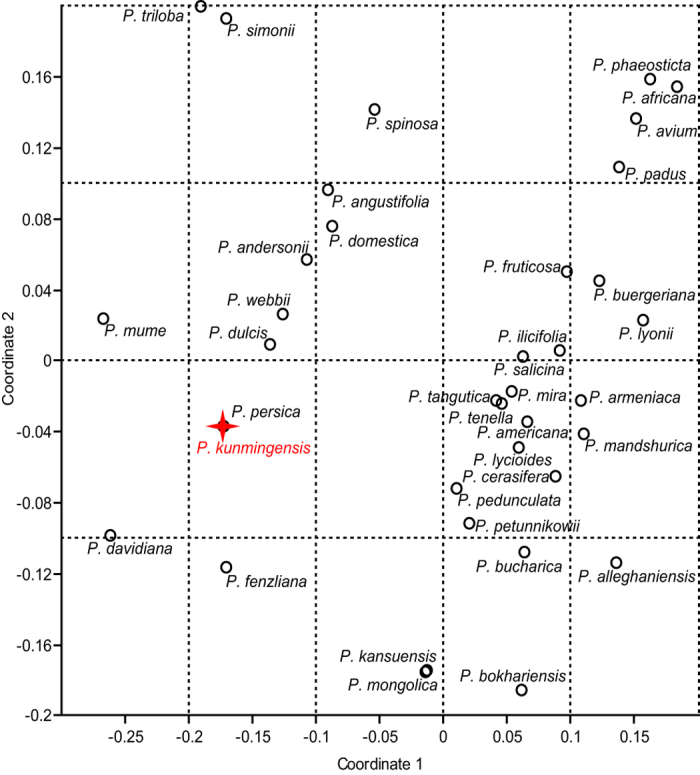
Prunus kunmingensis and modern peach share the same character scores. Data listed in Supplementary Table 1.
The well-preserved specimens reported here comprise the earliest record of peach, from the late Pliocene (i.e., by ca. 2.6 million years ago), as well as the only occurrence that predates archaeological evidence. The oldest reliable evidence for the genus Prunus comes from the Eocene of the Northern Hemisphere as endocarps, leaves, and wood, as recently reviewed by DeVore and Pigg22. Nevertheless, there are no reliable fossils that show close morphological similarities to peach8,15 except for subfossils that are mostly from Chinese archaeological sites3,4, because the typical characters of endocarps in peach, i.e., the presence of deep pits and furrows, as well as the apiculate apex, are absent in all these fossils.
The associated flora from the fossil-bearing layer, as well as global and regional paleoclimate reconstructions, all indicate that the ancient peach trees lived in a warmer, wetter regional climate than today23,24,25. The associated flora includes abundant fruits of the ring-cupped oak (Quercus subgenus Cyclobalanopsis, Fagaceae; Supplementary Figure 2), whose extant species are evergreen trees that principally inhabit tropical and subtropical Asian forests. Both ring-cupped oak and Prunus davidiana, a species with close affinity to peach, are found naturally today in subtropical forests of central Yunnan Province26.
Prunus kunmingensis demonstrates the early presence of peach in southwestern China and dramatically increases the region’s established significance for the evolutionary origins and cultivation history of the fruit. Southwestern China holds high species diversity in rosaceous genera with agricultural significance such as Malus (apple), Prunus (almond, apricot, cherry, peach and plum), and Pyrus (pear) (Supplementary Figure 3). In Prunus section Persica, all species except for Prunus mongolica are native to the region16, and a natural population of Prunus mira (section Persica), with some individual trees more than 1000 years old, exists in Linzhi County, eastern Tibet27. That region is also especially rich in local peach cultivars27,28.
The endocarps of modern peach cultivars show much more morphological variation and generally larger sizes than both Prunus kunmingensis and archaeological subfossils (Supplementary Figure 4), presumably reflecting the subsequent selection of varieties under cultivation. However, the size of Prunus kunmingensis is within the lower range of modern peach cultivars (Fig. 5, Supplementary Table 2), as are peach endocarps from some archaeological sites3,4. In modern peaches, endocarp size positively correlates with fruit size (Fig. 5); if this correlation existed in the past, the fossil endocarps indicate mean fruit diameters of ~5.2 cm (Fig. 5).
Figure 5. Size correlation between endocarps and fruits in modern peach cultivars and estimated fruit size in the fossil Prunus kunmingensis.
