The anti-inflammatory effects of θ-defensin RTD-1 are mediated by cell signaling pathways that down-regulate expression of pro-inflammatory cytokines.
Keywords: TLR, Akt, IκBα, THP-1, monocytes
Abstract
θ-Defensins are pleiotropic, macrocyclic peptides that are expressed uniquely in Old World monkeys. The peptides are potent, broad-spectrum microbicides that also modulate inflammatory responses in vitro and in animal models of viral infection and polymicrobial sepsis. θ-Defensins suppress proinflammatory cytokine secretion by leukocytes stimulated with diverse Toll-like receptor (TLR) ligands. Studies were performed to delineate anti-inflammatory mechanisms of rhesus θ-defensin 1 (RTD-1), the most abundant θ-defensin isoform in macaque granulocytes. RTD-1 reduced the secretion of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-8 in lipopolysaccharide (LPS)-stimulated human blood monocytes and THP-1 macrophages, and this was accompanied by inhibition of nuclear factor κB (NF-κB) activation and mitogen-activated protein kinase (MAPK) pathways. Peptide inhibition of NF-κB activation occurred following stimulation of extracellular (TLRs 1/2 and 4) and intracellular (TLR9) receptors. Although RTD-1 did not inhibit MAPK in unstimulated cells, it induced phosphorylation of Akt in otherwise untreated monocytes and THP-1 cells. In the latter, this occurred within 10 min of RTD-1 treatment and produced a sustained elevation of phosphorylated Akt (pAkt) for at least 4 h. pAkt is a negative regulator of MAPK and NF-κB activation. RTD-1 inhibited IκBα degradation and p38 MAPK phosphorylation, and stimulated Akt phosphorylation in LPS-treated human primary monocytes and THP-1 macrophages. Specific inhibition of phosphatidylinositol 3-kinase (PI3K) blocked RTD-1-stimulated Akt phosphorylation and reversed the suppression of NF-κB activation by the peptide. These studies indicate that the anti-inflammatory properties of θ-defensins are mediated by activation of the PI3K/Akt pathway and suppression of proinflammatory signals in immune-stimulated cells.
Introduction
Mammalian defensins are cationic, tridisulfide-containing peptides comprising 3 subfamilies denoted as α-defensin, β-defensin, and θ-defensin. Defensins contribute to host defense as antimicrobial agents [1–6] and by regulating inflammatory [7–13] and adaptive immune responses [14–17]. α-Defensin and β-defensin have similar 3-dimensional topologies but differ in their disulfide linkages [18]. θ-Defensins are macrocyclic peptides expressed in Old World monkeys (e.g., macaques and baboons) and are the only known cyclic proteins in animals [19]. The θ-defensin backbone is produced by head-to-tail splicing of 2 nonapeptides excised from α-defensin-like precursors [20]. In rhesus macaques, alternate binary splicing of nonapeptides encoded by 3 precursor genes gives rise to 6 θ-defensin isoforms, RTD-1 to RTD-6 [20, 21]. In baboons, alternate nonapeptide splicing produces 10 θ-defensin isoforms [22]. θ-Defensins are expressed at high levels in granules of neutrophils and in monocytes [20], and RTD-1 is the most abundant θ-defensin in macaques, constituting ∼55% of the total θ-defensin content of rhesus neutrophils [23]. θ-Defensins have a major role in the antimicrobial activities of rhesus neutrophil-granule extracts [23]. Humans and other hominids lack θ-defensins because of a stop codon in the prepro-coding sequence of θ-defensin genes in these species [24]. It has been suggested that the expression of θ-defensins in Old World monkeys underlies differences in immune and inflammatory responses of these nonhuman primates from those of humans [19].
Although α-defensins, β-defensins, and θ-defensins were discovered based on their broad-spectrum antimicrobial properties, subsequent studies have disclosed immune regulatory roles for these peptides [25]. For example, some α-defensins and β-defensins are chemotactic for T cells, neutrophils, dendritic cells, and monocytes [14–17], and they induce secretion of proinflammatory cytokines from activated dendritic cells, peripheral blood mononuclear cells, and epithelial cells [7–13]. In contrast to these proinflammatory activities, we recently reported that θ-defensins have anti-inflammatory properties both in vitro and in vivo. RTD-1 was a potent inhibitor of cytokine secretion by human peripheral blood leukocytes stimulated with diverse TLR agonists [26]. Naturally occurring θ-defensin isoforms (RTDs 1–6) [23] possess variable potency in suppressing TNF expression by LPS-stimulated or Escherichia coli-stimulated leukocytes. RTD-1 suppressed inflammatory cytokines, including TNF-α, IL-1β, and several chemokines in mouse models of severe acute respiratory syndrome corona virus infection [27], E. coli peritonitis, and in polymicrobial sepsis [26], and these effects strongly correlated with reduced mortality in each model. These results suggest that θ-defensins modulate inflammation via regulation of pathways shared by diverse inflammatory stimuli. To identify regulatory pathways mediated by these novel macrocyclic peptides, we analyzed the effects of RTD-1 on stimulated THP-1 macrophages and human monocytes, evaluating effects of the peptide on cytokine expression and secretion and on signal transduction pathways involved in the inflammatory response.
MATERIALS AND METHODS
Reagents
PMA, marimastat, and PMSF were from Sigma Chemical Co. (St. Louis, MO, USA), E. coli K12 LPS, Pam3CSK4, ODN2006, and Quantiblue were from InvivoGen (San Diego, CA, USA). Anti-phospho-p38 MAPK, anti-IκBα, anti-phospho-SAPK/JNK, anti-NF-κB (p65), anti-phospho-Akt (Ser473), and anti-Akt pan Abs were from Cell Signaling Technology (Danvers, MA, USA). Marimastat and Ly294002 (Cell Signaling Technology) solutions were prepared at 1000× in DMSO. Carrier-free TNF-α (Cell Signaling Technology) was suspended at 2 μg/ml in PBS containing 5% HI FBS. Synthetic RTD-1 hydrochloride (>99%) was prepared as described previously [20]. Human α-defensin HNP-1 was purified from neutrophils [28]. Stock solutions of RTD-1, S7 peptide, and HNP-1 were prepared in sterile 0.01% acetic acid [26, 29].
Ethics committee approval
EDTA-anticoagulated blood was collected from healthy donors after prior written consent, according to protocols approved by the University of Southern California Institutional Review Board (approval number: HS-09-00280).
Cell culture
THP-1 monocytes (ATCC, Manassas, VA, USA) were cultured in RPMI 1640 + 10% FBS and P/S. THP-1 Dual Cells (InvivoGen), which express the NF-κB reporter, SEAP, were grown in RPMI 1640 + 10% HI-FBS and antibiotics. THP-1 cell differentiation was induced by treatment of cells (∼3.3 × 105 cells/ml) with 100 nM PMA and cultured for 2 d. HEK-Blue hTLR9 cells (InvivoGen) were grown in DMEM medium containing 10% HI-FBS, P/S, and normocin. Cells were cultured at 37°C in 5% CO2.
Cytokine assays
PMA-differentiated THP-1 cells (∼8 × 105 cells) cultured in 6-well plates were washed twice with complete medium and grown in fresh medium containing 1% FBS for 24 h. Cells were washed twice with fresh medium and incubated for 2 h before further manipulation. Cells were stimulated with 100 ng/ml LPS in the presence or absence of 10 μg/ml RTD-1 or a vehicle (0.01% acetic acid) for 2 h. In some experiments, cells were first incubated for 1 h with 10 μg/ml marimastat or vehicle (DMSO), after which LPS, RTD-1, or both were added and incubated for 2 h as above. Peripheral blood CD14+ monocytes (Lonza, Walkersville, MD, USA) were thawed, rinsed with RPMI 1640 medium containing 10% HI FBS, P/S, and normocin, and then, resuspended in RPMI 1640 medium containing 1% HI FBS plus antibiotics as above (106 cells/ml) for 2 h. The cells were then stimulated for 4 h as described in Fig. 1. Medium was collected, clarified by centrifugation, first at 250 g for 8 min, and then at 5000 g for 5 min. TNF-α, IL-1β, and IL-8 were quantified by ELISA (Life Technologies, Carlsbad, CA, USA).
