Review of transcription factors that regulate MDSC expansion and accumulation.
Keywords: STAT3, S100A9, STAT6, Rb1
Abstract
Myeloid-derived suppressor cells are a heterogeneous group of pathologically activated immature cells that play a major role in the negative regulation of the immune response in cancer, autoimmunity, many chronic infections, and inflammatory conditions, as well as in the regulation of tumor angiogenesis, tumor cell invasion, and metastases. Accumulation of myeloid-derived suppressor cells is governed by a network of transcriptional regulators that could be combined into 2 partially overlapping groups: factors promoting myelopoiesis and preventing differentiation of mature myeloid cells and factors promoting pathologic activation of myeloid-derived suppressor cells. In this review, we discuss the specific nature of these factors and their impact on myeloid-derived suppressor cell development.
Introduction
Accumulation of nonlymphoid suppressive cells in cancer was first reported in the late 1970s [1, 2]. However, these cells received very little attention until 20 y later with the accumulation of information about their potential contribution to tumor progression. These cells were named MDSCs in 2007 to reflect their origin and major functional trait, i.e., the ability to suppress T cell activation and function [3]. MDSCs were studied initially in the context of cancer. In recent years, it has become clear that these cells also play an important role in the regulation of immune responses in chronic infections, inflammation, autoimmune diseases, and other pathologic conditions [4]. MSDC frequency has also been reported to increase with aging [5, 6]. MDSCs are phenotypically distinct from terminally differentiated DCs and MΦ and represent a heterogeneous population of immature myeloid cells that include cells with granulocytic and monocytic morphology and phenotype. MDSCs are known for their ability to suppress immune responses [7–9] and to promote tumor growth by supporting angiogenesis, tumor cell survival, metastases, and formation of premetastatic niches [10]. Extensive studies in recent years have provided ample evidence of the clinical relevance of MDSCs [11, 12].
MDSCs are now divided into 2 major populations: granulocytic or PMN-MDSC and mononuclear or M-MDSC [13, 14]. In most types of cancer, PMN-MDSCs represent 70–80% of the total MDSC population, whereas M-MDSCs usually represent no more than 20%. In some chronic infections, M-MDSCs are predominant population of MDSCs [8]. In mice, PMN-MDSCs are commonly defined as CD11b+Ly6G+Ly6Clo cells and M-MDSCs as CD11b+Ly6G−Ly6Chi. In humans, MDSCs are purified from the mononuclear fraction after Ficoll gradient cenrifugation. PMN-MDSCs are defined as CD11b+CD33+CD14−CD15+ cells and M-MDSC as CD14+HLA-DR−/lo or as CD11b+CD33+CD15−CD14−. Another commonly used combination of markers includes lineage (CD3, CD14, CD19, CD56)−HLA-DR−CD33+. These cells represent a mixed population of PMN-MDSCs and M-MDSCs, as well as early myeloid progenitors. As there is substantial overlap between the cells defined by these markers, they are often all used to provide for the most accurate evaluation of MDSCs in clinical samples. This issue has been reviewed recently in detail elsewhere [15].
PMN-MDSCs and neutrophils share a similar phenotype and morphology. However, in contrast to neutrophils, PMN-MDSCs suppress T cell functions and have a distinct gene-expression profile. Moreover, PMN-MDSCs have distinct functional characteristics, including lower phagocytic activity and higher activity of arginase-1, MPO and ROS [16]. M-MDSCs resemble normal monocytes in regard to their phenotype and morphology. In contrast to spleen monocytes in naïve mice and blood monocytes in healthy individuals, M-MDSCs have a potent ability to suppress T cell function, which is mediated by arginase-1, NO, and different soluble factors [17].
MDSC DIFFERENTIATION MODEL
MDSCs arise from a CMP. Their development is supported by the same growth factors that are responsible for normal myelopoiesis: GM-CSF [18–21], G-CSF [22–24], and M-CSF [25, 26]. However, simple expansion of myeloid cells is not sufficient to generate bona fide MDSCs, which are pathologically activated—a state of activation that is different from the “normal” activation of PMNs and monocytes. Normal activation is evolutionarily designed to protect the host from bacteria and viruses. It is characterized by active phagocytosis, respiratory burst, and release of proinflammatory cytokines. It is relatively short lived and quickly terminated upon cessation of the stimulus. Normal activation is also characterized by the generation of mature myeloid cells (primarily MΦ) that are able to support the remodeling of tissues after injury or after resolved inflammation. In contrast, pathologic activation is the result of persistent stimulation of the myeloid compartment with relatively low-strength signals coming from tumors or sites of chronic inflammation. Myeloid cells generated under these conditions are unable to differentiate effectively into mature myeloid cells, are poorly phagocytic, and produce high levels of ROS, MPO, NO, and mostly anti-inflammatory cytokines. As a result, these cells are not able to perform normal functions of myeloid cells effectively and acquire potent immune-suppressive potential. It is believed that the evolutionary role of MDSC is in the termination of persistent immune responses and the protection of the host from extensive tissue damage caused by an uncontrolled immune response associated with unresolved inflammation or infection. However, tumors hijack and amplify this activity to protect themselves from elimination by the immune system. In cancer, MDSCs play an important role, not only in facilitating tumor evasion from the immune system but also in limiting the effect of cancer immunotherapeutics.
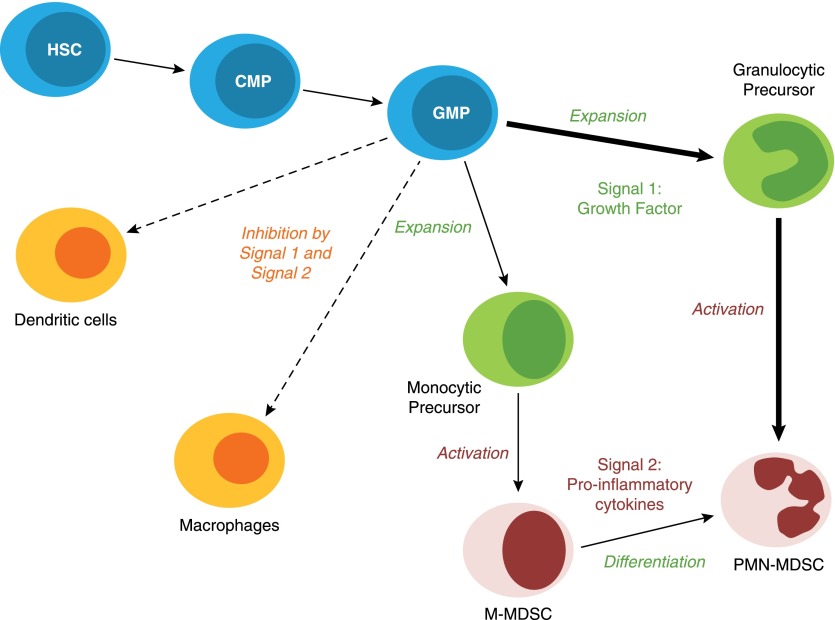
One of the major questions facing the MDSC field is to understand the mechanisms by which normal immature myeloid cells become immune-suppressive MDSCs. Several years ago, we proposed a 2-signal model to describe the differentiation of MDSC [27]. This model includes 2 phases: the expansion of immature myeloid cells associated with inhibition of their terminal differentiation and the activation phase converting immature myeloid cells to MDSC (Fig. 1). We assert that these phases partially overlap but are governed by sets of different transcription factors and intermediates. The 1st phase is mostly driven by tumor-derived growth factors, whereas the 2nd phase is driven by proinflammatory cytokines produced by tumor stroma. Recent studies refined this model by providing molecular mechanisms regulating these phases of MDSC development and by adding additional factors critically important for this process. Most of these studies have been done in tumor models, and the relevance of these findings to other pathologic conditions needs to be confirmed further. These new data may help to develop strategies for targeted disruption of these mechanisms and thus, help to control MDSC accumulation.
