Abstract
An improved and efficient method for the synthesis of thioamides is presented. For this transformation, dimethylamine as the key intermediate is generated in situ from dimethylformamide (DMF). All the tested substrates produced the desired products with excellent isolated yields.
Keywords: aldehydes, dimethylformamide (DMF), elemental sulfur, ketones, thioamides
Introduction
Thioamides, a well-known structural element of many sulfur-containing molecules, synthetic agents [1–2], heterocycles, natural products and pharmaceuticals [3–8], have attracted considerable attention for their construction and use in organic synthesis [9–10]. Many compounds containing a thioamide motif are of medicinal significance and exhibit potent biological activities. These include for example opioid activity [11], immunosuppressive activity and DHODH inhibitory properties [12], activity against parasitic nematodes [13], and antituberculotic activity [14].
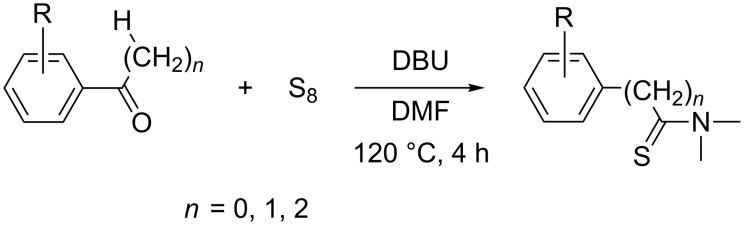
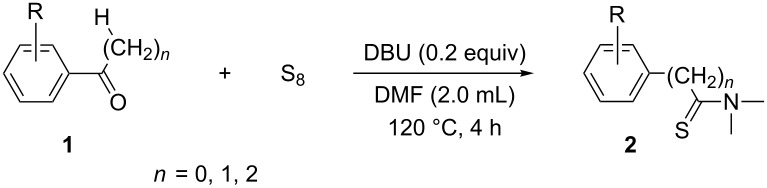
Consequently, a number of synthetic methods for the construction of this important unit have been established over the past decades [15–21]. However, some of these methods have limited applications, because of harsh conditions, low yields or the need of noble-metal catalysts. Therefore the development of novel and efficient methods for the construction of the thioamide motif is highly desirable. To avoid the disadvantages of the traditional methods, our group has developed an improved synthetic procedure to construct thioamides (Scheme 1).
Scheme 1.
Synthesis of thioamide derivatives.
Results and Discussion
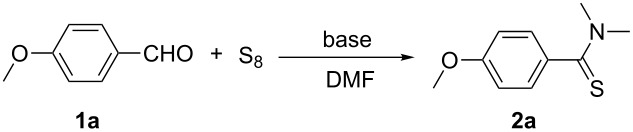
Our initial efforts focused on the optimization of the reaction conditions by employing 4-methoxybenzaldehyde (1a) as a model reactant to interact with elemental sulfur and DMF (Table 1). The reaction is completed after 4 h at 120 °C, providing 4-methoxy-N,N-dimethylbenzothioamide (2a) with 64% yield by using sodium acetate (AcONa) as the base (Table 1, entry 2). With regard to the catalytic activity of different bases (Table 1, entries 4–8), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) showed the highest activity and afforded the desired product with 96% yield (Table 1, entry 7). It is worth mentioning that this conversion does not take place in the absence of a base (Table 1, entry 9). Lowering the temperature from 120 °C to 100 °C decreased the yield considerably (Table 1, entry 10). When the reaction was performed at room temperature no product was obtained at all (Table 1, entry 11).
Table 1.
Optimization studies.a
 | ||||
| Entry | Temp. ( °C) | Base (0.2 equiv) | Time (h) | Yield (%)b |
| 1 | 120 | AcONa | 3 | 57 |
| 2 | 120 | AcONa | 4 | 64 |
| 3 | 120 | AcONa | 5 | 64 |
| 4 | 120 | Na2CO3 | 4 | 25 |
| 5 | 120 | KOH | 4 | 15 |
| 6 | 120 | DABCO | 4 | 21 |
| 7 | 120 | DBU | 4 | 96 |
| 8 | 120 | EtONa | 4 | 17 |
| 9 | 120 | none | 4 | none |
| 10 | 100 | DBU | 4 | 79 |
| 11 | rt | DBU | 4 | none |
aReaction conditions: 1a (0.25 mmol), S8 (1.2 equiv, based on 1/8 S8), DMF (2.0 mL); bGC yield.
Once the optimal conditions had been identified (Table 1, entry 7), next the scope of the reaction was investigated. Sixteen substrates including 12 substituted aldehydes and 4 ketones were screened and the results are presented in Table 2. As can be seen from the Table, all reactions proceeded smoothly and gave the corresponding thioamides 2a–p exclusively in good to excellent isolated yields. It was also found that unsubstituted, mono-, di- and trisubstituted substrates regardless of the position (o-, m-, or p-) and the electronic properties of the substituents (electron-donating or –withdrawing) on the benzene ring were all compatible with the standard conditions. For example, methoxy-, o-, m-, and p-methyl-, fluoro-, chloro-, or hydroxy-substituted aromatic aldehydes and ketones were all converted to their corresponding thioamides with excellent isolated yields. Finally, some aliphatic aldehydes such as phenylacetaldehyde (Table 2, entry 9), butyraldehyde (Table 2, entry 15) and pentanal (Table 2, entry 16) were subjected to the reaction. They were also found compatible with the standard conditions, and the corresponding products were isolated in moderate to good yields.
Table 2.
Scope of the reaction.a
 | |||
| Entry | Educt 1a–p | Product 2a–p | Yield (%)b |
| 1 |
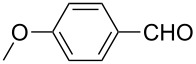 1a |
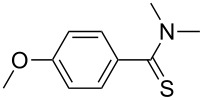 2a |
90 (88) |
| 2 |
 1b |
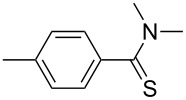 2b |
84 |
| 3 |
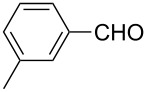 1c |
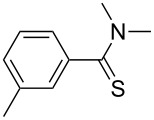 2c |
82 |
| 4 |
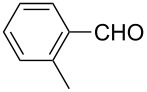 1d |
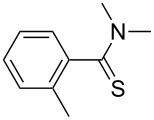 2d |
82 |
| 5 |
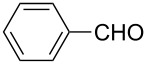 1e |
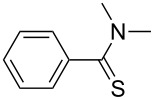 2e |
83 |
| 6 |
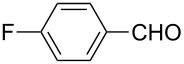 1f |
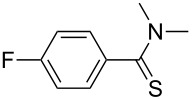 2f |
80 |
| 7 |
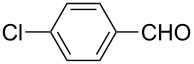 1g |
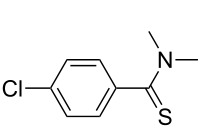 2g |
80 |
| 8 |
 1h |
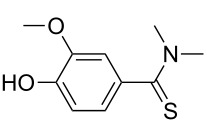 2h |
89 |
| 9 |
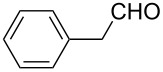 1i |
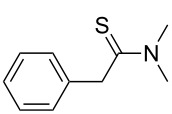 2i |
83 |
| 10 |
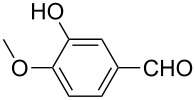 1j |
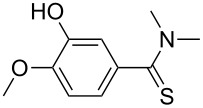 2j |
88 |
| 11 |
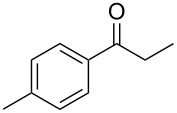 1k |
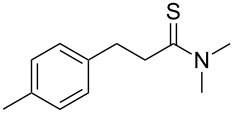 2k |
80 |
| 12 |
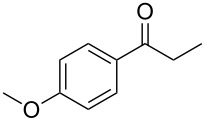 1l |
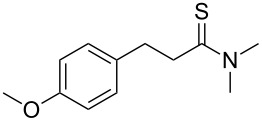 2l |
84 |
| 13 |
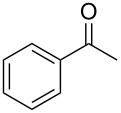 1m |
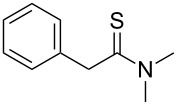 2i |
77 |
| 14 |
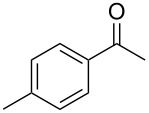 1n |
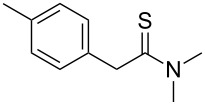 2n |
79 |
| 15 |
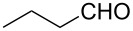 1o |
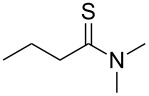 2o |
63 |
| 16 |
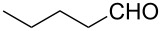 1p |
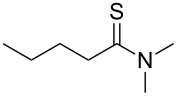 2p |
67 |
aReaction conditions: 1 (1.0 mmol), S8 (1.2 equiv, based on 1/8 S8); bisolated yield. Number in parentheses is the isolated yield of 1 (10.0 mmol scale) after purification by column chromatography.
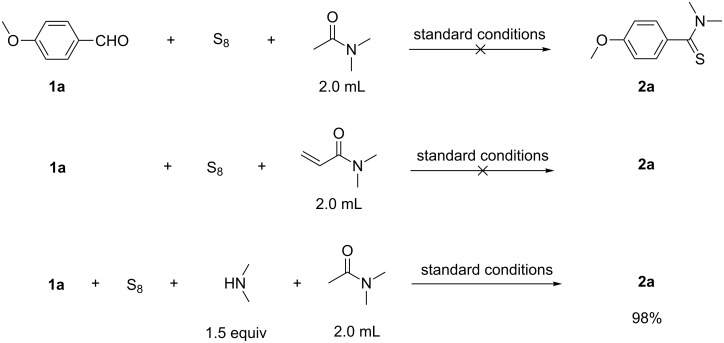
In order to expand the scope and to get insight into the reaction mechanism of this protocol, three control experiments were conducted (Scheme 2). In the first two experiments the reaction was performed with N,N-dimethylacetamide (DMA) and N,N-dimethylacrylamide instead of DMF under the standard conditions. In both cases no product formation was observed, suggesting that neither DMA nor N,N-dimethylacrylamide is able to promote the reaction. However, repeating the reaction in DMA in the presence of N,N-dimethylamine the desired product 2a was obtained in 98% yield. These results are proving the involvement of dimethylamine in this transformation and that it is in situ generated from N,N-dimethylformamide under the reaction conditions.
Scheme 2.
Control experiments.
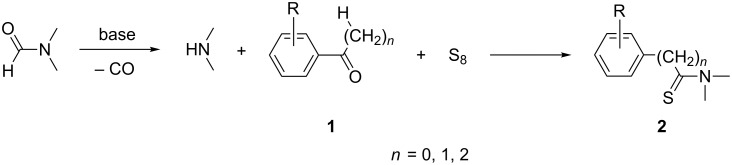
Based on the above experiments and the existing literature [22–24], a suggested mechanism is outlined in Scheme 3. The reaction starts with a base-induced cleavage of DMF to form the required dimethylamine according to the mechanism suggested by Van der Eycken, Hallberg and co-workers [25–26]. The subsequent step involves the classical Willgerodt–Kindler reaction as described by Amupitan and Darabi [27–28].
Scheme 3.
A mechanistic rationale for the synthesis of thioamides.
Conclusion
In summary, an improved synthetic procedure for the synthesis of thioamides has been established. This protocol is applicable to a wide range of aldehydes and ketones yielding the thioamides with excellent isolated yields. For this transformation, DMF works not only as the solvent but also as the source of dimethylamine. The present method is more practical compared to the traditional strategies and complements the classical methods for the rapid construction of thioamides.
Supporting Information
Full experimental details and copies of NMR spectra.
Acknowledgments
We thank the Excellent Young Scientist Fund of Guangdong Province Education Department (No.2013LYM_0059) for financial support.
Contributor Information
Weibing Liu, Email: liuhailing@bnu.edu.cn.
Hailing Liu, Email: liuhailing@bnu.edu.cn.
References
- 1.Goldberg J M, Speight L C, Fegley M W, Petersson E J. J Am Chem Soc. 2012;134:6088–6091. doi: 10.1021/ja3005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu C T, Maxwell C I, Pipe S G, Neverov A A, Mosey N J, Brown R S. J Am Chem Soc. 2011;133:20068–20071. doi: 10.1021/ja209605r. [DOI] [PubMed] [Google Scholar]
- 3.Batjargal S, Wang Y J, Goldberg J M, Wissner R F, Petersson E J. J Am Chem Soc. 2012;134:9172–9182. doi: 10.1021/ja2113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murai T, Hori F, Maruyama T. Org Lett. 2011;13:1718–1721. doi: 10.1021/ol200231z. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki Y, Iwata M, Yazaki R, Kumagai N, Shibasaki M. J Org Chem. 2012;77:4496–4500. doi: 10.1021/jo300566p. [DOI] [PubMed] [Google Scholar]
- 6.Okano A, James R C, Pierce J G, Xie J, Boger D L. J Am Chem Soc. 2012;134:8790–8793. doi: 10.1021/ja302808p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beattie D E, Crossley R, Curran A C W, Dixon G T, Hill D G, Lawrence A E, Shepherd R G. J Med Chem. 1977;20:714–718. doi: 10.1021/jm00215a019. [DOI] [PubMed] [Google Scholar]
- 8.Zacharie B, Lagraoui M, Dimarco M, Penney C L, Gagnon L. J Med Chem. 1999;42:2046–2052. doi: 10.1021/jm9900467. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg J M, Chen X, Meinhardt N, Greenbaum D C, Petersson E J. J Am Chem Soc. 2014;136:2086–2093. doi: 10.1021/ja412297x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg J M, Batjargal S, Chen B S, Petersson E J. J Am Chem Soc. 2013;135:18651–18658. doi: 10.1021/ja409709x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherman D B, Spatola A F, Wire W S, Burks T F, Nguyen T M-D, Schiller P W. Biochem Biophys Res Commun. 1989;162:1126–1132. doi: 10.1016/0006-291X(89)90790-0. [DOI] [PubMed] [Google Scholar]
- 12.Albert A, Knecht H, Andersen E, Hungerford V, Schreier M H, Papageorgiou C. Bioorg Med Chem. 1998;8:2203–2208. doi: 10.1016/S0960-894X(98)00365-5. [DOI] [PubMed] [Google Scholar]
- 13.Jeschke P, Harder A, Etzel W, Dau W, Thielking G, Bonse G, Linuma K. Pest Manage Sci. 2001;57:1000–1006. doi: 10.1002/ps.382. [DOI] [PubMed] [Google Scholar]
- 14.Cynamon M H, Gimi R, Gyenes F, Sharpe C A, Bergmann K E, Han H J, Gregor L B, Rapolu R, Luciano G, Welch J T. J Med Chem. 1995;38:3902–3907. doi: 10.1021/jm00020a003. [DOI] [PubMed] [Google Scholar]
- 15.Guntreddi T, Vanjari R, Singh K N. Org Lett. 2014;16:3624–3627. doi: 10.1021/ol501482g. [DOI] [PubMed] [Google Scholar]
- 16.Bergman J, Pettersson B, Hasimbegovic V, Svensson P H. J Org Chem. 2011;76:1546–1553. doi: 10.1021/jo101865y. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Jiang H, Wu W, Zeng W, Li J. Org Biomol Chem. 2014;12:700–707. doi: 10.1039/C3OB42275E. [DOI] [PubMed] [Google Scholar]
- 18.Priebbenowa D L, Bolm C. Chem Soc Rev. 2013;42:7870–7880. doi: 10.1039/c3cs60154d. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Ji M, Lim S, Jang H-Y. J Org Chem. 2014;79:7256–7260. doi: 10.1021/jo501378v. [DOI] [PubMed] [Google Scholar]
- 20.Patra M, Hess J, Konatschnig S, Spingler B, Gasser G. Organometallics. 2013;32:6098–6105. doi: 10.1021/om400715m. [DOI] [Google Scholar]
- 21.Nguyen T B, Tran M Q, Ermolenko L, Al-Mourabit A. Org Lett. 2014;16:310–313. doi: 10.1021/ol403345e. [DOI] [PubMed] [Google Scholar]
- 22.Čechová L, Jansa P, Šála M, Dračínský M, Holý A, Janeba Z. Tetrahedron. 2011;67:866–871. doi: 10.1016/j.tet.2010.12.040. [DOI] [Google Scholar]
- 23.Wang J, Hou J-T, Wen J, Zhang J, Yu X-Q. Chem Commun. 2011;47:3652–3654. doi: 10.1039/c0cc05811d. [DOI] [PubMed] [Google Scholar]
- 24.Kumagai T, Anki T, Ebi T, Konishi A, Matsumoto K, Kurata H, Kubo T, Katsumoto K, Kitamura C, Kawase T. Tetrahedron. 2010;66:8968–8973. doi: 10.1016/j.tet.2010.09.037. [DOI] [Google Scholar]
- 25.Wan Y, Alterman M, Larhed M, Hallberg A. J Org Chem. 2002;67:6232–6235. doi: 10.1021/jo025965a. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Mehta V P, Van der Eycken E. Tetrahedron. 2008;64:2605–2610. doi: 10.1016/j.tet.2008.01.030. [DOI] [Google Scholar]
- 27.Amupitan J O. Synthesis. 1983:730–735. doi: 10.1055/s-1983-30490. [DOI] [Google Scholar]
- 28.Nooshabadi M, Aghapoor K, Darabi H R, Mojtahedi M M. Tetrahedron Lett. 1999;40:7549–7552. doi: 10.1016/S0040-4039(99)01600-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full experimental details and copies of NMR spectra.