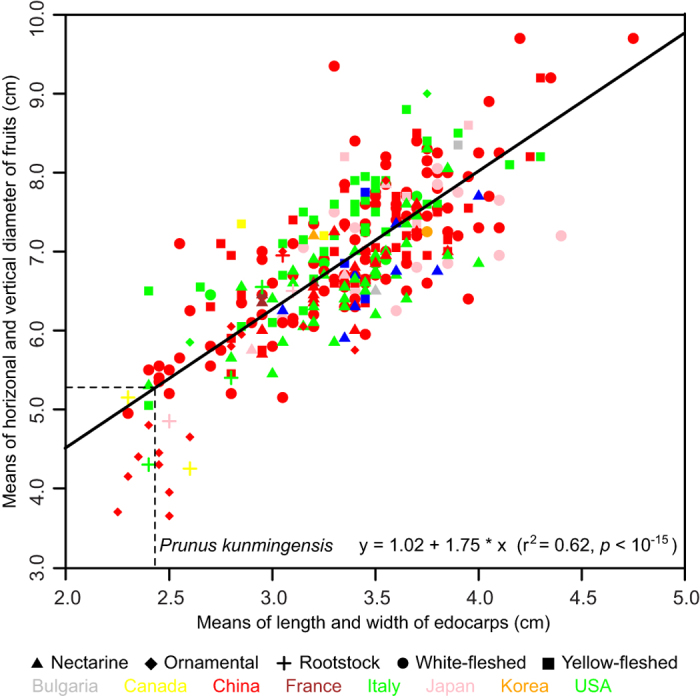
Data from modern cultivars are measured from photographs in Wang et al. 201228 and listed in Supplementary Table 2. Lines indicate the fossil endocarps of Prunus kunmingensis, whose inferred fruit diameter, based on the correlation shown here (solid black line), is ~5.2 cm.
Presumably, the fleshy ancient peaches would have been a desirable food source for large-bodied frugivores such as primates. Of special interest, the fossils show that peaches were already present in SW China before the Pleistocene arrivals of Homo erectus and Homo sapiens29. The universally known seed-dispersal mutualism between hominids and peaches, in all likelihood, is very ancient.
Methods
Geological setting
During August, 2010, eight fossil fruit endocarps were collected near North Terminal Bus Station of Kunming, Yunnan Province, southwestern China (25° 06'19.77"N, 102°45'52.45"E, 1974 m a.s.l.; Fig. 1a) by Paleoecology Group members of Xishuangbanna Tropical Botanical Garden. The fossiliferous strata, recently exposed by new road construction (Fig. 1b), are assigned to the Ciying Formation30. The stratigraphy of the Ciying Formation has been extensively described31. The geological age of the formation is considered to be late Pliocene32 based on a combination of lithostratigraphic correlations30, paleomagnetic data33, and regional palynology34. The fossils studied here came from organic-rich silty mudstones in the upper layer of the formation.
Morphological observation
Fossils were soaked in distilled water and cleaned with an ultrasonic cleaner (UC KO-50M) at a frequency of 40 kHz for 10 minutes to remove sand grains on the surface. Macrophotography was done with a Nikon D700 on a Kaiser 5510 stand, with the fossils placed on glass to eliminate shadows and improve contrast. Measurements were taken with a digital vernier caliper (Mitutoyo 500–351). The surface features of the fossils were observed under a stereo microscope (Zeiss REO Discovery V20). Detailed morphology was obtained under a scanning electron microscope (Zeiss EVO LS10) in the Central Laboratory, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences. To observe three-dimensional internal structures, the fossils were scanned with a HD-600 CT scanner in the Center for Quantitative X-Ray Imaging at Pennsylvania State University.
Morphology of modern Prunus endocarps was examined at the U.S. National Seed Herbarium (located at the U.S. National Arboretum, Washington, DC) and the Herbarium of Kunming Institute of Botany (KUN). For each species, one to five fruit endocarps (average of three) were observed, depending on the number of specimens available. Twelve morphological characters were scored for the endocarps of the fossils and for 36 living Prunus species (Supplementary Table 1). Nonmetric Multidimentional Scaling with euclidean distance measure, using the software PAST (Version 1.75 b, Oslo, Norway) was applied to analyze morphological similarity among species (Fig. 4).
Exclusion of modern sample contamination
Fossils were extracted from freshly exposed, well-bedded strata on a steep slope, in a layer containing many other species of fossil plants. However, the fossils look so strikingly modern that the possibility of modern contamination needed to be rigorously excluded. We measured the elemental composition of a seed in one of the endocarps, using Energy Dispersive analysis (EDS) with a scanning electron microscope (FEI Nova NanoSEM 630) in the Materials Characterization Laboratory at Pennsylvania State University. The seed is mostly replaced by iron oxides (Supplementary Table 3). In addition, we analyzed the 14C age of one endocarp at Beta Analytic (Miami, USA), using the AMS-Standard delivery method (Supplementary Table 3). Results indicate that the age of the fossils is beyond the range of radiocarbon dating (>43,500 years, Supplementary Table 3). The endocarps are flattened, presumably from compression, and the sclereid morphology is diagenetically altered (Fig. 3e), which further confirmed that the fossils are ancient and do not represent recent human activities at the site. All these lines of evidence show that the fossils were preserved within the Pliocene strata and do not represent more recent additions.
Additional Information
How to cite this article: Su, T. et al. Peaches Preceded Humans: Fossil Evidence from SW China. Sci. Rep. 5, 16794; doi: 10.1038/srep16794 (2015).
Supplementary Material
Acknowledgments
We thank J. H. Kirkbride for assistance at the U.S. National Seed Herbarium; T. Ryan for CT scanning and analyses; and L. Wang for SEM assistance. The Central Laboratory in Xishuangbanna Tropical Botanical Garden provided technical support for imaging; R. Wilf assisted with drafts; D. Layne, W.-C. Fang, G.-R. Zhu, and Y.-F. Zheng provided helpful advice on modern cultivars; Q.-J. Li gave constructive suggestions; and S.-H. Li provided geological information. This work is supported by National Natural Science Foundation of China (No. 31470325 to T.S. and 41372035 to Z.K.Z.); the 973 Project (No. 2012CB821900 to Z.K.Z.); the Foundation of the State Key Laboratory of Paleobiology and Stratigraphy, Nanjing Institute of Geology and Paleontology, CAS (No. 143107 to T.S. and No. 123107 to Y.J.H.); the CAS 135 program (No. XTBG-T01 and XTBG-F01 to Z.K.Z.); and the David and Lucile Packard Foundation (to P.W.). This work is a contribution to NECLIME (Neogene Climate Evolution in Eurasia).
Footnotes
Author Contributions T.S., P.W. and Z.Z. designed the research plan. T.S. and Y.H. discovered the fossils. S.Z. provided geological information. T.S. and P.W. performed analyses. T.S. and P.W. wrote the manuscript. All authors discussed and commented on the manuscript.
References
- Food and Agriculture Organization of the United Nations Statistics Division (FAOSTAT), Data of Annual Crop Production, http://faostat3.fao.org. Date of access: 12th November, 2013.
- Verde I. et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nature Genet. 45, 487–494 (2013). [DOI] [PubMed] [Google Scholar]
- Layne D. R. & Bassi D. The Peach: Botany, Production and Uses (CABI, 2008). [Google Scholar]
- Zheng Y.-F., Crawford G. W. & Chen X.-G. Archaeological evidence for peach (Prunus persica) cultivation and domestication in China. PLoS One 9, e106595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai D. H. & Walther H. Die Flora der Haselbacher Serie um Weißlster-Becken (Bezirk Leipzig, DDR). Abh. Staatl. Mus. Mineral. Geol. Dresden 28, 1–200 (1978). [Google Scholar]
- Wheeler E. Scott R. A. & Barghoorn E. S. Fossil dicotyledonous woods from Yellowstone National Park, part II. J. Arnold Arbor. 58, 280–306 (1978). [Google Scholar]
- Muller J. Fossil pollen records of extant angiosperms. Bot. Rev. 47, 1–142 (1981). [Google Scholar]
- Mai D. H. Karpologische Untersuchungen der Steinkerne fossiler und rezenter Amygdalaceae (Rosales). Fedd. Repert. 95, 299–322 (1984). [Google Scholar]
- Cevallos-Ferriz S. R. S. & Stockey R. A. Fruits and Seeds from the Princeton Chert (Middle Eocene) of British Columbia: Rosaceae (Prunoideae). Bot. Ga z. 152, 369–379 (1991). [Google Scholar]
- Manchester S. R. Fruits and seeds of the middle Eocene Nut Beds flora, Clarno Formation, Oregon. Palaeontogr. Amer. 58, 1–205 (1994). [Google Scholar]
- Mai D. H. Tertiäre Vegetationsgeschichte Europas, Methoden und Ergebnisse. (Gustav Fischer Verlag, 1995). [Google Scholar]
- Pollmann B. Jacomet S. & Schlumbaum A. Morphological and genetic studies of waterlogged Prunus species from the Roman vicus Tasgetium (Eschenz, Switzerland). J. Archaeol. Sci. 32, 1471–1480 (2005). [Google Scholar]
- Martinetto E. Palaeoenvironmental significance of plant macrofossils from the Piànico Formation, Middle Pleistocene of Lombardy, North Italy. Quat. Int. 204, 20–30 (2009) [Google Scholar]
- Benedict J. C. DeVore M. L.& Pigg K. B. Prunus and Oemleria (Rosaceae) Flowers from the Late Early Eocene Republic Flora of Northeastern Washington State, U.S.A. Int. J. Plant Sci. 172, 948–958 (2011). [Google Scholar]
- Li Y. et al. Endocarps of Prunus (Rosaceae: Prunoideae) from the early Eocene of Wutu, Shandong Province, China. Taxon 60, 555–564 (2011). [Google Scholar]
- Gu C.-Z. et al. Flora of China (Vol. 9). (Science Press & Missouri Botanical Garden, 2003). [Google Scholar]
- Yazbek M. & Al-Zein M. Wild almonds gone wild: revisiting Darwin’s statement on the origin of peaches. Genet. Resour. Crop Evol. 61, 1319–1328 (2014). [Google Scholar]
- Chin S.-W., Shaw J., Haberle R., Wen J. & Potter D. Diversification of almonds, peaches, plums and cherries – Molecular systematics and biogeographic history of Prunus (Rosaceae). Mol. Phylogenet. Evol. 76, 34–48 (2014). [DOI] [PubMed] [Google Scholar]
- Rehder A. North America Exclusive of the Subtropical and Warmer Temperate Regions 2nd edn. (MacMillan, 1940). [Google Scholar]
- Bojňanský V. & Fargašová A. Atlas of Seeds and Fruits of Central and East-European Flora. The Carpathian Mountains Region. (Springer, 2007). [Google Scholar]
- Yazbek M. & Oh S. H. Peaches and almonds: phylogeny of Prunus subg. Amygdalus (Rosaceae) based on DNA sequences and morphology. Plant Syst. Evol. 299, 1403–1418 (2013). [Google Scholar]
- DeVore M. L. & Pigg K. B. A brief review of the fossil history of the family Rosaceae with a focus on the Eocene Okanogan Highlands of eastern Washington State, USA, and British Columbia, Canada. Plant Syst. Evol. 266, 45–57 (2007). [Google Scholar]
- Tao J.-R. The Evolution of the Late Cretaceous-Cenozoic Flora in China. (Science Press, 2000). [Google Scholar]
- Zachos J., Pagani M., Sloan L., Thomas E. & Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001). [DOI] [PubMed] [Google Scholar]
- Su T. et al. Post-Pliocene establishment of the present monsoonal climate in SW China: evidence from the late Pliocene Longmen megaflora. Clim. Past. 9, 1911–1920 (2013). [Google Scholar]
- Wu Z.-Y. & Zhu Y.-C. Vegetation of Yunnan. (Science Press, 1987). [Google Scholar]
- Wang Z.-H. & Zhuang E.-J. China Fruit Monograph-Peach Flora. (China Forestry Press, 2001). [Google Scholar]
- Wang L.-R., Zhu G.-R. & Fang W.-C. Peach Genetic Resources in China. (China Agriculture Press, 2012). [Google Scholar]
- Wu X. & Poirier F. E. Human Evolution in China: A Metric Description of the Fossils and a Review of the Sites. (Oxford University Press, 1995). [Google Scholar]
- Yunnan Bureau of Geology and Mineral Resources Resources. Regional Geology of Yunnan Province. (Geological Publishing House, 1990). [Google Scholar]
- Hu Q. et al. Evolution of stomatal and trichome density of the Quercus delavayi complex since the late Miocene. Chin. Sci. Bull. 59, 310–319 (2014). [Google Scholar]
- Zhang S.-X. Geological Formation Names of China (1866-2000). (Springer, 2009). [Google Scholar]
- Jiang C.-S., Zhou R.-Q. & Hu Y.-X. Features of geological structure for Kunming Basin. J. Seismol. Res. 26, 67–74 (2003). [Google Scholar]
- Wang W.-M. & Shu J.-W. Late Cenozoic palynofloras from Qujing Basin, Yunnan, China. Acta Palaeontol. Sin. 43, 254–261 (2004). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.