Figure 1. MDSC differentiation is regulated by different signals.
MDSCs arise from CMP and GMP. However, the presence of tumor-derived growth factors (Signal 1) drives the expansion of monocytic and granulocytic precursors. These precursors require an activation signal (Signal 2) to acquire a suppressive phenotype to give rise to bona fide PMN-MDSC and M-MDSC. These 2 types of signals also inhibit myeloid cell differentiation into terminally differentiated cells, such as DC and MΦ. HSC, Hematopoietic stem cell.
TRANSCRIPTION FACTORS AND SIGNALING PATHWAYS INVOLVED IN MDSC EXPANSION
STAT3
The STAT family of transcription factors mediates signals from various cytokine receptors and growth factors. Dimerization of the receptors results in trans-phosphorylation of their associated JAK and tyrosine kinase 2. Activated kinases can then directly phosphorylate the cytoplasmic tail of the receptors, generating a docking site for STAT proteins to recruit and phosphorylate them. pSTAT sequentially forms homo- or heterodimers, translocates to the nucleus, binds to DNA, and induces the transcription of multiple target genes. STAT3 was the first transcription factor implicated in MDSC expansion in cancer [28–31]. Recent studies provided additional evidence supporting its major role in MDSC accumulation in humans and mice. For example, myeloid cell-specific ablation of the negative regulator of STAT3 activation, suppressor of cytokine signaling 3, led to an accumulation of MDSC in transplantable and orthotopic murine models of prostate cancer [32].
In addition to the myeloid-specific growth factors GM-CSF and G-CSF, several other factors have been implicated in the activation of STAT3 in MDSCs (Fig. 2). IL-6 is 1 of these factors [33, 34]. In mice, myeloid-specific expression of a dominant-negative form of peroxisome proliferator-activated receptor γ led to an increased production of proinflammatory cytokines, including IL-6, which resulted in the activation of STAT3 and the expansion of MDSC [35]. Interestingly, these mice spontaneously developed adenocarcinoma at 9–12 mo of age, further confirming the role of inflammation and MDSC during tumorigenesis. The involvement of IL-6 was also confirmed in cancer patients. In a recent study, IL-6 plasma concentrations were shown to correlate positively with the level of circulating MDSCs in patients with esophageal squamous carcinomas [36]. Remarkably, IL-6 was involved in the crosstalk between MDSC and tumor cells. Murine MDSC recruited to metastatic sites secreted high levels of IL-6, which led to pSTAT3 expression in tumor cells, thus increasing tumor invasiveness and metastatic potential [37]. Similar results were observed in a spontaneous murine model of prostate cancer, where in vivo IL-6 silencing resulted in decreased tumor aggressiveness associated with a decreased accumulation of MDSC [38].
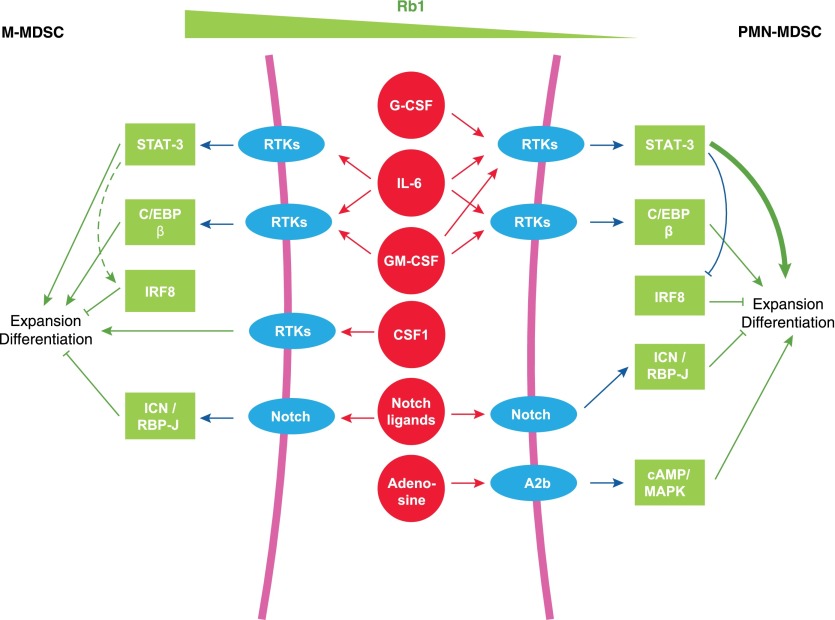
Figure 2. Transcription factors and signaling pathways involved in MDSC expansion.
There are several cytokines and transcriptional factors/regulators involved in the expansion and differentiation of MDSC. The transcription factors have a certain specificity in regulating the MDSC subsets. Details are provided in the text. RTKs, Receptor tyrosine kinases.
STAT3 activation in MDSC was reported in response to several others factors, including the hepatocyte growth factor [39], tumor-derived exosomes through TLR2 [40–42], and IL-11 [43]. In the latter study, IL-11-induced STAT3 activation was implicated in MDSC accumulation in a spontaneous murine model of stomach cancer. Interestingly, Yan et al. [44] recently reported that exposure to a diet rich in PUFAs significantly increased the accumulation of MDSC in tumor-bearing mice in a STAT3-dependent manner. However, the mechanism by which PUFAs can induce STAT3 activation and MDSC expansion requires further investigation.
Although the critical role of STAT3 in MDSC accumulation is well established, experiments targeting the STAT3 pathway raised questions regarding its potential role in the fate of MDSC within the tumor tissue. It is well established that inhibition of STAT3 results in decreased MDSC expansion. Treatment of tumor-bearing mice with different STAT3 inhibitors cucurbitacin I [45, 46], nifuroxazide [47], niclosamide [48], and tyrosine kinase inhibitors axitinib [49] or sunitinib [50, 51] resulted in a decrease of MDSC accumulation in the spleen, blood, and lungs and to a lower extent, in the primary tumor. Several studies reported that intratumoral GM-CSF was able to protect MDSC from STAT3 inhibition with sunitinib through an alternative STAT5-dependent survival mechanism [51, 52]. In most of the studies, the effect on different MDSC subsets was not established. However, recently published reports suggest that the accumulation of PMN-MDSC was decreased significantly within the tumor tissue upon STAT3 inhibition, whereas the percentage of M-MDSC remained unaffected or even increased [53, 54]. The efficacy of STAT3 targeting in cancer patients remains to be proven [55]. Therefore, the role of STAT3 in regulation of MDSC expansion depends on the cell subsets and the local microenvironment.
STAT3 is 1 of the transcription factors involved in the suppressive activity of these cells [33, 42, 56]. For example, it has been shown that STAT3 up-regulated the expression of several components of the NADPH complex (p47, gp96, S100A9). This up-regulation led to increased ROS production, which was associated with the suppressive ability of PMN-MDSC [57, 58].
