Myeloid-derived suppressor cells produce IL-10 to attenuate monocyte and macrophage proinflammatory properties that favors the establishment of implant-associated biofilm infection.
Keywords: MDSC, IL-10, macrophage
Abstract
Staphylococcus aureus is known to establish biofilms on medical devices. We recently demonstrated that Ly6GhighLy6C+ myeloid-derived suppressor cells are critical for allowing S. aureus biofilms to subvert immune-mediated clearance; however, the mechanisms whereby myeloid-derived suppressor cells promote biofilm persistence remain unknown. Interleukin-10 expression was significantly increased in a mouse model of S. aureus orthopedic implant biofilm infection with kinetics that mirrored myeloid-derived suppressor cell recruitment. Because myeloid-derived suppressor cells produce interleukin-10, we explored whether it was involved in orchestrating the nonproductive immune response that facilitates biofilm formation. Analysis of interleukin-10–green fluorescent protein reporter mice revealed that Ly6GhighLy6C+ myeloid-derived suppressor cells were the main source of interleukin-10 during the first 2 wk of biofilm infection, whereas monocytes had negligible interleukin-10 expression until day 14. Myeloid-derived suppressor cell influx into implant-associated tissues was significantly reduced in interleukin-10 knockout mice at day 14 postinfection, concomitant with increased monocyte and macrophage infiltrates that displayed enhanced proinflammatory gene expression. Reduced myeloid-derived suppressor cell recruitment facilitated bacterial clearance, as revealed by significant decreases in S. aureus burdens in the knee joint, surrounding soft tissue, and femur of interleukin-10 knockout mice. Adoptive transfer of interleukin-10 wild-type myeloid-derived suppressor cells into S. aureus–infected interleukin-10 knockout mice restored the local biofilm-permissive environment, as evidenced by increased bacterial burdens and inhibition of monocyte proinflammatory activity. These effects were both interleukin-10-dependent and interleukin-10-independent because myeloid-derived suppressor cell–derived interleukin-10 was required for promoting biofilm growth and anti-inflammatory gene expression in monocytes but was not involved in monocyte recruitment to biofilm-infected tissues. These results demonstrate that interleukin-10 production by myeloid-derived suppressor cells contributes to the persistence of S. aureus orthopedic biofilm infections.
Introduction
Staphylococcus aureus is a major cause of health care and community-associated infections, and because of the increased prevalence of methicillin-resistant S. aureus strains, this pathogen has become an even greater therapeutic challenge [1–4]. The risk of infection increases in the presence of a foreign body, and S. aureus is known for its ability to colonize and form biofilms on medical devices, such as indwelling catheters and orthopedic implants [5–8]. Biofilm-associated bacteria exhibit distinct properties compared with planktonic growth phases of the same species, and it is becoming clear that the composition and kinetics of the host immune response to S. aureus biofilms inadvertently facilitates biofilm persistence, whereas planktonic infections, such as abscesses, are often resolved [9, 10]. Our laboratory was the first to identify MDSCs during S. aureus biofilm formation, which represents a key immunosuppressive mechanism that supports chronic infection [11].
MDSCs are a heterogeneous population of immature monocytes and granulocytes that are intermediates of normal myeloid development and differentiation [12]. Under typical conditions, MDSCs differentiate at the site of inflammation or injury to generate mature myeloid populations, including neutrophils, Mϕs, and dendritic cells [12–14]. However, in pathologic situations, such as tumors, chronic inflammation, and bacterial biofilm infection, MDSCs become arrested in an immature state, where they negatively regulate inflammatory mechanisms through their suppressive actions [15–17]. In cancer, MDSC expansion can be induced by various cytokines and growth factors, such as IL-6, G-CSF, GM-CSF, and VEGF; however, it is currently unknown what host or bacterial products promote MDSC propagation during S. aureus biofilm infection or their arrest in the immature state. Following MDSC expansion, inflammatory stimuli provide activation signals and induce the acquisition of immunosuppressive properties [18]. Our recent report [19] demonstrated that IL-12 facilitates MDSC accumulation at the site of S. aureus biofilm infection, which is likely an indirect effect because the cytokine is not a chemoattractant. However, IL-12 is not required for MDSC activation during S. aureus biofilm infection because MDSCs from both IL-12 p40 and p35 KO mice still inhibited CD4+ T cell proliferation. Therefore, other inflammatory factors must be involved in inducing the expression Arg-1, IL-10, and other anti-inflammatory mediators expressed by MDSCs that contribute to their immunosuppressive functions during S. aureus biofilm infection.
IL-10 is an anti-inflammatory cytokine known for its role in controlling inflammatory responses [20, 21], including inhibiting T cell activation and polarization, and IL-10 secretion by MDSCs has been implicated in programming Mϕs toward an anti-inflammatory phenotype [22–26]. Previously, we have shown that IL-10 expression is increased in FACS-purified MDSCs recovered from S. aureus biofilms in a mouse orthopedic infection model [11]. Here, we sought to identify the role of IL-10 in MDSC-mediated immune suppression during biofilm infection and to determine whether its actions contribute to bacterial persistence. The use of IL-10-GFP reporter mice revealed that Ly6GhighLy6C+ MDSCs were the main source of IL-10 during S. aureus biofilm infection. To demonstrate the functional importance of IL-10 in shaping the inflammatory milieu typical of biofilm infection, we performed studies in IL-10 KO mice. Fewer MDSCs infiltrated implant-associated tissues of IL-10 KO mice at d 14 postinfection concomitant with enhanced monocyte and Mϕ infiltrates. The reduction in MDSCs translated into bacterial clearance, as revealed by significant decreases in S. aureus burdens in the knee joint, surrounding soft tissue, and femur of IL-10 KO mice, which coincided with increased monocyte proinflammatory gene expression. The adoptive transfer of IL-10 WT MDSCs into IL-10 KO mice during S. aureus infection resulted in fewer monocyte infiltrates and attenuated proinflammatory gene expression, which consequently restored bacterial burdens to levels reminiscent of WT animals. In contrast, the adoptive transfer of IL-10 KO MDSCs into IL-10 KO mice did not augment bacterial biofilm growth, indicating that MDSC-derived IL-10 is important for promoting biofilm persistence. Collectively, these data demonstrate that IL-10 production by MDSCs is one mechanism to promote S. aureus orthopedic biofilm formation by limiting monocyte/Mϕ recruitment and proinflammatory activity.
MATERIALS AND METHODS
Mice
Male IL-10-GFP reporter mice as well as IL-10 KO and age-matched C57BL/6 WT mice (8 wk old) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the U.S. National Institutes of Health. The animal use protocol was approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center.
Mouse model of S. aureus orthopedic implant biofilm infection
To model infectious complications in patients following surgical device placement, a mouse model of S. aureus orthopedic implant biofilm infection was used as previously described [11, 19]. Briefly, mice were anesthetized with a ketamine/xylazine cocktail (Hospira, Lake Forest, IL, USA, and Akorn, Decatur, IL, USA; 100 mg/kg and 5 mg/kg, respectively), and a medial parapatellar arthrotomy was performed with lateral displacement of the quadriceps-patella to access the distal femur. A 26-gauge needle was used to create a burr hole in the femoral intercondylar notch extending into the intramedullary canal, whereupon a precut 0.8 cm long, orthopedic-grade K-wire (0.6 mm diameter, nitinol nickel-titanium; Custom Wire Technologies, Port Washington, WI, USA) was inserted into the intramedullary canal, leaving approximately 1 mm protruding into the joint space. A total of 1000 CFU of the bioluminescent S. aureus USA300 LAC::lux isolate [27] was inoculated at the implant tip. In some experiments, control mice received sterile implants using an identical procedure. For pain relief, animals received Buprenex (0.1 mg/kg s.c.; Reckitt Benckiser Health Care, Hull, North Humberside, United Kingdom) immediately after infection and 24 h later. After this interval, all mice exhibited normal ambulation and no discernable pain behaviors.
ELISA
IL-10 levels in tissue homogenates associated with S. aureus infected and sterile implants were quantitated by a sandwich ELISA (BD OptEIA; BD Biosciences, San Diego, CA, USA). Results were normalized to the total amount of protein to account for differences in tissue sampling size. The lower limit of detection for the assay was 31.3 pg/ml.
Flow cytometry
To characterize leukocyte infiltrates associated with inflamed soft tissues surrounding the knee joint during S. aureus biofilm infection, tissues were excised, dissociated using the blunt end of a plunger from a 3 cc syringe, and passed through a 35 μm filter (BD Falcon; BD Biosciences). The resulting filtrate was washed with 1× PBS containing 2% FBS, and cells were collected by centrifugation (300 g, 10 min), whereupon RBCs were lysed using BD Pharm Lyse (BD Biosciences). After lysis, cells were resuspended in PBS containing 2% FBS and incubated with Fc Block (BD Biosciences) to minimize nonspecific Ab binding. Cells were then stained with CD45-allophycocyanin, Ly6G-PE, Ly6C-PerCP-Cy5.5, and F4/80-PE-Cy7. All fluorochrome-conjugated Abs were purchased from BD Biosciences or eBioscience (San Diego, CA, USA). An aliquot of cells was stained with isotype-matched control Abs to assess the degree of nonspecific staining. The number of events analyzed ranged from 20,000 to 100,000 per sample depending on the experimental setup. Analysis was performed using BD FACSDiva software with cells gated on the total CD45+ leukocyte population.
Recovery of orthopedic implant and surrounding tissues for S. aureus enumeration
Inflamed soft tissue surrounding the infected knee joint was collected after removing the skin, where the subcutaneous tissue dorsal to the patellar tendon was excised, weighed, and processed for flow cytometry as described above. Muscle and tendon tissues were excluded from the analysis. A small aliquot was removed after processing for quantification of bacterial burdens. The knee joint (including cartilage and ligaments) and femur were homogenized using 2 sequential procedures because of the resilient nature of these tissues: first a 30 s dispersal using a hand-held homogenizer, followed by disruption in a Bullet Blender (Next Advance, Averill Park, NY, USA) using 100 μm stainless steel beads (0.9–2.0 mm stainless steel blend). Serial, 10-fold dilutions of tissue, knee, or femur homogenates were plated on trypticase soy agar with 5% sheep blood (Remel Products, Lenexa, KS, USA) with titers expressed as CFUs per gram of tissue. Remaining homogenates were centrifuged (20,000 g, 20 min) and frozen at −80°C until further analysis by Milliplex bead arrays (EMD Millipore, Billerica, MA, USA) as described below.
Multianalyte microbead arrays
To evaluate the effects of IL-10 on inflammatory mediator production during S. aureus orthopedic implant biofilm infection, a custom-designed mouse microbead array was used (Milliplex), which detects the following mediators: G-CSF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, CCL2, CCL3, CCL5, CXCL1, CXCL2, CXCL9, CXCL10, TNF-α, and VEGF. A Bio-Plex workstation (Bio-Rad, Hercules, CA, USA) was used to analyze results, and values were normalized to the total amount of protein recovered from each sample to correct for differences in tissue sizes.
qRT-PCR
Ly6G−Ly6C+ monocytes from S. aureus-infected tissues were purified by FACS, whereupon total RNA was immediately isolated using the TaqMan gene expression Cells-to-CT kit (Ambion, Austin, TX, USA). qRT-PCR was performed using TaqMan primer/probe mixes (Applied Biosystems, Foster City, CA, USA) for the following genes: iNOS, Arg-1, IL-1β, IL-12p40, TNF-α, and IL-6. Gene expression levels were normalized to GAPDH and are presented as the fold-induction (2−ΔΔCt) for IL-10 KO relative to IL-10 WT Ly6G−Ly6C+ monocytes.
In vitro generation of MDSCs and adoptive transfer experiments
MDSCs were expanded from the bone marrow of IL-10 KO and WT mice as previously described [19]. Briefly, 107 bone marrow cells were plated into 175-mm dishes and incubated for 4 d in medium supplemented with GM-CSF and G-CSF (both at 40 ng/ml) at 37°C. Cells were collected, washed, and stained for flow cytometry as described above. The Ly6G+Ly6C+ population was purified by FACS, washed, and resuspended in PBS at 2.5 × 106 cells/5 μl. IL-10 KO or WT MDSCs (2.5 × 106 cells) were injected subcutaneously into IL-10 KO mice at the site of implant-associated infection at d 7 postinfection. The suppressive activity of in vitro-derived MDSCs was confirmed using polyclonal CD4+ T cell proliferation assays as previously described [19]. Implant-associated tissues were collected at d 14 postinfection for FACS analysis and quantification of bacterial burdens as described above.
Statistics
Significant differences between experimental groups were determined using an unpaired 2-tailed Student's t test using GraphPad Prism version 4 (GraphPad Software, La Jolla, CA, USA). For all analyses, P < 0.05 was considered statistically significant.
RESULTS
MDSCs are the main source of IL-10 during S. aureus orthopedic implant biofilm infection
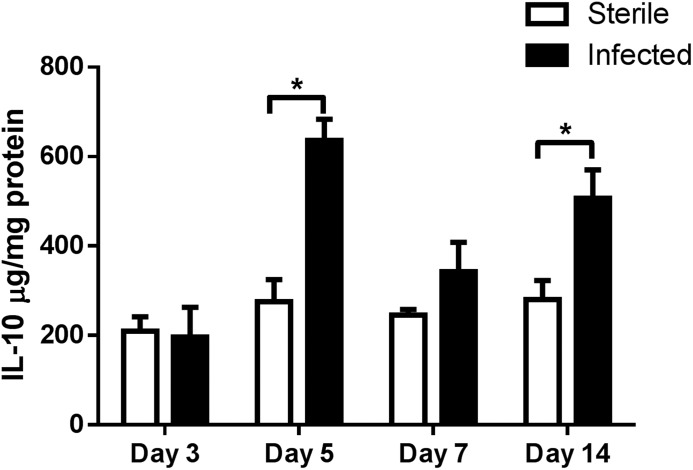
IL-10 has been shown to inhibit T cell activation and polarize Mϕs toward an anti-inflammatory phenotype [23–26]. We recently reported that FACS-purified Ly6GhighLy6C+ MDSCs recovered from S. aureus biofilm infections had increased IL-10 gene expression compared with Ly6G−Ly6C+ monocytes from the same region [11]. However, it remained unclear how much IL-10 was present at the infection site and whether MDSCs or other leukocytes were responsible for its production. In the present study, we used a mouse model of S. aureus orthopedic implant biofilm infection [11, 19, 28] to determine the contribution of MDSC-derived IL-10 in shaping the anti-inflammatory biofilm milieu. We first evaluated the kinetics of IL-10 production associated with sterile and S. aureus-infected implants (Fig. 1). IL-10 levels were similar at d 3, the earliest time point examined; however, by d 5, IL-10 expression was significantly increased in biofilm-infected tissues and remained elevated at d 14 (Fig 1).
Figure 1. IL-10 production is increased during S. aureus orthopedic implant biofilm infection.
Implant-associated tissues from sterile and S. aureus infected mice (n = 4–5/group) were collected at the indicated time points, whereupon IL-10 expression was quantitated by ELISA. Results were normalized to the amount of total protein recovered to correct for variances in tissue sampling and statistical differences are denoted by asterisks (*P < 0.05; unpaired 2-tailed Student’s t test).
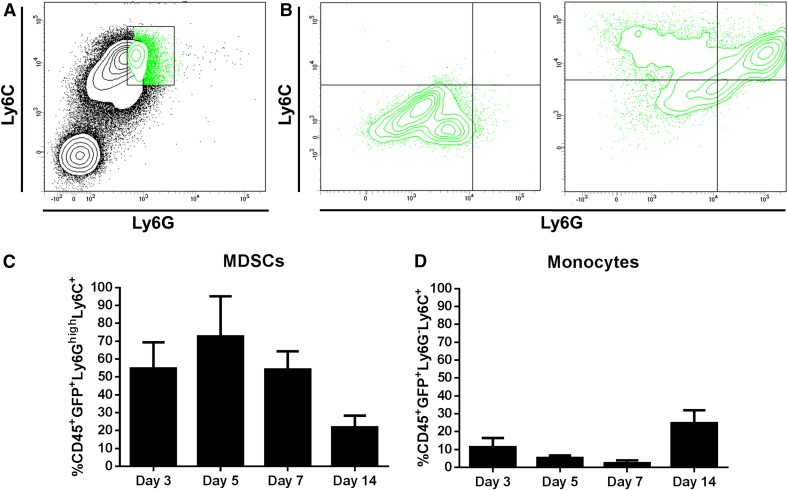
To determine which cell types were producing IL-10 in infected tissues, IL-10-GFP reporter mice were used because our previous study only compared IL-10 mRNA expression in sorted MDSCs and monocytes, and the FACS panels here were designed to also monitor neutrophils, Mϕs, and T cells in addition to MDSCs and monocytes. FACS analysis revealed that Ly6GhighLy6C+ MDSCs were the main source of IL-10 because nearly 70% were GFP+ at d 5 postinfection (Fig. 2A–C). The frequency of IL-10-GFP+ MDSCs progressively decreased to approximately 55 and 20% by d 7 and 14 postinfection, respectively (Fig. 2C). Previous studies from our laboratory have shown that MDSC infiltrates progressively increase in S. aureus-infected tissues from d 3 to 14 and remain relatively stable as the infection persists [19]. Interestingly, GFP levels in the Ly6G−Ly6C+ monocyte population were low throughout the first week of S. aureus infection (Fig. 2D); however, as the percentage of IL-10-GFP+ MDSCs decreased, there was a significant increase in IL-10-GFP+ monocytes at d 14 postinfection (Fig. 2D). These results establish MDSCs as the main source of IL-10 during early S. aureus orthopedic implant biofilm infection.
Figure 2. Ly6GhighLy6C+ MDSCs are the main source of IL-10 during S. aureus orthopedic implant biofilm infection.
Implant-associated tissues were collected from IL-10-GFP reporter mice at the indicated intervals following infection and processed for flow cytometry. The CD45+GFP+ leukocyte population from S. aureus biofilm infected tissues (A) was gated to identify IL-10-GFP expressing Ly6C+ and Ly6G+ cells (B). The percentage of CD45+GFP+Ly6GhighLy6C+ MDSCs (C) and CD45+GFP+ Ly6G−Ly6C+ monocytes (D) is shown. Results are representative of 2 independent experiments (n = 10 mice/time point). Statistical differences are denoted by asterisks (*P < 0.05, **P < 0.01; unpaired 2-tailed Student’s t test).
IL-10 is critical for organizing the anti-inflammatory biofilm milieu and maintaining S. aureus orthopedic implant infection
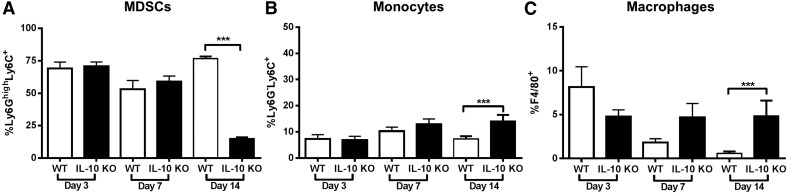
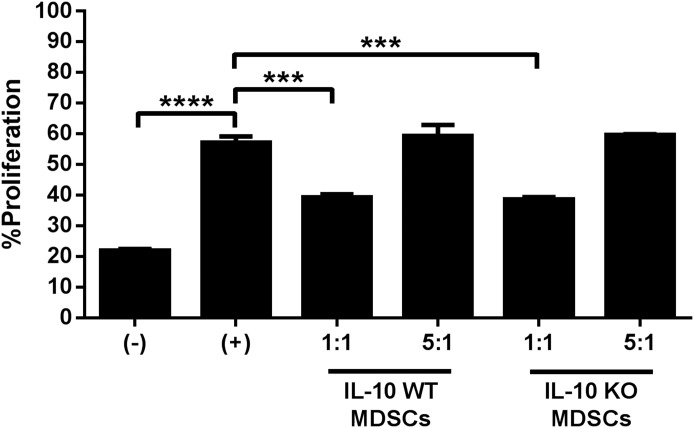
Because MDSCs are the primary cell type responsible for IL-10 production, we next determined the importance of this cytokine to the immunosuppressive function of MDSCs and its role in regulating the anti-inflammatory milieu that allows for biofilm persistence. Examination of leukocyte recruitment in S. aureus–infected IL-10 KO animals revealed a significant decrease in Ly6GhighLy6C+MDSCs at d 14 postinfection compared with WT mice (Fig. 3A) concomitant with increased Ly6G−Ly6C+ monocytes (Fig. 3B) and F4/80+ Mϕs (Fig. 3C). No differences in neutrophil or T cell infiltrates were observed in the absence of IL-10, and both leukocyte populations represented a minor fraction of the CD45+ infiltrate (i.e., <5%; data not shown). Biofilm-associated MDSCs from IL-10 KO mice maintained their ability to inhibit CD4+ T cell proliferation (Fig 4.), demonstrating that MDSC-mediated T cell suppression during S. aureus orthopedic infection is IL-10-independent.
Figure 3. IL-10 loss augments monocyte and macrophage recruitment during S. aureus orthopedic implant biofilm infection.
Implant-associated tissues from IL-10 KO and WT mice (n = 10/group) were collected at the indicated time points after infection and analyzed by flow cytometry. Quantitation of Ly6GhighLy6C+ MDSCs (A), Ly6G−Ly6C+ monocytes (B), and F4/80+ macrophages (C) from the total CD45+ leukocyte infiltrate. Results are presented from 2 independent experiments where significant differences between IL-10 KO and WT animals are denoted with asterisks (***P < 0.001; unpaired 2-tailed Student’s t test).
Figure 4. Staphylococcus aureus biofilm-associated MDSCs inhibit T cell activation in an IL-10-independent manner.
MDSCs were purified by FACS from infected IL-10 KO and WT mice at d 14 postinfection for T cell proliferation assays at a 1:1 or 5:1 ratio (T cell:MDSC). Results are expressed as the percentage of proliferation with T cells alone (−) and CD3/CD28-stimulated T cells (+) as controls. Results represent 2 independent experiments with significant differences denoted by asterisks (***P < 0.001, ****P < 0.0001; unpaired Student’s t test).
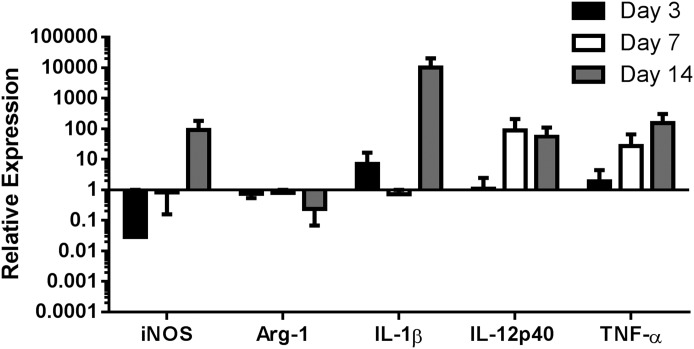
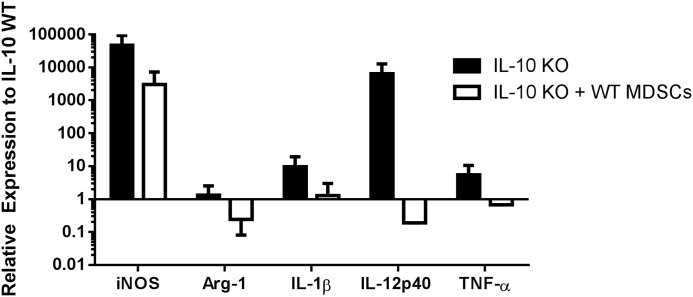
Our recent report demonstrated that MDSC depletion with the Ly6G Ab 1A8 led to significant increases in Ly6C+ monocyte infiltrates with heightened proinflammatory activity (11). By extension, we predicted that the reduced MDSC population in IL-10 KO mice at d 14 postinfection would also promote monocyte proinflammatory attributes. This possibility was assessed by monitoring gene expression profiles of FACS-purified IL-10 KO and WT monocytes immediately ex vivo by qRT-PCR. Ly6G−Ly6C+ cells recovered from IL-10 KO tissues at d 14 postinfection displayed increased expression of iNOS, IL-1β, IL-12p40, and TNF-α and decreased Arg-1 compared with Ly6G−Ly6C+ monocytes from WT tissues (Fig. 5). Because MDSCs are a main source of IL-10 at d 14 postinfection when monocyte proinflammatory attributes were heightened, this finding suggests that both the presence of MDSCs as well as their expression of IL-10 polarizes infiltrating monocytes toward an anti-inflammatory phenotype during S. aureus biofilm formation.
Figure 5. IL-10 loss augments proinflammatory gene expression in Ly6C+ monocytes during S. aureus biofilm infection.
Ly6G−Ly6C+ monocytes were purified from tissues surrounding the infected joints of IL-10 KO and WT mice (n = 10/group) at d 3, 7, and 14 postinfection by FACS, whereupon RNA was immediately isolated for qRT-PCR analysis. Gene expression levels in IL-10 KO monocytes were calculated after normalizing signals against GAPDH and are presented as the fold-change relative to WT monocytes.
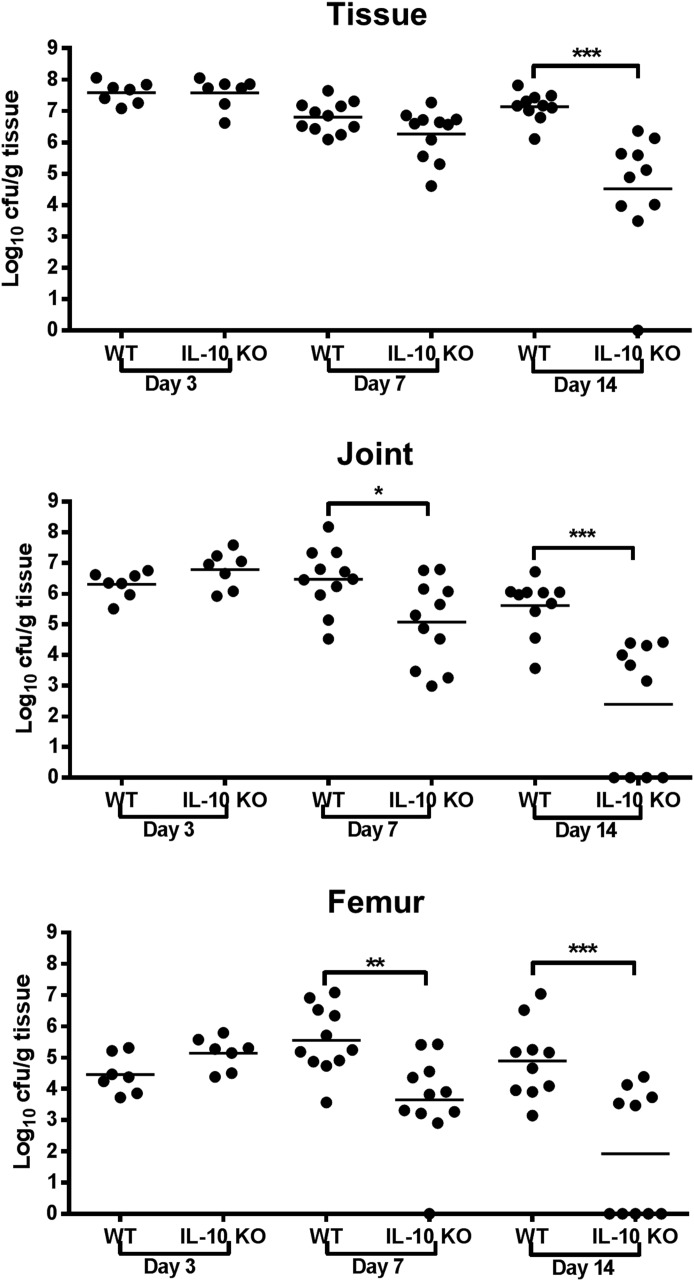
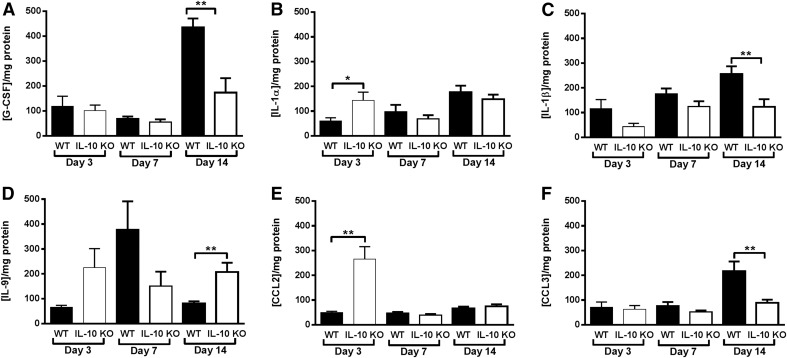
The ability of IL-10 to promote biofilm persistence was confirmed by significant reductions in S. aureus burdens in the tissue, knee joint, and femur of IL-10 KO animals by d 7 to 14 postinfection, depending on the site (Fig. 6). In addition, IL-10 KO mice displayed altered cytokine/chemokine expression patterns during S. aureus biofilm infection. For example, G-CSF levels in IL-10 KO animals were significantly decreased at d 14 (Fig. 7A). G-CSF has been implicated in initiating granulocytic MDSC accumulation [29, 30], and the decrease in G-CSF in IL-10 KO tissues at d 14 was concomitant with reduced MDSC infiltrates (Fig. 3A), indicating that G-CSF may have a critical role in the expansion and accumulation of this population. In addition, IL-1β and CCL3 levels were significantly decreased at d 14 postinfection (Fig. 7C and F), whereas IL-1α and CCL2 were significantly increased in IL-10 KO tissues at d 3 (Fig. 7B and E). Interestingly, IL-9 levels were significantly elevated in IL-10 KO mice at d 14 postinfection (Fig. 7D) even though there is a paucity of T cell infiltrates in this model as determined by CD3+CD4+ or CD3+CD8+ staining (data not shown) [11, 19, 27].
Figure 6. IL-10 is critical for S. aureus persistence during orthopedic implant biofilm infection.
Bacterial burdens associated with the implant-associated tissue, knee joint, and femur of IL-10 KO and WT mice (n = 10/group) were determined at d 3, 7, and 14 postinfection. Results are expressed as CFUs per gram of tissue to correct for alterations in tissue sampling size. Significant differences in bacterial burdens between IL-10 KO and WT mice are denoted by asterisks (*P < 0.05, **P < 0.01, ***P < 0.001; unpaired 2-tailed Student’s t test) and are from 2 independent experiments.
Figure 7. IL-10 KO mice have altered cytokine and chemokine expression patterns.
Tissues surrounding the knee joint of IL-10 KO or WT mice (n = 10/group) with S. aureus-infected implants were collected at the indicated time points, whereupon G-CSF (A), IL-1α (B), IL-1β (C), IL-9 (D), CCL2 (E), and CCL3 (F) production was measured by multianalyte bead arrays. Results are normalized to the amount of total protein to correct for differences in tissue sampling size and are representative of 2 independent experiments. Significant differences are denoted by asterisks (*P < 0.05, **P < 0.01; unpaired 2-tailed Student’s t test).
MDSCs influence bacterial burdens and monocyte infiltrates during S. aureus orthopedic implant biofilm infection via both IL-10-dependent and IL-10-independent mechanisms
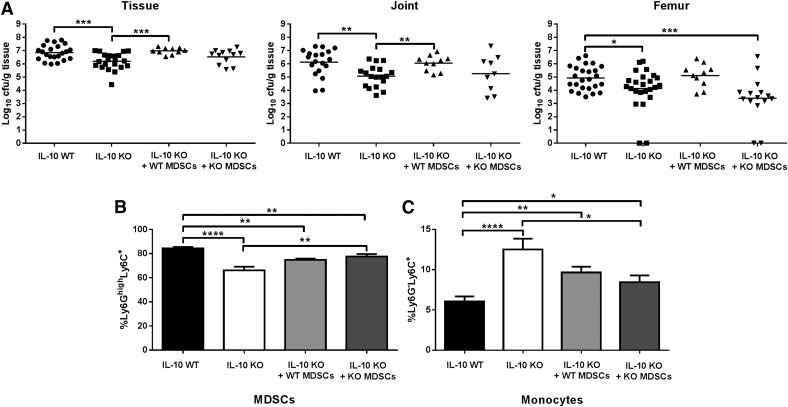
To directly assess the contribution of MDSC-derived IL-10 in inhibiting innate immune cell function and promoting S. aureus persistence during orthopedic implant biofilm infection, bone marrow-derived MDSCs from either WT or IL-10 KO mice were adoptively transferred into IL-10 KO animals 7 d after infection, whereupon bacterial burdens were assessed at d 14. This timing strategy was selected since most differences in IL-10 KO mice were observed at d 14. In vitro-derived MDSCs have been previously used in our laboratory and were confirmed to inhibit T cell proliferation [19]. The adoptive transfer of WT MDSCs into WT mice failed to significantly alter bacterial burdens or immune cell infiltrates at the site of S. aureus biofilm infection (data not shown), which eliminated this approach as a control. This is likely because MDSCs already represent approximately 50% of the total CD45+ population at d 7 [19], and the adoptive transfer of additional MDSCs does not exacerbate S. aureus infection. Instead, the reduced numbers of MDSCs in IL-10 KO mice at d 14 postinfection allowed us to detect the effect of adoptively transferred MDSCs in these animals. Therefore, WT MDSCs represented the control cell population, whereas IL-10 KO MDSCs were the experimental group, with both populations being transferred to IL-10 KO mice to monitor effects on bacterial burdens and leukocyte infiltrates. Indeed, the utility of WT MDSCs as a control was demonstrated by the finding that bacterial burdens were restored to levels typically observed in WT mice following the adoptive transfer of WT MDSCs into IL-10 KO animals (Fig. 8A), which coincided with an increase in Ly6GhighLy6C+ MDSCs (Fig. 8B) and reduced Ly6G−Ly6C+ monocytes compared with IL-10 KO mice that did not receive MDSCs (Fig. 8C).
Figure 8. MDSCs influence bacterial burdens and monocyte infiltrates during S. aureus orthopedic implant biofilm infection via both IL-10-dependent and IL-10-independent mechanisms.
S. aureus orthopedic implant infection was established in IL-10 KO and WT mice, whereupon IL-10 KO animals received an adoptive transfer of 2.5 × 106 purified IL-10 WT or IL-10 KO MDSCs subcutaneously at the implant site on d 7 postinfection, whereas separate groups of WT and IL-10 KO animals received subcutaneous injections of PBS (n = 10/group). (A) Implant-associated tissue, knee joint, and femur were collected on d 7 following MDSC transfer (d 14 after infection) for quantitation of bacterial burdens. Quantitation of Ly6GhighLy6C+ MDSCs (B) and Ly6G−Ly6C+ monocytes (C) on d 7 following MDSC adoptive transfer (d 14 postinfection). Results were calculated after gating on the CD45+ population and represent 2 independent experiments. Significant differences are denoted by asterisks (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; unpaired 2-tailed Student’s t test).
To determine whether IL-10 produced by MDSCs is solely responsible for promoting biofilm growth and inhibiting monocyte recruitment, we also performed adoptive transfers of IL-10 KO MDSCs into S. aureus-infected tissues of IL-10 KO mice at d 7 postinfection. The results demonstrated the complex involvement of both IL-10-dependent and IL-10-independent mechanisms. Regarding the former, MDSC-derived IL-10 was required for promoting bacterial biofilm growth in the tissue, joint, and femur because the transfer of IL-10 KO MDSCs had no effect on these measures compared with IL-10 KO animals that did not receive MDSCs (Fig. 8A). However, the effect of MDSCs on monocyte recruitment was found to be IL-10-independent because the adoptive transfer of both WT and IL-10 KO MDSCs into IL-10 KO mice resulted in similar changes in monocyte infiltrates (Fig. 8C).
Monocytes recovered from infected tissues of IL-10 KO animals have increased proinflammatory gene expression (Fig. 5), which is likely not only due to the absence of IL-10 but also to decreased MDSCs. To further validate these findings, gene expression profiles of FACS-purified Ly6G−Ly6C+ monocytes following adoptive transfer were performed. The adoptive transfer of WT MDSCs into IL-10 KO mice decreased monocyte proinflammatory gene expression, with reductions in iNOS, IL-1β, IL-12p40, and TNF-α, demonstrating a direct effect of MDSCs on monocyte activation state (Fig. 9).
Figure 9. Monocyte proinflammatory gene expression is reduced in IL-10 KO mice following adoptive transfer of WT MDSCs.
Ly6G−Ly6C+ monocytes were purified from infected tissues of WT and IL-10 KO mice ± WT MDSC adoptive transfer on d 14 postinfection by FACS (n = 10/group), whereupon RNA was immediately isolated for qRT-PCR analysis. Gene expression levels in monocytes from IL-10 KO animals or IL-10 KO mice receiving WT MDSCs were calculated after normalizing signals against GAPDH and are presented as the fold-change relative to monocytes recovered from IL-10 WT animals.
DISCUSSION
MDSCs are emerging as critical in the anti-inflammatory response to S. aureus and to promote chronic infection [11, 19, 31, 32]. Biofilm infections are known to skew the host innate immune response toward an anti-inflammatory phenotype [27, 33, 34]. In this setting, IL-10 could facilitate the establishment of persistent infection and allow organisms to subvert traditional mechanisms of bacterial clearance. Although IL-10 production has been implicated as an immunosuppressive mechanism by MDSCs [26, 35], to date there are no reports, to our knowledge, examining whether MDSC function is dependent on IL-10 during biofilm infection. This study demonstrates that MDSCs express significant amounts of IL-10 in response to biofilm-associated bacteria, which limits monocyte proinflammatory gene expression and directly contributes to S. aureus biofilm persistence during later stages of infection. Of note, the consequences of IL-10 action are likely context dependent because several studies have reported a beneficial role for IL-10 during S. aureus sepsis by controlling damaging inflammation and minimizing pathology [21, 36–40]. In contrast, our study suggests a deleterious role for IL-10 in preventing the genesis of an effective microbicidal response to facilitate biofilm clearance.
In terms of kinetics, IL-10 levels in S. aureus biofilm tissues were not significantly increased compared with animals receiving sterile orthopedic implants until d 5. This delay in IL-10 elevation implies that the biofilm is directing cytokine production, and our results indicate that MDSCs are responsible for IL-10 synthesis. Here, we demonstrate that nearly 70% of the MDSCs recruited to the site of S. aureus biofilm infection at d 5 expressed IL-10, whereas monocytes represented a minor fraction in comparison. MDSC-derived IL-10 began to decline by d 7 postinfection, which coincided with the decrease in total IL-10 measured in infected tissues by ELISA. However, tissue IL-10 levels exhibited a biphasic increase at d 14, which may reflect cytokine production by both MDSCs and monocytes, the latter which exhibited a late rise in IL-10 expression. It is currently unclear what causes MDSC-derived IL-10 to decrease after d 5 postinfection whereas the percentage of MDSCs remains constant (data not shown). Although we currently have no evidence of Tregs at the site of S. aureus biofilm infection as determined by CD4 and FoxP3 staining (data not shown), IL-10 production by MDSCs has been shown to induce Tregs that can produce IL-10 [35], which could perpetuate the anti-inflammatory circuit. It remains possible that the number of Tregs associated with S. aureus biofilms in our orthopedic model remains below the limit of detection by FACS. Alternatively, synovial cells could contribute to sustained IL-10 levels during S. aureus biofilm infection when IL-10-producing MDSCs have begun to decline (i.e., d 14 postinfection). It has been shown that cultured fibroblast-like synoviocytes constitutively express IL-10 along with functional IL-10 receptors and could modulate cellular responses in the joint [41]. However, these and other tissue-resident populations would need to be analyzed during S. aureus biofilm infection to determine whether they contribute to IL-10 production and potentially assist in bacterial persistence.
A direct role for MDSC-derived IL-10 in setting the stage for S. aureus biofilm persistence at later time points was supported by our observations in IL-10 KO mice. In general, in the absence of IL-10, infiltrating monocytes acquired a proinflammatory gene expression profile that translated into improved biofilm clearance at d 7 and 14 postinfection. These changes coincided with significant decreases in MDSC infiltrates and the adoptive transfer of MDSCs from WT, but not IL-10 KO, mice were capable of reversing these changes. In terms of mechanism, S. aureus could be co-opting MDSCs to promote their immunosuppressive activity during acute infection, which occurs via an IL-10-independent manner. When IL-10 expression peaks in MDSCs (i.e., d 5), we begin to see a reduction in biofilm burdens in IL-10 KO mice at d 7 and an inability to effectively skew monocytes toward an anti-inflammatory phenotype, which, altogether, demonstrate that MDSC-derived IL-10 is critical for the chronicity of S. aureus biofilm infection.
Currently, the signals responsible for eliciting IL-10 production by MDSCs during biofilm infection have yet to be identified. It is known that IL-10 can be induced by TLR stimulation [42–44], that MDSCs express TLRs [17, 45], and that TLR ligands can induce MDSC accumulation in tumor-bearing and septic mice [46–48]. However, this possibility appears less plausible in the context of biofilm infection because we and others have reported that S. aureus biofilms circumvent recognition by TLR2 [9, 27, 28], although MDSCs were not examined in these studies. Regardless of the inciting signal that triggers IL-10 production, the cytokine can then signal through the IL-10R to activate STAT3, a critical factor for driving MDSC development as well as polarizing monocytes/Mϕs toward an anti-inflammatory phenotype [13, 49–51]. In addition, STAT3 activation can augment IL-10 production [52]. The role of IL-10 in inducing STAT3 activation during S. aureus biofilm infection and its subsequent contribution to the anti-inflammatory biofilm milieu are current topics of investigation in our laboratory.
G-CSF preferentially signals through STAT3, where it induces MDSC expansion and accelerates the proliferation and release of granulocytic precursors in tumor-bearing mice, in addition to its well-known ability to direct neutrophilic granulocyte differentiation [29, 53]. We have previously reported that G-CSF is significantly increased in S. aureus-infected tissues during the time when MDSCs represent the main cellular infiltrate in implant-associated tissue [19]. Interestingly, in the current study, G-CSF levels were reduced in IL-10 KO mice at d 14 postinfection, concomitant with reduced MDSC infiltrates. By extension, it is possible that G-CSF contributes to MDSC expansion and accumulation during S. aureus biofilm infection and that IL-10 loss may limit STAT3 activation and subsequent G-CSF production. However, this possibility remains speculative.
Both IL-10 and STAT3 are known to inhibit IL-12 production, and MDSCs are traditionally thought to secrete IL-10 to down-regulate IL-12 release from monocytes/Mϕs. This relationship appears to be operative during S. aureus biofilm infection; however, unlike planktonic infections, MDSCs persist and maintain the biofilm milieu in an anti-inflammatory state. For example, our recent report showed that IL-12 was increased in S. aureus-infected tissues over a 1 mo period and IL-12p40 and p35 KO animals had significantly fewer MDSC infiltrates concomitant with reduced bacterial burdens as early as d 7 postinfection [19]. In the current study, because IL-10 was not elevated in infected tissues until d 5 and MDSC infiltrates were not significantly reduced in IL-10 KO animals until d 14, IL-10 most likely operates downstream of MDSC recruitment and IL-12 action (Fig. 10). Indeed, IL-12p40 gene expression was increased in monocytes from IL-10 KO mice, beginning on d 7 postinfection, revealing a negative effect of MDSC-derived IL-10 on monocyte IL-12 expression. This was also directly demonstrated by the ability of adoptively transferred WT MDSCs to inhibit IL-12 in monocytes from IL-10 KO animals. Despite these cell type–specific changes, IL-12p40 levels in tissue homogenates of both IL-10 KO and WT animals were similar on d 3, 7, and 14 postinfection (data not shown) and because MDSC recruitment was unaffected during the first week of S. aureus orthopedic biofilm infection, this suggests the delayed action of an alternative chemoattractant that is IL-12-independent. Taken together, it appears that IL-12 produced during early S. aureus biofilm infection is key for MDSC recruitment, which is likely an indirect effect mediated by chemoattractants that remain to be defined, whereupon MDSCs produce IL-10 that dampens the proinflammatory immune response of monocytes/macrophages and contributes to biofilm persistence (Fig. 10). These studies assessing the role of IL-10 in regulating innate immune responses have focused on monocytes, as they represent the most numerous population after MDSCs in our S. aureus orthopedic biofilm infection model. It remains possible that MDSCs could be influencing neutrophil responses; however, neutrophils represent a very minor infiltrate and no significant differences were observed between IL-10 KO and WT animals. Therefore, we did not explore the possible effects of MDSCs and IL-10 on neutrophil function.
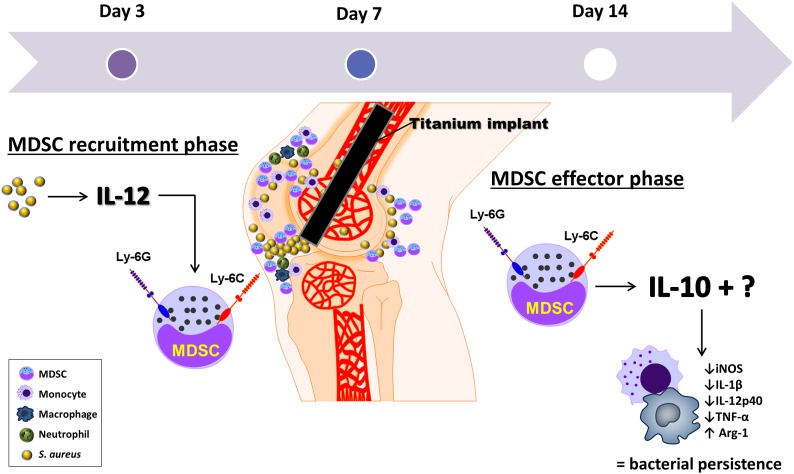
Figure 10. Temporal relationship between IL-10 and IL-12 actions during S. aureus orthopedic implant biofilm infection.
IL-12 has a key role in MDSC recruitment during early biofilm infection via a chemoattractant that remains to be identified. IL-10 is produced by infiltrating MDSCs at the site of S. aureus biofilm infection, whereupon it has a critical role in polarizing monocytes toward an anti-inflammatory phenotype, thereby promoting bacterial persistence. Loss of either IL-12 or IL-10 during the early MDSC recruitment or effector phases, respectively, promotes biofilm clearance, implicating key roles for each cytokine at distinct stages of infection.
Recently, we and others have shown that the adoptive transfer of MDSCs significantly exacerbates S. aureus infection [19, 31]; however, the mediators released by MDSCs that are responsible for this effect remain to be defined. Here, we show that IL-10 is one factor because the adoptive transfer of MDSCs from IL-10 KO mice did not exacerbate biofilm growth, whereas WT MDSCs significantly increased biofilm burdens in the joint, surrounding soft tissue, and femur. However, it is apparent that IL-10 is not the only immunosuppressive mechanism of MDSCs during S. aureus biofilm infection because IL-10 KO MDSCs still affected monocyte recruitment during biofilm infection, reflecting an IL-10-independent mechanism of action. Originally, IL-10 was defined by its ability to inhibit Th1 activation and cytokine production; however, it is now recognized that the biologic effects of IL-10 are also directed at monocytes/Mϕs [54]. Indeed, we found that MDSC inhibition of CD4+ T cell proliferation was IL-10-independent, in agreement with another recent report [31], again indicating the existence of additional inhibitory effector mechanisms for MDSCs. A potential candidate is Arg-1, which we have previously shown is elevated in MDSCs recovered from the site of S. aureus orthopedic biofilm infection in both our mouse model and in tissue specimens from humans with prosthetic joint infections [11, 19, 27]. A role for Arg-1 in the anti-inflammatory response to S. aureus biofilms is a topic of ongoing investigation in our laboratory. Similarly, in addition to the percentages of MDSC infiltrates, their activation status is also influenced by biofilm infection. Indeed, we previously reported that only MDSCs recovered from S. aureus biofilm-infected animals, but not those receiving sterile implants, were capable of attenuating T cell proliferation, reflecting the inhibitory nature of MDSCs specifically recruited to the biofilm infection site. Likewise, only MDSCs recruited to the biofilm site and not the spleen of S. aureus-infected mice displayed suppressive activity [11]. Therefore, MDSC levels are only one aspect of the equation, with their inhibitory capacity and gene expression profiles representing other important attributes; both of which were studied in the current report.
Inflammatory mediator analysis in infected IL-10 KO mice revealed some interesting disparities, particularly in the timing of when differences became apparent. For example, both IL-1α and CCL2 were significantly increased in IL-10 KO animals at d 3 postinfection but not at later time points. In contrast, more differences were evident at d 14, which coincided with the significant decrease in biofilm burdens in IL-10 KO mice, namely, reductions in G-CSF, IL-1β, and CCL3. However, not all mediators were reduced because IL-9 production was significantly elevated in IL-10 KO animals at this later interval. The detection of IL-9 is intriguing because this cytokine is mainly produced by select T cell subsets; however, minimal T cell infiltrates (CD3+CD4+ or CD3+CD8+) were observed in IL-10 KO or WT mice in either this or our prior reports, making it difficult to predict the source of IL-9 production. Another interesting finding is that IL-9 has been reported to promote Treg expansion, yet we have not been able to detect CD4+FoxP3+ cells in any of our S. aureus biofilm-infection models (data not shown) [11, 19, 27]. However, IL-9 is known to stimulate mast cell expansion from the bone marrow [55–57], and mast cells have been shown to release several cytokines and nitric oxide into the knee joint during osteoarthritis [58] and to drive tissue metaplasia and heterotrophic ossification in patients following total knee arthroplasty [59]. It is possible that the absence of IL-10 at later time points allows the proinflammatory activities of IL-9 to be heightened, although the presence of mast cells and their role during S. aureus orthopedic biofilm infection have not yet been examined.
The role of MDSCs during S. aureus biofilm infection and the mechanisms involved in their expansion, accumulation, and effector functions are only beginning to be explored. By manipulating the ability of these cells to exert their immunosuppressive pressure, we have demonstrated that they directly attenuate monocyte proinflammatory properties. However, there are still many areas of MDSC-biofilm interaction that remain to be examined. For instance, we know that IL-12 is involved in MDSC recruitment to biofilm infections, but it is unclear whether biofilm-derived products directly contribute to MDSC accumulation by interfering with myeloid precursor differentiation. In addition, whether MDSCs can recognize S. aureus pathogen-associated molecular patterns through TLRs or other PRRs to activate genes essential for their effector functions could contribute to our understanding of their role during infection. Our findings to date do not exclude the possibility that S. aureus biofilms cooperate with MDSCs to inhibit monocyte/Mϕ effector functions directly, and studies are ongoing in our laboratory to address these interactions. However, preventing the immunosuppressive action of infiltrating MDSCs may offer a novel therapeutic strategy to treat chronic biofilm infections.
AUTHORSHIP
C.E.H. contributed to the experimental design and data analysis, performed all of the experiments, and wrote the manuscript. D.V. assisted in experimental procedures. T.K. designed the overall project, analyzed data, and edited the manuscript.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health National Institute of Allergy and Infectious Disease (Grant 2P01AI083211; project 4 to T.K.) and by an American Heart Association Predoctoral Fellowship (Grant 13PRE16910040 to C.E.H.). The authors thank Dr. Philip Hexley, Victoria Smith, and Samantha Wall in the University of Nebraska Medical Center Flow Cytometry Research Facility for assistance with FACS analysis and cell sorting. We also thank Tyler Scherr for assistance with mouse procedures and Rachel Fallet for maintenance of the mice used in these studies.
Glossary
- KO
knockout
- K-wire
Kirschner wire
- MDSC
myeloid-derived suppressor cell
- qRT-PCR
quantitative real-time polymerase chain reaction
- S. aureus
Staphylococcus aureus
- STAT3
signal transducer and activator of transcription 3
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
- WT
wild-type
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Boucher H., Miller L. G., Razonable R. R. (2010) Serious infections caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 51(Suppl 2), S183–S197. [DOI] [PubMed] [Google Scholar]
- 2.Grundmann H., Hellriegel B. (2006) Mathematical modelling: a tool for hospital infection control. Lancet Infect. Dis. 6, 39–45. [DOI] [PubMed] [Google Scholar]
- 3.Shorr A. F. (2007) Epidemiology and economic impact of meticillin-resistant Staphylococcus aureus: review and analysis of the literature. Pharmacoeconomics 25, 751–768. [DOI] [PubMed] [Google Scholar]
- 4.Parry M. C., Duncan C. P. (2014) The challenge of methicillin resistant staphylococcal infection after total hip replacement: overlooked or overstated? Bone Joint J. 96-B(11, Suppl A)60–65. [DOI] [PubMed] [Google Scholar]
- 5.Otto M. (2008) Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 322, 207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzsimmons K., Bamber A. I., Smalley H. B. (2010) Infective endocarditis: changing aetiology of disease. Br. J. Biomed. Sci. 67, 35–41. [DOI] [PubMed] [Google Scholar]
- 7.Del Pozo J. L., Patel R. (2009) Clinical practice. Infection associated with prosthetic joints. N. Engl. J. Med. 361, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmerli W., Trampuz A., Ochsner P. E. (2004) Prosthetic-joint infections. N. Engl. J. Med. 351, 1645–1654. [DOI] [PubMed] [Google Scholar]
- 9.Cho J. S., Guo Y., Ramos R. I., Hebroni F., Plaisier S. B., Xuan C., Granick J. L., Matsushima H., Takashima A., Iwakura Y., Cheung A. L., Cheng G., Lee D. J., Simon S. I., Miller L. S. (2012) Neutrophil-derived IL-1β is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 8, e1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feuerstein R., Seidl M., Prinz M., Henneke P. (2015) MyD88 in macrophages is critical for abscess resolution in staphylococcal skin infection. J. Immunol. 194, 2735–2745. [DOI] [PubMed] [Google Scholar]
- 11.Heim C. E., Vidlak D., Scherr T. D., Kozel J. A., Holzapfel M., Muirhead D. E., Kielian T. (2014) Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J. Immunol. 192, 3778–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai J., El Gazzar M., Li G. Y., Moorman J. P., Yao Z. Q. (2015) Myeloid-derived suppressor cells: paradoxical roles in infection and immunity. J. Innate Immun. 7, 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrilovich D. I., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sica A., Bronte V. (2007) Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 117, 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youn J. I., Collazo M., Shalova I. N., Biswas S. K., Gabrilovich D. I. (2012) Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J. Leukoc. Biol. 91, 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brudecki L., Ferguson D. A., McCall C. E., El Gazzar M. (2012) Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infect. Immun. 80, 2026–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostrand-Rosenberg S., Sinha P. (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 182, 4499–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Condamine T., Gabrilovich D. I. (2011) Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heim C. E., Vidlak D., Scherr T. D., Hartman C. W., Garvin K. L., Kielian T. (2015) IL-12 Promotes Myeloid-Derived Suppressor Cell Recruitment and Bacterial Persistence during Staphylococcus aureus orthopedic implant infection. J. Immunol. 194, 3861–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray P. J. (2005) The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc. Natl. Acad. Sci. U. S. A. 102, 8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couper K. N., Blount D. G., Riley E. M. (2008) IL-10: the master regulator of immunity to infection. J. Immunol. 180, 5771–5777. [DOI] [PubMed] [Google Scholar]
- 22.Bunt S. K., Clements V. K., Hanson E. M., Sinha P., Ostrand-Rosenberg S. (2009) Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J. Leukoc. Biol. 85, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrin G. Q., Johnson H. M., Subramaniam P. S. (1999) Mechanism of interleukin-10 inhibition of T-helper cell activation by superantigen at the level of the cell cycle. Blood 93, 208–216. [PubMed] [Google Scholar]
- 24.Taga K., Mostowski H., Tosato G. (1993) Human interleukin-10 can directly inhibit T-cell growth. Blood 81, 2964–2971. [PubMed] [Google Scholar]
- 25.Letterio J. J., Roberts A. B. (1998) Regulation of immune responses by TGF-β. Annu. Rev. Immunol. 16, 137–161. [DOI] [PubMed] [Google Scholar]
- 26.Sinha P., Clements V. K., Bunt S. K., Albelda S. M., Ostrand-Rosenberg S. (2007) Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 179, 977–983. [DOI] [PubMed] [Google Scholar]
- 27.Thurlow L. R., Hanke M. L., Fritz T., Angle A., Aldrich A., Williams S. H., Engebretsen I. L., Bayles K. W., Horswill A. R., Kielian T. (2011) Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 186, 6585–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernthal N. M., Stavrakis A. I., Billi F., Cho J. S., Kremen T. J., Simon S. I., Cheung A. L., Finerman G. A., Lieberman J. R., Adams J. S., Miller L. S. (2010) A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One 5, e12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waight J. D., Hu Q., Miller A., Liu S., Abrams S. I. (2011) Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One 6, e27690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawanobori Y., Ueha S., Kurachi M., Shimaoka T., Talmadge J. E., Abe J., Shono Y., Kitabatake M., Kakimi K., Mukaida N., Matsushima K. (2008) Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111, 5457–5466. [DOI] [PubMed] [Google Scholar]
- 31.Tebartz C., Horst S. A., Sparwasser T., Huehn J., Beineke A., Peters G., Medina E. (2015) A major role for myeloid-derived suppressor cells and a minor role for regulatory T cells in immunosuppression during Staphylococcus aureus infection. J. Immunol. 194, 1100–1111. [DOI] [PubMed] [Google Scholar]
- 32.Skabytska Y., Wölbing F., Günther C., Köberle M., Kaesler S., Chen K. M., Guenova E., Demircioglu D., Kempf W. E., Volz T., Rammensee H. G., Schaller M., Röcken M., Götz F., Biedermann T. (2014) Cutaneous innate immune sensing of Toll-like receptor 2-6 ligands suppresses T cell immunity by inducing myeloid-derived suppressor cells. Immunity 41, 762–775. [DOI] [PubMed] [Google Scholar]
- 33.Hanke M. L., Heim C. E., Angle A., Sanderson S. D., Kielian T. (2013) Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. J. Immunol. 190, 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prabhakara R., Harro J. M., Leid J. G., Keegan A. D., Prior M. L., Shirtliff M. E. (2011) Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect. Immun. 79, 5010–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimura T., Kambayashi Y., Aiba S. (2012) Crosstalk between regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) during melanoma growth. Oncoimmunology 1, 1433–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corsetti P. P., de Almeida L. A., Carvalho N. B., Azevedo V., Silva T. M., Teixeira H. C., Faria A. C., Oliveira S. C. (2013) Lack of endogenous IL-10 enhances production of proinflammatory cytokines and leads to Brucella abortus clearance in mice. PLoS One 8, e74729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J., Roderiquez G., Norcross M. A. (2012) Control of adaptive immune responses by Staphylococcus aureus through IL-10, PD-L1, and TLR2. Sci. Rep. 2, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki H., Hou L., Belani A., Wang C. Y., Uchiyama T., Müller R., Stashenko P. (2000) IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J. Immunol. 165, 3626–3630. [DOI] [PubMed] [Google Scholar]
- 39.García-Alvarez F., Monzón M., Grasa J. M., Laclériga A., Amorena B., García-Alvarez I., Navarro-Zorraquino M., Alvarez F. G. (2006) Interleukin-1, interleukin-6, and interleukin-10 responses after antibiotic treatment in experimental chronic Staphylococcus aureus osteomyelitis. J. Orthop. Sci. 11, 370–374. [DOI] [PubMed] [Google Scholar]
- 40.Gjertsson I., Jonsson I. M., Peschel A., Tarkowski A., Lindholm C. (2012) Formylated peptides are important virulence factors in Staphylococcus aureus arthritis in mice. J. Infect. Dis. 205, 305–311. [DOI] [PubMed] [Google Scholar]
- 41.Ritchlin C., Haas-Smith S. A. (2001) Expression of interleukin 10 mRNA and protein by synovial fibroblastoid cells. J. Rheumatol. 28, 698–705. [PubMed] [Google Scholar]
- 42.Redford P. S., Murray P. J., O’Garra A. (2011) The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 4, 261–270. [DOI] [PubMed] [Google Scholar]
- 43.Frodermann V., Chau T. A., Sayedyahossein S., Toth J. M., Heinrichs D. E., Madrenas J. (2011) A modulatory interleukin-10 response to staphylococcal peptidoglycan prevents Th1/Th17 adaptive immunity to Staphylococcus aureus. J. Infect. Dis. 204, 253–262. [DOI] [PubMed] [Google Scholar]
- 44.Peres A. G. and Madrenas J. (2013) The broad landscape of immune interactions with Staphylococcus aureus: from commensalism to lethal infections. Burns 39, 380–388. [DOI] [PubMed] [Google Scholar]
- 45.Ray A., Chakraborty K., Ray P. (2013) Immunosuppressive MDSCs induced by TLR signaling during infection and role in resolution of inflammation. Front. Cell. Infect. Microbiol. 3, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delano M. J., Scumpia P. O., Weinstein J. S., Coco D., Nagaraj S., Kelly-Scumpia K. M., O’Malley K. A., Wynn J. L., Antonenko S., Al-Quran S. Z., Swan R., Chung C. S., Atkinson M. A., Ramphal R., Gabrilovich D. I., Reeves W. H., Ayala A., Phillips J., Laface D., Heyworth P. G., Clare-Salzler M., Moldawer L. L. (2007) MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 204, 1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagaraj S., Gabrilovich D. I. (2007) Myeloid-derived suppressor cells. Adv. Exp. Med. Biol. 601, 213–223. [DOI] [PubMed] [Google Scholar]
- 48.Maruyama A., Shime H., Takeda Y., Azuma M., Matsumoto M., Seya T. (2015) Pam2 lipopeptides systemically increase myeloid-derived suppressor cells through TLR2 signaling. Biochem. Biophys. Res. Commun. 457, 445–450. [DOI] [PubMed] [Google Scholar]
- 49.Bromberg J. (2002) Stat proteins and oncogenesis. J. Clin. Invest. 109, 1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nefedova Y., Nagaraj S., Rosenbauer A., Muro-Cacho C., Sebti S. M., Gabrilovich D. I. (2005) Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the Janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 65, 9525–9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nefedova Y., Huang M., Kusmartsev S., Bhattacharya R., Cheng P., Salup R., Jove R., Gabrilovich D. (2004) Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 172, 464–474. [DOI] [PubMed] [Google Scholar]
- 52.Yu H., Kortylewski M., Pardoll D. (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 7, 41–51. [DOI] [PubMed] [Google Scholar]
- 53.Chakraborty A., Tweardy D. J. (1998) Stat3 and G-CSF-induced myeloid differentiation. Leuk. Lymphoma 30, 433–442. [DOI] [PubMed] [Google Scholar]
- 54.Hedrich C. M., Bream J. H. (2010) Cell type-specific regulation of IL-10 expression in inflammation and disease. Immunol. Res. 47, 185–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Townsend J. M., Fallon G. P., Matthews J. D., Smith P., Jolin E. H., McKenzie N. A. (2000) IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 13, 573–583. [DOI] [PubMed] [Google Scholar]
- 56.Matsuzawa S., Sakashita K., Kinoshita T., Ito S., Yamashita T., Koike K. (2003) IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J. Immunol. 170, 3461–3467. [DOI] [PubMed] [Google Scholar]
- 57.Goswami R., Kaplan M. H. (2011) A brief history of IL-9. J. Immunol. 186, 3283–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renoux M., Hilliquin P., Galoppin L., Florentin I., Menkes C. J. (1996) Release of mast cell mediators and nitrites into knee joint fluid in osteoarthritis–comparison with articular chondrocalcinosis and rheumatoid arthritis. Osteoarthritis Cartilage 4, 175–179. [DOI] [PubMed] [Google Scholar]
- 59.Freeman T. A., Parvizi J., Dela Valle C. J., Steinbeck M. J. (2010) Mast cells and hypoxia drive tissue metaplasia and heterotopic ossification in idiopathic arthrofibrosis after total knee arthroplasty. Fibrogenesis Tissue Repair 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]