Abstract
Introduction
In order to investigate the role of roe deer in the maintenance and transmission of infectious animal and human diseases in Flanders, we conducted a serologic screening in 12 hunting areas.
Materials and methods
Roe deer sera collected between 2008 and 2013 (n=190) were examined for antibodies against 13 infectious agents, using indirect enzyme-linked immunosorbent assay, virus neutralisation, immunofluorescence, or microagglutination test, depending on the agent.
Results and discussion
High numbers of seropositives were found for Anaplasma phagocytophilum (45.8%), Toxoplasma gondii (43.2%) and Schmallenberg virus (27.9%), the latter with a distinct temporal distribution pattern following the outbreak in domestic ruminants. Lower antibody prevalence was found for Chlamydia abortus (6.7%), tick-borne encephalitis virus (5.1%), Neospora caninum (4.8%), and Mycobacterium avium subsp paratuberculosis (4.1%). The lowest prevalences were found for Leptospira (1.7%), bovine viral diarrhoea virus 1 (1.3%), and Coxiella burnetii (1.2%). No antibodies were found against Brucella sp., bovine herpesvirus 1, and bluetongue virus. A significant difference in seroprevalence between ages (higher in adults >1 year) was found for N. caninum. Four doubtful reacting sera accounted for a significant difference in seroprevalence between sexes for C. abortus (higher in females).
Conclusions
Despite the more intensive landscape use in Flanders, the results are consistent with other European studies. Apart from maintaining C. abortus and MAP, roe deer do not seem to play an important role in the epidemiology of the examined zoonotic and domestic animal pathogens. Nevertheless, their meaning as sentinels should not be neglected in the absence of other wild cervid species.
Keywords: roe deer, serology, screening, antibodies, infection, pathogens, exposure, sentinel
Wild cervids can carry several infectious agents known to affect human or animal health. Often spillover from domestic animals is assumed and, conversely, wild cervids may constitute a reservoir for pathogens causing disease in domestic ruminants or zoonoses in man. Screening in wild ruminants is essential to gain insight into the relations between sylvatic and domestic cycles of pathogens, and provides basic information for risk assessment for animal and human health in the region concerned.
Roe deer (Capreolus capreolus) are the only native cervid species living in the wild in Flanders (northern Belgium). Their population has increased over the last decennia and is currently estimated at about 25,000 with highest densities (up to 30/100 ha) in the eastern provinces Limburg, Antwerp and Flemish Brabant (1). The small landscape structure of Flanders with intermixed nature, cultured land and urbanisation allows regular contact between roe deer and livestock or human dwellings. In Europe, roe deer are important feeding hosts for Ixodes ricinus ticks by which their population number and density is related to tick abundance and indirectly to the incidence of tick-borne infections.
Before the present study, only sporadic data about the prevalence of infectious agents in roe deer were generated in Flanders. Serologic methods allow to screen for multiple agents in one single blood sample in a cost-effective way, even after clearance of the agent from the host. Blood samples of wild cervids are usually collected during hunting activities. In contrast to the battue method used in Wallonia (southern Belgium), in Flanders roe deer are hunted one by one, hampering the continuous presence of an experienced sampler and resulting in a much slower sample collection. In such conditions and in the absence of a continued surveillance program, an optimal use of the sera is desirable by screening for a broad range of infectious agents. The objective of the present study was to find serologic evidence on the exposure of roe deer in Flanders to 13 infectious agents with economic impact in animals or zoonotic impact in man. Former studies in Europe in roe and red deer reported a broad range of serologic results for the 13 agents examined (Supplementary file 1).
Materials and methods
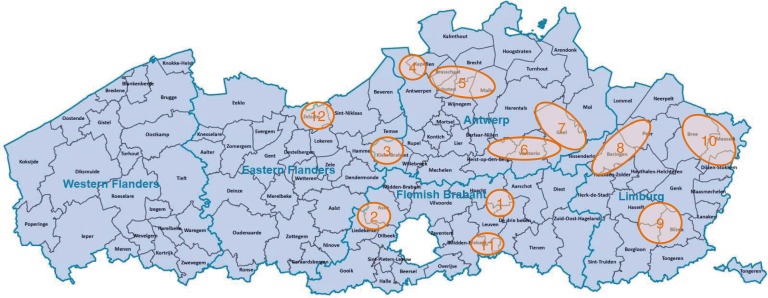
In 12 Flemish hunting areas (Fig. 1), from October 2008 to March 2013, instructed hunters collected 195 blood samples from the v. jugularis, v. cava caudalis, or hearth of roe deer, soon after the killing. If sampling from the veins was impossible, free blood from the body cavities was collected. Nine more samples were obtained during necropsy of culled or found-dead roe deer. One last sample came from a sick roe deer fawn in a rescue centre. For each animal sampled, the sex, the estimated age based on the tooth wear, the area of origin, and the date of sampling were recorded. The samples were cooled at 2–4°C, and on arrival at the lab were centrifuged for 10 min at 4,000 rpm. Heavily contaminated or decomposed samples were discarded. After dividing each serum in multiple Eppendorff tubes, the sera were kept at −20°C until analysis. The sera were examined for antibodies to 13 infectious agents by means of the tests listed in Table 1. Different numbers of sera were tested for the various pathogens depending on the available volumes.
Fig. 1.
Geographic origin (12 sampling areas) of roe deer sera examined for antibodies against 13 infectious agents in Flanders (Belgium). (Modified from: VDAB, 2015)
Table 1.
Serologic methods applied for the detection of antibodies against 13 infectious agents in roe deer sera
| Cut-off | |||||
|---|---|---|---|---|---|
| Test | Conjugate | Units | Neg | Pos | |
| MAP | iELISA (Pourquier) | G-HRP | SP | <60 | >70 |
| iELISA (IDEXX) | G-HRP | SP | <45 | >55 | |
| BRU | iELISA | G-HRP | IU | <2 | >2 |
| LEPT | MAT | – | – | – | – |
| COX | iELISA | G-HRP | SP | <40 | >40 |
| ANA | IFA | Anti-deer IgG FITC | Titre | <1/64 | >1/64 |
| CHL | iELISA | Anti-bov IgG HRP | SP | <50 | >60 |
| BVDV1 | VNT | – | Titre | >1/10 | <1/10 |
| BHV1 | VNT | – | Titre | >1/2 | <1/2 |
| BTV | cELISA | – | PN | >75 | <66 |
| TBEV | VNT:RFFIT | – | DIL50 | <10 | >15 |
| SBV | VNT | – | Titre | >1/8 | <1/8 |
| TOX | iELISA | Anti-deer IgG HRP | OD per plate | ||
| NEO | iELISA | G-HRP | SP | <41 | >50 |
G-HRP: protein G horseradish peroxidase (all species); FITC: fluorescein isothiocyanate; S/P: sample/positive control optical density ratio at 450 nm (OD450), determined as: 100× (sample OD450 – negative control OD450/average 2 positive controls OD450 – negative control OD450); IU: international units, corresponding to dilutions (standard curve based on six dilutions of a reference serum: 1/270 to 1/8340); PN: percent negativity compared to the negative kit control; DIL50: dilution at which 50% of the virus is neutralised; OD per plate: for each plate separately: mean corrected OD450 of three negative reference sera + 3× standard deviation OD450.
MAP: Mycobacterium avium subsp. paratuberculosis; BRU: Brucella sp.; COX: Coxiella burnetii; LEPT: Leptospira; ANA: Anaplasma phagocytophilum; CHL: Chlamydia abortus; BVDV1: bovine viral diarrhoea virus 1; BHV1: bovine herpesvirus 1; BTV: bluetongue virus; TBEV: tick-borne encephalitis virus; SBV: Schmallenberg virus; TOX: Toxoplasma gondii; NEO: Neospora caninum.