IRF8
Although initially discovered for their involvement in type I IFN signaling, the IRF family of transcription factors was implicated in the regulation of the cell cycle, apoptosis, and cellular differentiation [59–61]. Within the murine hematopoietic system, IRF8 is expressed at the CMP stage, especially in cells that will differentiate into GMPs [62]. IRF8 expression coincides with the commitment to the granulocytic and monocytic lineages and the repression of the megakaryocyte-erythrocyte developmental axis. GMPs with high expression of IRF8 are more prone to give rise to MΦ colonies [62]. Yanez et al. [63] refined this idea by showing that cells with high IRF8 expression in mice were not GMPs per se but rather, further committed progeny. Mechanistically, IRF8 interacts with the transcription factor C/EBPα in mice to prevent its binding to DNA, resulting in the inhibition of the granulocytic differentiation program [64]. IRF8 regulates the differentiation and survival of GMPs and favor monocytic differentiation at the expense of granulocytes.
According to this concept, IRF8 could negatively regulate the differentiation of MDSC and their granulocytic subset, in particular. Mice deficient for IRF8 developed a chronic myeloid leukemia-like disease with 100% penetrance at ∼20 wk of age [65]. This pathology was associated with an increase in CD11b+Gr-1+ cells in the lymph nodes. Likewise, in the B16F10 melanoma model, IRF8-deficient mice had an increased accumulation of MDSC, both in spleens and tumors, compared with WT mice [66]. The molecular mechanisms driving this expansion are not fully understood, but a recent study suggested that IRF8 deletion in T cells induced GM-CSF production, which could promote MDSC expansion [67]. Interestingly, the silencing of IRF8 in MDSC isolated from mammary or colon carcinoma-bearing mice was associated with decreased, spontaneous apoptosis of these cells, mediated by down-regulation of Fas [68]. This allowed MDSC to escape Fas-mediated elimination by cytotoxic T cells, suggesting that IRF8 was not only a negative regulator of MDSC differentiation but could also indirectly inhibit their survival. Consistent with these results, overexpression of IRF8 resulted in a decreased accumulation of MDSC in a spontaneous mammary carcinoma model [69, 70]. A decrease of both MDSC subsets was reported, but the decrease in PMN-MDSC was much greater. This observation is consistent with the fact that IRF8 negatively regulates granulopoiesis. Surprisingly, the same group also reported that the frequency of splenic MDSC remained unchanged in a transplantable tumor model (4T1 mammary carcinoma). However, in this model, splenic MDSC had decreased protumorigenic ability [71].
Irf8 could be negatively regulated by tumor-derived G-CSF and GM-CSF, mediated through STAT3 and STAT5, respectively [70] (Fig. 2). Recently, Papaspyridonos et al. [72] showed that Irf8 down-regulation took place when murine hematopoietic progenitors were cultured in vitro in the presence of TCM. This down-regulation favored the development of MDSC at the expense of DCs. This effect was mediated by the TGF-β, present in the TCM, which induced the expression of the ID1. Overexpression of ID1 finally led to Irf8 down-regulation. High levels of ID1 were detected in peripheral blood CD11b+ cells from cancer patients, suggesting that this mechanism could also occur in humans. These results were consistent with data reported by Waight et al. [70], who found an inverse correlation between IRF8 expression levels and frequency of circulating MDSC in breast cancer patients.
C/EBPβ
C/EBPs encompass a family of basic-region-leucine zipper transcription factors. These factors form homo- or heterodimers (except for C/EBPζ, also known as CHOP) and bind to DNA [73]. Whereas 4 members (α, β, δ, and ε) of C/EBP are expressed in the cells of myeloid lineage, only C/EBPβ has been implicated in MDSC expansion [33]. C/EBPβ exists as 3 different isoforms: LAP*, LAP, and LIP. Whereas LAP* and LAP contain the DNA-binding domain and the activation domain, LIP lacks the latter. Therefore, LIP can act as a dominant-negative form for C/EBPβ [74]. The balance of different isoforms can affect the functional properties of C/EBPβ. In the steady state, C/EBPβ-deficient mice showed only defects in MΦ activation following stimulation with bacteria or LPS [75]. The granulocyte lineage remained unaltered. However, C/EBPβ KO mice were unable to mount an efficient emergency granulopoiesis response [76]. MDSC accumulation and emergency granulopoiesis are very similar phenomena. Indeed, mice lacking C/EBPβ in the hematopoietic system had lower frequencies of splenic CD11bhi/Gr-1hi, CD11bhi/Gr-1int, and CD11bhi/Gr-1lo MDSC in MCA203 fibrosarcoma-bearing mice. Surprisingly, the CD11b+ Gr-1int MDSC subset, mostly composed of M-MDSC, was the most affected population, suggesting that C/EBPβ deficiency affects mostly the differentiation of M-MDSC [33].
RB1
The RB family includes 3 members: RB1 (p105), RB2 (p130), and p107. They are not bona fide transcription factors but are recruited to DNA through interactions with other transcription factors. The main role of the RB proteins is to inhibit cell proliferation by repressing the activity of the E2F transcription factors. Although the RB proteins have similar structure and mostly overlapping activity, they can also display distinct functions, depending on cell type and context [77, 78].
RB1 was implicated recently in MDSC expansion in mice and humans [79]. Heterogeneous expression of Rb1 was found in M-MDSC. Immunofluorescence microscopy revealed a subpopulation of M-MDSC with low levels of Rb1, similar to PMN-MDSC. Whereas Rb1hi M-MDSCs mainly gave rise to MΦ and DC in vitro, the vast majority of Rb1lo M-MDSCs differentiated toward Ly6G+ granulocytic cells with potent immunosuppressive abilities (PMN-MDSC). Likewise, M-MDSC from the bone marrow of patients with multiple myeloma generated CD66b+ granulocytic cells in vitro. Rb1 down-regulation in M-MDSC was mediated largely by transcriptional silencing via recruitment of histone deacetylase 2. Recently, the accumulation of Rb1lo Ly6G+ PMN-MDSC was confirmed in the polyoma middle T antigen transgenic model of breast cancer [80]. Accumulation of PMN-MDSC resulted from a biased expansion of the early hematopoietic progenitors toward the granulocytic lineage. In this model, this process was mediated by an increased level of G-CSF [80]. These results suggest that tumor-derived G-CSF may reprogram the early hematopoietic compartment, and therefore, it is possible that RB1lo M-MDSCs are an intermediate stage of this process (Fig. 2).
The Notch pathway
The Notch pathway is an evolutionary conserved pathway, critically important for embryogenesis. In addition, it is directly involved in the regulation of innate and adaptive immunity. Down-regulation of Notch signaling was found in Gr-1+ splenocytes from tumor-bearing mice [81]. This was the result of ICN phosphorylation by activated CKII. Phosphorylation of ICN prevented its interaction with transcriptional partner RBPJκ. Overexpression of CKII in bone marrow progenitors shifted their differentiation toward CD11b+Gr-1+ cells at the expense of DCs. When tumor-bearing animals were treated with a CKII inhibitor, expression of the Notch target gene hairy and enhancer of split-1 was restored in bone marrow and spleens, and the differentiation of DC was improved [81]. These results suggested that Notch inhibition skewed the differentiation of the hematopoietic progenitors toward MDSC instead of DC. Interestingly, sustained activation of the Notch pathway in mice devoid of FBXW7, an E3 ubiquitin protein ligase required for ICN degradation, promoted MDSC migration. In these mice, M-MDSC frequencies were increased in lung metastases in a breast cancer model [82]. This effect was not observed in the bone marrow, suggesting that FBXW7 regulates the recruitment of M-MDSC to the tumor site rather than their production in the bone marrow. Indeed, enhanced M-MDSC migration in this study was found to be a result of increased production of CCL2 by bone marrow-derived stromal cells [82].