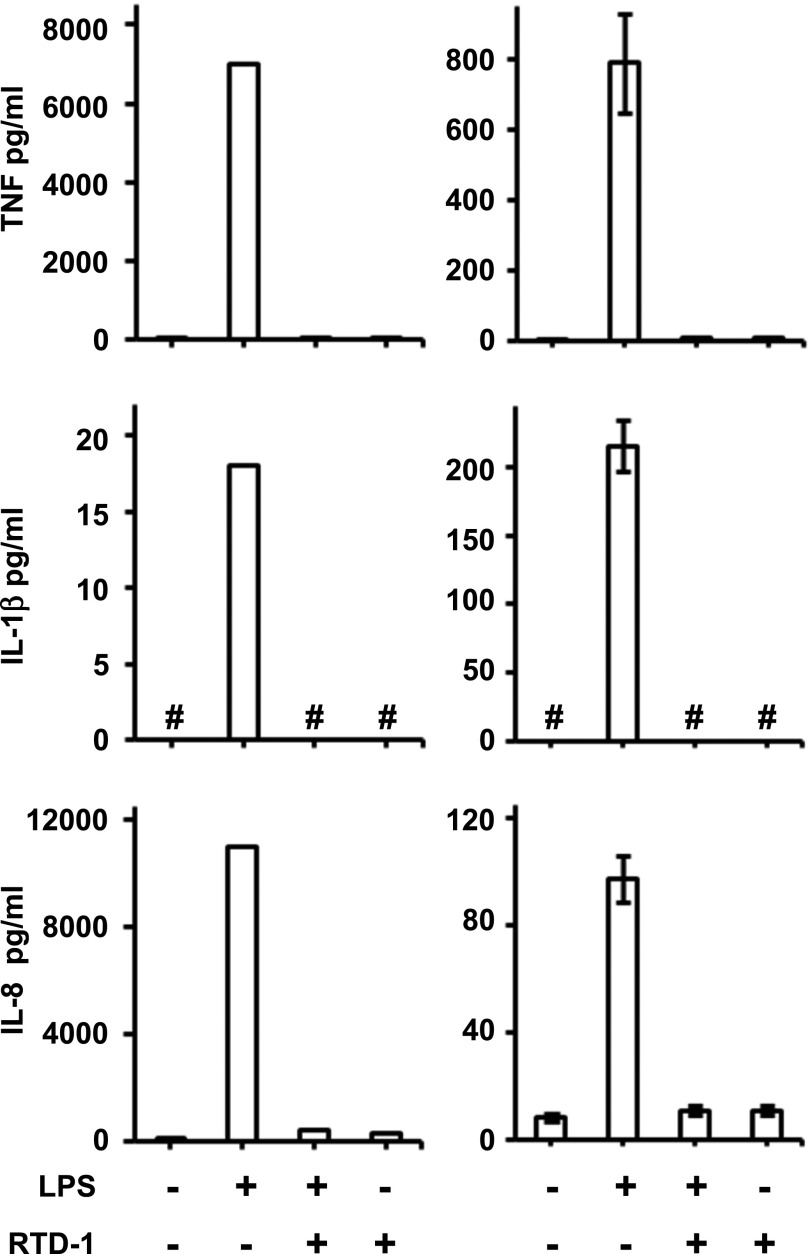
Fig. 1. RTD-1 inhibits secretion of proinflammatory cytokines.
TNF-α, IL-1β, and IL-8 secreted in the medium were quantified by ELISA. (Left panels) Medium from human monocytes stimulated for 4 h with 20 ng/ml LPS or from unstimulated cells ± RTD-1 (10 μg/ml). Results are from 1 of 2 similar, independent experiments. (Right panels) Medium from differentiated THP-1 cells treated with LPS (100 ng/ml) for 2 h or from untreated cells ± RTD-1 (10 μg/ml). Results shown are means ± sd from 3 independent experiments. # indicates cytokine was below detection limit.
Quantitative real-time PCR
Differentiated THP-1 cells were treated with LPS and RTD-1 for 2 h as above and washed with PBS, and RNA was isolated using an RNA mini kit (Zymo Research, Irvine, CA, USA) or with RNeasy mini kit (Qiagen, Valencia, CA, USA). RNA integrity was confirmed on agarose gels, and RNA samples with A260/280 and A260/230 ratios ≥1.7 were used to generate cDNA. In brief, 400 ng of RNA was incubated with gDNA elimination buffer for 5 min at 4°C and reverse transcribed using the RT2 first strand synthesis kit (Qiagen). Custom PCR arrays (SA Biosciences, Frederick, MD, USA) containing human genes of interest and controls (for reverse transcriptase and PCR efficiency, RTC and PPC, respectively) were used for simultaneous, real-time PCR analysis. Master mix containing cDNA and RT2 SYBR Green was added to the array wells. PCR cycling parameters were 1 cycle for 10 min at 95°C, 40 cycles of 15 s at 95°C, 1 min at 60°C on a C1000 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA) equipped with a CFX96 real-time system. Melting curve analysis confirmed a single amplicon with CtPPC = 20 ± 2 and ΔCt(RTC-PPC) < 5 for all samples. The ACTB gene was used for normalization of gene expression and fold stimulation (compared with untreated cells) was calculated using the ΔΔCt method. Absence of gDNA in cDNA samples (Ct > 35) was confirmed using a human gDNA primer (SA Biosciences).
NF-κB activity assay
Aliquots of 1 ml of PMA-differentiated THP-1 Dual cells (∼3.3 × 105 cells/ml) in 24-well tissue culture plates were starved for 1 d in complete medium with 1% HI FBS as described above. For experiments with S7 peptide, 0.5 ml cultures in 48-well plates were used. Fresh medium containing 1% HI FBS was added to the cells and, after 2 h, cells were stimulated with 100 ng/ml LPS or 25 ng/ml Pam3CSK4 in the presence of RTD-1 or 0.01% acetic acid (vehicle). In some experiments, cells were preincubated with marimastat, Ly294002, or vehicle for 1 h before addition of LPS or RTD-1 or both. Following overnight incubation, medium was clarified by centrifugation, and SEAP activity was determined using Quantiblue reagent at 37°C and measurement of absorbance at 625 nm. To test the effect of RTD-1 on TLR9-dependent NF-κB activation, 2 × 105 HEK-Blue hTLR9 cells/ml were cultured for 1 d in 24-well plates and stimulated overnight with ODN2006 with or without RTD-1. Medium was harvested, and SEAP activity was assayed using Quantiblue as above. NF-κB activation was defined as fold stimulation of SEAP activity compared with control cells.
NF-κB DNA-binding activity
Nuclear extracts were prepared (nuclear extraction kit; Cayman Chemical Co., Ann Arbor, MI, USA) from differentiated THP-1 cells treated for 30 min with vehicle, LPS (100 ng/ml) or TNF-α (2 ng/ml) in the presence or absence of 10 μg/ml RTD-1. NF-κB DNA binding was quantified by p65 ELISA (Cayman Chemical). Nonspecific DNA-binding, positive-control extract and positive-control extract treated with competitor DNA control included with ELISA were used to confirm the specificity of DNA-binding.
Isolation of monocytes from blood
EDTA-anticoagulated blood was incubated with human monocyte enrichment cocktail (StemCell Technologies, Vancouver, BC, Canada) for 20 min and layered on Ficoll-Paque in a SepMate 50 tube (Stem Cell Technologies). The cells were centrifuged at 1200 g for 10 min, and the monocyte-containing layer of cells was isolated and washed with PBS containing 2% HI FBS and 1 mM EDTA.
Immunoblot analyses
Differentiated THP-1 cells (∼8 × 105 cells/2.5 ml medium) were starved in complete medium containing 1% FBS for 1 d as above, and fresh 2.5 ml RPMI 1640, 1% FBS, and P/S were added for 2 h. For experiments with fresh monocytes, cells (>80% CD14+) were resuspended (∼106 cells/ml) in monocyte-attachment medium (PromoCell, Heidelberg, Germany) in a 12-well plate for 2 h at 37°C in 5% CO2. The cells were then washed 2 times with RPMI 1640 medium containing 1% HI FBS and suspended overnight in RPMI 1640 medium containing 1% HI FBS. Cells were stimulated with agonists with or without RTD-1, as described in the figure legends, after which cells were washed with PBS and extracts were prepared in cell lysis buffer and 1 mM PMSF. Protein content was determined using the bicinchoninic acid method (Bio-Rad), and extracts were resolved on SDS-tricine gels and transferred to nitrocellulose membrane. The membranes were probed with the indicated antibodies and developed using anti-mouse or anti-rabbit HRP-conjugated secondary antibodies and detected by chemiluminescence. A phosphoprotein antibody array (Phospho-MAPK; R&D systems, Minneapolis, MI, USA) was used to analyze activation of phosphoprotein kinases. Differentiated THP-1 cells (1.6 × 106 cells) were treated with 100 ng/ml LPS, 10 μg/ml RTD1, 100 ng/ml LPS, and 10 μg/ml RTD-1, or with the vehicle for 30 min. Arrays were probed with 200 μg of cell-extract protein and chemiluminescence of spots from arrays or bands from Western blotting was quantified using U.S. National Institutes of Health ImageJ software (Bethesda, MD, USA), with the background subtracted, and was normalized and plotted.