Mycobacterium avium subsp. paratuberculosis (MAP) antibodies were detected by means of an indirect enzyme-linked immunosorbent assay (iELISA) in which the sera are first absorbed with Mycobacterium phlei antigen (2). Two commercial tests, ELISA paratuberculosis antibody screening (Institut Pourquier, Monpellier, France) and IDEXX paratuberculosis screening Ab test (IDEXX Laboratories, Westbrook, Maine, USA), were used successively, due to a stock rupture of the first. Sera were tested for Brucella abortus antibodies with iELISA, using strain Weybridge 99 (B. abortus biotype 1) as antigen (3). For Coxiella burnetii antibodies, samples were tested with the iELISA kit LSI Fièvre Q Ruminants Serum® (Laboratoire Service International, Lissieu, France), according to the manufacturer's instructions (4). Antibodies to Leptospira were looked up with the microscopic agglutination test (MAT), using live cultures as antigens (5). A panel of 12 antigens was used, representing the most prevalent serogroups in Belgium: grippotyphosa, canicola, pomona, ballum, icterohaemorrhagiae, javanica (syn. poi), australis, autumnalis, bataviae, pyrogenes, tarassovi, sejroe. Results were expressed as positive/negative at dilution 1/100. For Anaplasma phagocytophilum, sera were tested with an indirect fluorescent antibody test (IFA) (A. phagocytophilum IFA IgG (OUS) (Focus Diagnostics, Cypress, California, USA) according to the manufacturer's specifications, but with an anti-deer IgG fluorescein isothyocyanate (FITC)-labelled conjugate (KPL Inc., Gaithersburg, Maryland, USA). For Chlamydia abortus antibodies, sera were analysed with iELISA (ID Screen Chlamydia abortus, ID Vet Innovative Diagnostics, Montpellier, France) according to the manufacturer's instructions. In-house virus neutralisation tests (VNTs) were used to detect antibodies against the bovine viral diarrhoea virus 1 (BVDV1), strain NADL (6), and against bovine herpes virus 1 (BHV1) (7). Titres were expressed as the reciprocal of the highest serum dilution yielding virus growth neutralisation. Antibodies to the VP7 group antigen of bluetongue virus (BTV) were looked up by means of the ID Screen Bluetongue Competition assay (ID VET, Montpellier, France), according to the manufacturer's instructions. Antibodies to tick-borne encephalitis virus (TBEV) were searched for with an in-house rapid fluorescent focus inhibition (RFFIT) seroneutralisation test (8). Neutralising antibody titres were expressed in DIL50 which represents the dilution at which 50% of the virus, dosed at 0.8–1.4 log TCID50 (tissue culture infective dose), is neutralised (9). An in-house VNT was used to detect antibodies against Schmallenberg virus (SBV). Titres were expressed as the reciprocal of the highest serum dilution yielding virus growth neutralisation (10). For Toxoplasma gondii, an in-house iELISA detecting antibodies against the SAG1 tachyzoite surface antigen (IBL Int., Hamburg, Germany) was used as described before (11). Sera were tested for the presence of anti-Neospora caninum IgG using the ID Screen N. caninum indirect ELISA (IDVet, Montpellier, France) according to the manufacturer's instructions (12). The antigen used in this test is a whole N. caninum extract.
For C. abortus, anti-bovine IgG horseradish peroxidase (HRP) and, for T. gondii, anti-deer IgG HRP were used as the conjugates. For all the other iELISA tests, ‘all species’ protein G-HRP was used. The conjugates were a component of the test kits mentioned, except for the in-house tests for Brucella (Thermo Scientific, Erembodegem, Belgium) and T. gondii (KPL Inc., Gaithersburg, Maryland, USA). In the ELISA's for MAP, B. abortus, C. burnetii, C. abortus, BTV, N. caninum and the VNTs for BVDV1, BHV1 and SBV, the cut-off values for domestic ruminants were applied. For the A. phagocytophilum IFA test, the cut-off value for human sera, as supplied by the manufacturer, was used. For BTV, the cut-off as described by Vandenbussche et al. (13) was used. For the TBEV RFFIT, sera with DIL50<10 were considered negative, between 10 and 15 borderline, and >15 positive (8). For T. gondii, cut-off values were determined for each plate separately after comparison with three negative and two positive sera as determined by the Sabin Feldman lysis test.
For all the results, confidence intervals (Table 2) for proportion of number positive (or suspected) versus number tested were calculated. For the agents showing a high seroprevalence, the log-likelihood ratio (Tables 3 and 4) test for contingency tables was used to analyse whether seropositivity was dependent on sex, age, and geographical origin of the samples; p<0.05 indicates significant difference between sexes, ages, or sampling regions.
Table 2.
Numbers of roe deer sera examined (N), absolute numbers and percentages of seropositive (+) and doubtful (susp) results, and 95% confidence intervals (CIs)
| MAP | BRU | COX | LEPT | ANA | CHL | BVDV1 | BHV 1 | BTV | TBEV | SBV | TOX | NEO | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 171 | 129 | 171 | 177 | 190 | 178 | 159 | 130 | 165 | 98 | 111 | 169 | 168 |
| + | 7 | 0 | 2 | 3 | 87 | 12 | 2 | 0 | 0 | 5 | 31 | 73 | 8 |
| % + | 4.1 | 0.0 | 1.2 | 1.7 | 45.8 | 6.7 | 1.3 | 0.0 | 0.0 | 5.1 | 27.9 | 43.2 | 4.8 |
| 95% CI low |
1.7 | 0.0 | 0.1 | 0.4 | 38.6 | 3.5 | 0.2 | 0.0 | 0.0 | 0.0 | 19.8 | 35.6 | 2.1 |
| 95% CI high |
8.3 | 2.8 | 4.2 | 4.9 | 53.2 | 11.5 | 4.5 | 2.8 | 2.2 | 11.5 | 37.2 | 51.0 | 9.2 |
| Susp | 2 | 4 | 5 | ||||||||||
| % susp | 1.2 | 2.2 | 3.0 | ||||||||||
| 95% CI low |
0.1 | 0.6 | 1.0 | ||||||||||
| 95% CI high |
4.2 | 5.7 | 6.9 |
MAP: Mycobacterium avium subsp. paratuberculosis; BRU: Brucella sp.; COX: Coxiella burnetii; LEPT: Leptospira; ANA: Anaplasma phagocytophilum; CHL: Chlamydia abortus; BVDV1: bovine viral diarrhoea virus 1; BHV1: bovine herpesvirus 1; BTV: bluetongue virus; TBEV: tick-borne encephalitis virus; SBV: Schmallenberg virus; TOX: Toxoplasma gondii; NEO: Neospora caninum.
Table 3.
Comparison in seroprevalence between sexes, ages, and geographical origin of roe deer
| ANA | TBEV | SBV | TOX | NEO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 85/184 | 46.2% | 5/95 | 5.3% | 30/108 | 27.8% | 69/164 | 42.1% | 8/163 | 4.9% |
| LR: p=0.641 | LR: p=0.304 | LR: p=0.828 | LR: p=0.733 | LR: p=0.477 | ||||||
| M | 45/94 | 47.9% | 2/59 | 3.4% | 17/63 | 27.0% | 33/81 | 40.7% | 3/81 | 3.7% |
| F | 40/90 | 44.4% | 3/36 | 8.3% | 13/45 | 28.9% | 36/83 | 43.4% | 5/82 | 6.1% |
| Age (year) | 83/182 | 45.6% | 5/96 | 5.2% | 31/108 | 28.7% | 67/161 | 41.6% | 8/160 | 5.0% |
| LR: p=0.528 | LR: p=0.07 | LR: p=0.734 | LR: p=0.417 | LR: p=0.026 | ||||||
| <1 | 21/43 | 48.8% | 2/15 | 13.3% | 6/21 | 28.6% | 16/38 | 42.1% | 0/38 | 0.0% |
| 1 | 27/54 | 50.0% | 0/35 | 0.0% | 9/37 | 24.3% | 16/47 | 34.0% | 1/47 | 2.1% |
| >1 | 35/85 | 41.2% | 3/46 | 6.5% | 16/50 | 32.0% | 35/76 | 46.1% | 7/75 | 9.3% |
| Region | 81/181 | 44.8% | 5/93 | 5.4% | 31/106 | 29.2% | 69/160 | 43.1% | 6/159 | 3.8% |
| LR: p=0.221 | LR: p=0.635 | LR: p=0.068 | LR: p=0.472 | LR: p=0.843 | ||||||
| 1 | 0/2 | 0.0% | 1/2 | 50.0% | 0/2 | 0.0% | ||||
| 2 | 8/16 | 50.0% | 1/4 | 25.0% | 1/3 | 33.3% | 6/16 | 37.5% | 2/16 | 12.5% |
| 3 | 4/7 | 57.1% | 0/4 | 0.0% | 0/4 | 0.0% | 2/5 | 40.0% | 0/5 | 0.0% |
| 4 | 3/4 | 75.0% | 0/1 | 0,0% | 1/2 | 50.0% | 2/3 | 66.7% | 0/3 | 0.0% |
| 5 | 3/11 | 27.3% | 0/3 | 0.0% | 7/11 | 63.6% | 1/11 | 9.1% | ||
| 6 | 0/2 | 0.0% | 0/2 | 0,0% | 1/2 | 50.0% | 0/2 | 0.0% | ||
| 7 | 25/50 | 50.0% | 2/24 | 8.3% | 4/30 | 13.3% | 15/49 | 30.6% | 2/49 | 4.1% |
| 8 | 5/7 | 71.4% | 0/2 | 0.0% | 1/3 | 33.3% | 3/7 | 42.9% | 0/7 | 0.0% |
| 9 | 12/33 | 36.4% | 1/11 | 9.1% | 8/18 | 44.4% | 14/26 | 53.8% | 0/25 | 0.0% |
| 10 | 21/47 | 44.7% | 1/47 | 2.1% | 16/41 | 39.0% | 16/37 | 43.2% | 1/37 | 2.7% |
| 11 | 0/1 | 0.0% | 1/1 | 100.0% | 0/1 | 0.0% | ||||
| 12 | 0/1 | 0.0% | 1/1 | 100.0% | 0/1 | 0.0% | ||||
ANA: Anaplasma phagocytophilum; TBEV: tick-borne encephalitis virus; SBV: Schmallenberg virus; TOX: Toxoplasma gondii; NEO: Neospora caninum; LR=log-likelihood ratio.