Adenosine receptors A2b
Extracellular AMP can be converted to adenosine through the action of the membrane-anchored ectonucleotidases CD39 and CD73. Extracellularly generated adenosine can then bind to adenosine receptors. Subsequent signaling downstream of the adenosine receptors is mediated by increased concentrations of cAMP and activation of the MAPK pathways [83]. Among the adenosine receptors, A2b has been specifically involved in MDSC expansion in cancers. Lewis lung carcinoma tumor-bearing mice, deficient for A2b, had decreased PMN-MDSC in the tumor site [84]. A2b expression specifically affected PMN-MDSC, as the levels of M-MDSC were unchanged. This was a result of the higher expression of CD73 on PMN-MDSC compared with M-MDSC. With the use of agonists and antagonists of the A2bR, Iannone et al. [85] demonstrated an associated increase or decrease in the frequency of MDSC in the melanoma lesions of tumor-bearing mice.
NLRP3
NLRP3 is an intracellular sensor found in some types of inflammasomes, which consist of macromolecular complexes, activated mainly in the presence of bacteria-derived compounds. Upon inflammasome activation, procaspase-1 is processed into its active form, caspase I, which then generates the fully matured inflammatory cytokines IL-1β and IL-18 through proteolytic cleavage [86, 87]. Van Deventer et al. [88] reported NLRP3 expression in tumor MDSCs. Subsequent experiments revealed that Nlrp3-deficient mice had decreased MDSC in the tumor site, implicating NLRP3 in MDSC recruitment to the tumors. In a metastatic melanoma model, Chow et al. [89] demonstrated that tumor-bearing mice had less metastases in the lungs in the absence of Nlrp3. This effect depended on NK cells and was potentiated by a CD11b+Gr-1int myeloid population present in the tumors. These cells displayed an eosinophil-staining pattern and were not found in the lymphoid organs of the tumor-bearing animals. This is an intriguing observation, as CD11b+Gr-1int cells usually have characteristics of M-MDSC in tumor-bearing hosts [90]. In addition, these cells were shown to suppress NK activity rather than to potentiate their activity [91]. More studies are needed to clarify the role of NLRP3 in MDSC function.
TRANSCRIPTION FACTORS AND SIGNALING PATHWAYS INVOLVED IN MDSC ACTIVATION
The NF-κB pathway
The NF-κB pathway is activated in MDSC by various proinflammatory factors, such as TLR ligands, IL-1β or TNF-α. All of these factors have been shown to increase the suppressive activity of MDSC [27] (Fig. 3). Several studies reported that TLR-mediated activation of NF-κB through MyD88 could contribute to the suppressive activities of MDSC [41, 92, 93]. These findings were confirmed by a more recent study showing that MyD88-deficient MDSCs lost their suppressive abilities and even acquired immunostimulatory activity. At the same time, MDSC accumulation was only slightly affected and was mainly a result of the decreased tumor size rather than the involvement of MyD88 in MDSC expansion [94]. More recently, activation of NF-κB through MyD88 has been linked to the engagement of TLR2 by exosomal HSP70 [95]. The authors demonstrated that tumor-derived exosomes were enriched for HSP70, which triggered an up-regulation of STAT3 activity in a TLR2/MyD88-dependent manner. However, several studies challenged the hypothesis that TLR could drive the suppressive function of MDSC and instead, suggested that it could inhibit their suppressive activity [96, 97]. Several studies also linked IL-1β with recruitment and activation of MDSC in an NF-κB-dependent manner [93, 98, 99] (Fig. 3). For example, overexpression of IL-1β by tumor cells resulted in an increased number of peroxynitrite-producing MDSCs [100].
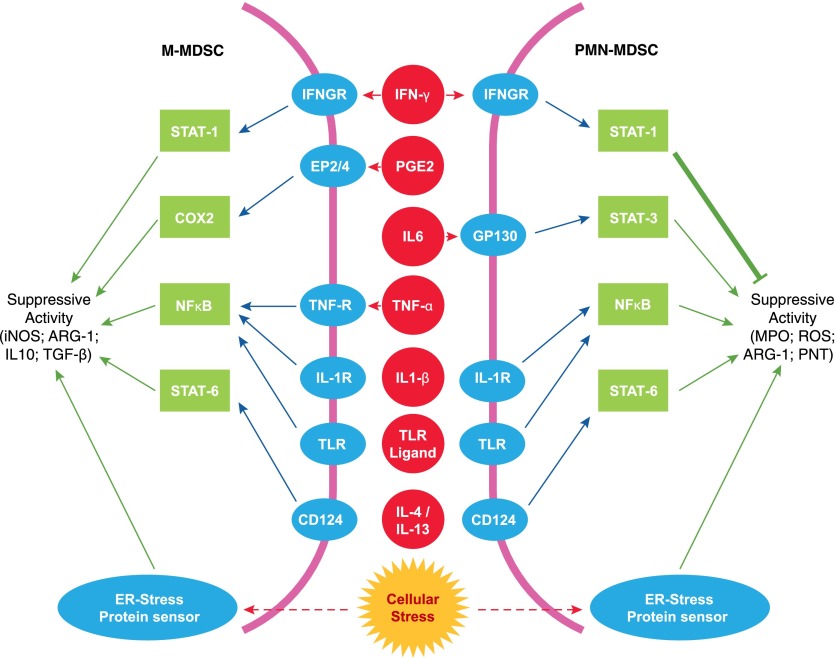
Figure 3. Pathways involved in the acquisition of a suppressive phenotype by MDSC.
A large array of proinflammatory factors is involved in the conversion of immature myeloid cells into suppressive cells. Several pathways have been involved in the conversion of PMN-MDSC and M-MDSC (NF-κB, STAT6, and ER stress pathways), whereas some are more specific to PMN-MDSC (STAT3) or M-MDSC (COX2). The STAT1 pathway may have opposite effects on MDSC subsets, promoting M-MDSC-suppressive activity while inhibiting the activity of PMN-MDSC. ARG-1, Arginase-1; PNT, peroxynitrite.
TNF-α, another well-known activator of the NF-κB pathway, was implicated in the suppressive activity of MDSC. Hu et al. showed that activation of MDSC through tmTNF-α could increase the suppressive activity of these cells. tmTNF-α was able to regulate iNOS expression in an NF-κB- and p38 MAPK-dependent manner [101] (Fig. 3). TNF-α enhanced the suppressive activity of MDSC in a model of chronic inflammation induced by repetitive injections of inactivated Bacillus Calmette–Guérin vaccines [102]. One study restricted the activity of TNF-α to M-MDSC in a model of cecal ligation and puncture [103]. Activation of NF-κB through TNFR2 could promote the survival of MDSC via NF-κB-mediated expression of C-FLIP, which inhibited caspase-8 [104].
The Stat1 pathway
Several studies have linked the activation of STAT1 with MDSC-suppressive activity [13, 105, 106]. STAT1 is activated upon IFN-γ stimulation and is implicated in the up-regulation of iNOS and arginase-1 expression. More recently, the involvement of STAT1 was restricted to M-MDSC [107]. In that study, the IFN-γ-STAT1-IRF1 axis was shown to be crucial for M-MDSC-suppressive activity, whereas PMN-MDSCs were only moderately affected. Furthermore, a more recent study demonstrated that activation of STAT1 through the IFN-γR could actually decrease PMN-MDSC survival and suppressive activity by repressing the expression of the antiapoptotic Bcl-2-related protein A1 [108]. This suggests that STAT1 signaling may have opposite effects on different MDSC subsets (Fig. 3). This could explain why a previous study did not observe an involvement of IFN-γ and/or STAT1 in MDSC-suppressive activity. In that study, Sinha et al. [109] analyzed the total MDSC population and did not find differences between WT and STAT1-deficient MDSCs. A plausible explanation would be that the loss of suppressive activity by M-MDSC was compensated for by the increased suppressive activity of PMN-MDSC.
