Review on the decline of immune response with age.
Keywords: immunosenescence, Toll-like receptor, polymorphonuclear leukocyte, dendritic cell, systems immunology
Abstract
Immunosenescence, describing alterations, including decline of immune responses with age, is comprised of inappropriate elevations, decreases, and dysregulated immune responses, leading to more severe consequences of bacterial and viral infections and reduced responses to vaccination. In adaptive immunity, these changes include increased proportions of antigen-experienced B and T cells at the cost of naïve cell populations. Innate immune changes in aging are complex in spanning multiple cell types, activation states, and tissue context. Innate immune responses are dampened in aging, yet there is also a paradoxical increase in certain signaling pathways and cytokine levels. Here, we review recent progress and highlight novel directions for expected advances that can lead the aging field to a new era of discovery that will embrace the complexity of aging in human populations.
Introduction
Aging of the human population is a global phenomenon, with the number of individuals over age 60 projected to increase from 841 million in 2013 to over 2 billion by 2050. Notably, whereas at present, 2/3 of these older adults live in developed regions, by 2050, it is estimated that 80% will reside in less-developed regions of the world [1]. Immunosenescence or alterations, including decline of immune responses with age, are comprised of inappropriate elevations, decreases, and dysregulated immune responses, leading to more severe consequences of bacterial and viral infections and reduced responses to vaccination. In adaptive immunity, these changes include increased proportions of antigen-experienced B and T cells at the cost of naïve cell populations. These changes reflect diminished thymic and bone marrow lymphopoiesis; diminished antigen receptor repertoire diversity, in part, reflecting control of chronic viral infections, such as cytomegalovirus; and developmental and signal transduction alterations, resulting in impaired generation of protective antibodies and CD4 and CD8 T cell responses [2, 3]. Recent studies have advanced our understanding of innate immune changes in aging, which are complex in spanning multiple cell types, activation states, and tissue contexts [4]. Aging of the innate immune system in humans is also notable for a paradoxical increase in levels of proinflammatory cytokines, such as IL-6 and TNF-α, as well as acute-phase reactants, such as C-reactive protein and blood clotting factors; this state of low-grade, chronic inflammation has been termed inflamm-aging [4, 5]. We will summarize recent findings on such aspects of age-related innate immune dysregulation and will highlight novel directions for expected advances that will enable more complete understanding and new avenues for therapeutic approaches.
DECREASED RESPONSES IN INNATE IMMUNITY
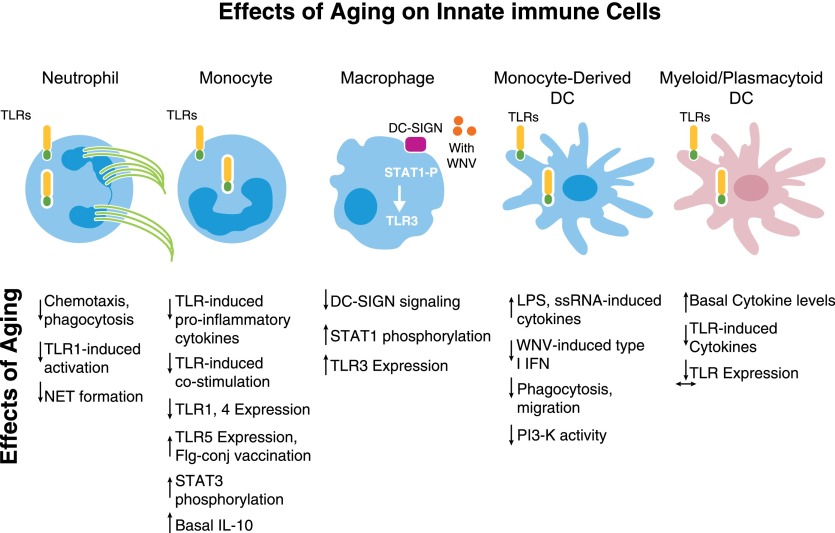
TLRs are highly conserved germ-line-encoded receptors that recognize repeated patterns of pathogens and trigger immune-response pathways. With age, expression and function of TLRs decline in several cell lineages, including monocytes [6], DCs [7], and PMN (Fig. 1) [8]. Deficiencies of PMN functions in aging include reduced chemotaxis, phagocytosis, and intracellular killing and release of cytokines and granules, resulting from alterations in multiple pathways, including inappropriate elevation of PI3K signaling [9]. PMN from older donors produce fewer NETs, extracellular networks of chromatin and antimicrobial proteins that trap and facilitate killing of pathogens [10, 11]. PMN also show reduced expression of TLR1, and stimulation of PMN from older donors through TLR1 led to lower expression of activation markers, lower production of chemokine mediators, lower phosphorylation of signaling intermediates, and reduced rescue of PMN from apoptosis compared with PMN from younger donors. PMN energy use was dramatically lower in cells from older adults, and such reduced bioenergetics likely contributes to reduced PMN function in aging and suggests potential therapeutic opportunities [8]. Such age-related defects in chemotaxis, cytokine production, and NET formation have been associated with impaired wound healing and enhanced systemic dissemination of Staphylococcus aureus infection from the skin in mouse models [10, 12]; notably, alterations in chemotaxis may result in impaired recruitment and delayed egress from sites of infection, resulting in impaired responses to pathogen as well as inappropriate persistence of chronic inflammation.
Figure 1. Effects of aging on innate immune cells.
In aging, innate immune cells show altered responses and up-regulated and down-regulated functions. DC-SIGN, DC-specific ICAM-3-grabbing nonintegrin; STAT1-P, phosphorylated STAT1; Flg-conj, flagellin-conjugated.
In monocytes from older humans, an age-related decrease in TLR1/TLR2-mediated cytokine production was associated with decreased TLR1 surface expression [6, 13]. Changes in TLR-induced expression of costimulatory molecules in monocytes and of proinflammatory cytokines in primary mDC and pDCs were also associated with impaired influenza vaccine antibody response [7, 14]. Notably, substantial elevations in basal cytokine production were found in mDCs and pDCs, implying that such inflammatory dysregulation could contribute to impaired responses to newly encountered antigens. Recent gene expression microarray analyses of PBMCs stimulated with agonists engaging TLR4, TLR7/8, and cytoplasmic innate immune PRR Retinoic acid-inducible gene-I suggests an age-associated delay in downstream signaling pathways, particularly those related to IFN-dependent signaling, which may contribute to decreases in cytokine production [15].
In contrast, TLR5 expression appears to be increased in monocytes from older subjects, suggesting a possible opportunity for increasing targeting for vaccination of older subjects [16]. Moreover, tissue context and cellular activation state may also play a role in paradoxical increases in TLR function with aging, as suggested by age-associated increases in TLR-induced cytokine production found in monocyte-derived DCs obtained following treatment with IL-4 plus GM-CSF [13, 17]. In addition to vaccine responsiveness, the extent of TLR-induced cytokine production in monocytes has been found to relate to muscle mass and strength in older adults, providing an additional functional consequence of innate immunosenescence [18]. Furthermore, alterations in TLR-dependent signal transduction in innate immune cells from older donors correspond to reduced efficiency against infecting pathogens. DCs from older donors produce reduced levels of type I IFN in response to infection with WNV [19], whereas macrophages from older donors, when infected in vitro with WNV, show inappropriate persistence of TLR3 expression and concomitant increases in proinflammatory cytokines that may contribute to the more severe clinical WNV disease associated with advanced age [20] (Fig. 1).
Studies of age-associated changes in murine TLR function have shown decreased TLR-induced cytokine production in macrophages from older versus young mice, with differences in the extent of involved TLRs that may, in part, reflect differences in genetic background [21–23]. However, these studies all suggest that TLR2 and TLR4 function is diminished in macrophages from older compared with young mice; in this regard, decreased expression of TLR1, TLR2, and TLR4 protein was found in lung homogenates from older compared with young mice, with decreased proinflammatory cytokine production in response to treatment with pneumococcal bacteria and bacterial pathogen-associated molecular patterns and impaired NF-κB activation [24]. At the same time, elevated basal levels of proinflammatory cytokines were found in lung homogenates from older versus young mice, providing evidence in a model system of pneumococcal pneumonia (with increased mortality seen in older mice) for the juxtaposition of age-related chronic inflammation and innate immune dysfunction [25]. In this context, enhanced inflammation has been found in several cell types and tissues beyond hematopoietic cells. For example, increased age-associated basal inflammation has been reported in murine vascular smooth muscle cells (with increased TLR4 expression), with potential for increased atherogenesis [26], and extensive activation of inflammatory pathways has been found in analyses of gene-expression microarrays from older versus young brain tissue [27]. These findings add additional complexity to understanding the effects of aging on innate immune responses and their consequences for disease.
Recent findings have demonstrated age-associated changes in the innate immune response to the inactivated influenza vaccine [28]. Intracellular production of TNF-α and IL-6 assessed following vaccination was diminished substantially in classic and CD14+CD16+ monocyte populations from older compared with young adults—and remained elevated at 28 d postvaccine. The extent of this cytokine production was strongly associated with vaccine antibody response in young and older adults. Notably, intracellular production of the anti-inflammatory cytokine IL-10 was markedly elevated in monocyte populations from older but not young adults and was linked to altered signaling pathways regulating IL-10 production—implicating dysregulated expression of IL-10 in the impaired response to vaccination [28]. The mechanism of innate immune activation by the inactivated seasonal influenza vaccine remains incompletely understood. TLR recognition could play a role, as residual ssRNA has been found in some vaccine preparations [29]. However, a recent report used mass cytometry (CyTOF) to profile signaling pathways activated by in vitro stimulation of whole blood by influenza vaccine and implicated immune complex formation and Fcγ-dependent signaling in vaccine-associated innate immune activation [30]; it will be of interest to evaluate the effects of age on this signaling pathway.
It is likely that multiple factors contribute to age-associated dysregulation of innate immune responses. These may include hormonal influences, including those arising from alterations in adipose tissue in the context of aging or obesity [31]; nutrients, such as vitamin D [32]; and release of endogenous innate immune PRR ligands from damaged cells [33]. Chronic viral infections, such as with HIV, may also contribute to a heightened proinflammatory environment [34]; indeed, whether chronic age-associated medical conditions, such as diabetes and metabolic syndrome, cardiovascular disease, or chronic obstructive pulmonary disease, can be viewed as individual factors contributing to basal inflammation or are differential manifestations of an age-related, generalized chronic inflammatory syndrome remains an intriguing issue [35]. In this regard, the contribution of other innate immune PRRs, such as NLRP3 inflammasome activation, may well play an important role, as NLRP3 appears to be activated by numerous endogenous, so-called damage-associated molecular patterns, such as urate crystals, amyloid, hyaluronan, and ATP [36]. In older mice, lower induction of NLRP3-dependent cytokines, following influenza infection, was reported [37], and NLRP3 appears to play a role in mediating the inflammatory and cognitive changes associated with aging [38]. A recent report implicating the β-hydroxybutyrate ketone body as an inhibitor of NLRP3 suggests an additional mechanism for modulation of inflammation via nutritional or metabolic status [39]. In this regard, the effects of mTOR signaling on the regulation of anabolic versus catabolic metabolism should also be considered for their effects on age-related inflammation. For example, treatment with the mTOR inhibitor rapamycin appears to diminish chronic inflammation in models of ischemic stroke [40] and age-associated cardiac dysfunction [41]. Notably, a recent report indicated that a brief course of rapamycin treatment in older humans resulted in improved (20% increase) antibody titers to influenza vaccine, although whether this effect was, in part, mediated by changes in innate immune responses remains incompletely understood [42].
NEW DIRECTIONS FOR DISCOVERIES IN IMMUNE AGING
We expect advances relevant to immune aging based on growing appreciation of the genetic, functional, and medical heterogeneity of human cohorts; studies extending to tissues beyond the blood; and multidimensional systems profiling. Accordingly, we focus here on emerging studies relevant to function in aging, including polymorphisms and methylation in DNA, regulatory miRNAs; newly accessible tissues and tissue examination methods; and the burgeoning arena of holistic "systems"-based studies that coordinately examine multiple aspects of immunity.
Genetic and epigenetic changes in aging
There is considerable host genetic variation that in inflammation-related genes, can control the balance between pro- and anti-inflammatory networks [43]. The genetic background of immune-related genes, such as the highly polymorphic HLA and NK cell Ig-like receptor genes, is associated with successful aging and longevity. A high frequency of proinflammatory polymorphisms or haplotypes in these inflammation-related genes may influence susceptibility to age-related diseases [44]. Advances in our understanding of host genetic variation related to aging and the genetic determinants of immunosenescence offer insight into the effects of aging and could transform development of novel approaches to overcoming aging defects [45]. Interestingly, genomic integrity has been identified as a key alteration in recent comparisons of gene-expression signatures associated with human aging by use of analyses of blood or PBMCs. Immunologic pathways associated with processes, such as T cell activation and NF-κB activity, have been identified, but it is striking that at least 2 studies have implicated DNA damage or metabolism and mitochondrial pathways [46, 47]. Other analyses of the effects of aging on influenza vaccine response revealed a role for prevaccine expression of immune pathways, including those mediating apoptosis [48]. A recent gene-expression analysis of young and older adults (evaluated pre- and postvaccine), selected for strong or weak influenza vaccine antibody responses, also revealed a mitochondrial signature associated with vaccine response that appeared to reflect mitochondrial biogenesis [49]. The metabolic functions of mitochondria in modulating innate and adaptive immune responses via reactive oxygen species generation and in facilitating innate immune signaling via mitochondrial membrane-associated proteins, such as mitochondrial anti-viral signaling protein, further illuminate potential links among inflammation, metabolism, and immunosenescence [50].
Over a lifespan, changes to DNA accumulate that can affect gene expression include chromosomal instability, shortening of telomeres, and reversible methylation. DNA methylation is a stable modification of adding or removing a 5-methyl group on cytosine residues that can lead to silencing of a gene locus [51]. Overall, methylation is highest in early life, decreases with aging, and can be reliably used to predict age [51, 52], although tissue-specific methylation changes over a lifespan include hyper- and hypomethylation of DNA [53]. A meta-analysis of 4000 diverse tissue types from studies available in public resources identified signatures that could be used as an age predictor, reflecting hypermethylation at CpG islands, regions of GC-rich DNA correlated with promoter regions, and hypomethylation in non-CpG regions [53, 54]. DNA hypermethylation during aging has been shown to occur at gene promoters enriched in repressive histone marks, such as H3K9me3 and H3K27me3. In this context, the potential function of Polycomb group proteins should be considered; PRC2, in conjunction with PRC1, generates the H3K27me-repressive mark, and it is notable that studies of age-associated methylation patterns in mice have revealed over-representation of Polycomb target genes among hypermethylated loci [55, 56]. In humans, functional age-associated methylation in CpG-enriched regions of DNA was cell-type specific in monocytes and CD4+ T cells from subjects aged 55–94 [54]. Additional studies in humans are needed, but such increases in methylation of inflammatory genes may be relevant to heightened levels of age-associated inflammation. However, it should also be noted that in the context of aging and malignancy, hypomethylated CpG sites were strongly enriched in the active chromatin mark H3K4me1 [52]. Moreover, ablation of methyltransferase enzymes in mice led to myeloid skewing and reduced self-renewal of stem cells, both changes that are also noted with aging [57], as well as altered transcription in progeny cells [58]. Taken together, these findings make clear the importance of changes in genomic methylation in understanding the biology and potential therapeutic opportunities for aging and age-related diseases.
Noncoding miRNA regulation of gene expression
Recent studies have identified noncoding miRNAs, typically 21–23 nucleotide sequences encoded by DNA, which have broad functions in regulation of gene expression, including regulating immune responses [59]. Aging-associated miRNAs are largely negative regulators of the immune innate response and target, in particular, NF-κB, a downstream effector of TLR signals that leads to induction of proinflammatory responses. In innate immune cells, miR-146, whose expression is increased in aging, represses endothelial cell and macrophage activation and cytokine production by inhibiting NF-κB proinflammatory pathways [60–63]. The age-related up-regulation of certain miRNAs with potential anti-inflammatory activity, such as miR-21, miR-146, miR-223, and miR-29, may be a compensatory mechanism enacted by senescent and/or activated immune cells to restrain the excessive proinflammatory activity associated with normal aging [64, 65].
Investigations of new tissues relevant to aging
An age-associated decline in HSC function leads to a decline in the regenerative capacity and furthermore, a skewing in favor of myeloid lineages over lymphoid cell types [57]. Age-related changes in HSCs may be mediated by a reduced ability to adhere to the stromal niche cells that maintain stem cell quiescence in the bone marrow [66]. In some studies, specific pathways have been identified as playing a potential role in HSC aging phenotypes. For example, expression of SIRT3, which regulates acetylation of mitochondrial proteins, is down-regulated in murine HSCs from older compared with young animals; notably, SIRT3 overexpression improved the function of aged HSCs [67]. In addition, expression of the Wnt5a-dependent small RhoGTPase Cdc42 is increased in aged HSCs and may mediate HSC immunosenescence by affecting HSC cell polarity [57, 68]. Studies in humans remain incomplete, but these findings suggest that rejuvenation of stem cells and improved regenerative capacity can be achieved through modulation of these pathways [57].
In the lung, airway neutrophilia is related to age in adults, with an elevated neutrophilic phenotype found in older adults with asthma [69]. However, in-depth investigation of this less-accessible tissue site has been challenging. Recent advances in technology support transcriptional analysis of cells from induced sputum [70, 71] that will allow meaningful investigation of age-related differences in airway disease. Further progress may also result from studies of lymphoid and mucosal tissues from organ donors that has allowed tissue-specific analysis of immune cell subtypes and functional status in healthy individuals of many ages. The ability to assess activity from cells in situ in healthy tissue will likely reveal previously unknown tissue- and cell lineage-specific innate immune interactions [72, 73].
New approaches for immune profiling in aging
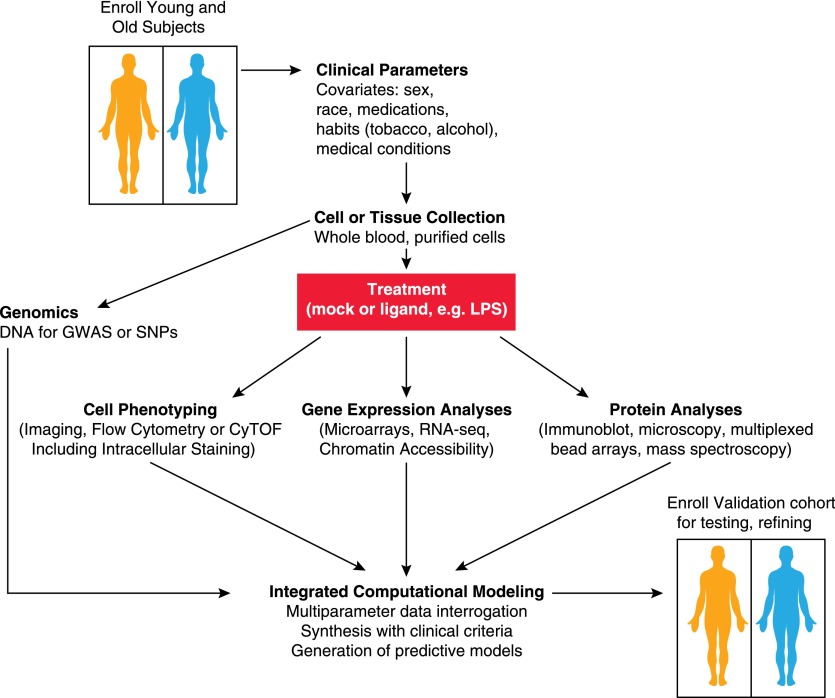
The complexity of the immune response includes many interacting cell types, tissues, genes, and signaling pathways. The approaches detailed here have been applied in individual studies, but the next level of progress in understanding age-related changes in immunity will require coordinated investigations. Systems-based approaches investigate multiple interacting elements of the immune response simultaneously [74], facilitating generation of interacting networks rather than individual measurements (Fig. 2). Such a systems-based evaluation is particularly critical in view of the high degree of heterogeneity found in human cohorts, which should consider important covariates, such as sex; race; associated medical conditions, such as cardiovascular disease or diabetes; medication use; and smoking or alcohol consumption. To date, relatively few systems biology studies of immune system aging take such variables into account.
Figure 2. Systems investigation of effects of aging.
Schematic view of systems investigation integrating multiple components of immune function. Collection of blood or tissue samples from enrolled subjects (older and younger) can be assessed for multiple assays, including DNA polymorphisms and genotyping; phenotyping of surface receptor expression; signaling pathway activity; and ligand-stimulated responses, which may be correlated with clinical parameters of study subjects for a more comprehensive view of immune status. Computational models can be tested and refined in a validation cohort. GWAS, genome-wide association studies; SNP, single nucleotide polymorphism; RNA-seq, RNA sequencing.
Central to these advances is the multiparameter assessment of cell types and functions of multiple cell lineages simultaneously through use of mass cytometry or CyTOF, a new technology for multiparameter single-cell analysis. CyTOF uses heavy metal ions as antibody labels and thus, overcomes many of the limitations of fluorescence-based flow cytometry, such as background and overlapping channels. Experiments can now combine 40 or so antibody specificities to facilitate unprecedented multidimensional cellular and proteomic analyses of immune subsets [75–77]. An equally valuable feature of CyTOF is its excellent sensitivity for studies from a small number of cells, as sample quantities are frequently limiting, particularly in human studies. The main cell lineages in PBMC samples can be detected from as few as 10,000 cells in a highly sensitive and quantitative manner [78], and successful phenotyping of immune cell types may be assessed in tissue, such as from skin biopsies. In the not-too-distant future, CyTOF technology may be routinely applied to other tissues as well [79, 80], offering tremendous power to detect relevant cellular changes in situ. Mass spectroscopy has also been used in analyses of serum or plasma to derive metabolic signatures associated with longevity in humans [81–84]. Taken together with methods for single-cell transcriptomic analyses [85, 86] or for genome-wide assessments of chromatin accessibility [87], these approaches should lead the aging field to a new era of discovery that will embrace the complexity of aging in human populations.
ACKNOWLEDGMENTS
This work was supported, in part, by the U.S. National Institutes of Health (HHS N272201100019C, U19AI089992, K24 AG042489). The authors regret omission of many important articles as a result of space and scope limitations and thank many colleagues for insightful discussions.
Glossary
- CyTOF
cytometry by time-of-flight
- DC
dendritic cell
- H3K4me1
histone H3 monomethyl Lys4
- H3K9/27me3
histone H3 trimethyl Lys9/27
- HSC
hematopoietic stem cell
- mDC
myeloid dendritic cell
- miR/miRNA
microRNA
- mTOR
mammalian target of rapamycin
- NET
neutrophil extracellular trap
- NLRP3
nucleotide-binding oligomerization domain-like receptor family, pyrin domain containing 3
- pDC
plasmacytoid dendritic cell
- PMN
polymorphonuclear leukocyte(s)
- PRC1/2
Polycomb repressor complex 1/2
- PRR
pattern recognition receptor
- SIRT3
sirtuin 3
- WNV
West Nile virus
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.United Nations, Department of Economic and Social Affairs. (2013) World Population Ageing 2013, ST/ESA/SER.A/348, United Nations, New York. [Google Scholar]
- 2.Goronzy J. J., Weyand C. M. (2013) Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14, 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frasca D., Blomberg B. B. (2014) B Cell function and influenza vaccine responses in healthy aging and disease. Curr. Opin. Immunol. 29, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw A. C., Goldstein D. R., Montgomery R. R. (2013) Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 13, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. (2000) Inflamm-aging. an evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254. [DOI] [PubMed] [Google Scholar]
- 6.Van Duin D., Mohanty S., Thomas V., Ginter S., Montgomery R. R., Fikrig E., Allore H. G., Medzhitov R., Shaw A. C. (2007) Age-associated defect in human TLR-1/2 function. J. Immunol. 178, 970–975. [DOI] [PubMed] [Google Scholar]
- 7.Panda A., Qian F., Mohanty S., van Duin D., Newman F. K., Zhang L., Chen S., Towle V., Belshe R. B., Fikrig E., Allore H. G., Montgomery R. R., Shaw A. C. (2010) Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 184, 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian F., Guo X., Wang X., Yuan X., Chen S., Malawista S. E., Bockenstedt L. K., Allore H. G., Montgomery R. R. (2014) Reduced bioenergetics and Toll-like receptor 1 function in human polymorphonuclear leukocytes in aging. Aging (Albany, N.Y.) 6, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng C. W., Liu G. Y. (2014) Expanding roles of neutrophils in aging hosts. Curr. Opin. Immunol. 29, 43–48. [DOI] [PubMed] [Google Scholar]
- 10.Tseng C. W., Kyme P. A., Arruda A., Ramanujan V. K., Tawackoli W., Liu G. Y. (2012) Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin-resistant S. aureus. PLoS One 7, e41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazeldine J., Harris P., Chapple I. L., Grant M., Greenwood H., Livesey A., Sapey E., Lord J. M. (2014) Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell 13, 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brubaker A. L., Rendon J. L., Ramirez L., Choudhry M. A., Kovacs E. J. (2013) Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J. Immunol. 190, 1746–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyugen J., Agrawal S., Gollapudi S., Gupta S. (2010) Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 30, 806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Duin D., Allore H. G., Mohanty S., Ginter S., Newman F. K., Belshe R. B., Medzhitov R., Shaw A. C. (2007) Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J. Infect. Dis. 195, 1590–1597. [DOI] [PubMed] [Google Scholar]
- 15.Metcalf T. U., Cubas R. A., Ghneim K., Cartwright M. J., Grevenynghe J. V., Richner J. M., Olagnier D. P., Wilkinson P. A., Cameron M. J., Park B. S., Hiscott J. B., Diamond M. S., Wertheimer A. M., Nikolich-Zugich J., Haddad E. K. (2015) Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 14, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian F., Wang X., Zhang L., Chen S., Piecychna M., Allore H., Bockenstedt L., Malawista S., Bucala R., Shaw A. C., Fikrig E., Montgomery R. R. (2012) Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell 11, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agrawal A., Agrawal S., Cao J. N., Su H., Osann K., Gupta S. (2007) Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 178, 6912–6922. [DOI] [PubMed] [Google Scholar]
- 18.Beenakker K. G., Westendorp R. G., de Craen A. J., Slagboom P. E., van Heemst D., Maier A. B. (2013) Pro-inflammatory capacity of classically activated monocytes relates positively to muscle mass and strength. Aging Cell 12, 682–689. [DOI] [PubMed] [Google Scholar]
- 19.Qian F., Wang X., Zhang L., Lin A., Zhao H., Fikrig E., Montgomery R. R. (2011) Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J. Infect. Dis. 203, 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong K.-F., Delroux K., Wang X., Qian F., Arjona A., Malawista S. E., Fikrig E., Montgomery R. R. (2008) Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J. Virol. 82, 7613–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehmer E. D., Goral J., Faunce D. E., Kovacs E. J. (2004) Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J. Leukoc. Biol. 75, 342–349. [DOI] [PubMed] [Google Scholar]
- 22.Boehmer E. D., Meehan M. J., Cutro B. T., Kovacs E. J. (2005) Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech. Ageing Dev. 126, 1305–1313. [DOI] [PubMed] [Google Scholar]
- 23.Renshaw M., Rockwell J., Engleman C., Gewirtz A., Katz J., Sambhara S. (2002) Cutting edge: impaired Toll-like receptor expression and function in aging. J. Immunol. 169, 4697–4701. [DOI] [PubMed] [Google Scholar]
- 24.Hinojosa E., Boyd A. R., Orihuela C. J. (2009) Age-associated inflammation and Toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J. Infect. Dis. 200, 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shivshankar P., Boyd A. R., Le Saux C. J., Yeh I. T., Orihuela C. J. (2011) Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell 10, 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Y., Shen H., Schenten D., Shan P., Lee P. J., Goldstein D. R. (2012) Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 32, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cribbs D. H., Berchtold N. C., Perreau V., Coleman P. D., Rogers J., Tenner A. J., Cotman C. W. (2012) Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J. Neuroinflammation 9, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohanty S., Joshi S. R., Ueda I., Wilson J., Blevins T. P., Siconolfi B., Meng H., Devine L., Raddassi K., Tsang S., Belshe R. B., Hafler D. A., Kaech S. M., Kleinstein S. H., Trentalange M., Allore H. G., Shaw A. C. (2015) Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults. J. Infect. Dis. 211, 1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeisy-Scott V., Kim J. H., Davis W. G., Cao W., Katz J. M., Sambhara S. (2012) TLR7 recognition is dispensable for influenza virus A infection but important for the induction of hemagglutinin-specific antibodies in response to the 2009 pandemic split vaccine in mice. J. Virol. 86, 10988–10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Gorman W. E., Huang H., Wei Y. L., Davis K. L., Leipold M. D., Bendall S. C., Kidd B. A., Dekker C. L., Maecker H. T., Chien Y. H., Davis M. M. (2014) The split virus influenza vaccine rapidly activates immune cells through Fcγ receptors. Vaccine 32, 5989–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg S. K., Delaney C., Shi H., Yung R. (2014) Changes in adipose tissue macrophages and T cells during aging. Crit. Rev. Immunol. 34, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Rodriguez L., Lopez-Hoyos M., Garcia-Unzueta M., Amado J. A., Cacho P. M., Martinez-Taboada V. M. (2012) Age and low levels of circulating vitamin D are associated with impaired innate immune function. J. Leukoc. Biol. 91, 829–838. [DOI] [PubMed] [Google Scholar]
- 33.Kapetanovic R., Bokil N. J., Sweet M. J. (2015) Innate immune perturbations, accumulating DAMPs and inflammasome dysregulation: a ticking time bomb in ageing. Ageing Res. Rev. doi:10.1016/j.arr.2015.02.005 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Zapata H. J., Shaw A. C. (2014) Aging of the human innate immune system in HIV infection. Curr. Opin. Immunol. 29, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabbri L. M., Rabe K. F. (2007) From COPD to chronic systemic inflammatory syndrome? Lancet 370, 797–799. [DOI] [PubMed] [Google Scholar]
- 36.Abderrazak A., Syrovets T., Couchie D., El Hadri K., Friguet B., Simmet T., Rouis M. (2015) NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 4, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stout-Delgado H. W., Vaughan S. E., Shirali A. C., Jaramillo R. J., Harrod K. S. (2012) Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with nigericin. J. Immunol. 188, 2815–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youm Y. H., Grant R. W., McCabe L. R., Albarado D. C., Nguyen K. Y., Ravussin A., Pistell P., Newman S., Carter R., Laque A., Münzberg H., Rosen C. J., Ingram D. K., Salbaum J. M., Dixit V. D. (2013) Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youm Y. H., Nguyen K. Y., Grant R. W., Goldberg E. L., Bodogai M., Kim D., D’Agostino D., Planavsky N., Lupfer C., Kanneganti T. D., Kang S., Horvath T. L., Fahmy T. M., Crawford P. A., Biragyn A., Alnemri E., Dixit V. D. (2015) The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie L., Sun F., Wang J., Mao X., Xie L., Yang S. H., Su D. M., Simpkins J. W., Greenberg D. A., Jin K. (2014) mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia. J. Immunol. 192, 6009–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flynn J. M., O’Leary M. N., Zambataro C. A., Academia E. C., Presley M. P., Garrett B. J., Zykovich A., Mooney S. D., Strong R., Rosen C. J., Kapahi P., Nelson M. D., Kennedy B. K., Melov S. (2013) Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12, 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannick J. B., Del Giudice G., Lattanzi M., Valiante N. M., Praestgaard J., Huang B., Lonetto M. A., Maecker H. T., Kovarik J., Carson S., Glass D. J., Klickstein L. B. (2014) mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 6, 268ra179. [DOI] [PubMed] [Google Scholar]
- 43.Naumova E., Ivanova M., Pawelec G., Constantinescu I., Bogunia-Kubik K., Lange A., Oguz F., Ozdilli K., Franceschi C., Caruso C., Mishra M., Middleton D. (2013) 16(th) IHIW: immunogenetics of aging. Int. J. Immunogenet. 40, 77–81. [DOI] [PubMed] [Google Scholar]
- 44.Balistreri C. R., Candore G., Accardi G., Bova M., Buffa S., Bulati M., Forte G. I., Listì F., Martorana A., Palmeri M., Pellicanò M., Vaccarino L., Scola L., Lio D., Colonna-Romano G. (2012) Genetics of longevity. Data from the studies on Sicilian centenarians. Immun. Ageing 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruan Q., Qian F., Yu Z. (2014) Effects of polymorphisms in immunity-related genes on the immune system and successful aging. Curr. Opin. Immunol. 29, 49–55. [DOI] [PubMed] [Google Scholar]
- 46.Jylhävä J., Raitanen J., Marttila S., Hervonen A., Jylhä M., Hurme M. (2014) Identification of a prognostic signature for old-age mortality by integrating genome-wide transcriptomic data with the conventional predictors: the Vitality 90+ Study. BMC Med. Genomics 7, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Akker E. B., Passtoors W. M., Jansen R., van Zwet E. W., Goeman J. J., Hulsman M., Emilsson V., Perola M., Willemsen G., Penninx B. W., Heijmans B. T., Maier A. B., Boomsma D. I., Kok J. N., Slagboom P. E., Reinders M. J., Beekman M. (2014) Meta-analysis on blood transcriptomic studies identifies consistently coexpressed protein-protein interaction modules as robust markers of human aging. Aging Cell 13, 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furman D., Jojic V., Kidd B., Shen-Orr S., Price J., Jarrell J., Tse T., Huang H., Lund P., Maecker H. T., Utz P. J., Dekker C. L., Koller D., Davis M. M. (2013) Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol. Syst. Biol. 9, 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thakar J., Mohanty S., West A. P., Joshi S. R., Ueda I., Wilson J., Meng H., Blevins T. P., Tsang S., Trentalange M., Siconolfi B., Park K., Gill T. M., Belshe R. B., Kaech S. M., Shadel G. S., Kleinstein S. H., Shaw A. C. (2015) Aging-dependent alterations in gene expression and a mitochondrial signature of responsiveness to human influenza vaccination. Aging (Albany, N.Y.) 7, 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinberg S. E., Sena L. A., Chandel N. S. (2015) Mitochondria in the regulation of innate and adaptive immunity. Immunity 42, 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung M., Pfeifer G. P. (2015) Aging and DNA methylation. BMC Biol. 13, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernández A. F., Bayón G. F., Urdinguio R. G., Toraño E. G., García M. G., Carella A., Petrus-Reurer S., Ferrero C., Martinez-Camblor P., Cubillo I., García-Castro J., Delgado-Calle J., Pérez-Campo F. M., Riancho J. A., Bueno C., Menéndez P., Mentink A., Mareschi K., Claire F., Fagnani C., Medda E., Toccaceli V., Brescianini S., Moran S., Esteller M., Stolzing A., de Boer J., Nisticò L., Stazi M. A., Fraga M. F. (2015) H3K4me1 marks DNA regions hypomethylated during aging in human stem and differentiated cells. Genome Res. 25, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J., Kim K., Kim H., Yoon G., Lee K. (2014) Characterization of age signatures of DNA methylation in normal and cancer tissues from multiple studies. BMC Genomics 15, 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reynolds L. M., Taylor J. R., Ding J., Lohman K., Johnson C., Siscovick D., Burke G., Post W., Shea S., Jacobs D. R. Jr., Stunnenberg H., Kritchevsky S. B., Hoeschele I., McCall C. E., Herrington D. M., Tracy R. P., Liu Y. (2014) Age-related variations in the methylome associated with gene expression in human monocytes and T cells. Nat. Commun. 5, 5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beerman I., Bock C., Garrison B. S., Smith Z. D., Gu H., Meissner A., Rossi D. J. (2013) Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell 12, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maegawa S., Hinkal G., Kim H. S., Shen L., Zhang L., Zhang J., Zhang N., Liang S., Donehower L. A., Issa J. P. (2010) Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 20, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geiger H., Denkinger M., Schirmbeck R. (2014) Hematopoietic stem cell aging. Curr. Opin. Immunol. 29, 86–92. [DOI] [PubMed] [Google Scholar]
- 58.Beerman I., Rossi D. J. (2014) Epigenetic regulation of hematopoietic stem cell aging. Exp. Cell Res. 329, 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung H. J., Suh Y. (2012) MicroRNA in aging: from discovery to biology. Curr. Genomics 13, 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olivieri F., Procopio A. D., Montgomery R. R. (2014) Effect of aging on microRNAs and regulation of pathogen recognition receptors. Curr. Opin. Immunol. 29, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olivieri F., Spazzafumo L., Santini G., Lazzarini R., Albertini M. C., Rippo M. R., Galeazzi R., Abbatecola A. M., Marcheselli F., Monti D., Ostan R., Cevenini E., Antonicelli R., Franceschi C., Procopio A. D. (2012) Age-related differences in the expression of circulating microRNAs: miR-21 as a new circulating marker of inflammaging. Mech. Ageing Dev. 133, 675–685. [DOI] [PubMed] [Google Scholar]
- 62.Cobos Jiménez V., Bradley E. J., Willemsen A. M., van Kampen A. H., Baas F., Kootstra N. A. (2014) Next-generation sequencing of microRNAs uncovers expression signatures in polarized macrophages. Physiol. Genomics 46, 91–103. [DOI] [PubMed] [Google Scholar]
- 63.Olivieri F., Lazzarini R., Recchioni R., Marcheselli F., Rippo M. R., Di Nuzzo S., Albertini M. C., Graciotti L., Babini L., Mariotti S., Spada G., Abbatecola A. M., Antonicelli R., Franceschi C., Procopio A. D. (2013) MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age (Dordr.) 35, 1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ugalde A. P., Ramsay A. J., de la Rosa J., Varela I., Mariño G., Cadiñanos J., Lu J., Freije J. M., López-Otín C. (2011) Aging and chronic DNA damage response activate a regulatory pathway involving miR-29 and p53. EMBO J. 30, 2219–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fenn A. M., Smith K. M., Lovett-Racke A. E., Guerau-de-Arellano M., Whitacre C. C., Godbout J. P. (2013) Increased micro-RNA 29b in the aged brain correlates with the reduction of insulin-like growth factor-1 and fractalkine ligand. Neurobiol. Aging 34, 2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walter D., Lier A., Geiselhart A., Thalheimer F. B., Huntscha S., Sobotta M. C., Moehrle B., Brocks D., Bayindir I., Kaschutnig P., Muedder K., Klein C., Jauch A., Schroeder T., Geiger H., Dick T. P., Holland-Letz T., Schmezer P., Lane S. W., Rieger M. A., Essers M. A., Williams D. A., Trumpp A., Milsom M. D. (2015) Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature 520, 549–552. [DOI] [PubMed] [Google Scholar]
- 67.Brown K., Xie S., Qiu X., Mohrin M., Shin J., Liu Y., Zhang D., Scadden D. T., Chen D. (2013) SIRT3 reverses aging-associated degeneration. Cell Reports 3, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Florian M. C., Dörr K., Niebel A., Daria D., Schrezenmeier H., Rojewski M., Filippi M. D., Hasenberg A., Gunzer M., Scharffetter-Kochanek K., Zheng Y., Geiger H. (2012) Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell 10, 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brooks C. R., Gibson P. G., Douwes J., Van Dalen C. J., Simpson J. L. (2013) Relationship between airway neutrophilia and ageing in asthmatics and non-asthmatics. Respirology 18, 857–865. [DOI] [PubMed] [Google Scholar]
- 70.Baines K. J., Simpson J. L., Wood L. G., Scott R. J., Fibbens N. L., Powell H., Cowan D. C., Taylor D. R., Cowan J. O., Gibson P. G. (2014) Sputum gene expression signature of 6 biomarkers discriminates asthma inflammatory phenotypes. J. Allergy Clin. Immunol. 133, 997–1007. [DOI] [PubMed] [Google Scholar]
- 71.Yan X., Chu J. H., Gomez J., Koenigs M., Holm C., He X., Perez M. F., Zhao H., Mane S., Martinez F. D., Ober C., Nicolae D. L., Barnes K. C., London S. J., Gilliland F., Weiss S. T., Raby B. A., Cohn L., Chupp G. L. (2015) Noninvasive analysis of the sputum transcriptome discriminates clinical phenotypes of asthma. Am. J. Respir. Crit. Care Med. 191, 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sathaliyawala T., Kubota M., Yudanin N., Turner D., Camp P., Thome J. J., Bickham K. L., Lerner H., Goldstein M., Sykes M., Kato T., Farber D. L. (2013) Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thome J. J., Yudanin N., Ohmura Y., Kubota M., Grinshpun B., Sathaliyawala T., Kato T., Lerner H., Shen Y., Farber D. L. (2014) Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159, 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poland G. A., Ovsyannikova I. G., Kennedy R. B., Lambert N. D., Kirkland J. L. (2014) A systems biology approach to the effect of aging, immunosenescence and vaccine response. Curr. Opin. Immunol. 29, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bendall S. C., Simonds E. F., Qiu P., Amir A. D., Krutzik P. O., Finck R., Bruggner R. V., Melamed R., Trejo A., Ornatsky O. I., Balderas R. S., Plevritis S. K., Sachs K., Pe’er D., Tanner S. D., Nolan G. P. (2011) Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bandura D. R., Baranov V. I., Ornatsky O. I., Antonov A., Kinach R., Lou X., Pavlov S., Vorobiev S., Dick J. E., Tanner S. D. (2009) Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 81, 6813–6822. [DOI] [PubMed] [Google Scholar]
- 77.Ornatsky O., Bandura D., Baranov V., Nitz M., Winnik M. A., Tanner S. (2010) Highly multiparametric analysis by mass cytometry. J. Immunol. Methods 361, 1–20. [DOI] [PubMed] [Google Scholar]
- 78.Yao Y., Liu R., Shin M. S., Trentalange M., Allore H., Nassar A., Kang I., Pober J. S., Montgomery R. R. (2014) CyTOF supports efficient detection of immune cell subsets from small samples. J. Immunol. Methods 415, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giesen C., Wang H. A., Schapiro D., Zivanovic N., Jacobs A., Hattendorf B., Schüffler P. J., Grolimund D., Buhmann J. M., Brandt S., Varga Z., Wild P. J., Günther D., Bodenmiller B. (2014) Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–422. [DOI] [PubMed] [Google Scholar]
- 80.Angelo M., Bendall S. C., Finck R., Hale M. B., Hitzman C., Borowsky A. D., Levenson R. M., Lowe J. B., Liu S. D., Zhao S., Natkunam Y., Nolan G. P. (2014) Multiplexed ion beam imaging of human breast tumors. Nat. Med. 20, 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu Z., Zhai G., Singmann P., He Y., Xu T., Prehn C., Römisch-Margl W., Lattka E., Gieger C., Soranzo N., Heinrich J., Standl M., Thiering E., Mittelstraß K., Wichmann H. E., Peters A., Suhre K., Li Y., Adamski J., Spector T. D., Illig T., Wang-Sattler R. (2012) Human serum metabolic profiles are age dependent. Aging Cell 11, 960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collino S., Montoliu I., Martin F. P., Scherer M., Mari D., Salvioli S., Bucci L., Ostan R., Monti D., Biagi E., Brigidi P., Franceschi C., Rezzi S. (2013) Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One 8, e56564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montoliu I., Scherer M., Beguelin F., DaSilva L., Mari D., Salvioli S., Martin F. P., Capri M., Bucci L., Ostan R., Garagnani P., Monti D., Biagi E., Brigidi P., Kussmann M., Rezzi S., Franceschi C., Collino S. (2014) Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity. Aging (Albany, N.Y.) 6, 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez-Covarrubias V. (2013) Lipidomics in longevity and healthy aging. Biogerontology 14, 663–672. [DOI] [PubMed] [Google Scholar]
- 85.Shalek A. K., Satija R., Adiconis X., Gertner R. S., Gaublomme J. T., Raychowdhury R., Schwartz S., Yosef N., Malboeuf C., Lu D., Trombetta J. J., Gennert D., Gnirke A., Goren A., Hacohen N., Levin J. Z., Park H., Regev A. (2013) Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature 498, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sandberg R. (2014) Entering the era of single-cell transcriptomics in biology and medicine. Nat. Methods 11, 22–24. [DOI] [PubMed] [Google Scholar]
- 87.Buenrostro J. D., Wu B., Chang H. Y., Greenleaf W. J. (2015) ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]