Statistical analysis
sd and sem were calculated for experiments repeated 3 and 4 times, respectively, and the Student’s t test was performed to evaluate the significance of peptide effects; as indicated in the figure legends, difference was considered significant if P < 0.05. Microsoft (Redmond, WA, USA) Excel was used for all statistical analyses.
RESULTS
RTD-1 suppresses expression and release of proinflammatory cytokines
In a previous study, we found that RTD-1 suppressed secretion of several inflammatory cytokines by peripheral blood leukocytes stimulated by agonists of TLRs 2, 4, 5, and 8, and the inhibition of TNF-α, IL-1β, and IL-8 release was most notable [26]. In this study, RTD-1 treatment markedly (>90%) suppressed secretion of TNF-α, IL-1β, and IL-8 by LPS-stimulated primary human monocytes and THP-1 macrophages (Fig. 1). RTD-1 alone had no effect on THP-1 cytokine secretion (Fig. 1), consistent with previous findings [26]. The cytokine inhibitory effects in THP-1 macrophages closely resemble the responses obtained with human-blood buffy coat cells [26] and human monocytes, indicating that THP-1 cells provide an appropriate model for dissecting RTD-1-mediated anti-inflammatory mechanisms.
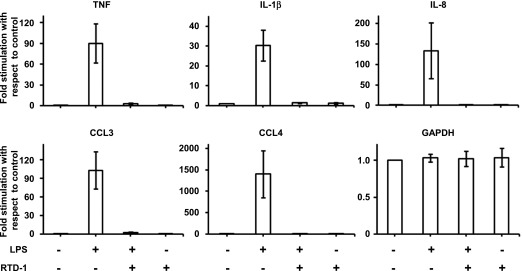
In experiments similar to those described in Figure 1, we analyzed the effect of RTD-1 on cytokine mRNA expression by LPS-stimulated THP-1 cells, focusing on TNF-α, IL-1β, IL-8, CCL3, and CCL4 because each of these cytokines was suppressed by θ-defensin treatment of LPS-stimulated blood leukocytes [26]. As shown in Fig. 2, RTD-1 markedly inhibited LPS-induced mRNA expression of each of the 5 cytokines. The addition of RTD-1 had no effect on the expression of GAPDH, and the peptide alone had no effect on cytokine mRNA expression. These results (Figs. 1 and 2) demonstrate potent regulation of proinflammatory cytokine expression and release by RTD-1 in LPS-stimulated macrophages.
Fig. 2. RTD-1 down-regulates mRNA of proinflammatory cytokines.
Real-time PCR analysis of cDNA from differentiated THP-1 cells treated with LPS (100 ng/ml) ± RTD-1 (10 μg/ml) for 2 h or from control (untreated) cells. Results are means ± sd from 3 independent experiments.
Regulation of NF-κB pathway by RTD-1
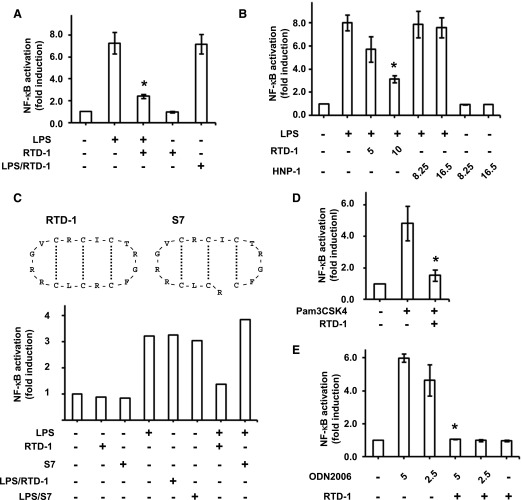
Given the central role of NF-κB signaling in inflammation, we hypothesized that RTD-1 modulates this pathway in THP-1 cells stimulated with different TLR agonists. LPS (TLR4 agonist) treatment of THP-1 cells induced a ∼7-fold activation of NF-κB (Fig. 3A). Simultaneous treatment with RTD-1 and LPS suppressed NF-κB activation by ∼70%, whereas RTD-1 alone had no effect (Fig. 3A). In addition, RTD-1 had no direct effect on the enzymatic activity of the SEAP reporter (LPS/RTD-1 in Fig. 3A) and inhibition of NF-κB activation by RTD-1 was dose dependent (Fig. 3B). It is noteworthy that no inhibition of NF-κB activity was observed when equimolar concentrations of human neutrophil α-defensin HNP-1 (Fig. 3B) or an acyclic variant of RTD-1 (S7; Fig. 3C) were used in place of RTD-1.
Fig. 3. RTD-1 inhibits NF-κB activation in cells stimulated with TLR agonists.
SEAP activity in the medium of cells stimulated with TLR agonists in the presence or absence of RTD-1 was assayed using Quantiblue. SEAP activity is expressed as fold-induction compared with untreated control cells. (A) Differentiated THP-1 Dual cells were treated with or without LPS (100 ng/ml) in the presence or absence of 10 μg/ml RTD-1. (B) Cells were treated with or without LPS (100 ng/ml) and in the presence or absence of either RTD-1 or HNP-1 (numbers indicate concentrations of RTD-1 and HNP-1 in μg/ml; 2.4 and 4.8 μM correspond to 5 and 10 μg/ml RTD-1 and 8.25 and 16.5 μg/ml HNP-1, respectively). (C, upper panel) The structures of RTD-1 and S7 peptide; the dotted lines indicate disulfide linkages. (C, lower panel) THP-1 Dual cells were stimulated with 100 ng/ml LPS in the presence or absence of 10 μg/ml RTD-1 or the acyclic S7 peptide. One out of 2 independent experiments is shown. (D) Differentiated THP-1 Dual cells were treated with Pam3CSK4 (25 ng/ml) in the presence or absence of 10 μg/ml RTD-1. (E) HEK-Blue hTLR9 cells were treated with or without ODN2006 (ODN) and ±10 μg/ml RTD-1. The numbers indicate the concentration of ODN2006 in μg/ml. Results are shown as means ± sd from 3 independent experiments. *P < 0.05 when compared with treatment with agonist alone.
To test for the effects of RTD-1 on NF-κB activity induced by other stimuli, we analyzed responses of THP-1 cells stimulated with the TLR1/2 agonist Pam3CSK4 and HEK Blue hTLR9 cells stimulated with the TLR9 agonist ODN2006 with and without addition of RTD-1. Both ligands stimulated NF-κB activation, 5- and 6-fold, respectively. Addition of 10 µg/ml RTD-1 inhibited Pam3CSK4-induced NF-κB activity by ∼70%, and the peptide suppressed ODN2006-mediated activation to baseline levels (Fig. 3D and E, respectively).
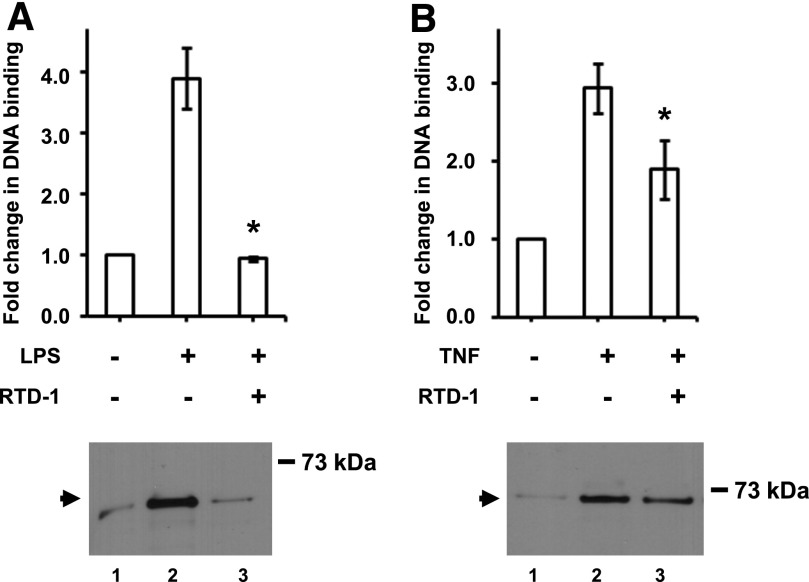
We further characterized the effect of RTD-1 on LPS-stimulation of THP-1 cells by analyzing the effect of the peptide on DNA binding by NF-κB. LPS treatment induced a 4-fold increase in NF-κB DNA binding (Fig. 4A). However, coincubation of LPS and RTD-1 reduced NF-κB DNA binding to baseline levels (Fig. 4A), which correlated with the levels of NF-κB p65 subunit levels in nuclear extracts from cells treated with LPS and RTD-1 as detected by Western blotting (Fig. 4A).