The STAT6 pathway
In MDSC, the STAT6 pathway can be activated through engagement of CD124 (IL-4Rα) by IL-4 or IL-13. This led to the up-regulation of arginase-1 expression [110–113], as well as increased production of TGF-β [114]. More recently, Highfill et al. [115] reported that IL-13 increased the suppressive activity of in vitro-generated M-MDSC by increasing arginase-1 activity. The STAT6 pathway has also been involved in the survival and accumulation of MDSC via CD124 [116, 117] (Fig. 3).
PGE2 and COX2
PGE2 was implicated in the ability of MDSC to suppress T cells by up-regulating arginase-1 via EP4 in MDSC [118]. The role of PGE2 in MDSC-suppressive activity was confirmed further by the observation that COX2 expression correlated with the expression of arginase-1 and iNOS in murine tumor-infiltrating leukocytes [119]. More recently, a series of reports further investigating the role of PGE2 in MDSC described a positive-feedback loop, where PGE2 increased the expression of COX2 in monocytes, converting them to M-MDSC, and further increased the production of PGE2 [120, 121]. The involvement of PGE2 in MDSC-suppressive function was confirmed in melanoma patients, where inhibition of COX2 was found to down-regulate the suppressive activity of M-MDSC [122, 123]. Likewise, the COX2 inhibitor celecoxib blocked MDSC accumulation and function in 2 different mouse models of glioma and mesothelioma [124, 125]. However, PGE2 was also implicated in the accumulation and the recruitment of MDSC. One study demonstrated that PGE2 could induce MDSC accumulation via EP2R [126]. EP2 KO mice, as well as mice treated with an inhibitor of COX2 (SC58236), had decreased tumor growth associated with a reduced accumulation of MDSC. PGE2 was involved in the recruitment of MDSC to the tumor site by inducing the expression of CXCL12 [127, 128].
The ER stress response pathway
The ER stress response is an evolutionary conserved mechanism developed to protect cells from various stress conditions, including hypoxia, nutrient deprivation, low pH, and others. In eukaryotes, three major signaling cascades are involved in the ER stress response and are initiated by 3 protein sensors, i.e., PERK, IRE-1, and ATF6 [129]. PERK phosphorylates eIF2α, which controls the initiation of mRNA translation and inhibits the flux of synthesized proteins. eIF2α induces the expression of ATF4 and its downstream targets, including the proapoptotic transcription factor CHOP. IRE-1 cleaves the mRNA encoding for the transcription factor XBP1 [130]. sXBP1 mRNA is then ligated by a RNA ligase and translated to produce sXBP1 transcription factor [131]. ATF6 is transported to the nucleus through the Golgi and induces the transcription of ER chaperone genes, such as binding Ig protein and several major targets, including XBP1.
The ER stress response pathway has been shown to regulate inflammation by activating the NF-κB pathway [131–133]. The ability of the ER stress response to modulate myeloid cell function was first shown by Dr. Zanetti’s group [134–136]. They demonstrated that tumor cells are able to transmit ER stress to DCs and MΦ. In MΦ, it led to the up-regulation of arginase-1 activity. Our group reported that the ER stress response pathway was activated in MDSC [137]. We demonstrated that both MDSC isolated from tumor-bearing mice or cancer patients overexpressed several markers of ER stress, including sXBP1 and CHOP, and displayed an enlarged ER, 1 of the hallmarks of ER stress. In a different study, administration of an ER stress inducer to tumor-bearing mice was shown to increase the accumulation of MDSCs and their suppressive activity [138]. More recently, the transcription factor CHOP was implicated in the suppressive activity of MDSC. Intratumoral CHOP-deficient MDSCs lost the ability to suppress T cells stimulated in an antigen-nonspecific manner and were even able to stimulate T cells [139]. However, CHOP-deficient MDSC retained the ability to suppress the T cell response to an antigen-specific stimulation [140]. More studies will be necessary to clarify the role of the specific mechanisms of ER stress responses in MDSC function.
CONCLUDING REMARKS
Accumulation of MDSC is controlled by a network of transcription factors and regulators that could be broadly ascribed to 2 large groups. One group is responsible for expansion of immature myeloid cells and the other group for pathologic activation of these immature cells. Both of these groups are indispensable for MDSC accumulation. However, the precise role and potential redundancy among different factors need to be elucidated. With better understanding of the nature of cells that comprise MDSC, it became clear that the biology and regulatory mechanisms of PMN-MDSC and M-MDSC are different. PMN-MDSC is the population that extensively expands in cancer. In contrast, M-MDSCs expand very modestly. It is likely that molecular mechanisms regulating these 2 populations are different. However, most of the studies to date were performed by use of total population of MDSC. The specific nature of those mechanisms only recently started to emerge and need further elucidation.
A similar challenge exists for understanding the mechanisms regulating activation of MDSC. It appears that in M-MDSC, this process depends on STAT1, STAT6, and NF-κB. The acquisition of suppressive abilities by PMN-MDSC is less clear. STAT3 could play a major role, not only in expansion of these cells but also in their acquisition of suppressive activity. One of the attractive hypotheses of MDSC activation is the effect of proinflammatory signals coupled with ER stress response. Cooperation of the ER stress response with TLR signaling has been demonstrated [141–144]. However, more studies will be needed to clarify this issue. The understanding of specific molecular mechanisms responsible for MDSC accumulation would enable more precise therapeutic targeting of these cells.
AUTHORSHIP
T.C. , J.M. and D.I. wrote the paper.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants CA 100062, CA 84488, and CA141438. The authors thank Lev Gabrilovich and Sima Patel for help in preparation of the manuscript.
Glossary
- ATF6
activating transcription factor 6
- CHOP
C/EBP homologous protein
- CKII
casein kinase II
- CMP
common myeloid progenitor
- COX2
cyclooxygenase 2
- DC
dendritic cell
- eIF2α
eukaryotic protein synthesis initiation factor 2 α
- EP2/4
PGE2/4R
- ER
endoplasmic reticulum
- FBXW7
F-box and WD repeat domain containing 7
- GMP
granulocyte-monocyte progenitor
- HSP70
heat shock protein 70
- ICN
intracellular Notch 1
- ID1
inhibitor of differentiation 1
- IRE-1
inositol-requiring enzyme 1
- IRF
IFN regulatory factor
- KO
knockout
- LAP
liver-enriched activating protein
- LIP
liver-enriched inhibitory protein
- M-MDSC
monocytic myeloid-derived suppressor cell
- MΦ
macrophage(s)
- MDSC
myeloid-derived suppressor cell
- MPO
myeloperoxidase
- NLRP3
nucleotide-binding oligomerization domain-like receptor family, pyrin domain-containing 3
- PERK
protein kinase RNA-like ERK
- PMN
polymorphonuclear cell
- pSTAT
phosphorylated STAT
- PUFA
polyunsaturated fatty acid
- RB
retinoblastoma protein
- RBPJ
recombining binding protein suppressor of hairless
- ROS
reactive oxygen species
- sXBP1
spliced X-box-binding protein-1
- TCM
tumor conditioned media
- tmTNF-α
transmembrane TNF-α
- WT
wild-type
- XBP–1
X-box-binding protein-1
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Lee M. Y., Rosse C. (1982) Depletion of lymphocyte subpopulations in primary and secondary lymphoid organs of mice by a transplanted granulocytosis-inducing mammary carcinoma. Cancer Res. 42, 1255–1260. [PubMed] [Google Scholar]
- 2.Tsuchiya Y., Igarashi M., Suzuki R., Kumagai K. (1988) Production of colony-stimulating factor by tumor cells and the factor-mediated induction of suppressor cells. J. Immunol. 141, 699–708. [PubMed] [Google Scholar]
- 3.Gabrilovich D. I., Bronte V., Chen S. H., Colombo M. P., Ochoa A., Ostrand-Rosenberg S., Schreiber H. (2007) The terminology issue for myeloid-derived suppressor cells. Cancer Res. 67, 425, author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goh C., Narayanan S., Hahn Y. S. (2013) Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol. Rev. 255, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verschoor C. P., Johnstone J., Millar J., Dorrington M. G., Habibagahi M., Lelic A., Loeb M., Bramson J. L., Bowdish D. M. (2013) Blood CD33(+)HLA-DR(-) myeloid-derived suppressor cells are increased with age and a history of cancer. J. Leukoc. Biol. 93, 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurez V., Daniel B. J., Sun L., Liu A. J., Ludwig S. M., Kious M. J., Thibodeaux S. R., Pandeswara S., Murthy K., Livi C. B., Wall S., Brumlik M. J., Shin T., Zhang B., Curiel T. J. (2012) Mitigating age-related immune dysfunction heightens the efficacy of tumor immunotherapy in aged mice. Cancer Res. 72, 2089–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haile L. A., Greten T. F., Korangy F. (2012) Immune suppression: the hallmark of myeloid derived suppressor cells. Immunol. Invest. 41, 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagaraj S., Youn J. I., Gabrilovich D. I. (2013) Reciprocal relationship between myeloid-derived suppressor cells and T cells. J. Immunol. 191, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schouppe E., Van Overmeire E., Laoui D., Keirsse J., Van Ginderachter J. A. (2013) Modulation of CD8(+) T-cell activation events by monocytic and granulocytic myeloid-derived suppressor cells. Immunobiology 218, 1385–1391. [DOI] [PubMed] [Google Scholar]
- 10.Talmadge J. E., Gabrilovich D. I. (2013) History of myeloid-derived suppressor cells. Nat. Rev. Cancer 13, 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messmer M. N., Netherby C. S., Banik D., Abrams S. I. (2015) Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer Immunol. Immunother. 64, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solito S., Marigo I., Pinton L., Damuzzo V., Mandruzzato S., Bronte V. (2014) Myeloid-derived suppressor cell heterogeneity in human cancers. Ann. N. Y. Acad. Sci. 1319, 47–65. [DOI] [PubMed] [Google Scholar]
- 13.Movahedi K., Guilliams M., Van den Bossche J., Van den Bergh R., Gysemans C., Beschin A., De Baetselier P., Van Ginderachter J. A. (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111, 4233–4244. [DOI] [PubMed] [Google Scholar]
- 14.Youn J. I., Nagaraj S., Collazo M., Gabrilovich D. I. (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damuzzo V., Pinton L., Desantis G., Solito S., Marigo I., Bronte V., Mandruzzato S. (2015) Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry B Clin. Cytom. 88, 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youn J. I., Collazo M., Shalova I. N., Biswas S. K., Gabrilovich D. I. (2012) Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J. Leukoc. Biol. 91, 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabrilovich D. I., Ostrand-Rosenberg S., Bronte V. (2012) Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayne L. J., Beatty G. L., Jhala N., Clark C. E., Rhim A. D., Stanger B. Z., Vonderheide R. H. (2012) Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21, 822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bronte V., Chappell D. B., Apolloni E., Cabrelle A., Wang M., Hwu P., Restifo N. P. (1999) Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J. Immunol. 162, 5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 20.Dolcetti L., Peranzoni E., Ugel S., Marigo I., Fernandez Gomez A., Mesa C., Geilich M., Winkels G., Traggiai E., Casati A., Grassi F., Bronte V. (2010) Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 40, 22–35. [DOI] [PubMed] [Google Scholar]
- 21.Morales J. K., Kmieciak M., Knutson K. L., Bear H. D., Manjili M. H. (2010) GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res. Treat. 123, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowanetz M., Wu X., Lee J., Tan M., Hagenbeek T., Qu X., Yu L., Ross J., Korsisaari N., Cao T., Bou-Reslan H., Kallop D., Weimer R., Ludlam M. J., Kaminker J. S., Modrusan Z., van Bruggen N., Peale F. V., Carano R., Meng Y. G., Ferrara N. (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. USA 107, 21248–21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okazaki T., Ebihara S., Asada M., Kanda A., Sasaki H., Yamaya M. (2006) Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int. Immunol. 18, 1–9. [DOI] [PubMed] [Google Scholar]
- 24.Waight J. D., Hu Q., Miller A., Liu S., Abrams S. I. (2011) Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One 6, e27690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusmartsev S., Cheng F., Yu B., Nefedova Y., Sotomayor E., Lush R., Gabrilovich D. (2003) All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 63, 4441–4449. [PubMed] [Google Scholar]
- 26.Menetrier-Caux C., Montmain G., Dieu M. C., Bain C., Favrot M. C., Caux C., Blay J. Y. (1998) Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood 92, 4778–4791. [PubMed] [Google Scholar]
- 27.Condamine T., Gabrilovich D. I. (2011) Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabrilovich D. I., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Condamine T., Ramachandran I., Youn J. I., Gabrilovich D. I. (2015) Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 66, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nefedova Y., Huang M., Kusmartsev S., Bhattacharya R., Cheng P., Salup R., Jove R., Gabrilovich D. (2004) Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 172, 464–474. [DOI] [PubMed] [Google Scholar]
- 31.Rébé C., Végran F., Berger H., Ghiringhelli F. (2013) STAT3 activation: a key factor in tumor immunoescape. JAK-STAT 2, e23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H., Liu Y., McFarland B. C., Deshane J. S., Hurst D. R., Ponnazhagan S., Benveniste E. N., Qin H. (2015) SOCS3 deficiency in myeloid cells promotes tumor development: involvement of STAT3 activation and myeloid-derived suppressor cells. Cancer Immunol. Res. 3, 727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marigo I., Bosio E., Solito S., Mesa C., Fernandez A., Dolcetti L., Ugel S., Sonda N., Bicciato S., Falisi E., Calabrese F., Basso G., Zanovello P., Cozzi E., Mandruzzato S., Bronte V. (2010) Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 32, 790–802. [DOI] [PubMed] [Google Scholar]
- 34.Lechner M. G., Liebertz D. J., Epstein A. L. (2010) Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 185, 2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu L., Yan C., Czader M., Foreman O., Blum J. S., Kapur R., Du H. (2012) Inhibition of PPARγ in myeloid-lineage cells induces systemic inflammation, immunosuppression, and tumorigenesis. Blood 119, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen M. F., Kuan F. C., Yen T. C., Lu M. S., Lin P. Y., Chung Y. H., Chen W. C., Lee K. D. (2014) IL-6-stimulated CD11b+ CD14+ HLA-DR− myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget 5, 8716–8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh K., Lee O. Y., Shon S. Y., Nam O., Ryu P. M., Seo M. W., Lee D. S. (2013) A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 15, R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C. T., Hsieh C. C., Lin C. C., Chen W. C., Hong J. H., Chen M. F. (2012) Significance of IL-6 in the transition of hormone-resistant prostate cancer and the induction of myeloid-derived suppressor cells. J. Mol. Med. 90, 1343–1355. [DOI] [PubMed] [Google Scholar]
- 39.Yen B. L., Yen M. L., Hsu P. J., Liu K. J., Wang C. J., Bai C. H., Sytwu H. K. (2013) Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Rep. 1, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J. M., Kim E. K., Seo H., Jeon I., Chae M. J., Park Y. J., Song B., Kim Y. S., Kim Y. J., Ko H. J., Kang C. Y. (2014) Serum amyloid A3 exacerbates cancer by enhancing the suppressive capacity of myeloid-derived suppressor cells via TLR2-dependent STAT3 activation. Eur. J. Immunol. 44, 1672–1684. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y., Xiang X., Zhuang X., Zhang S., Liu C., Cheng Z., Michalek S., Grizzle W., Zhang H. G. (2010) Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am. J. Pathol. 176, 2490–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang X., Liu Y., Zhuang X., Zhang S., Michalek S., Taylor D. D., Grizzle W., Zhang H. G. (2010) TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am. J. Pathol. 177, 1606–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buzzelli J. N., Pavlic D. I., Chalinor H. V., O’Connor L., Menheniott T. R., Giraud A. S., Judd L. M. (2015) IL-1RT1 signaling antagonizes IL-11 induced STAT3 dependent cardiac and antral stomach tumor development through myeloid cell enrichment. Oncotarget 6, 679–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan D., Yang Q., Shi M., Zhong L., Wu C., Meng T., Yin H., Zhou J. (2013) Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur. J. Immunol. 43, 2943–2955. [DOI] [PubMed] [Google Scholar]
- 45.Nefedova Y., Nagaraj S., Rosenbauer A., Muro-Cacho C., Sebti S. M., Gabrilovich D. I. (2005) Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 65, 9525–9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita M., Zhu X., Sasaki K., Ueda R., Low K. L., Pollack I. F., Okada H. (2008) Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J. Immunol. 180, 2089–2098. [DOI] [PubMed] [Google Scholar]
- 47.Yang F., Hu M., Lei Q., Xia Y., Zhu Y., Song X., Li Y., Jie H., Liu C., Xiong Y., Zuo Z., Zeng A., Li Y., Yu L., Shen G., Wang D., Xie Y., Ye T., Wei Y. (2015) Nifuroxazide induces apoptosis and impairs pulmonary metastasis in breast cancer model. Cell Death Dis. 6, e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye T., Xiong Y., Yan Y., Xia Y., Song X., Liu L., Li D., Wang N., Zhang L., Zhu Y., Zeng J., Wei Y., Yu L. (2014) The anthelmintic drug niclosamide induces apoptosis, impairs metastasis and reduces immunosuppressive cells in breast cancer model. PLoS One 9, e85887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan H., Cai P., Li Q., Wang W., Sun Y., Xu Q., Gu Y. (2014) Axitinib augments antitumor activity in renal cell carcinoma via STAT3-dependent reversal of myeloid-derived suppressor cell accumulation. Biomed. Pharmacother. 68, 751–756. [DOI] [PubMed] [Google Scholar]
- 50.Xin H., Zhang C., Herrmann A., Du Y., Figlin R., Yu H. (2009) Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 69, 2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko J. S., Rayman P., Ireland J., Swaidani S., Li G., Bunting K. D., Rini B., Finke J. H., Cohen P. A. (2010) Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 70, 3526–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finke J., Ko J., Rini B., Rayman P., Ireland J., Cohen P. (2011) MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int. Immunopharmacol. 11, 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abad C., Nobuta H., Li J., Kasai A., Yong W. H., Waschek J. A. (2014) Targeted STAT3 disruption in myeloid cells alters immunosuppressor cell abundance in a murine model of spontaneous medulloblastoma. J. Leukoc. Biol. 95, 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tu S. P., Jin H., Shi J. D., Zhu L. M., Suo Y., Lu G., Liu A., Wang T. C., Yang C. S. (2012) Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev. Res. (Phila.) 5, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu H., Lee H., Herrmann A., Buettner R., Jove R. (2014) Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat. Rev. Cancer 14, 736–746. [DOI] [PubMed] [Google Scholar]
- 56.Chalmin F., Ladoire S., Mignot G., Vincent J., Bruchard M., Remy-Martin J. P., Boireau W., Rouleau A., Simon B., Lanneau D., De Thonel A., Multhoff G., Hamman A., Martin F., Chauffert B., Solary E., Zitvogel L., Garrido C., Ryffel B., Borg C., Apetoh L., Rébé C., Ghiringhelli F. (2010) Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 120, 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corzo C. A., Cotter M. J., Cheng P., Cheng F., Kusmartsev S., Sotomayor E., Padhya T., McCaffrey T. V., McCaffrey J. C., Gabrilovich D. I. (2009) Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng P., Corzo C. A., Luetteke N., Yu B., Nagaraj S., Bui M. M., Ortiz M., Nacken W., Sorg C., Vogl T., Roth J., Gabrilovich D. I. (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 205, 2235–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abrams S. I. (2010) A multi-functional role of interferon regulatory factor-8 in solid tumor and myeloid cell biology. Immunol. Res. 46, 59–71. [DOI] [PubMed] [Google Scholar]
- 60.Savitsky D., Tamura T., Yanai H., Taniguchi T. (2010) Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol. Immunother. 59, 489–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tamura T., Kurotaki D., Koizumi S. I. (2015) Regulation of myelopoiesis by the transcription factor IRF8. Int. J. Hematol. 101, 342–351. [DOI] [PubMed] [Google Scholar]
- 62.Wang H., Yan M., Sun J., Jain S., Yoshimi R., Abolfath S. M., Ozato K., Coleman W. G. Jr., Ng A. P., Metcalf D., DiRago L., Nutt S. L., Morse H. C. III (2014) A reporter mouse reveals lineage-specific and heterogeneous expression of IRF8 during lymphoid and myeloid cell differentiation. J. Immunol. 193, 1766–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yáñez A., Ng M. Y., Hassanzadeh-Kiabi N., Goodridge H. S. (2015) IRF8 acts in lineage-committed rather than oligopotent progenitors to control neutrophil vs monocyte production. Blood 125, 1452–1459. [DOI] [PubMed] [Google Scholar]
- 64.Kurotaki D., Yamamoto M., Nishiyama A., Uno K., Ban T., Ichino M., Sasaki H., Matsunaga S., Yoshinari M., Ryo A., Nakazawa M., Ozato K., Tamura T. (2014) IRF8 inhibits C/EBPα activity to restrain mononuclear phagocyte progenitors from differentiating into neutrophils. Nat. Commun. 5, 4978. [DOI] [PubMed] [Google Scholar]
- 65.Holtschke T., Löhler J., Kanno Y., Fehr T., Giese N., Rosenbauer F., Lou J., Knobeloch K. P., Gabriele L., Waring J. F., Bachmann M. F., Zinkernagel R. M., Morse H. C. III, Ozato K., Horak I. (1996) Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87, 307–317. [DOI] [PubMed] [Google Scholar]
- 66.Mattei F., Schiavoni G., Sestili P., Spadaro F., Fragale A., Sistigu A., Lucarini V., Spada M., Sanchez M., Scala S., Battistini A., Belardelli F., Gabriele L. (2012) IRF-8 controls melanoma progression by regulating the cross talk between cancer and immune cells within the tumor microenvironment. Neoplasia 14, 1223–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paschall A. V., Zhang R., Qi C. F., Bardhan K., Peng L., Lu G., Yang J., Merad M., McGaha T., Zhou G., Mellor A., Abrams S. I., Morse H. C. III, Ozato K., Xiong H., Liu K. (2015) IFN regulatory factor 8 represses GM-CSF expression in T cells to affect myeloid cell lineage differentiation. J. Immunol. 194, 2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu X., Bardhan K., Paschall A. V., Yang D., Waller J. L., Park M. A., Nayak-Kapoor A., Samuel T. A., Abrams S. I., Liu K. (2013) Deregulation of apoptotic factors Bcl-xL and Bax confers apoptotic resistance to myeloid-derived suppressor cells and contributes to their persistence in cancer. J. Biol. Chem. 288, 19103–19115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stewart T. J., Greeneltch K. M., Reid J. E., Liewehr D. J., Steinberg S. M., Liu K., Abrams S. I. (2009) Interferon regulatory factor-8 modulates the development of tumour-induced CD11b+Gr-1+ myeloid cells. J. Cell. Mol. Med. 13, 3939–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waight J. D., Netherby C., Hensen M. L., Miller A., Hu Q., Liu S., Bogner P. N., Farren M. R., Lee K. P., Liu K., Abrams S. I. (2013) Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J. Clin. Invest. 123, 4464–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart T. J., Liewehr D. J., Steinberg S. M., Greeneltch K. M., Abrams S. I. (2009) Modulating the expression of IFN regulatory factor 8 alters the protumorigenic behavior of CD11b+Gr-1+ myeloid cells. J. Immunol. 183, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papaspyridonos M., Matei I., Huang Y., do Rosario Andre M., Brazier-Mitouart H., Waite J. C., Chan A. S., Kalter J., Ramos I., Wu Q., Williams C., Wolchok J. D., Chapman P. B., Peinado H., Anandasabapathy N., Ocean A. J., Kaplan R. N., Greenfield J. P., Bromberg J., Skokos D., Lyden D. (2015) Id1 suppresses anti-tumour immune responses and promotes tumour progression by impairing myeloid cell maturation. Nat. Commun. 6, 6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramji D. P., Foka P. (2002) CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J. 365, 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nerlov C. (2007) The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 17, 318–324. [DOI] [PubMed] [Google Scholar]
- 75.Huber R., Pietsch D., Günther J., Welz B., Vogt N., Brand K. (2014) Regulation of monocyte differentiation by specific signaling modules and associated transcription factor networks. Cell. Mol. Life Sci. 71, 63–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manz M. G., Boettcher S. (2014) Emergency granulopoiesis. Nat. Rev. Immunol. 14, 302–314. [DOI] [PubMed] [Google Scholar]
- 77.Burkhart D. L., Sage J. (2008) Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer 8, 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Genovese C., Trani D., Caputi M., Claudio P. P. (2006) Cell cycle control and beyond: emerging roles for the retinoblastoma gene family. Oncogene 25, 5201–5209. [DOI] [PubMed] [Google Scholar]
- 79.Youn J. I., Kumar V., Collazo M., Nefedova Y., Condamine T., Cheng P., Villagra A., Antonia S., McCaffrey J. C., Fishman M., Sarnaik A., Horna P., Sotomayor E., Gabrilovich D. I. (2013) Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat. Immunol. 14, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casbon A. J., Reynaud D., Park C., Khuc E., Gan D. D., Schepers K., Passegué E., Werb Z. (2015) Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc. Natl. Acad. Sci. USA 112, E566–E575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng P., Kumar V., Liu H., Youn J. I., Fishman M., Sherman S., Gabrilovich D. (2014) Effects of Notch signaling on regulation of myeloid cell differentiation in cancer. Cancer Res. 74, 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yumimoto K., Akiyoshi S., Ueo H., Sagara Y., Onoyama I., Ueo H., Ohno S., Mori M., Mimori K., Nakayama K. I. (2015) F-Box protein FBXW7 inhibits cancer metastasis in a non-cell-autonomous manner. J. Clin. Invest. 125, 621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen J. F., Eltzschig H. K., Fredholm B. B. (2013) Adenosine receptors as drug targets–what are the challenges? Nat. Rev. 12, 265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryzhov S., Novitskiy S. V., Goldstein A. E., Biktasova A., Blackburn M. R., Biaggioni I., Dikov M. M., Feoktistov I. (2011) Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J. Immunol. 187, 6120–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iannone R., Miele L., Maiolino P., Pinto A., Morello S. (2013) Blockade of A2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia 15, 1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saxena M., Yeretssian G. (2014) NOD-like receptors: master regulators of inflammation and cancer. Front. Immunol. 5, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vanaja S. K., Rathinam V. A., Fitzgerald K. A. (2015) Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 25, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Deventer H. W., Burgents J. E., Wu Q. P., Woodford R. M., Brickey W. J., Allen I. C., McElvania-Tekippe E., Serody J. S., Ting J. P. (2010) The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res. 70, 10161–10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chow M. T., Sceneay J., Paget C., Wong C. S., Duret H., Tschopp J., Möller A., Smyth M. J. (2012) NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 72, 5721–5732. [DOI] [PubMed] [Google Scholar]
- 90.Youn J. I., Gabrilovich D. I. (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 40, 2969–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H., Han Y., Guo Q., Zhang M., Cao X. (2009) Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 182, 240–249. [DOI] [PubMed] [Google Scholar]
- 92.Arora M., Poe S. L., Oriss T. B., Krishnamoorthy N., Yarlagadda M., Wenzel S. E., Billiar T. R., Ray A., Ray P. (2010) TLR4/MyD88-induced CD11b+Gr-1 int F4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol. 3, 578–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bunt S. K., Clements V. K., Hanson E. M., Sinha P., Ostrand-Rosenberg S. (2009) Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J. Leukoc. Biol. 85, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hong E. H., Chang S. Y., Lee B. R., Kim Y. S., Lee J. M., Kang C. Y., Kweon M. N., Ko H. J. (2013) Blockade of Myd88 signaling induces antitumor effects by skewing the immunosuppressive function of myeloid-derived suppressor cells. Int. J. Cancer 132, 2839–2848. [DOI] [PubMed] [Google Scholar]
- 95.Diao J., Yang X., Song X., Chen S., He Y., Wang Q., Chen G., Luo C., Wu X., Zhang Y. (2015) Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3. Med. Oncol. 32, 453. [DOI] [PubMed] [Google Scholar]
- 96.Wu H., Tao N., Liu X., Li X., Tang J., Ma C., Xu X., Shao H., Hou B., Wang H., Qin Z. (2012) Polysaccharide from Lentinus edodes inhibits the immunosuppressive function of myeloid-derived suppressor cells. PLoS One 7, e51751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang Y., Zhang R., Xia F., Zou T., Huang A., Xiong S., Zhang J. (2013) LPS converts Gr-1(+)CD115(+) myeloid-derived suppressor cells from M2 to M1 via P38 MAPK. Exp. Cell Res. 319, 1774–1783. [DOI] [PubMed] [Google Scholar]
- 98.Tu S., Bhagat G., Cui G., Takaishi S., Kurt-Jones E. A., Rickman B., Betz K. S., Penz-Oesterreicher M., Bjorkdahl O., Fox J. G., Wang T. C. (2008) Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14, 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elkabets M., Ribeiro V. S., Dinarello C. A., Ostrand-Rosenberg S., Di Santo J. P., Apte R. N., Vosshenrich C. A. (2010) IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur. J. Immunol. 40, 3347–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu T., Ramakrishnan R., Altiok S., Youn J. I., Cheng P., Celis E., Pisarev V., