Abstract
Rationale
Because of lack of information regarding timing of stroke, patients who suffer stroke during sleep are generally ineligible for intravenous thrombolysis, although many of these patients could potentially recover with this treatment. Magnetic resonance image findings with positive diffusion-weighted imaging and no marked parenchymal hyperintensity on fluid-attenuated inversion recovery (negative pattern) can identify acute ischemic stroke patients within 4·5 h from symptom onset.
Aims
The THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0·6 mg/kg trial aims to determine the efficacy and safety of intravenous thrombolysis with alteplase at 0·6 mg/kg body weight, the approved dose for Japanese stroke patients, using magnetic resonance image-based selection in ischemic stroke patients with unclear time of symptom onset, and compare findings with standard treatment.
Design
This is an investigator-initiated, multicenter, prospective, randomized, open-treatment, blinded-end-point clinical trial. The design is similar to the Efficacy and Safety of MRI-based Thrombolysis in Wake-up Stroke trial. Patients with unclear-onset time of stroke symptoms beyond 4·5 h and within 12 h after the time of the last-known-well period and within 4·5 h after symptom recognition, who showed a negative fluid-attenuated inversion recovery pattern, are randomized to either intravenous thrombolysis or standard treatment.
Study outcomes
The primary efficacy end-point is modified Rankin Scale 0–1 at 90 days. The safety outcome measures are symptomatic intracranial hemorrhage at 22–36 h, and major bleeding and mortality at 90 days.
Discussion
This trial may help determine if low-dose alteplase at 0·6 mg/kg should be recommended as a routine clinical strategy for ischemic stroke patients with unclear-onset time.
Keywords: acute ischemic stroke, clinical trials, diffusion-weighted imaging, fluid-attenuated inversion recovery imaging, thrombolysis, unclear-onset time
Introduction and rationale
Currently, intravenous (i.v.) thrombolysis with recombinant tissue-type plasminogen activator (rt-PA) is the only evidence-based effective treatment for acute ischemic stroke within 4·5 h after symptom onset (1,2). Because the therapeutic time range for effective i.v. rt-PA therapy is limited, onset-to-treatment time is vital. Delayed thrombolysis may cause a life-threatening condition such as symptomatic intracranial hemorrhage (sICH). Because accurate onset-to-treatment time is not determined for patients with unclear-onset time of stroke symptoms or those who wake up with stroke symptoms, i.v. rt-PA therapy is usually contraindicated for these patients.
According to previous reports, about one-fourth of acute stroke patients suffer from stroke symptoms with unclear-onset time or onset during sleep (3–5). These patients were reported to have similar early ischemic findings on initial computed tomography (CT) or magnetic resonance imaging (MRI) compared with those presenting within three-hours (6) or six-hours of symptom recognition (7). Because onset of all subtypes of stroke has an early morning peak (8), a large number of patients who wake up with stroke symptoms may still be within the time window for i.v. thrombolysis when they arrive at the hospital.
Recently, stroke MRI findings with positive diffusion-weighted imaging (DWI) and no marked parenchymal hyperintensity on fluid-attenuated inversion recovery (FLAIR) (negative FLAIR pattern) was proposed to act as a ‘brain clock’ that indicates the stroke occurred within 3–4·5 h from stroke symptom onset (9–11). DWI depicts a reduced apparent diffusion coefficient indicating cytotoxic edema caused by ischemia within minutes of stroke (12). FLAIR is characterized by strong T2 weighting together with suppression of cerebrospinal fluid signal and is often positive 3–4·5 h after stroke onset (9–11,13).
There are several reports of nonrandomized patients on thrombolysis based on CT or MRI findings in acute stroke patients with unclear-onset time. Most reports had retrospective study designs and suggested the safety and feasibility of thrombolysis for this subgroup of stroke patients (14–19). In Japan, a single-center, nonrandomized, prospective study involving 20 patients with unclear-onset time and negative FLAIR pattern on initial MRI showed no occurrence of sICH after i.v. rt-PA at 0·6 mg/kg alteplase; at 90 days, 47% of patients showed modified Rankin Scale (mRS) scores of 0–2 (15). However, there is no established data from randomized, controlled trials of i.v. rt-PA in patients with unclear-onset time. To our knowledge, there are six ongoing clinical trials to test the safety and efficacy of thrombolysis in acute stroke patients with unclear-onset time worldwide: the Safety of Intravenous Thrombolytics in Stroke on Awakening (SAIL-ON) (ClinicalTrials.gov Identifier NCT01643902), the Safety of Intravenous Thrombolysis for Wake-up Stroke (Wake-Up Stroke) (ClinicalTrials.gov Identifier NCT01183533), the Efficacy and Safety of MRI-based Thrombolysis in Wake-up Stroke (WAKE-UP) (ClinicalTrials.gov Identifier NCT01525290) (20), the Wake up Symptomatic Stroke in Acute Brain Ischemia (WASSABI) Trial (ClinicalTrials.gov Identifier NCT01455935), and WUS-rTPA (EudraCT no. 2010–019359-23). All of the trials use alteplase at 0·9 mg/kg. We hypothesized that stroke patients with unclear-onset time and a negative FLAIR pattern on MRI will improve more by i.v. thrombolysis than by standard treatment using alteplase at 0·6 mg/kg, a dose that is unique to Japanese patients. Thus, we planned a multicenter trial to test this hypothesis.
Methods
Design
The THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0·6 mg/kg (THAWS) Trial is an investigator-initiated, multicenter, prospective, randomized, open-treatment, blinded-end-point clinical trial comparing i.v. rt-PA (alteplase) and standard treatment in unclear-onset stroke. This trial is registered with the ClinicalTrials.gov (Clinical Trials.gov Identifier NCT02002325) and the UMIN clinical trial (ID: UMIN000011630). Figure 1 shows a flowchart of the trial design. Table 1 shows a schedule of the trial. The design is similar to the WAKE-UP trial (20).
Fig. 1.
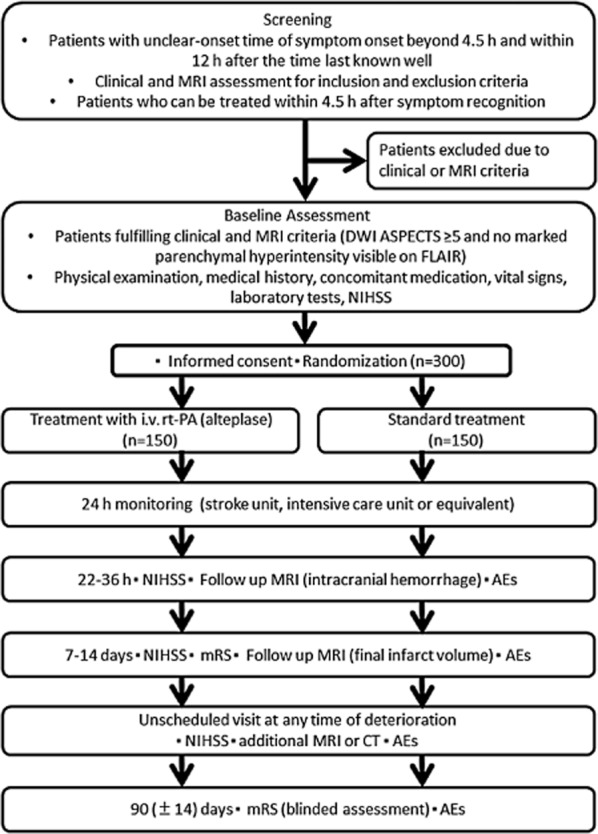
Thrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0·6 mg/kg (THAWS) trial flow chart. AEs, adverse events; ASPECTS, the Alberta Stroke Program Early CT Score; CT, computed tomography; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; i.v., intravenous; MRI, magnetic resonance imaging; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; rt-PA, recombinant tissue-type plasminogen activator.
Table 1.
Schedule of assessments for THAWS trial
| Baseline |
Administration of treatment |
Observational period |
|||
|---|---|---|---|---|---|
| Timing | Enrollment | 0 h | 24 h* ± 3 h | Day 7 or discharge ± 1 day† | Day 90 ± 14 days |
| Consent | ○ | ||||
| Demographics/Baseline information/Medical history/Prior medication | ○ | ||||
| Screening/Eligibility | ○ | ||||
| Randomization | ○ | ||||
| Physical examination | |||||
| NIHSS | ○ | ○ | ○ | ||
| mRS | Premorbid | ○ | ○ | ||
| Height/Weight | ○ | ||||
| Blood pressure/Pulse rate | ○ | ○ | ○ | ||
| Body temperature | ○ | ||||
| Laboratory tests/Imaging | |||||
| Blood test | ○ | ○ | |||
| Urine test | ○ | ||||
| Electrocardiogrphy | ○ | ||||
| MRI | ○ | ○* | ○† | ||
| Adverse events | ○ | ○ | ○ | ○ | |
| Alteplase administration | ○ | ||||
Follow-up MRI at 24 h is allowed to be obtained between 22 and 36 h after the treatment administration.
Follow-up MRI at day 7 is allowed to be obtained between 7 and 14 days after the treatment administration or at discharge (whichever is first).
MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Patient population
Patients with acute ischemic stroke whose onset time of stroke symptoms cannot be determined represent the target population for the THAWS. Inclusion and exclusion criteria are listed in Table 2. As clinical inclusion criteria for timing of treatment initiation, time from last-known-well period without neurological symptoms to treatment initiation should be between 4·5 and 12 h, and time from symptom recognition to treatment initiation should be within 4·5 h. Patients with posterior circulation stroke including brain stem stroke are not excluded because they are reported to potentially have a longer therapeutic time window than those with anterior stroke (21). The negative FLAIR pattern as an acute ischemic lesion visible on DWI, but no marked parenchymal hyperintensity visible on FLAIR is a vital imaging inclusion (Fig. 2). Exclusion criteria mainly follow the prescribing information for alteplase in Japan. A detailed imaging guidebook kindly provided by the WAKE-UP steering committee will confer extensive examples illustrating inclusion and exclusion criteria on MRI (20).
Table 2.
Inclusion and exclusion criteria
| Clinical inclusion criteria |
| • Clinical diagnosis of acute ischemic stroke with unknown symptom onset (e.g., acute wake-up ischemic stroke and acute ischemic stroke with unknown time of symptom onset) |
| • Age 20 years or older |
| • Last-known-well period without neurological symptoms > 4·5 h and <12 h of treatment initiation |
| • Treatment can be started within 4·5 h of symptom recognition (e.g., awakening) |
| • Initial NIHSS ≥ 5 and ≤25 |
| • Written informed consent by patient or next of kin |
| Imaging inclusion criteria |
| • Acute stroke MRI including DWI and FLAIR completed |
| • ASPECTS on initial DWI ≥ 5 |
| • Pretreatment MRI showing a pattern of ‘negative FLAIR’, that is, acute ischemic lesion visible (or normally visible) on DWI but no marked parenchymal hyperintensity visible on FLAIR indicative of an acute ischemic lesion ≤ 4·5 h of age |
| Clinical exclusion criteria |
| • Prestroke mRS > 1 (patients who have inability to carry out all daily activities and require some help or supervision) |
| • Contraindications in the Japanese guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase) |
| √ History of nontraumatic intracranial hemorrhage |
| √ History of stroke within the last one-month (excluding transient ischemic attack) |
| √ History of significant head/spinal injury or surgery within the last three-months |
| √ History of gastrointestinal or urinary tract bleeding within the last 21 days |
| √ History of major surgery or significant trauma other than head injury within the last 14 days |
| √ Hypersensitivity to alteplase or any of the excipients |
| √ Suspected subarachnoid hemorrhage |
| √ Concurrent acute aortic dissection |
| √ Concurrent hemorrhage (e.g., intracranial, gastrointestinal, urinary tract, or retroperitoneal) |
| √ Systolic blood pressure ≥ 185 mmHg despite antihypertensive therapy |
| √ Diastolic blood pressure ≥ 110 mmHg despite antihypertensive therapy |
| √ Significant hepatic disorder |
| √ Acute pancreatitis |
| √ Blood glucose < 50 or >400 mg/dL (<2·8 or >22·2 mmol/L) |
| √ Platelet count ≤ 100 000/mm3 |
| √ PT-INR > 1·7 or prolonged aPTT [>1·5 times the baseline value ( > approximately 40 s only as a guide)] for patients on anticoagulation therapy or those with abnormal coagulation |
| • Any contraindication to MRI (e.g., cardiac pacemaker) |
| • Planned or anticipated treatment with surgery or endovascular reperfusion strategies (e.g., intra-arterial thrombolysis, mechanical recanalization techniques) |
| • Pregnant, lactating, or potentially pregnant |
| • Life expectancy six-months or less by judgment of the investigator |
| • Inappropriate for study enrollment by judgment of the investigator |
| Imaging exclusion criteria |
| • Poor MRI quality precluding interpretation according to the study protocol |
| • Large DWI lesion volume > 50% of the anterior cerebral artery or posterior cerebral artery territory (visual inspection) |
| • Large DWI lesion in brain stem or cerebellum (e.g., more than half of brain stem or more than half of unilateral cerebellar hemisphere) |
| • Any sign of intracranial hemorrhage on baseline MRI |
| • FLAIR showing marked parenchymal hyperintensity corresponding to the acute DWI lesion indicative of an acute ischemic lesion with a high likelihood of being >4·5 h old (‘positive FLAIR’) |
| • Any MRI findings indicative of a high risk of symptomatic intracranial hemorrhage related to potential intravenous alteplase treatment in the judgment of the investigator |
aPTT, activated partial thromboplastin time; ASPECTS, Alberta Stroke Program Early CT score; DWI, diffusion weighted imaging; FLAIR, fluid attenuated inversion recovery; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; PT-INR, prothrombin time international normalized ratio.
Fig. 2.
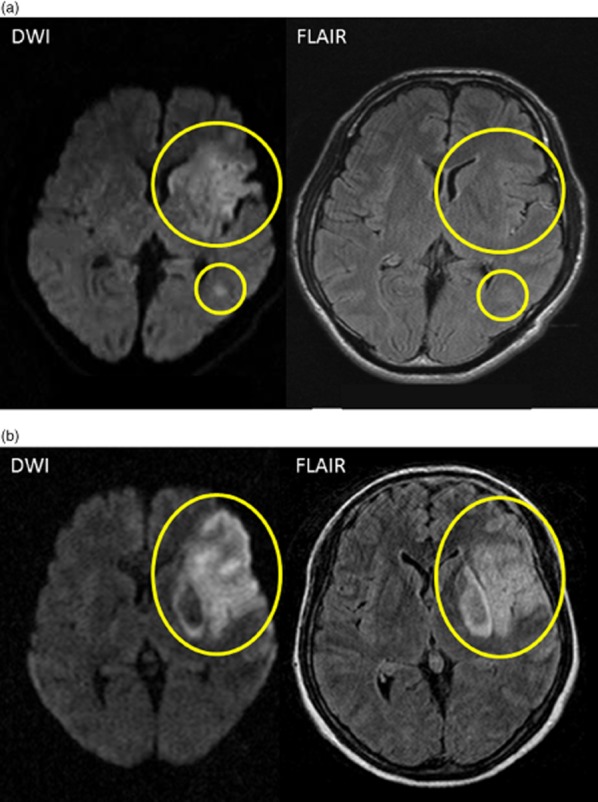
Examples of magnetic resonance imaging (MRI) inclusion and exclusion criteria. (a) A negative fluid-attenuated inversion recovery (FLAIR) pattern shows an acute ischemic lesion clearly visible on diffusion-weighted imaging (DWI), but no marked parenchymal hyperintensity visible on fluid-attenuated inversion recovery (FLAIR) corresponding to the DWI lesion (yellow circles). (b) A positive FLAIR pattern shows an acute ischemic lesion clearly visible on DWI and clear parenchymal hyperintensity on FLAIR corresponding to the acute DWI lesion (yellow circle).
MRI sequence and baseline assessment
DWI (spin-echo echo planar imaging), FLAIR (fast spin echo), T2* and magnetic resonance angiography (MRA) sequences are mandatory acquired. Perfusion imaging with dynamic susceptibility contrast is optionally added for baseline imaging. The details of DWI and FLAIR parameters are as follows: DWI with field of view (FOV) of 240 mm, acquisition matrix of 128 × 128, slice thickness of ≈5 mm, gap of 0–1 mm, repetition time (TR) of ≈8000 ms, and echo time (TE) of ≤100 ms; FLAIR on 1·5 Tesla MRI with FOV of 240 mm, acquisition matrix of 256 × 256, slice thickness of ≈5 mm, gap of 0–1 mm, TR of ≥8000 ms, TE of 100–140 ms, and inversion time (TI) of ≈2300 ms; and FLAIR on 3 Tesla MRI with FOV of 240 mm, acquisition matrix of 256 × 256, slice thickness of ≈5 mm, gap of 0–1 mm, TR of ≥10000 ms, TE of 95–125 ms, and TI of ≈2600 ms.
Basically, DWI and FLAIR lesions are visually assessed according to the WAKE-UP imaging guidebook. For DWI assessment, apparent diffusion coefficient map can be used to exclude a T2 shine through effect. If DWI lesion is extensively overlapping with a previous stroke lesion or extensive white matter change, such patient needs to be excluded. The guidebook optionally provides the objective guidance to include patients with relative signal intensity of <1·2 on FLAIR lesion corresponding to acute DWI lesion as compared with contralateral normal signal intensity.
Randomization and data management
Eligible patients are randomized 1:1 to either i.v. rt-PA (alteplase, the rt-PA group) or standard treatment (the control group). Both patients and investigators are open to treatment allocation. However, primary and secondary outcomes are assessed without information regarding treatment allocation by independent neurologists, neurosurgeons, or nurses. The Research Electronic Data Capture (REDCap) system is used for data entering and management.
Treatment
Alteplase is supplied in glass vials. Labeling and packaging of study medication are conducted according to good manufacturing practice, good clinical practice, and local and national regulatory requirements. Patients randomized to the rt-PA group receive alteplase 0·6 mg/kg body weight i.v. up to a maximum of 60 mg, 10% as bolus, and 90% as continuous infusion over one-hour. Patients randomized to the control group do not receive i.v. rt-PA but are treated with one to three antithrombotic drugs, including aspirin (160–300 mg/day), clopidogrel (75 mg/day), argatroban, and unfractionated heparin, except for the combination of argatroban and heparin according to attending physician's decisions. Such antithrombotics are prohibited for use in the rt-PA group within the initial 25 h. Treatment has to be initiated as soon as possible within 60 min of the end of the MRI examination. In addition, i.v. edaravone (free radical scavenger) is routinely given before or soon after trial enrollment for both groups, for a maximum of 14 days, unless contraindicated or inappropriate. Other antiplatelets (e.g., cilostazol) and anticoagulants (e.g., warfarin) are prohibited within the initial 25 h, and thrombolytic agents such as urokinase and monteplase are prohibited during the 90-day study period in both groups.
Clinical and radiological assessments
Trained neurologists, neurosurgeons, or nurses will perform clinical assessment at baseline, at 22–36 h, at 7–14 days or at hospital discharge, and at 90 days after stroke onset. Neurological severity is evaluated using the National Institutes of Health Stroke Scale (NIHSS). At 90 days, a physician, nurse, or clinical research coordinator who is not aware of treatment assignment assesses mRS and adverse events at the clinic or by telephone interview. Investigators are recommended to complete a training and certification program for NIHSS and mRS.
In addition to the initial MRI prior to randomization, follow-up MRI is performed after 22–36 h to identify ICH, and after 7–14 days to delineate final infarct volume. All images are judged at the MRI examination scanner or the display for reading or viewing purposes by the local investigators. All investigators are recommended to pass a standardized training program for image judgment provided by the WAKE-UP committee. A central image reading board continuously monitors the fulfillment of the prespecified MRI standards in each participating center and the compliance of patients randomized with the imaging inclusion and exclusion criteria.
Primary and secondary outcomes
Efficacy and safety end-points are listed in Table 3. Primary efficacy end-point is favorable outcome defined by mRS score 0–1 at 90 days after stroke onset. The safety end-points are sICH at 22–36 h and major bleeding (22), and death due to any cause at 90 days.
Table 3.
Efficacy and safety assessment
| Primary efficacy end-point |
| • Favorable outcome defined by mRS score 0–1 at 90 days after stroke onset |
| Secondary efficacy end-points |
| • Categorical shift in NIHSS score at 24 h after the initiation of treatment |
| • Categorical shift in NIHSS score at seven-days after the initiation of treatment |
| • mRS score 0–2 at 90 days after stroke onset |
| • Categorical shift in mRS score at 90 days after stroke onset |
| • Recanalization of culprit artery on MRA 22–36 h after the initiation of treatment |
| • Infarct volume on FLAIR 7–14 days after the initiation of treatment |
| Safety end-points |
| • sICH as defined by an increase of NIHSS score of ≥4 from baseline and parenchymal hematoma type II (PH-2) on MRI 22–36 h after the initiation of treatment |
| • Major bleeding as defined by fatal bleeding, symptomatic bleeding in a critical area or organ, such as intraspinal, intraocular, retroperitoneal, intraarticular, or pericardial, or intramuscular with compartment syndrome, or bleeding causing a fall in hemoglobin level ≥ 2 g/dL, or leading to transfusion of ≥4·5 units (≈1125 mL) of whole blood or red cells according to the definition of the International Society on Thrombosis and Haemostasis (22) within 90 days after stroke onset |
| • Death (mRS 6) due to any cause at 90 days after stroke onset |
FLAIR, fluid attenuated inversion recovery; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; sICH, symptomatic intracerebral hemorrhage.
Data monitoring body
Data monitoring is centrally conducted by the members of Department of Advanced Medical Technology Development, National Cerebral and Cardiovascular Center. According to the order from the steering committee, the members occasionally visit collaborating hospitals to review source materials, including related medical records and documents for consent forms. Responsible authorities, related ethics committees, or directors of collaborating hospitals have the right to review source material if necessary.
Data and safety monitoring board
An independent data and safety monitoring board (DSMB) oversees the conduct of the trial. The occurrence of any safety end-point is immediately reported to the DSMB by the responsible physician via the central office, along with the accumulated number of safety end-points and total patient enrollment numbers. All safety end-points are mandatorily analyzed after inclusion of 150 and 300 patients. The proportion of sICH in the i.v. rt-PA group is compared with those of previous reports (23–27), and the proportions of major bleeding and death in the i.v. rt-PA group are compared with those in the control group. In any case of concern about the safety of participants, the DSMB makes a recommendation to the steering committee about continuing, stopping, or modifying the trial.
Sample size calculation
A total of 278 patients (139 per group) is required to ensure 1 − β = 90% probability to demonstrate that the relative effect of i.v. rt-PA to standard treatment for ischemic stroke patients is more than a fraction of 0·5 of the combined relative effect of i.v. rt-PA across the stroke thrombolysis studies (15–17,28), by using one-sided chi-square test of significant level of 2·5%, where the effects of i.v. rt-PA and standard treatment are assumed 30% and 20% commonly for Japanese patients and comparable combined studies. Accounting for possible treatment failures, protocol violations, and dropouts, a total of 300 patients (150 per treatment group) will be recruited.
Statistical analysis
Analyses will be done according to an intention-to-treat (ITT) principle. A secondary per-protocol sensitivity analysis will be done to assess the robustness of conclusion from ITT basis analysis. Patient demographic data will be analyzed descriptively; categorical variables will be assessed with the chi-square test or Fisher's exact test, whereas continuous variables will be assessed with the Student's t-test or the Wilcoxon rank-sum test, as appropriate. The primary outcome is the proportion of patients with mRS 0–1 (i.e., primary end-point) at 90 days between the i.v. rt-PA and control groups, analyzed by the chi-square test. Relative risk (RR) for the primary outcome will be calculated with the corresponding 95% confidence interval. The secondary outcome is the change in NIHSS from baseline to at 24 h or 7 days, analyzed by analysis of covariance (ANCOVA), where the model includes treatment group as factor and NIHSS at baseline as a covariate. Safety data will be analyzed descriptively for the treated set, which consists of all randomized patients who receive at least one study treatment. The statistical analysis plan, which includes more technical and detailed elaboration of the principal features stated in the protocol, will separately be prepared and be finalized before breaking the blind.
Study organization and funding
The THAWS is organized by a central coordinating center located at the National Cerebral and Cardiovascular Center, and conducted in approximately 35 centers in Japan after the approval of Advanced Medical Technology Development authentication system by the Ministry of Health, Labour and Welfare. The steering committee manages the trial. The THAWS receives funding support from the Award from Charitable Trust Mihara Cerebrovascular Disorder Research Promotion Fund (to Minematsu) and the Intramural Research Fund for Cardiovascular Diseases of National Cerebral and Cardiovascular Center (H23-4-3).
Discussion and conclusion
The THAWS is a randomized, controlled trial of stroke thrombolysis with alteplase 0·6 mg/kg based on the presence of negative FLAIR pattern on initial MRI in patients with unclear-onset time of stroke symptom, that is, wake-up stroke. The negative FLAIR will ensure the enrollment of patients with ischemic lesions likely to be less than 4·5 h after stroke onset who are likely to benefit from thrombolysis. The THAWS may trigger approval of low-dose i.v. thrombolysis using MRI-based selection as a routine clinical practice for ischemic stroke patients with unclear-onset time. Furthermore, 0·6 mg/kg of alteplase is expected to have similar efficacy and higher safety than 0·9 mg/kg in Asian countries (29), and it is now investigated in the Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED) (Clinical Trials.gov Identifier NCT01422616).
Of several ongoing clinical trials on thrombolysis in acute stroke patients with unclear-onset time in worldwide, the WAKE-UP trial is the largest. We are in contact with the WAKE-UP group and they kindly provided us their detailed imaging guidebook and standardized training program for image judgment to share the same imaging inclusion criteria. We may conduct a meta-analysis with the ongoing trials for unclear-onset stroke, including the WAKE-UP trial.
THAWS boards and institutions
Senior advisor: Kazuo Minematsu
Steering committee: Kazunori Toyoda (Chair), Kazumi Kimura (vice-chair), Haruko Yamamoto (data monitoring), Masatoshi Koga (central office)
Protocol committee: Junya Aoki, Toshimitsu Hamasaki, Kazumi Kimura, Masatoshi Koga, Shoichiro Sato, Kazunori Toyoda
Statistician: Toshimitsu Hamasaki
Independent safety monitoring board: Takanari Kitazono (Chair), Toshisuke Otsuki, Wataru Shimizu, Takashi Sozu
Central imaging reading board: Makoto Sasaki (Chair), Teruyuki Hirano, Kohsuke Kudo, Naomi Morita
Central pharmacy: Ken Kuwahara
Coordinating investigators: Sohei Yoshimura, Shoichiro Sato, Kazunari Homma, Kenta Seki
Secretariats: Haruka Kanai, Azusa Tokunaga
THAWS consortium
National Cerebral and Cardiovascular Center, Suita, Osaka – Kazunori Toyoda, Kazuyuki Nagatsuka, Haruko Yamamoto, Masatoshi Koga, Hiroshi Yamagami, Shoichiro Sato, Kazunari Homma, Kenta Seki
Kawasaki Medical School, Kurashiki – Kazumi Kimura, Junya Aoki
Hokkaido P.W.F.A.C. Obihiro-Kosei General Hospital, Obihiro – Masafumi Ohtaki
Nakamura Memorial Hospital, Sapporo – Kamiyama Kenji
Hirosaki Stroke and Rehabilitation Center, Hirosaki – Metoki Norifumi
Kohnan Hospital, Sendai – Eisuke Furui
Yamagata City Hospital SAISEIKAN, Yamagata – Rei Kondo
South Miyagi Medical Center, Shibata – Hiroshi Mochizuki
Niigata City General Hospital, Niigata – Shuichi Igarashi
Mihara Memorial Hospital, Isesaki – Ban Mihara
Jichi Medical University, Shimotsuke – Tomoaki Kameda
Saitama Medical University International Medical Center, Hidaka – Norio Tanahashi, Ichiro Deguchi
Juntendo University Urayasu Hospital, Urayasu – Takao Urabe
Juntendo University Hospital, Tokyo – Nobutaka Hattori
Jikei University School of Medicine, Tokyo – Yasuyuki Iguchi
Toranomon Hospital, Tokyo – Yoshikazu Uesaka
Kyorin University, Mitaka – Yoshiaki Shiokawa, Denbo Norihisa
St. Marianna University School of Medicine, Kawasaki – Yasuhiro Hasegawa
Tokai University, Isehara – Shunya Takizawa
Shizuoka Saiseikai General Hospital, Shizuoka – Jin Yoshii
Toyota Memorial Hospital, Toyota – Yasuhiro Ito
National Hospital Organization Nagoya Medical Center, Nagoya – Satoshi Okuda
Japanese Red Cross Kyoto Daini Hospital, Kyoto – Yoshinari Nagakane
Hyogo College of Medicine, Nishinomiya – Shinichi Yoshimura
Kobe City Medical Center Central Hospital, Kobe – Nobuyuki Sakai, Kenichi Todo.
Ohnishi Neurological Center, Akashi – Hideyuki Ohnishi
Kawasaki Hospital, Okayama – Tsuyoshi Inoue
Osaka Neurosurgical Hospital, Takamatsu – Hideo Ohyama
Kokura Memorial Hospital, Kokura – Ichiro Nakahara
Steel Memorial Yawata Hospital, Kitakyushu – Shigeru Fujimoto
Japanese Red Cross Fukuoka Hospital, Fukuoka – Kenichiro Fujii, Sohei Yoshimura
National Hospital Organization Kyushu Medical Center, Fukuoka – Yasushi Okada, Takeshi Uwatoko
Nagasaki University Hospital, Nagasaki – Akira Tsujino
Saiseikai Kumamoto Hospital, Kumamoto – Toshiro Yonehara
Japanese Red Cross Kumamoto Hospital, Kumamoto – Tadashi Terasaki
References
- 1.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 3.Mackey J, Kleindorfer D, Sucharew H, et al. Population-based study of wake-up strokes. Neurology. 2011;76:1662–1667. doi: 10.1212/WNL.0b013e318219fb30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang DW, Kwon JY, Kwon SU, Kim JS. Wake-up or unclear-onset strokes: are they waking up to the world of thrombolysis therapy? Int J Stroke. 2012;7:311–320. doi: 10.1111/j.1747-4949.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- 5.Koton S, Tanne D, Bornstein NM. Ischemic stroke on awakening: patients' characteristics, outcomes and potential for reperfusion therapy. Neuroepidemiology. 2012;39:149–153. doi: 10.1159/000341242. [DOI] [PubMed] [Google Scholar]
- 6.Todo K, Moriwaki H, Saito K, Tanaka M, Oe H, Naritomi H. Early CT findings in unknown-onset and wake-up strokes. Cerebrovasc Dis. 2006;21:367–371. doi: 10.1159/000091545. [DOI] [PubMed] [Google Scholar]
- 7.Serena J, Davalos A, Segura T, Mostacero E, Castillo J. Stroke on awakening: looking for a more rational management. Cerebrovasc Dis. 2003;16:128–133. doi: 10.1159/000070592. [DOI] [PubMed] [Google Scholar]
- 8.Marsh EE, 3rd, Biller J, Adams HP, Jr, et al. Circadian variation in onset of acute ischemic stroke. Arch Neurol. 1990;47:1178–1180. doi: 10.1001/archneur.1990.00530110032012. [DOI] [PubMed] [Google Scholar]
- 9.Aoki J, Kimura K, Iguchi Y, Shibazaki K, Sakai K, Iwanaga T. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39–44. doi: 10.1016/j.jns.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Thomalla G, Rossbach P, Rosenkranz M, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65:724–732. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- 11.Petkova M, Rodrigo S, Lamy C, et al. MR imaging helps predict time from symptom onset in patients with acute stroke: implications for patients with unknown onset time. Radiology. 2010;257:782–792. doi: 10.1148/radiol.10100461. [DOI] [PubMed] [Google Scholar]
- 12.Minematsu K, Li L, Fisher M, Sotak CH, Davis MA, Fiandaca MS. Diffusion-weighted magnetic resonance imaging: rapid and quantitative detection of focal brain ischemia. Neurology. 1992;42:235–240. doi: 10.1212/wnl.42.1.235. [DOI] [PubMed] [Google Scholar]
- 13.Ebinger M, Galinovic I, Rozanski M, Brunecker P, Endres M, Fiebach JB. Fluid-attenuated inversion recovery evolution within 12 hours from stroke onset: a reliable tissue clock? Stroke. 2009;41:250–255. doi: 10.1161/STROKEAHA.109.568410. [DOI] [PubMed] [Google Scholar]
- 14.Aoki J, Kimura K, Iguchi Y, et al. Intravenous thrombolysis based on diffusion-weighted imaging and fluid-attenuated inversion recovery mismatch in acute stroke patients with unknown onset time. Cerebrovasc Dis. 2011;31:435–441. doi: 10.1159/000323850. [DOI] [PubMed] [Google Scholar]
- 15.Aoki J, Kimura K, Shibazaki K, Sakamoto Y. Negative fluid-attenuated inversion recovery- based intravenous thrombolysis using recombinant tissue plasminogen activator in acute stroke patients with unknown onset time. Cerebrovasc Dis Extra. 2013;3:35–45. doi: 10.1159/000348552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barreto AD, Martin-Schild S, Hallevi H, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40:827–832. doi: 10.1161/STROKEAHA.108.528034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho AH, Sohn S-I, Han M-K, et al. Safety and efficacy of MRI-based thrombolysis in unclear-onset stroke. Cerebrovasc Dis. 2008;25:572–579. doi: 10.1159/000132204. [DOI] [PubMed] [Google Scholar]
- 18.Breuer L, Schellinger PD, Huttner HB, et al. Feasibility and safety of magnetic resonance imaging-based thrombolysis in patients with stroke on awakening: initial single-centre experience. Int J Stroke. 2010;5:68–73. doi: 10.1111/j.1747-4949.2010.00410.x. [DOI] [PubMed] [Google Scholar]
- 19.Ebinger M, Scheitz JF, Kufner A, Endres M, Fiebach JB, Nolte CH. MRI-based intravenous thrombolysis in stroke patients with unknown time of symptom onset. Eur J Neurol. 2012;19:348–350. doi: 10.1111/j.1468-1331.2011.03504.x. [DOI] [PubMed] [Google Scholar]
- 20.Thomalla G, Fiebach JB, Østergaard L, et al. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP) Int J Stroke. 2014;9:829–836. doi: 10.1111/ijs.12011. [DOI] [PubMed] [Google Scholar]
- 21.Ostrem JL, Saver JL, Alger JR, et al. Acute basilar artery occlusion: diffusion-perfusion MRI characterization of tissue salvage in patients receiving intra-arterial stroke therapies. Stroke. 2004;35:e30–34. doi: 10.1161/01.STR.0000113783.45745.BE. [DOI] [PubMed] [Google Scholar]
- 22.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 23.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 24.Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–954. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 25.Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. The Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Mori E, Minematsu K, et al. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT) Stroke. 2006;37:1810–1815. doi: 10.1161/01.STR.0000227191.01792.e3. [DOI] [PubMed] [Google Scholar]
- 27.Toyoda K, Koga M, Naganuma M, et al. Routine use of intravenous low-dose recombinant tissue plasminogen activator in Japanese patients: general outcomes and prognostic factors from the SAMURAI register. Stroke. 2009;40:3591–3595. doi: 10.1161/STROKEAHA.109.562991. [DOI] [PubMed] [Google Scholar]
- 28.Kang DW, Sohn SI, Hong KS, et al. Reperfusion therapy in unclear-onset stroke based on MRI evaluation (RESTORE): a prospective multicenter study. Stroke. 2012;43:3278–3283. doi: 10.1161/STROKEAHA.112.675926. [DOI] [PubMed] [Google Scholar]
- 29.Chao AC, Hsu HY, Chung CP, et al. Outcomes of thrombolytic therapy for acute ischemic stroke in Chinese patients: the Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) study. Stroke. 2010;41:885–890. doi: 10.1161/STROKEAHA.109.575605. [DOI] [PubMed] [Google Scholar]