Table 4.
Comparison of numbers positive and doubtful reacting sera between sexes, ages, and geographical origin of roe deer
| MAP | CHL | |||||||
|---|---|---|---|---|---|---|---|---|
| Positive | Doubtful | Positive | Doubtful | |||||
| Sex | 7/165 | 4.2% | 2/165 | 1.2% | 10/173 | 5.8% | 4/173 | 2.3% |
| LR: p=0.529 | LR: p=0.029 | |||||||
| M | 5/84 | 6.0% | 1/84 | 1.2% | 4/92 | 4.3% | 0/92 | 0.0% |
| F | 2/81 | 2.5% | 1/81 | 1.2% | 6/81 | 7.4% | 4/81 | 4.9% |
| Age | 7/163 | 4.3% | 2/163 | 1.2% | 11/172 | 6.4% | 4/172 | 2.3% |
| LR: p=0.725 | LR: p=0.786 | |||||||
| <1 | 1/39 | 2.6% | 1/39 | 2.6% | 4/38 | 10.5% | 1/38 | 2.6% |
| 1 | 2/47 | 4.3% | 0/47 | 0.0% | 2/53 | 3.8% | 1/53 | 1.9% |
| >1 | 4/77 | 5.2% | 1/77 | 1.3% | 5/81 | 6.2% | 2/81 | 2.5% |
| Region | 7/166 | 4.2% | 2/166 | 1.2% | 12/171 | 7.0% | 4/171 | 2.3% |
| LR: p=0.430 | LR: p=0.979 | |||||||
| 1 | 0/2 | 0.0% | 0/2 | 0.0% | ||||
| 2 | 0/13 | 0.0% | 1/13 | 7.7% | 2/15 | 13.3% | 0/15 | 0.0% |
| 3 | 0/7 | 0.0% | 0/7 | 0.0% | 0/5 | 0.0% | 0/5 | 0.0% |
| 4 | 0/3 | 0.0% | 0/3 | 0.0% | 0/3 | 0.0% | 0/3 | 0.0% |
| 5 | 1/10 | 10.0% | 0/10 | 0.0% | 1/10 | 10.0% | 0/10 | 0.0% |
| 6 | 0/2 | 0.0% | 1/2 | 50.0% | 0/2 | 0.0% | 0/2 | 0.0% |
| 7 | 2/49 | 4.1% | 0/49 | 0.0% | 2/50 | 4.0% | 1/50 | 2.0% |
| 8 | 0/7 | 0.0% | 0/7 | 0.0% | 0/7 | 0.0% | 0/7 | 0.0% |
| 9 | 0/33 | 0,0% | 0/33 | 0.0% | 2/29 | 6.9% | 2/29 | 6.9% |
| 10 | 4/40 | 10.0% | 0/40 | 0.0% | 5/47 | 10.6% | 1/47 | 2.1% |
| 11 | 0/1 | 0.0% | 0/1 | 0.0% | ||||
| 12 | 0/1 | 0.0% | 0/1 | 0.0% | 0/1 | 0.0% | 0/1 | 0.0% |
MAP: Mycobacterium avium subsp. paratuberculosis; CHL: Chlamydia abortus; LR: log-likelihood ratio.
Results
From 205 sera collected, 15 (7%) had to be discarded due to putrefaction. A total of 190 sera useful for analysis were obtained over the whole sampling period 2008–2013. Table 2 shows the numbers and percentages of positive/doubtful reacting sera. As our results did not allow normal approximation, exact confidence intervals were determined, explaining the asymmetric intervals.
The highest prevalences (>25%) found were for A. phagocytophilum, SBV, and T. gondii. A lower prevalence (3–10%) was found for MAP (7/171: 2/60 Pourquier, 5/111 IDEXX), C. abortus (12/178), TBEV (5/98), and N. caninum (8/168). The lowest prevalences (1–2%) were found for C. burnetii (2/171), Leptospira (3/177), and BVDV1 (2/159). Doubtful results, i.e. between the negative and positive cut-off value, were obtained for MAP (2/171), C. abortus (4/178), and BTV (5/165). For BTV, no seropositives were found. No antibodies were found against B. abortus and BHV1.
For the agents with a seroprevalence higher than 2%, comparison between sexes, ages, and regions of sampling is shown in Tables 3 and 4. Sera with unknown sex, age or region were not included.
Concerning the doubtful results for C. abortus, a significant difference between sexes could be noticed (higher seroprevalence in females). For N. caninum, the difference between ages is significant. An age dependence for TBEV and a region dependence for SBV are suggested by the results, albeit below the threshold of significance. For SBV, two sampling periods yielding different results could be distinguished: period 1: 02/2009-09/2011; period 2: 01/2012-03/2013 (no sera were obtained from October to January due to the legal hunting periods in Flanders): all the seropositive sera were found in period 2 exclusively (period 1: 0/63 or 0%; period 2: 31/49 or 63%).
Discussion
None of the serologic tests used has been validated for use in roe deer, and exact information about their performances in species other than domestic ruminants is lacking. The use of all species IgG in the conjugate of the iELISAs enables the use of these tests in wild cervids, but for lack of species-specific cut-off values, those for domestic ruminants were applied, like in many similar studies. In most of the results, a clear distinction could be made between negative and positive reacting sera. Nevertheless, a small number of doubtful results were obtained for MAP, C. abortus, and BTV (Tables 2 and 4). In a competitive binding assay, Tryland et al. (14) found for roe deer and red deer IgG a respectively higher and lower affinity for protein G as compared to cattle IgG. Also elk (Cervus canadensis) IgG shows a higher protein G affinity (15). This implies that when protein G is used in the conjugate for the iELISA tests, cut-off values for roe deer sera could be slightly adapted as compared to cattle sera (see below).
The iELISA tests commonly used for MAP cross-react with other mycobacteria. By absorbing the sera with M. phlei antigen, genetically related to MAP, cross-reactions are eliminated and an increased specificity is obtained (2). The sensitivity of the ELISA is very low as less than 50% of infected cattle (confirmed by isolation) were found seropositive (16). Poor sensitivity also limits the utility for surveillance in wild cervids (17). For example, Nebbia et al. (18) found only one seropositive among 10 PCR positive red deer in the Italian Alps, and in a serosurvey in a paratuberculosis affected fallow deer herd in Spain no seropositive animals could be detected (19). The distribution of optical densities we obtained allowed to distinguish positive and negative samples, except for two ‘doubtful’ results (IDEXX) between the negative and positive cut-off. The higher affinity of protein G for roe deer IgG can be taken into account (14): by slightly increasing the negative and positive cut-off values (expressed as sample/positive control optical density ratio), i.e. from 55 to 60% for the positives and from 45 to 50% for the negatives, the two doubtful sera become negative but one of the formerly positive sera becomes ‘doubtful’, reducing the final number of doubtful results to one. Comparison of serologic results for MAP in wild cervids in Europe is an approximation, given the use of different antigens, antibodies, and cut-off values and the lack of validation of the tests in species other than cattle (15, 20). The seroprevalence reported in roe deer in Europe is generally low, reaching up to 12% (14), in contrast to red deer where 30% seropositives and clinically affected animals are found in areas where MAP is endemic in cattle (20). In Wallonia, Linden et al. (21) found 1.13% seropositive roe deer and 7.26% red deer, only the latter showing signs and lesions of paratuberculosis. The herd seroprevalence in Belgian cattle is 22%, and at animal level in dairy cattle the prevalence is up to 3.9% (22). Given the poor sensitivity of the test, our results (4.1% positives) confirm that MAP is endemic in roe deer in Flanders and that maintenance of the infection in roe deer cannot be excluded.
B. abortus: Sensitivity of the iELISA for Brucella antibodies is high, but poor specificity and the non-validation for use in wildlife necessitate confirmation by the Rose Bengal Test for the seropositive results. For in-depth epidemiological studies, the isolation of Brucella remains the gold standard (23, 24). Brucella appears to be of little importance in cervids in Europe. Antibodies are generally absent in roe deer, while only 0.4% positives were found in red deer in Spain (25). The absence of anti-Brucella antibodies in Flemish roe deer accords with other European studies, and indicates also that transmission of B. suis from wild boar is unlikely (B. melitensis does not occur in Northwestern Europe).
Of the serologic tests commonly used for C. burnetii, the iELISA and immunofluorescence assay (IFA) are more sensitive than the complement fixation (CF) test (4). C. burnetii seroprevalence in wild cervids in Europe is poorly documented but can reach 15% in roe deer and 29% in red deer (26). In the Netherlands during the human epidemic (2007–2010), 23% of the roe deer examined were PCR positive, without signs of disease (27). The antibody prevalence (1.2%) we found in roe deer in Flanders is low compared to domestic ruminants: in Belgian dairy cattle, the within-herd prevalence is 3.9% while the herd prevalence is 77% in dairy cattle and 13% in goats (22, 28). Differences in virulence of C. burnetii strains for different host species could play a role in the different seroprevalence between roe deer and cattle: C. burnetii field isolates from goat and bovine origin in Belgium showed a different replication rate in bovine macrophages suggesting host specificity (29). Our findings do not indicate roe deer to be a reservoir for C. burnetii in Flanders.
The sensitivity of the MAT for Leptospira is limited as chronic carriers may have undetectable antibody titres. Moreover, in order to maximise the sensitivity, all the serogroups known to occur in the area studied have to be included. The specificity is good although significant cross-reactivity exists between serogroups (5). The seroprevalence to leptospirosis in wild cervids in Europe is poorly documented, but is generally low (up to 7%) (30, 31). Consistently, our results confirm that roe deer in Flanders are insignificant carriers of Leptospira: 3/177 (1.7%) were positive. Two of the positives were collected on dead-found roe deer, an adult hind showing mycotic abscesses in the lungs, and a fawn. The lesions were not suggestive for leptospirosis. The third sample came from a male roe deer, over 1 year old, that also proved seropositive for MAP and A. phagocytophilum. Immune suppression or concurrent infections may have played a role in the seropositive animals. The serovars we found were icterohaemorrhagiae+ballum (from the hind and the fawn) and grippotyphosa (from the male). However, due to serological cross-reactivity between serovars and serogroups of Leptospira, identifying correctly the serovar would require isolation of the agent.
A. phagocytophilum: Serological cross-reactivity between Anaplasma species is known in both tests commonly used, the competitive ELISA and the IFA (32, 33). Yet A. marginale, A. centrale, and A. ovis are restricted to Mediterranean and Central–Eastern Europe and have not been reported in North–Western Europe until now (33). False positives due to haemolysis can bias the test. Therefore, the high seroprevalence against A. phagocytophilum, although comparable to results of other European studies using the same method, can be questioned for field-collected samples being frequently haemolytic. Nevertheless, high seroprevalences mostly coincide with high PCR prevalences (up to 86%) in roe deer blood or spleen samples. In cervids, the seroprevalence reported is generally very high, and is higher in roe deer (up to 96%) than in red deer (up to 55%) (34–36). Like elsewhere in Europe, roe deer in Flanders appear to be highly exposed to A. phagocytophilum with a seroprevalence of 45.8%. Yet the importance for transmission to cattle has to be relativised as suggested by recent phylogenetic work. Three genotypic distinct groups of A. phagocytophilum have been identified, of which two include 98% of all the genotypes found in cattle, different of the genotypes found in roe deer (37).
C. abortus: Serological data about Chlamydia infections in ruminants often refer to CF antibodies against the lipopolysaccharide (LPS) antigen common to all Chlamydiaceae. Aspecific cross-reactions are possible (38). The antigen in the iELISA used here is a species-specific fragment of the major outer membrane protein (MOMP) of C. abortus. By using a MOMP- or polymorphic outer membrane protein (POMP)-based ELISA, a higher specificity for C. abortus and higher sensitivity are obtained (39). Up to 20% seroprevalence was reported in roe deer for C. abortus-specific antibodies and 28% for Chlamydiaceae (Supplementary file 1). Though the impact on European wild ungulates is unknown, there is no doubt about their capacity of maintaining the infection (39). We found 6.7% seropositives and 2.2% doubtful results for C. abortus antibodies. Even without including the doubtfuls, the seroprevalence in roe deer is higher than the highest prevalence found in domestic animals, being 4.05% in sheep (province of Limburg) and 4.23% in cattle (province of Walloon Brabant) (40). Interestingly, a majority of our sera came from the province of Limburg. Yet there was no statistic difference in seropositive results between geographical origin of the samples (p=0.98). A significant difference in seroprevalence between sexes can be noticed on account of the four doubtful results (p=0.029; higher in females). Between-sex differences were reported in sheep and goats (41) and in free-ranging yak (Poephagus grunniens), possibly linked to sexual transmission by males carrying C. abortus in their genital tract (42). Other studies found no sex difference in C. abortus antibody prevalence in red and roe deer (39, 43). The higher prevalence than in domestic ruminants we found in Flemish roe deer supports their reservoir role for C. abortus.
Antigenic variability in some of the epitopes of viral antigen contributes to the complexity in classification, clinical behaviour, and diagnosis of BVDV strains (44, 45). VNT is considered the gold standard for antibody testing against BVDV. It offers reliable information on the circulation of virus strains antigenically related to the NADL strain of BVDV1 we used. Antibodies to less related strains ((e.g. certain BVDV2 strains) may still cross-react, but low antibody levels may be missed. To broaden the reactivity, testing against more than one isolate, e.g. both a BVDV1 and a BVDV2 strain, is needed. In Belgian cattle, BVDV1 is the most prevalent virus, the herd seroprevalence being 47% and the prevalence at animal level 33% (46). Antibodies to BVDV in cervids are generally low or absent. A higher prevalence was found in roe deer in Germany (10%: 47) and Norway (12%: 48). The low antibody prevalence (1.3%) against BVDV1 we found does not allow us to suspect maintenance in roe deer and contrasts with the high seroprevalence in Belgian cattle. Spillover from the latter is probable. Distinct pestivirus strains, different from those in domestic animals, have been found in roe deer (49, 50) but need more specialised methods to be detected.
In cattle, VNT and various ELISA tests are used for BHV1 antibody detection (7, 51). These tests are unable to distinguish between antibodies against BHV1 or BHV1 related herpesviruses due to their antigenic similarity. Weak positive samples may score falsely negative. Combined VNTs against the different related viruses or non-serologic methods such as qPCR are needed for differentiation. The seroprevalence for alphaherpesviruses reported in roe deer in Europe is between 0 and 3% (48, 52). In roe deer, it is generally lower than in red deer, for which between 11 and 29% seropositives were found in Belgium (53, 54) and up to 40% in Scotland (55). Using combined neutralisation tests, Frölich et al. (56) found 10.55%, 2.6%, and 5.2% roe deer seropositive for BHV1, caprine herpesvirus 1 (CpHV1), and cervid herpesvirus 1 (CvHV1), respectively. Our results indicate that roe deer are not relevant in the epidemiology of BHV1 and related alphaherpesviruses in Flanders.
The commonly used ELISA tests for BTV antibodies all show high specificity and sensitivity in cattle (57). The competitive ELISA is species independent because the detection system is directed to a generic competitive monoclonal antibody (58). In a Swiss survey in wild ruminants using cELISA, increasing haemolysis lowered the mean optical density value, implicating a higher probability of false negative results. Yet confirmatory tests to rule out false negative and positive results showed that haemolysis did not significantly affect the outcome (59). Information about BTV seroprevalence in wild cervids in Europe is scarce but in roe deer up to 5% is reported in epizootic areas (60). This is much lower than in red deer (in Spain regionally up to 62%; France 47%) in which maintenance of infection is suspected while roe deer appear to be dead-end hosts (61, 62). During the northwestern European epizootic between 2006 and 2008, the seroprevalence in roe deer in Wallonia rose from 0% in 2005 to 2.75% in 2007 and went back to 1.67% in 2008, while in red deer it increased from 1.51% in 2006 to 52.33% in 2007 and back to 33.95% in 2008 (63). None of our sera was positive for antibodies against BTV, but 5/165 (3%) were ‘doubtful’. Based on the foregoing, all the sera can be considered seronegative, according to the fading seroprevalence in roe deer in Wallonia from 2008 on (63).
Compared to the ELISA test in which cross-reactions with other flaviviruses occur, the RFFIT seroneutralisation test for TBEV is highly specific. Neutralising titres obtained by RFFIT show a high correlation with standard tests (plaque reduction test; haemagglutination inhibition), indicating a good sensitivity (9). The TBEV seroprevalence reported in roe deer in endemic areas in Europe ranges between 9 and 26% (35, 64), with local maxima as high as 50% (65). In Wallonia, between 2007 and 2009, 12.4% roe deer were found seropositive with ELISA (including 2/5 highly reactive sera showing significant VNT titres) (66). For Flanders, we found weak positive titres, just above cut-off, in 5.1% of our sera. Disregarding a possible aspecific viral inhibition due to poor quality of the sera (S.Van Gucht, personal communication), this result suggests virus circulation, and accords with the serologic findings in cattle and wild boar. Autochthonous human infection is not reported in Belgium, but 2.6–3.5% of Belgian cattle proved seropositive (8). Recently, antibodies have also been found in wild boar in Flanders (Roelandt et al., personal communication). The nearly significant difference in seroprevalence between roe deer of different ages (p=0.07, highest in animals <1 year) is in agreement with Gerth et al. 1995 (64) who suggest most infections occur at an early age.
The massive circulation of SBV in 2011 in Belgium was evidenced by a between-herd seroprevalence of almost 100% in sheep and cattle at the end of the first vector season (67). In domestic ruminants, VNT for SBV antibodies has a high specificity and sensitivity (10, 68), but little is known about its performance in roe deer sera. By using a cut-off value of 8, 27.9% of the sera collected over the whole sampling period were positive. By its recent emergence, information about SBV in wild ruminants in Europe is scarce. In The Netherlands, VNT revealed 69% (cut-off 8) and 52% seropositives (cut-off 16) in roe deer submitted for post-mortems (69). An exponential increase in seropositivity was found in the fall of 2011 in Wallonia in wild cervids (red deer 40%, roe deer 46%) and in France (20%) using ELISA (70, 71). In Flanders, where the circulation of SBV in its Culicoides vectors was reported from September 2011 (72), all sera sampled before October 2011 (period 1) were negative. In the sera collected between January 2012 and March 2013 (period 2), the percentage of positives (63%) was similar to the Walloon sera collected in November 2011 (62.5%). Therefore, seroconversion in Flemish roe deer must have taken place between October and December 2011. In Wallonia, seropositive deer were already found in October 2011 and seropositivity rose to 88.9% in December 2011 (70). In Flanders, no further follow-up was carried out for SBV in roe deer after March 2013. The high seropositivity for SBV in Flemish roe deer, apparently without any clinical impact, like in Wallonia coincided temporally with the progress of the epizootic in domestic ruminants, and with the presence of infected vectors. Larska et al. (73) found a higher SBV seroprevalence in roe deer with increasing domestic ruminant density, suggesting spillover from the latter. The density distribution of domestic animals may provide a possible explanation for the close to significant difference in seroprevalence we found between regions (p=0.068).
The performances of the iELISA for antibodies to T. gondii in roe deer appear good because 100% agreement was found between the results of iELISA and MAT (11). Cross-reactivity with antibodies to the related N. caninum is rare (74). The T. gondii seroprevalence found in roe deer in Europe ranges from 13 to 63% (52, 75). In accordance, we found a high prevalence (43.2%) in Flemish roe deer. As cervids are herbivorous, infection with Toxoplasma occurs by ingesting oocysts, which supposes contact with cat faeces. Therefore, roe deer, among other wildlife, are important indicators of environmental contamination with Toxoplasma oocysts. The small landscape structure of densely populated Flanders and the many domestic and stray cats make heavy contamination probable and could explain the high seropositivity in roe deer sera. Flemish house cats show 20.4% seropositivity (Belgium: 27%) (76). Wild cats (Felis silvestris) are extinct in Flanders.
N. caninum is the most important cause of abortion in Flemish cattle, the herd seroprevalence being 62.5% (22). In cattle, more than 95% specificity and sensitivity are obtained with the IDVET test (12). Cross-reactions with T. gondii are negligible (cf above). In the few studies carried out in roe deer in Europe, the seroprevalence appears generally low, i.e. between 3 and 14% (52, 77). The 4.8% we found fits within this range. Low seroprevalence to N. caninum may reflect rare contacts with domestic dogs, being the end hosts. Contacts with red fox faeces are more probable for roe deer than contact with dog faeces, the red fox being omnipresent in Flanders with often high population densities in multiple small territoria ((regional estimates: up to 3.5/km2; K.Van Den Berge, personal communication). If the test is enough sensitive, the low seroprevalence of N. caninum antibodies we found in roe deer supports the non-relevance of foxes as N. caninum end hosts (78, 79). The significant difference in seroprevalence we observe between ages (p=0.026) accords with other studies in which seroprevalence increases with age (80)
Being browsers, roe deer feed on a broad range of wild plants and cultured crops, of which they select those parts with high protein and carbohydrate and low cellulose contents, such as buds, young twigs, and leaves (81). As food generalists, they take advantage of a broad diversity of natural and cultured biotopes, which explains their near-omnipresence in Europe. Flanders is characterised by an intensive landscape use and urbanisation pressure by which roe deer have adapted to feeding close to livestock and human dwellings, as confirmed by many observations. Still, the antibody prevalences we found in roe deer are largely comparable to those of other surveys in Europe and may depend on local environmental factors as suggested by Boadella et al. (82).
Briefly, our observations indicate that a reservoir function of roe deer for C. abortus and MAP is probable, that despite the enzootic situation in domestic animals, antibodies to C. burnetii and BVDV1 are very low and that antibodies to BHV1 are absent. Like elsewhere, roe deer appear important carriers of at least certain genotypes of the tick-borne A. phagocytophilum. Roe deer are the main feeding hosts for I. ricinus in Europe and, next to climate change (83), their expansion is thought to be a major driving force in tick abundance at least in some parts of Europe (84–86). Therefore, serologic screening in roe deer provides essential information about the circulation of many tick-borne infections in Europe, even those for which they are non-competent hosts like TBEV and Borellia burgdorfferi. For the infections transmitted by midges, unknown in northern Europe before the BTV8 epidemic despite the presence of endemic Culicoides species, there is no reason to assume that roe deer would play a key role. The seroprevalence to BTV or SBV coincided temporally with the epizootic in domestic ruminants, indicating simultaneous infection or spillover.
In conclusion, roe deer maintain C. abortus and MAP in Flanders, but do not seem to play an important role in the epidemiology of the other infectious agents examined. However, continued surveillance in roe deer as sentinels for newly emerging or existing diseases is recommended, because it is the principal large game species in Flanders and relatively easy to access for sampling.
Acknowledgements
Many people have contributed voluntarily to this work that was carried out without any financial support. We thank the roe deer hunters who kindly provided the roe deer sera. We also thank the technicians and the heads of the different laboratories for carrying out the analyses, the information provided and the fruitful discussions. Finally, we acknowledge all those who helped in completing the literature collection and in providing useful suggestions.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
To access the supplementary material for this article, please see Supplementary files under ‘Article Tools’
References
- 1.Scheppers T, Huysentruyt F, Neukermans A, Vercammen J, Verschaffel E, Casaer J. Grofwildjacht in Vlaanderen. Cijfers en statistieken over de periode 2002–2012. Report (in Dutch) INBO.R.2013.30 D/2013/3241/185. 2013. pp. 19–20. Available from: https://data.inbo.be/purews/files/4525047/Scheppers_etal_2013_GrofwildjachtVlaanderen.pdf [cited 7 September 2015].
- 2.Bech-Nielsen S, Berg Jorgensen J, Ahrens P, Feld NC. Diagnostic accuracy of a Mycobacterium phlei-absorbed serum enzyme-linked immunosorbent assay for diagnosis of bovine paratuberculosis in dairy cows. J Clin Microbiol. 1992;3:613–18. doi: 10.1128/jcm.30.3.613-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limet JN, Kerkhofs P, Wijffels R, Dekeyser P. Le diagnostic serologique de la brucellose bovine par ELISA. Ann Med Vet. 1988;132:565–75. [Google Scholar]
- 4.Guatteo R, Beaudeau F, Joly A, Seegers H. Performances of an ELISA applied to serum and milk for the detection of antibodies to Coxiella burnetii in dairy cattle. Rev Méd Vet. 2007;158:250–2. [Google Scholar]
- 5.OIE. Leptospirosis. OIE Terrestrial manual. 2014. Leptospirosis, Ch 2.1.9 http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.09_LEPTO.pdf [cited 7 September 2015].
- 6.Collet MS, Larson R, Gold C, Strick D, Anderson DK, Purchio AF. Molecular cloning and nucleotide sequence of the pestivirus bovine diarrhea virus. Virology. 1988;165:191–9. doi: 10.1016/0042-6822(88)90672-1. [DOI] [PubMed] [Google Scholar]
- 7.Kramps JA, Banks M, Beer M, Kerkhofs P, Perrin M, Wellenberg GJ, et al. Evaluation of tests for antibodies against bovine herpesvirus 1 performed in national reference laboratories in Europe. Vet Microbiol. 2004;102:169–81. doi: 10.1016/j.vetmic.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Roelandt S, Suin V, Riocreux F, Lamoral S, Van der Heyden S, Van der Stede Y, et al. Autochthonous tick-borne encephalitis virus (TBEV) - seropositive cattle in Belgium: a risk-based targeted serological survey. Parasit Vectors. 2014;7(Suppl 1):O35. doi: 10.1089/vbz.2014.1576. [DOI] [PubMed] [Google Scholar]
- 9.Vene S, Haglund M, Vapalahti O, Lundkvist Å. A rapid fluorescent focus inhibition test for detection of neutralizing antibodies to tick-borne encephalitis virus. J Virol Methods. 1998;73:71–5. doi: 10.1016/s0166-0934(98)00041-x. [DOI] [PubMed] [Google Scholar]
- 10.De Regge N, van den Berg T, Georges L, Cay B. Diagnosis of Schmallenberg virus infection in malformed lambs and calves and first indications for virus clearance in the fetus. Vet Microbiol. 2013;162:595–600. doi: 10.1016/j.vetmic.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 11.De Craeye S, Speybroeck N, Ajzenberg D, Dardé ML, Collinet F, Tavernier P, et al. Toxoplasma gondii and Neospora caninum in wildlife: common parasites in Belgian foxes and Cervidae? Vet Parasitol. 2011;178:64–9. doi: 10.1016/j.vetpar.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Álvarez-García G, García-Culebras A, Gutiérrez-Expósito D, Navarro-Lozano V, Pastor-Fernández I, Ortega-Mora LM. Serological diagnosis of bovine neosporosis: a comparative study of commercially available ELISA tests. Vet Parasitol. 2013;198:85–95. doi: 10.1016/j.vetpar.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Vandenbussche F, Vanbinst T, Verheyden B, Van Dessel W, Demeestere L, Houdart P, et al. Evaluation of antibody-ELISA and real-time RT-PCR for the diagnosis and profiling of bluetongue virus serotype 8 during the epidemic in Belgium in 2006. Vet Microbiol. 2008;129:15–27. doi: 10.1016/j.vetmic.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Tryland M, Olsen I, Vikøren T, Handeland K, Arnemo JM, Tharaldsen J, et al. Serologic survey for antibodies against Mycobacterium avium subsp. paratuberculosis in free-ranging cervids from Norway. J Wildl Dis. 2004;40:32–41. doi: 10.7589/0090-3558-40.1.32. [DOI] [PubMed] [Google Scholar]
- 15.Pruvot M, Forde TL, Steele J, Kutz SJ, De Buck J, van der Meer F, et al. The modification and evaluation of an ELISA test for the surveillance of Mycobacterium avium subsp. paratuberculosis infection in wild ruminants. BMC Vet Res. 2013;9:5. doi: 10.1186/1746-6148-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitlock RH, Wells SJ, Sweeney RW, Van Tiem J. ELISA and fecal culture for paratuberculosis (Johne's disease): sensitivity and specificity of each method. Vet Microbiol. 2000;77:387–98. doi: 10.1016/s0378-1135(00)00324-2. [DOI] [PubMed] [Google Scholar]
- 17.Woodbury MR, Chirino-Trejo M, Mihajlovic B. Diagnostic detection methods for Mycobacterium avium subsp. paratuberculosis in white-tailed deer. Can Vet J. 2008;49:683–88. [PMC free article] [PubMed] [Google Scholar]
- 18.Nebbia P, Robino P, Ferroglio E, Rossi L, Meneguz G, Rosati S. Paratuberculosis in red deer (Cervus elaphus hippelaphus) in the western Alps. Vet Res Commun. 2000;24:435–43. doi: 10.1023/a:1006482520336. [DOI] [PubMed] [Google Scholar]
- 19.Marco I, Ruiz M, Juste R, Garrido JM, Lavin S. Paratuberculosis in free-ranging fallow deer in Spain. J Wildl Dis. 2002;38:629–32. doi: 10.7589/0090-3558-38.3.629. [DOI] [PubMed] [Google Scholar]
- 20.Reyes-García R, Pérez-de-la-Lastra JM, Vicente J, Ruiz-Fons F, Garrido JM, Gortázar C. Large scale ELISA testing of Spanish red deer for paratuberculosis. Vet Immunol Immunopathol. 2008;124:75–81. doi: 10.1016/j.vetimm.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Linden A, Grégoire F, Mousset B, Hoyoux A, Canivet P, De Rijk P, et al. Monitoring of wild cervids paratuberculosis (Mycobacterium avium subspecies paratuberculosis) in southern Belgium; Proceedings 6th Int. Conference European Wildlife Disease Association; 8–12 September 2004; Uppsala. p. 33. [Google Scholar]
- 22.Vangeel I, Méroc E, Roelandt S, Welby S, Van Driessche EV, Czaplicky G, et al. Seroprevalence of Neospora caninum, paratuberculosis and Q Fever in cattle in Belgium in 2009–2010. Vet Rec. 2012;171:477. doi: 10.1136/vr.101016. [DOI] [PubMed] [Google Scholar]
- 23.Godfroid J. Brucellosis in wildlife. Rev Sci Tech. 2002;21:277–86. doi: 10.20506/rst.21.2.1333. [DOI] [PubMed] [Google Scholar]
- 24.Godfroid J, Nielsen K, Saegerman C. Diagnosis of brucellosis in livestock and wildlife. Croat Med J. 2010;51:296–305. doi: 10.3325/cmj.2010.51.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muñoz PM, Boadella M, Arnal M, de Miguel MJ, Revilla M, Martínez D, et al. Spatial distribution and risk factors of brucellosis in Iberian wild ungulates. BMC Infect Dis. 2010;10:46. doi: 10.1186/1471-2334-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz-Fons F, Rodríguez O, Torina A, Naranjo V, Gortázar C, de la Fuente J. Prevalence of Coxiella burnetti infection in wild and farmed ungulates. Vet Microbiol. 2008;126:282–6. doi: 10.1016/j.vetmic.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Rijks JM, Roest HIJ, van Tulden PW, Kik MJL, IJzer J, Gröne A. Coxiella burnetii infection in roe deer during Q Fever epidemic, The Netherlands. Emerg Infect Dis. 2011;17:2369–71. doi: 10.3201/eid1712.110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribbens S, De Bleecker K, Van Crombrugge J-M, De Meulemeester L, Mijten E, Stoops S, et al. Serosurvey of four emerging cattle diseases (Q-fever, Neosporosis, Leptospirosis and Salmonellosis) in northern-Belgian dairy herds using bulk-milk samples. Proceedings of the 17th Annual Meeting of the Flemish Society for Veterinary Epidemiology and Economics; Tervuren; 23 October 2009. pp. 51–5. [Google Scholar]
- 29.Mori M, Boarbi S, Michel P, Bakinahe R, Rits K, Wattiau P, et al. In vitro and in vivo infectious potential of Coxiella burnetii: a study on Belgian livestock isolates. PLoS One. 2013;8:e67622. doi: 10.1371/journal.pone.0067622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treml F, Nesnalová E. Occurrence of Leptospira antibodies in the blood of game animals. Vet Med (Praha) 1993;38:123–7. [PubMed] [Google Scholar]
- 31.Fennestadt KL, Borg-Petersen C. Leptospirosis in Danish wild mammals. J Wildl Dis. 1972;8:343–51. doi: 10.7589/0090-3558-8.4.343. [DOI] [PubMed] [Google Scholar]
- 32.Dreher UM, De La Fuente J, Hofmann-Lehmann R, Meli ML, Pusterla N, Kocan KM, et al. Serologic cross-reactivity between Anaplasma marginale and Anaplasma phagocytophilum . Clin Diagn Lab Immunol. 2005;12:1177–83. doi: 10.1128/CDLI.12.10.1177-1183.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dreher UM, Hofmann-Lehmann R, Meli ML, Regula G, Cagienard AY, Stärk KD, et al. Seroprevalence of anaplasmosis among cattle in Switzerland in 1998 and 2003: no evidence of an emerging disease. Vet Microbiol. 2005;107:71–9. doi: 10.1016/j.vetmic.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Petrovec M, Bidovec A, Summer JW, Nicholson WL, Childs JE, Avsic-Zupanc T. Infection with Anaplasma phagocytophila in cervids from Slovenia: evidence of two genotypic lineages. Wien Klin Wochenschr. 2002;114:641–7. [PubMed] [Google Scholar]
- 35.Skarphédinsson S, Jensen PM, Kristiansen K. Survey of tick-borne infections in Denmark. Emerg Infect Dis. 2005;11:1055–61. doi: 10.3201/eid1107.041265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuen S, Åkerstedt J, Bergström K, Handeland K. Antibodies to granulocytic Ehrlichia in moose, red deer, and roe deer in Norway. J Wildl Dis. 2002;38:1–6. doi: 10.7589/0090-3558-38.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Dugat T, Chastagner A, Lagrée AC, Petit E, Durand B, Thierry S, et al. A new multiple-locus variable-number tandem repeat analysis reveals different clusters for Anaplasma phagocytophilum circulating in domestic and wild ruminants. Parasit Vectors. 2014;7:439. doi: 10.1186/1756-3305-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nurminen M, Wahlström E, Kleemola M, Leinonen M, Saikku P, Mäkelä H. Immunologically related ketodeoxyoctonate-containing structures in Chlamydia trachomatis, Re mutants of Salmonella species and Acinetobacter calcoaceticus var. anitratus . Infect Immun. 1984;44:609–13. doi: 10.1128/iai.44.3.609-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salinas J, Caro MR, Vicente J, Cuello F, Reyes-García AR, Buendía AJ, et al. High prevalence of antibodies against Chlamydiaceae and Chlamidophila abortus in wild ungulates using two “in house” blocking-ELISA tests. Vet Microbiol. 2009;135:46–53. doi: 10.1016/j.vetmic.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Yin L, Schautteet K, Kalmar ID, Bertels G, Van Driessche E, Czaplicki G, et al. Prevalence of Chlamydia abortus in Belgian ruminants. Vlaams Diergeneeskd Tijdschr. 2014;83:164–70. [Google Scholar]
- 41.Aljumaah RS, Hussein MF. Serological prevalence of ovine and caprine chlamydophilosis in Riyadh region, Saudi Arabia. Afr J Microbiol Res. 2012;11:2654–8. [Google Scholar]
- 42.Bandyopadhyay S, Sasmal D, Biswas TK, Samanta I, Ghosh MK. Serological evidence of antibodies against Chlamydophila abortus in free-ranging yak (Poephagus grunniens) in Arunachal Pradesh, India. Rev Sci Tech. 2009;28:1051–5. doi: 10.20506/rst.28.3.1948. [DOI] [PubMed] [Google Scholar]
- 43.Cubero-Pablo MJ, Plaza M, Pérez L, González M, León-Vizcaíno L. Seroepidemiology of chlamydial infections of wild ruminants in Spain. J Wildl Dis. 2000;36:35–47. doi: 10.7589/0090-3558-36.1.35. [DOI] [PubMed] [Google Scholar]
- 44.Potgieter LND. Bovine viral diarrhoea and mucosal disease (Ch 76) In: Coetzer JAW, Tustin RC, editors. Infectious diseases of livestock II. Cape Town: Oxford University Press; 2004. pp. 946–69. [Google Scholar]
- 45.Lanyon SR, Hill FI, Reichel MP, Brownlie J. Bovine viral diarrhea: pathogenesis and diagnosis. Vet J. 2013;199:201–9. doi: 10.1016/j.tvjl.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 46.Sarrazin S, Veldhuis A, Méroc E, Vangeel I, Laureyns J, Dewulf J, et al. Serological and virological BVDV prevalence and risk factor analysis for herds to be BVDV seropositive in Belgian cattle herds. Prev Vet Med. 2013;108:28–37. doi: 10.1016/j.prevetmed.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Frölich K. Bovine viral diarrhea and mucosal disease in free-ranging and captive deer (Cervidae) in Germany. J Wildl Dis. 1995;31:247–50. doi: 10.7589/0090-3558-31.2.247. [DOI] [PubMed] [Google Scholar]
- 48.Lillehaug A, Vikøren T, Larsen I-L, Åkerstedt J, Tharaldsen J, Handeland K. Antibodies to ruminant alpha-herpesviruses and pestiviruses in Norwegian cervids. J Wildl Dis. 2003;39:779–86. doi: 10.7589/0090-3558-39.4.779. [DOI] [PubMed] [Google Scholar]
- 49.Frölich K, Hofmann M. Isolation of bovine viral diarrhea virus-like pestiviruses from roe deer (Capreolus capreolus) J Wildl Dis. 1995;31:243–6. doi: 10.7589/0090-3558-31.2.243. [DOI] [PubMed] [Google Scholar]
- 50.Fisher S, Weiland E, Frölich K. Characterization of a bovine viral diarrhea virus isolated from roe deer in Germany. J Wildl Dis. 1998;34:47–55. doi: 10.7589/0090-3558-34.1.47. [DOI] [PubMed] [Google Scholar]
- 51.OIE. OIE terrestrial manual, editor. Infectious Bovine Rhinotracheitis/Infectious Pustular Vulvovaginitis. 2010;Ch 2.4.13:1–22. Available from: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.04.13_IBR_IPV.pdf [cited 7 September 2015]. [Google Scholar]
- 52.Gaffuri A, Giacometti M, Massimo Tranquilo V, Magnino S, Cordioli P, Lanfranchi P. Serosurvey of roe deer, chamois and domestic sheep in the central Italian Alps. J Wildl Dis. 2006;42:685–90. doi: 10.7589/0090-3558-42.3.685. [DOI] [PubMed] [Google Scholar]
- 53.Thiry E, Vercouter M, Dubuisson J, Barrat J, Sepulchre C, Gerardy C, et al. Serological survey of herpesvirus infections in wild ruminants of France and Belgium. J Wildl Dis. 1988;24:268–73. doi: 10.7589/0090-3558-24.2.268. [DOI] [PubMed] [Google Scholar]
- 54.Thiry J, Widén F, Grégoire F, Linden A, Belák S, Thiry E. Isolation and characterization of a ruminant alphaherpesvirus closely related to bovine herpesvirus I in a free-ranging red deer. BMC Vet Res. 2007;3:26. doi: 10.1186/1746-6148-3-26. doi: http://dx.doi.org/10.1186/1746-6148-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nettleton PF, Sinclair JA, Herring JA, Inglis DM, Fletcher TJ, Ross HM, et al. Prevalence of herpesvirus infection in British red deer and investigations of further disease outbreaks. Vet Rec. 1986;118:267–70. doi: 10.1136/vr.118.10.267. [DOI] [PubMed] [Google Scholar]
- 56.Frölich K, Hamblin C, Parida S, Tuppurainen E, Schettler E. Serological survey for potential disease agents of free-ranging cervids in six selected national parks from Germany. J Wildl Dis. 2006;42:836–43. doi: 10.7589/0090-3558-42.4.836. [DOI] [PubMed] [Google Scholar]
- 57.Niedbalski W. Evaluation of commercial ELISA kits for the detection of antibodies against bluetongue virus. Pol J Vet Sci. 2011;14:615–19. doi: 10.2478/v10181-011-0091-y. [DOI] [PubMed] [Google Scholar]
- 58.Meyer T. Competitive ELISA. In: Kontermann and Dübel, editor. Antibody engineering, Vol.1. Ch.47. Vienna: Springer Protocols, Springer; 2010. pp. 739–42. [Google Scholar]
- 59.Casaubon J, Chaignat V, Vogt H-R, Michel AO, Thür B, Ryser-Degiorgis M-P. Survey of bluetongue virus infection in free-ranging wild ruminants in Switzerland. BMC Vet Res. 2013;9:166. doi: 10.1186/1746-6148-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruiz-Fons F, Reyes-García AR, Alcaide V, Gortázar C. Spatial and temporal evolution of bluetongue virus in wild ruminants, Spain. Emerg Infect Dis. 2008;14:951–3. doi: 10.3201/eid1406.071586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.García-Bocanegra I, Arenas-Montes A, Lorca-Oró, Pujols J, Ángel Gonzáles M, Napp S, et al. Role of wild ruminants in the epidemiology of bluetongue virus serotypes 1, 4 and 8 in Spain. Vet Res. 2011;42:88. doi: 10.1186/1297-9716-42-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossi S, Pioz M, Beard E, Durand B, Gibert P, Gauthier D, et al. Bluetongue dynamics in French wildlife: exploring the driving forces. Transbound Emerg Dis. 2013;61:e12–24. doi: 10.1111/tbed.12061. [DOI] [PubMed] [Google Scholar]
- 63.Linden A, Grégoire F, Nahayo A, Hanrez D, Mousset B, Massart L, et al. Bluetongue virus in wild deer, Belgium, 2005–2008. Emerg Infect Dis. 2010;16:833–6. doi: 10.3201/eid1605.091217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerth HJ, Grimshandl D, Stage B, Döller G, Kunz C. Roe deer as sentinels for endemicity of tick-borne encephalitis virus. Epidemiol Infect. 1995;115:355–65. doi: 10.1017/s0950268800058477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiffner C, Vor T, Hagedorn P, Niedrig M, Rühe F. Determinants of tick-borne encephalitis virus antibody presence in roe deer (Capreolus capreolus) sera. Med Vet Entomol. 2012;26:18–25. doi: 10.1111/j.1365-2915.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- 66.Linden A, Wirtgen M, Nahayo A, Heyman P, Niedrig M, Schulze Y. Tick-borne encephalitis virus antibodies in wild cervids in Belgium. Vet Rec. 2012;170:108. doi: 10.1136/vr.e646. [DOI] [PubMed] [Google Scholar]
- 67.Méroc E, Poskin A, Van Loo H, Quinet C, Van Driessche E, Delooz L, et al. Large-scale cross-sectional serological survey of Schmallenberg virus in Belgian cattle at the end of the first vector season. Transbound Emerg Dis. 2013;60:4–8. doi: 10.1111/tbed.12042. [DOI] [PubMed] [Google Scholar]
- 68.Loeffen W, Quak S, de Boer-Luijtze E, Hulst M, van der Poel W, Bouwstra R, et al. Development of a virus neutralisation test to detect antibodies against Schmallenberg virus and serological results in suspect and infected herds. Acta Vet Scand. 2012;54:44. doi: 10.1186/1751-0147-54-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DWHC, Dutch Wildlife Health Centre. Terugkoppeling bevindingen – aanwijzingen voor antistoffen tegen Schmallenberg virus infectie bij reeën (in Dutch) 2013. Available from: http://www.dwhc.nl/Diersoort/Zoogdieren/Ree/antistof.html [cited 7 September 2015].
- 70.Linden A, Desmecht D, Volpe R, Wirtgen M, Gregoire F, Pirson J, et al. Epizootic spread of Schmallenberg virus among wild cervids, Belgium, fall 2011. Emerg Infect Dis. 2012;18:2006–8. doi: 10.3201/eid1812.121067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laloy E, Bréard E, Sailleau C, Viarouge C, Desprat A, Zientara S, et al. Schmallenberg virus infection among red deer, France 2010–2012. Emerg Infect Dis. 2014;20:131–4. doi: 10.3201/eid2001.130411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Regge N, De Deken R, Fassotte C, Losson B, Deblauwe I, Madder M, et al. Culicoides monitoring in Belgium in 2011: analysis of spatio-temporal abundance, species diversity and Schmallenberg virus detection. Med Vet Entomol. 2015;29:263–75. doi: 10.1111/mve.12109. [DOI] [PubMed] [Google Scholar]
- 73.Larska M, Krzysiak MK, Kesic-Maliszkewska J, Rola J. Cross-sectional study of Schmallenberg virus seroprevalence in wild ruminants in Poland at the end of the vector season of 2013. BMC Vet Res. 2014;10:967. doi: 10.1186/s12917-014-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum . Clin Microbiol Rev. 2007;20:323–67. doi: 10.1128/CMR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kapperud G. Survey for toxoplasmosis in wild and domestic animals from Norway and Sweden. J Wildl Dis. 1978;14:157–62. doi: 10.7589/0090-3558-14.2.157. [DOI] [PubMed] [Google Scholar]
- 76.De Craeye S, Francart A, Chabauty J, Van Gucht S, Leroux I, Jongert E. Toxoplasmosis in Belgian pet cats: recommendations for owners. Vlaams Diergeneeskd Tijdschr. 2008;77:325–30. [Google Scholar]
- 77.Bartova E, Sedlak K, Pavlik I, Literak I. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in wild ruminants from the countryside or captivity in the Czech Republic. J Parasitol. 2007;93:1216–18. doi: 10.1645/GE-1126R.1. [DOI] [PubMed] [Google Scholar]
- 78.Laureyns J. Overdracht van Neospora caninum. Vraag en Antwoord. Vlaams Diergeneeskd Tijdschr. 2009;78:450. (in Dutch) [Google Scholar]
- 79.Constantin EM, Schares G, Grossmann E, Sauter K, Romig T, Hartmann S. Studies on the role of the red fox (Vulpes vulpes) as a potential definitive host of Neospora caninum . Berl Münch Tierärtzl Wochenschr. 2011;124:148–53. [PubMed] [Google Scholar]
- 80.Malmsten J, Jakubek EB, Björkman C. Prevalence of antibodies against Toxoplasma gondii and Neospora caninum in moose (Alces alces) and roe deer (Capreolus capreolus) in Sweden. Vet Parasitol. 2011;177:275–80. doi: 10.1016/j.vetpar.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 81.Casaer J, Neukermans A, Baert P. Ree. In: Verkem S, De Maeseneer J, Vandendriessche B, Verbeylen G, Yskout S, editors. Zoogdieren in Vlaanderen. Ecologie en Verspreiding van 1987 tot 2000. Mechelen: Natuurpunt.Studie en JNM Zoogdierenwerkgroep; 2003. pp. 383–88. [Google Scholar]
- 82.Boadella M, Carta T, Oleaga Á, Pajares G, Muñoz M, Gortázar C. Serosurvey for selected pathogens in Iberian roe deer. BMC Vet Res. 2010;6:51. doi: 10.1186/1746-6148-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis 2009. 2009 doi: 10.1155/2009/593232. 593232. doi: http://dx.doi.org/10.1155/2009/593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George J-C, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jaenson TGT, Hjertqvist M, Bergström T, Lundkvist Å. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit Vectors. 2012;5:184. doi: 10.1186/1756-3305-5-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaenson TGT, Jaenson DGE, Eisen L, Petersson E, Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit Vectors. 2012;5:8. doi: 10.1186/1756-3305-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]