Fig. 4. Inhibition of NF-κB DNA-binding activity by RTD-1.
Nuclear extracts containing 2.8 μg protein from control-differentiated THP-1 cells, stimulated with LPS (100 ng/ml) (A) or TNF-α (2 ng/ml) (B) and with or without 10 μg/ml RTD-1, were assayed for DNA binding. Results are means ± sd from 3 independent experiments. *P < 0.05; compared with LPS- or TNF-α-treated samples. (Lower panel) Western blot of THP-1 nuclear extracts containing 8.1 μg protein used for DNA-binding assay probed with anti-NF-κB p65 antibodies.
We also tested the effect of RTD-1 on TNF-α stimulation of NF-κB DNA binding. Compared with TNF-α alone, RTD-1 suppressed NF-κB DNA binding by ∼35%, substantially less inhibition than observed in LPS-stimulated cells. The decrease in RTD-1 inhibition of TNF-α-stimulated NF-κB DNA binding correlated with the levels of p65 in nuclear extracts of treated and untreated THP-1 cells (Fig. 4B).
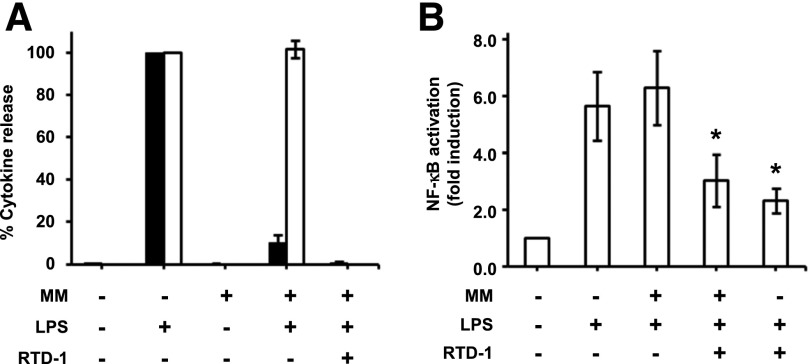
The anti-inflammatory effects of RTD-1 were compared with those of marimastat, a potent inhibitor of TNF-α converting enzyme (TACE/ADAM17), the sheddase that produces soluble TNF-α from the membrane-bound precursor. Marimastat efficiently blocked release of TNF-α by LPS-stimulated THP-1 cells but did not affect secretion of IL-1β (Fig. 5). This result was in contrast to the inhibitory activity of RTD-1, which blocked both cytokines effectively (Figs. 1 and 5A). Also, marimastat treatment had no effect on LPS-stimulated NF-κB activation (Fig. 5B), in contrast to RTD-1, which was a potent inhibitor in the presence or absence of marimastat (Fig. 5B).
Fig. 5. Differential effects of RTD-1 and TACE inhibitor marimastat (MM).
(A) THP-1 macrophages were treated with or without LPS (100 ng/ml) in the presence or absence of MM and RTD-1. TNF-α (black bars) and IL-1β (open bars) secreted in the medium were then measured by ELISA. Results are means ± sd from 3 independent experiments. (B) Differentiated THP-1 Dual cells were stimulated with 100 ng/ml LPS and coincubated with the indicated reagents and NF-κB activity was measured relative to control using the Quantiblue assay. Results shown are means ± sd from 3 independent experiments. *P < 0.05 when compared with MM + LPS treatment.
Signaling pathways affected by RTD-1
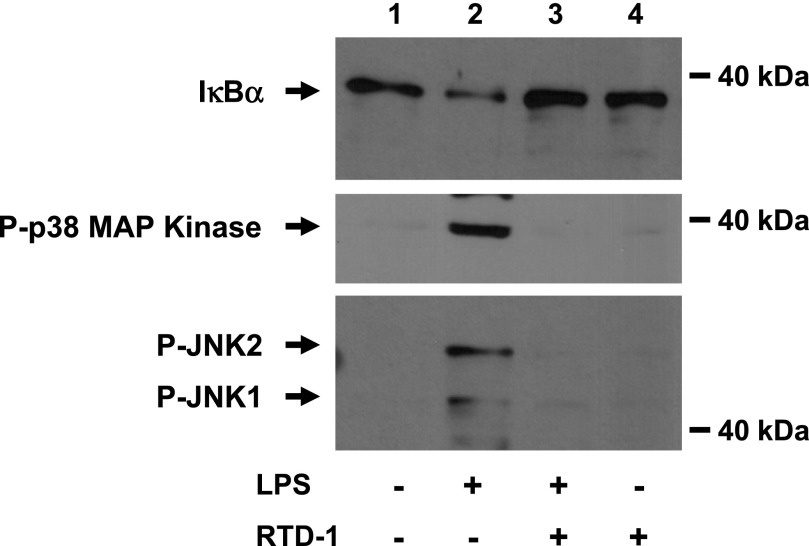
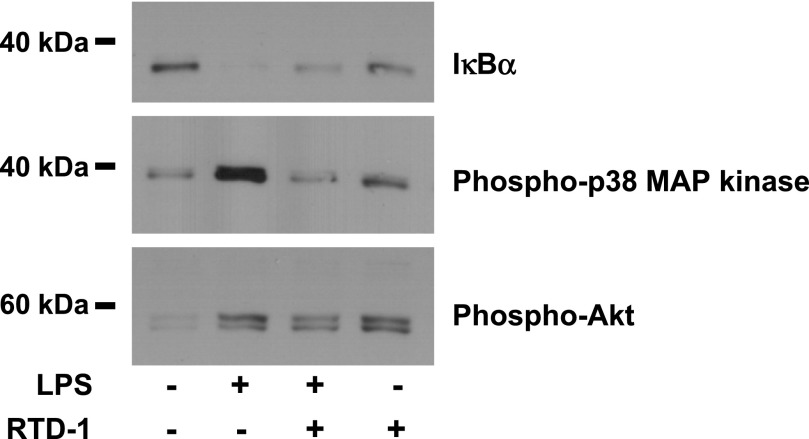
LPS binding to TLR4 initiates a cascade of signaling events that leads to activation of a complex containing TAK1, which phosphorylates IKK. Activated IKK phosphorylates the NF-κB inhibitor IκBα, triggering its degradation, which activates NF-κB for nuclear translocation (Fig. 6). LPS-induced activation of TAK1 also stimulates numerous kinase pathways [30]. RTD-1 was effective in blocking the degradation of IκBα in LPS-stimulated macrophages, and this was accompanied by inhibition of p38 MAPK and JNK1/2 phosphorylation. RTD-1 alone had no effect on IκBα levels or on phosphorylation of p38 MAPK and JNK1/2 (Fig. 6).
Fig. 6. RTD-1 stabilizes IκBα and blocks phosphorylation of p38 MAPK and JNK.
Cell extracts (15 µg protein) were obtained from THP-1 cells stimulated with diluent, 100 ng/ml LPS, LPS + 10 µg/ml RTD-1, or RTD-1 alone. Extracts were resolved on SDS-tricine gels, and Western blots were probed with anti-IκBα, anti-phospho p38 MAPK, and anti-phospho SAPK/JNK antibodies.
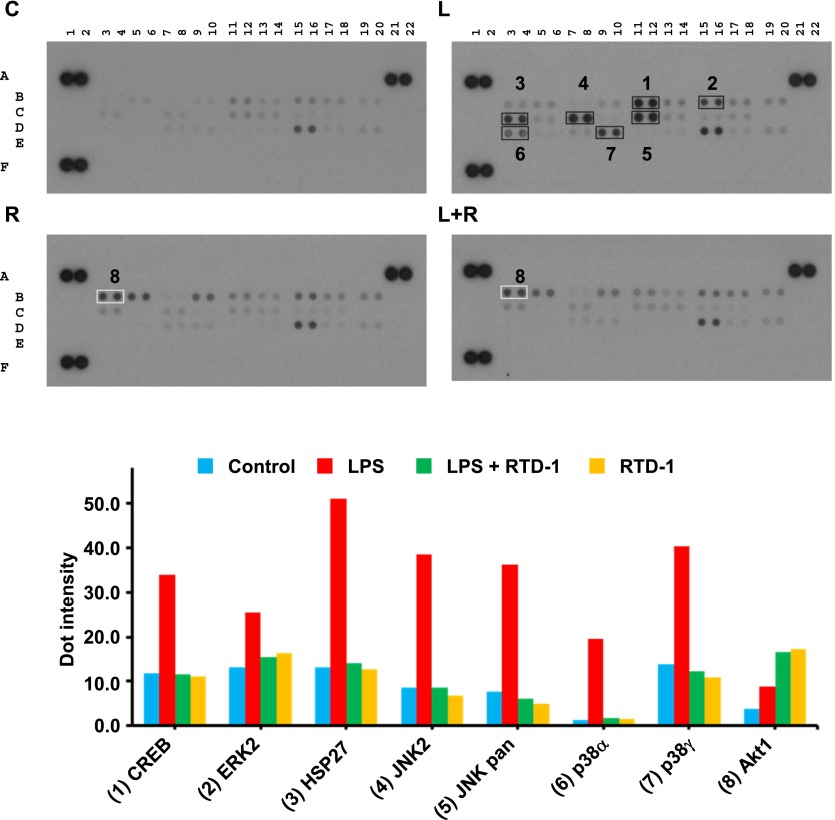
We used phosphoprotein antibody arrays to identify other potential signaling targets of RTD-1. Extracts of LPS-stimulated macrophages contained elevated levels of phosphorylated CREB, ERK2, HSP27, JNK2, p38α, and p38γ compared with control cells (Fig. 7). RTD-1 inhibited phosphorylation of each of these proteins to baseline levels (Fig. 7, lower panel), but treatment with RTD-1 alone did not suppress the phosphorylation of these proteins (Fig. 7).
Fig. 7. RTD-1 modulates phosphorylation of multiple inflammatory signaling proteins.
Extracts from control (C), 100 ng/ml LPS-treated (L), 100 ng/ml LPS + 10 μg/ml RTD-1 treated (L+R), or 10 μg/ml RTD-1-treated (R) THP-1 cells were used to probe a phospho-MAPK array as described in Materials and Methods (upper panel). Positive controls were spotted at (A1, A2), (A21, A22), and (F1, F2) and negative control at (E19, E20). Dot intensities from 2 independent experiments were quantified with ImageJ software and normalized, and the mean values were plotted (lower panel). RTD-1 suppressed LPS-induced phosphorylation of 7 proteins implicated in LPS-induced inflammatory signaling. Treatment of THP-1 macrophages with peptide alone increased pAkt.
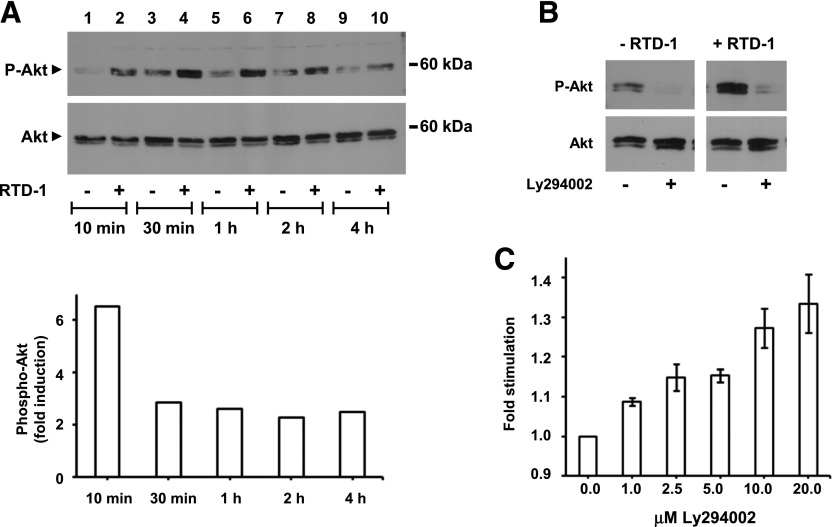
In contrast, RTD-1 alone or in combination with LPS stimulated phosphorylation of Akt1 (Fig. 7). The kinetics of RTD-1 effects on Akt expression and phosphorylation was determined by treating THP-1 cells with RTD-1 alone or with the vehicle and analyzing Akt and pAkt levels. RTD-1 treatment induced a 6.5-fold increase in pAkt within 10 min of peptide treatment and pAkt levels remained elevated by ∼2.5-fold for at least 4 h (Fig. 8A). Additional experiments were performed to identify the role of PI3K in the observed RTD-1-dependent stimulation of Akt phosphorylation. Addition of PI3K inhibitor Ly294002 markedly reduced RTD-1-induced pAkt levels (Fig. 8B), demonstrating that RTD-1 effect was upstream of Akt. Because the PI3K–Akt pathway negatively regulates activation of NF-κB and MAPK pathways [31], we tested the effect of Ly294002 on the RTD-1-mediated inhibition of NF-κB activation in LPS-stimulated THP-1 Dual macrophage cells. Ly294002 reversed the suppression of NF-κB activation by RTD-1 in dose-dependent manner (Fig. 8C), evidence that stimulation of Akt phosphorylation contributes to the observed anti-inflammatory effects of RTD-1.
Fig. 8. RTD-1 stimulation of Akt phosphorylation.
(A) THP-1 cells were treated with diluent or 10 μg/ml RTD-1 and extracts were prepared from cells harvested at the indicated times. Extracts were resolved on SDS-tricine gels and probed using anti-phospho-Akt (pAkt) and anti-Akt antibodies. Band intensities were quantified with ImageJ software, normalized relative to total Akt, and the average fold-increase of pAkt from 2 independent experiments was plotted as function of incubation time. (B) THP-1 macrophages were incubated with 10 μg/ml RTD-1 or diluent ± 10 µM Ly294002. Western blots were performed using Akt or pAkt antibodies. (C) THP-1 Dual cells were pretreated with Ly294002 for 60 min and then stimulated overnight with 100 ng/ml LPS in the presence of 10 µg/ml RTD-1. Medium was analyzed for SEAP activity, and fold stimulation was calculated for each Ly294002 concentration relative to cells treated with no Ly294002. Results are means ± sem from 4 independent experiments, and the effect of Ly294002 treatment on SEAP expression was significant (P < 0.05) at all concentrations tested.
We next tested the effects of RTD-1 on signaling pathways in primary human monocytes. RTD-1 alone did not induce degradation of IκBα, but it reduced the degradation of IκBα in LPS-stimulated monocytes (Fig. 9). RTD-1 inhibited p38 MAPK phosphorylation in LPS-stimulated monocytes, but the peptide alone had no effect on p38 MAPK phosphorylation (Fig. 9). However, as observed in experiments with THP-1 macrophages, increased phosphorylation of Akt was observed in monocytes treated with LPS, LPS and RTD-1, or with RTD-1 alone compared with control cells (Fig. 9). Thus, RTD-1 similarly regulates NF-κB, MAPK and PI3K-Akt signaling pathways in human blood monocytes and THP-1 cells.
Fig. 9. Effect of RTD-1 on signaling pathways in human monocytes.
Extracts (∼7 μg protein) from unstimulated monocytes or monocytes stimulated with 100 ng/ml LPS ± RTD-1 (10 μg/ml) for 30 min were resolved on SDS-tricine gels, and the immunoblots were probed with antibodies against IκBα, phospho-p38 MAPK, and phospho-Akt.
DISCUSSION
Studies on θ-defensins have revealed the pleiotropic properties of these macrocyclic host-defense molecules. Initially isolated based on their antibacterial and antifungal properties in vitro [18], subsequent studies have demonstrated broader host-defense properties mediated by their antiviral properties against herpes simplex virus [32], HIV [33, 34], and influenza [35], and arming of phagocytes for enhanced killing of Bacillus anthracis [36]. Also, studies revealed that the protective effects of systemically administered RTD-1 in mouse models of severe acute respiratory syndrome coronavirus infection [27] and sepsis [26] are mediated, at least partly, by suppression of immunopathological cytokine expression. To gain insights into the immunomodulatory mechanisms mediated by θ-defensins, we analyzed the effects of RTD-1 on inflammatory pathways in THP-1 macrophages.
RTD-1 inhibits secretion of several proinflammatory cytokines by human buffy coat cells stimulated with E. coli cells as well as with agonists for TLR2, 4, 5, and 8 with particularly marked suppression of TNF-α, IL-1α/β, IL-6, IL-8, CCL3, and CCL4 [26]. Consistent with these findings, 10 µg/ml RTD-1, a concentration that we previously found was very effective in blocking TNF secretion from stimulated blood cells, was highly effective in blocking secretion of TNF-α, IL-1β, and IL-8 by LPS-stimulated THP-1 cells and in human monocytes (Fig. 1). Because RTD-1 binds inefficiently to LPS, its immune regulatory effects are not mediated by neutralization of LPS [26]. Here, we also show that RTD-1 suppression of these cytokines, and of CCL3 and CCL4, corresponds to down-regulation of the corresponding mRNA levels, indicating, for the first time, to our knowledge, that the previously observed anti-inflammatory mechanisms of θ-defensins [26] are mediated by regulation of inflammatory gene expression as well. Of note, RTD-1 alone did not affect cytokine mRNA or protein levels in unstimulated cells (Figs. 1 and 2).
The stimulation of inflammatory gene expression by diverse TLR agonists is due to the activation of the NF-κB pathway [37, 38]. Because RTD-1 repressed cytokines stimulated by several TLR agonists [26], we analyzed the effect of peptide treatment on NF-κB activation in THP-1 cells treated with LPS and Pam3CSK4, agonists for TLRs 4 and 1/2 (cell surface), respectively, and in HEK-Blue hTLR9 cells stimulated with ODN2006, agonist for TLR9 (intracellular). RTD-1 treatment markedly inhibited NF-κB activation by each agonist (Fig. 3) and the inhibitory effect was dose dependent. Consistent with these findings, RTD-1 inhibited translocation of NF-κB p65 to the nucleus in LPS-stimulated macrophages (Fig. 4) and stabilized IκBα (Fig. 6). The human α-defensin HNP-1 had no inhibitory activity (Fig. 3B), in agreement with a previous report of human neutrophil α-defensins lacking anti-inflammatory properties [26]. It is noteworthy that an acyclic version of RTD-1 (S7; Fig. 3C) did not inhibit NF-κB activation, demonstrating that the cyclic structure of the peptide is essential for this activity.
Previously, we showed that RTD-1-mediated blockade of TNF-α secretion by E. coli-stimulated whole blood was extremely rapid, which had suggested that blockade of numerous cytokines resulted from TNF-α-regulated cytokine expression [26]. We now show that RTD-1 reduced TNF-α-mediated NF-κB activation in THP-1 cells (Fig. 4) but to a lesser extent than the peptide’s effect on LPS stimulation (Fig. 4). Consistent with this result, RTD-1 both suppressed TNF-α and IL-1β secretion by LPS-treated THP-1 cells in marked contrast to marimastat (Fig. 5), a potent inhibitor of the TACE/ADAM17 sheddase that generates soluble TNF-α, which did not inhibit IL-1β release (Fig. 5). Thus, the blockade of TNF-α release, and subsequent suppression of autocrine signaling, cannot alone account for the immunomodulatory effects of RTD-1.
We also analyzed the effect of RTD-1 on signaling kinases implicated in LPS-induced inflammatory responses in macrophages. RTD-1 inhibited LPS-induced phosphorylation of several inflammatory signaling proteins, including p38, JNK, ERK2, CREB, and HSP27 (Figs. 6 and 7). Inhibition of CREB and HSP27 phosphorylation is consistent with the suppression of p38 MAPK, which phosphorylates these proteins (Fig. 10). p38 MAPK is also implicated in stabilization of mRNAs containing AU-rich elements, including TNF-α [39, 40], IL-6 [41], CCL3 [42], IL-8 [41, 43], and several other immune response genes [44]. The MEK-ERK pathway is involved in nucleocytoplasmic transport of TNF-α mRNA in mouse macrophage cells [45]. Stimulation of JNK MAPK leads to activation of the AP-1 class of transcription factors, which are involved in activation of immune response genes [46]. Thus, RTD-1 appears to regulate inflammatory signaling by inhibiting the phosphorylation of several inflammatory signaling proteins.
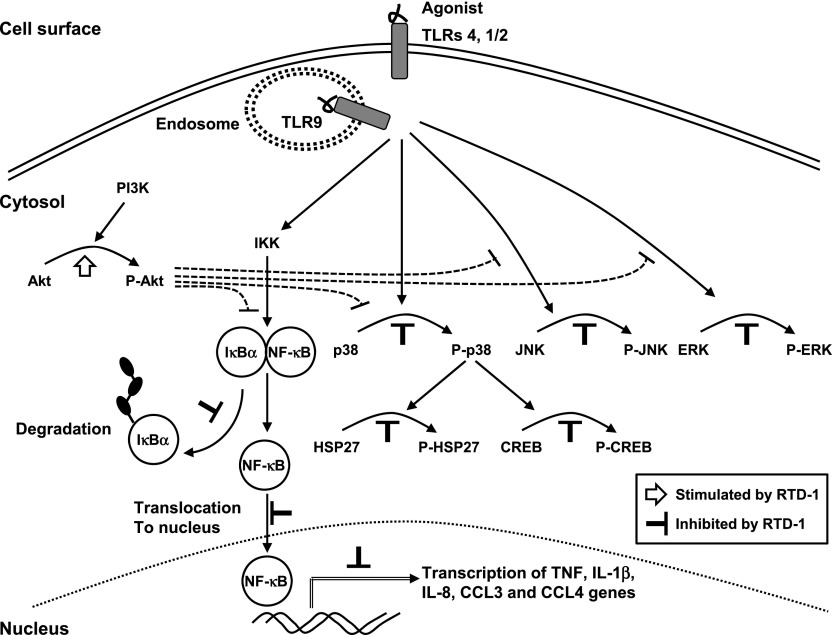
Fig. 10. Immunoregulatory effects of RTD-1.
The schematic summarizes findings in the current study, including 1) the suppression of NF-κB activation stimulated by extracellular (TLR1/2 and 4) and intracellular (TLR9) receptors; 2) inhibitory and stimulatory effects of RTD-1 on LPS-stimulated pathways; and 3) negative regulatory pathways mediated by pAkt identified previously [31].
THP-1 cell exposure to RTD-1 alone induced phosphorylation of Akt (Fig. 7), which occurred very rapidly after RTD-1 treatment and produced a sustained effect (Fig. 8). In contrast, RTD-1 did not increase baseline phosphorylation of the other kinase targets evaluated in otherwise untreated cells (Figs. 7 and 8). Consistent with these results from THP-1 cells, RTD-1 inhibited the degradation of IκBα, phosphorylation of p38 MAPK in LPS-stimulated human monocytes, and also stimulated Akt phosphorylation (Fig. 9) in these cells.
RTD-1-induced phosphorylation of Akt was blocked by a specific PI3K inhibitor, implicating the PI3K/Akt pathway in mediating the anti-inflammatory effects of RTD-1 (Fig. 8). PI3K-Akt is a known negative regulator of NF-κB and MAPK pathways and inhibits TNF-α gene expression in LPS-stimulated cells [31, 47, 48]. Consistent with this, resveratrol inhibits secretion of TNF-α and IL-1β in LPS-stimulated RAW264.7 cells by stimulating the PI3K-Akt pathway, which is accompanied by inhibition of p38 and JNK MAPK [49]. Inhibition of the PI3K-Akt pathway increases the mortality and circulating proinflammatory cytokine levels in endotoxemic mice [50], as well as in mice with polymicrobial sepsis induced by cecal ligation and puncture [51]. Additionally, glucan phosphate, which protects mice in the cecal ligation and puncture model, increased tissue PI3K activity in kidney, lung, and heart, correlating with survival of these animals [51]. Interestingly, both human α-defensin HNP1 and human β-defensins HBD2 and HBD3 stimulate expression and secretion of proinflammatory cytokines from human conjunctival epithelial cells, which are accompanied by phosphorylation of Akt [12]. Both α-defensins and β-defensins are known to regulate cellular responses through receptor-mediated pathways; human neutrophil peptides induce signaling pathways through the P2Y6 receptor [7]. The human β-defensins HBD2 and HBD3 induce chemotaxis in monocytes through the CCR2 receptor [52], and HBD2 induces chemotaxis in dendritic and T cells through the CCR6 receptors [15]. To date, there have been no reports of a receptor for θ-defensins. Nevertheless, studies are underway to identify RTD-1 targets upstream of the PI3K-Akt pathway and the relationship of RTD-1-induced pAkt to downstream signaling pathways. The suppression of TNF-α secretion by RTD-1 is extremely rapid, suggesting that RTD-1 may also regulate pathways distinct from those involved in signaling and expression of proinflammatory genes [26].
Semple et al. [53, 54] demonstrated anti-inflammatory properties of β-defensin. For example, human β-defensin-3 inhibited LPS-induced gene transcription and proinflammatory cytokine secretion by mouse RAW264.7 macrophages via inhibition of the NF-κB pathway involving signaling through MyD88 and TRIF [53]. It has been suggested that the contrasting proinflammatory and anti-inflammatory properties reported for β-defensins may reflect differences in structural features and/or in the purity of the peptide preparations [53]. Another possibility, as suggested by others, is that the proinflammatory vs. anti-inflammatory properties of a single peptide may be a function of local concentration [55–57]. In this context, we speculate the θ-defensins, expressed at very high levels in granules of circulating neutrophils and monocytes [23], provide a physiologic dampening of inflammatory processes following their release and/or secretion systemically or locally.
In summary, RTD-1 suppresses NF-κB activation and cytokine expression and release, and such immunomodulation occurs at both the TLR and TNF pathways. Evidence for a role for induction of Akt phosphorylation is suggested by RTD-1-induced pAkt in both naïve and LPS-stimulated cells. The immunomodulatory properties of the macrocyclic peptide are distinct from those of human α-defensins, and RTD-1 appears to suppress inflammation by mechanisms distinct from those of human β-defensin-3. Further, because RTD-1 and related θ-defensins differentially inhibit TNF secretion from stimulated blood cells [26], we propose that θ-defensin isoforms may be unique tools for dissecting regulatory pathways that determine whether the host response to inflammatory stimuli results in health or disease.
AUTHORSHIP
P.T. and K.K.T. designed and performed the experiments; J.B.S. and D.T. produced, analyzed, and provided reagents; P.T., A.J.O., P.S.G., and M.E.S. analyzed the data and wrote the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Angie E. Garcia for help with isolation and analysis of monocytes from peripheral blood. This work was supported by the Robert E. and May R. Wright Foundation Award (to P.T.); a U.S. Arthritis Foundation grant (to M.E.S.); and U.S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases Grants AI22931 (to M.E.S.) and AI105057 (to A.J.O.), NIH National Institute of Dental and Craniofacial Research Grant DE021341 (to M.E.S.), University of Southern California Norris Comprehensive Cancer Center NIH National Cancer Institute Support Grant P30 CA014089, and Southern California Clinical and Translational Science Institute NIH National Center for Advancing Translational Sciences Grant UL1RR031986.
Glossary
- Ct
cycle threshold
- gDNA
genomic DNA
- HI
heat inactivated
- HNP
human neutrophil peptide
- HSP
heat shock protein
- IKK
IκB kinase
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MM
marimastat
- ODN
oligodeoxynucleotide
- pAkt
phosphorylated Akt
- PPC
positive PCR control
- P/S
penicillin/streptomycin
- RTC
reverse transcription control
- RTD
rhesus θ-defensin
- SAPK
stress-activated protein kinase
- SEAP
secreted embryonic alkaline phosphatase
- TACE/ADAM17
TNF-α converting enzyme/A disintegrin and metalloproteinase domain17
- TAK1
TGF β-activated kinase 1
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Ericksen B., Wu Z., Lu W., Lehrer R. I. (2005) Antibacterial activity and specificity of the six human α-defensins. Antimicrob. Agents Chemother. 49, 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mambula S. S., Simons E. R., Hastey R., Selsted M. E., Levitz S. M. (2000) Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect. Immun. 68, 6257–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman S. L., Gootee L., Gabay J. E., Selsted M. E. (2000) Identification of constituents of human neutrophil azurophil granules that mediate fungistasis against Histoplasma capsulatum. Infect. Immun. 68, 5668–5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aley S. B., Zimmerman M., Hetsko M., Selsted M. E., Gillin F. D. (1994) Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect. Immun. 62, 5397–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daher K. A., Selsted M. E., Lehrer R. I. (1986) Direct inactivation of viruses by human granulocyte defensins. J. Virol. 60, 1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastian A., Schäfer H. (2001) Human α-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul. Pept. 101, 157–161. [DOI] [PubMed] [Google Scholar]
- 7.Khine A. A., Del Sorbo L., Vaschetto R., Voglis S., Tullis E., Slutsky A. S., Downey G. P., Zhang H. (2006) Human neutrophil peptides induce interleukin-8 production through the P2Y6 signaling pathway. Blood 107, 2936–2942. [DOI] [PubMed] [Google Scholar]
- 8.Boniotto M., Jordan W. J., Eskdale J., Tossi A., Antcheva N., Crovella S., Connell N. D., Gallagher G. (2006) Human β-defensin 2 induces a vigorous cytokine response in peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 50, 1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito T., Tanabe H., Ayabe T., Ishikawa C., Inaba Y., Maemoto A., Kono T., Ashida T., Fujiya M., Kohgo Y. (2012) Paneth cells regulate both chemotaxis of immature dendritic cells and cytokine production from epithelial cells. Tohoku J. Exp. Med. 227, 39–48. [DOI] [PubMed] [Google Scholar]
- 10.Yin L., Chino T., Horst O. V., Hacker B. M., Clark E. A., Dale B. A., Chung W. O. (2010) Differential and coordinated expression of defensins and cytokines by gingival epithelial cells and dendritic cells in response to oral bacteria. BMC Immunol. 11, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niyonsaba F., Ushio H., Nagaoka I., Okumura K., Ogawa H. (2005) The human β-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J. Immunol. 175, 1776–1784. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Zhu H. Y., Beuerman R. W. (2009) Stimulation of specific cytokines in human conjunctival epithelial cells by defensins HNP1, HBD2, and HBD3. Invest. Ophthalmol. Vis. Sci. 50, 644–653. [DOI] [PubMed] [Google Scholar]
- 13.Syeda F., Liu H. Y., Tullis E., Liu M., Slutsky A. S., Zhang H. (2008) Differential signaling mechanisms of HNP-induced IL-8 production in human lung epithelial cells and monocytes. J. Cell. Physiol. 214, 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chertov O., Michiel D. F., Xu L., Wang J. M., Tani K., Murphy W. J., Longo D. L., Taub D. D., Oppenheim J. J. (1996) Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 271, 2935–2940. [DOI] [PubMed] [Google Scholar]
- 15.Yang D., Chertov O., Bykovskaia S. N., Chen Q., Buffo M. J., Shogan J., Anderson M., Schröder J. M., Wang J. M., Howard O. M., Oppenheim J. J. (1999) β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286, 525–528. [DOI] [PubMed] [Google Scholar]
- 16.Grigat J., Soruri A., Forssmann U., Riggert J., Zwirner J. (2007) Chemoattraction of macrophages, T lymphocytes, and mast cells is evolutionarily conserved within the human α-defensin family. J. Immunol. 179, 3958–3965. [DOI] [PubMed] [Google Scholar]
- 17.Soruri A., Grigat J., Forssmann U., Riggert J., Zwirner J. (2007) β-Defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur. J. Immunol. 37, 2474–2486. [DOI] [PubMed] [Google Scholar]
- 18.Selsted M. E., Ouellette A. J. (2005) Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6, 551–557. [DOI] [PubMed] [Google Scholar]
- 19.Lehrer R. I., Cole A. M., Selsted M. E. (2012) θ-Defensins: cyclic peptides with endless potential. J. Biol. Chem. 287, 27014–27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y. Q., Yuan J., Osapay G., Osapay K., Tran D., Miller C. J., Ouellette A. J., Selsted M. E. (1999) A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science 286, 498–502. [DOI] [PubMed] [Google Scholar]
- 21.Leonova L., Kokryakov V. N., Aleshina G., Hong T., Nguyen T., Zhao C., Waring A. J., Lehrer R. I. (2001) Circular minidefensins and posttranslational generation of molecular diversity. J. Leukoc. Biol. 70, 461–464. [PubMed] [Google Scholar]
- 22.Garcia A. E., Osapay G., Tran P. A., Yuan J., Selsted M. E. (2008) Isolation, synthesis, and antimicrobial activities of naturally occurring θ-defensin isoforms from baboon leukocytes. Infect. Immun. 76, 5883–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tongaonkar P., Tran P., Roberts K., Schaal J., Osapay G., Tran D., Ouellette A. J., Selsted M. E. (2011) Rhesus macaque θ-defensin isoforms: expression, antimicrobial activities, and demonstration of a prominent role in neutrophil granule microbicidal activities. J. Leukoc. Biol. 89, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen T. X., Cole A. M., Lehrer R. I. (2003) Evolution of primate θ-defensins: a serpentine path to a sweet tooth. Peptides 24, 1647–1654. [DOI] [PubMed] [Google Scholar]
- 25.Yang D., Biragyn A., Hoover D. M., Lubkowski J., Oppenheim J. J. (2004) Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 22, 181–215. [DOI] [PubMed] [Google Scholar]
- 26.Schaal J. B., Tran D., Tran P., Ösapay G., Trinh K., Roberts K. D., Brasky K. M., Tongaonkar P., Ouellette A. J., Selsted M. E. (2012) Rhesus macaque theta defensins suppress inflammatory cytokines and enhance survival in mouse models of bacteremic sepsis. PLoS One 7, e51337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohlford-Lenane C. L., Meyerholz D. K., Perlman S., Zhou H., Tran D., Selsted M. E., McCray P. B. Jr (2009) Rhesus theta-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. J. Virol. 83, 11385–11390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. (1985) Defensins: natural peptide antibiotics of human neutrophils. J. Clin. Invest. 76, 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tongaonkar P., Golji A. E., Tran P., Ouellette A. J., Selsted M. E. (2012) High fidelity processing and activation of the human α-defensin HNP1 precursor by neutrophil elastase and proteinase 3. PLoS One 7, e32469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J., Mira-Arbibe L., Ulevitch R. J. (2000) TAK1 regulates multiple protein kinase cascades activated by bacterial lipopolysaccharide. J. Leukoc. Biol. 68, 909–915. [PubMed] [Google Scholar]
- 31.Guha M., Mackman N. (2002) The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 277, 32124–32132. [DOI] [PubMed] [Google Scholar]
- 32.Yasin B., Wang W., Pang M., Cheshenko N., Hong T., Waring A. J., Herold B. C., Wagar E. A., Lehrer R. I. (2004) Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78, 5147–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole A. M., Hong T., Boo L. M., Nguyen T., Zhao C., Bristol G., Zack J. A., Waring A. J., Yang O. O., Lehrer R. I. (2002) Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 99, 1813–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seidel A., Ye Y., de Armas L. R., Soto M., Yarosh W., Marcsisin R. A., Tran D., Selsted M. E., Camerini D. (2010) Cyclic and acyclic defensins inhibit human immunodeficiency virus type-1 replication by different mechanisms. PLoS One 5, e9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doss M., Ruchala P., Tecle T., Gantz D., Verma A., Hartshorn A., Crouch E. C., Luong H., Micewicz E. D., Lehrer R. I., Hartshorn K. L. (2012) Hapivirins and diprovirins: novel θ-defensin analogs with potent activity against influenza A virus. J. Immunol. 188, 2759–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welkos S., Cote C. K., Hahn U., Shastak O., Jedermann J., Bozue J., Jung G., Ruchala P., Pratikhya P., Tang T., Lehrer R. I., Beyer W. (2011) Humanized θ-defensins (retrocyclins) enhance macrophage performance and protect mice from experimental anthrax infections. Antimicrob. Agents Chemother. 55, 4238–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayden M. S., Ghosh S. (2011) NF-κB in immunobiology. Cell Res. 21, 223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton K., Dixit V. M. (2012) Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 4, pii:a006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahtani K. R., Brook M., Dean J. L., Sully G., Saklatvala J., Clark A. R. (2001) Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor α mRNA stability. Mol. Cell. Biol. 21, 6461–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutault K., Hazzalin C. A., Mahadevan L. C. (2001) Combinations of ERK and p38 MAPK inhibitors ablate tumor necrosis factor-α (TNF-α) mRNA induction. Evidence for selective destabilization of TNF-α transcripts. J. Biol. Chem. 276, 6666–6674. [DOI] [PubMed] [Google Scholar]
- 41.Winzen R., Kracht M., Ritter B., Wilhelm A., Chen C. Y., Shyu A. B., Müller M., Gaestel M., Resch K., Holtmann H. (1999) The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18, 4969–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S. W., Pawlowski J., Wathen S. T., Kinney S. D., Lichenstein H. S., Manthey C. L. (1999) Cytokine mRNA decay is accelerated by an inhibitor of p38-mitogen-activated protein kinase. Inflamm. Res. 48, 533–538. [DOI] [PubMed] [Google Scholar]
- 43.Holtmann H., Winzen R., Holland P., Eickemeier S., Hoffmann E., Wallach D., Malinin N. L., Cooper J. A., Resch K., Kracht M. (1999) Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol. Cell. Biol. 19, 6742–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frevel M. A., Bakheet T., Silva A. M., Hissong J. G., Khabar K. S., Williams B. R. (2003) p38 Mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol. Cell. Biol. 23, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dumitru C. D., Ceci J. D., Tsatsanis C., Kontoyiannis D., Stamatakis K., Lin J. H., Patriotis C., Jenkins N. A., Copeland N. G., Kollias G., Tsichlis P. N. (2000) TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103, 1071–1083. [DOI] [PubMed] [Google Scholar]
- 46.Karin M. (2005) Inflammation-activated protein kinases as targets for drug development. Proc. Am. Thorac. Soc. 2, 386–390, discussion 394–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park Y. C., Lee C. H., Kang H. S., Chung H. T., Kim H. D. (1997) Wortmannin, a specific inhibitor of phosphatidylinositol-3-kinase, enhances LPS-induced NO production from murine peritoneal macrophages. Biochem. Biophys. Res. Commun. 240, 692–696. [DOI] [PubMed] [Google Scholar]
- 48.Fukao T., Koyasu S. (2003) PI3K and negative regulation of TLR signaling. Trends Immunol. 24, 358–363. [DOI] [PubMed] [Google Scholar]
- 49.Zong Y., Sun L., Liu B., Deng Y. S., Zhan D., Chen Y. L., He Y., Liu J., Zhang Z. J., Sun J., Lu D. (2012) Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cells. PLoS One 7, e44107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schabbauer G., Tencati M., Pedersen B., Pawlinski R., Mackman N. (2004) PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler. Thromb. Vasc. Biol. 24, 1963–1969. [DOI] [PubMed] [Google Scholar]
- 51.Williams D. L., Li C., Ha T., Ozment-Skelton T., Kalbfleisch J. H., Preiszner J., Brooks L., Breuel K., Schweitzer J. B. (2004) Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J. Immunol. 172, 449–456. [DOI] [PubMed] [Google Scholar]
- 52.Röhrl J., Yang D., Oppenheim J. J., Hehlgans T. (2010) Human β-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J. Immunol. 184, 6688–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semple F., MacPherson H., Webb S., Cox S. L., Mallin L. J., Tyrrell C., Grimes G. R., Semple C. A., Nix M. A., Millhauser G. L., Dorin J. R. (2011) Human β-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur. J. Immunol. 41, 3291–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Semple F., Webb S., Li H. N., Patel H. B., Perretti M., Jackson I. J., Gray M., Davidson D. J., Dorin J. R. (2010) Human β-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur. J. Immunol. 40, 1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Y., Niyonsaba F., Ushio H., Nagaoka I., Ikeda S., Okumura K., Ogawa H. (2007) Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br. J. Dermatol. 157, 1124–1131. [DOI] [PubMed] [Google Scholar]
- 56.Kahlenberg J. M., Kaplan M. J. (2013) Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J. Immunol. 191, 4895–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mookherjee N., Brown K. L., Bowdish D. M., Doria S., Falsafi R., Hokamp K., Roche F. M., Mu R., Doho G. H., Pistolic J., Powers J. P., Bryan J., Brinkman F. S., Hancock R. E. (2006) Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 176, 2455–2464. [DOI] [PubMed] [Google Scholar]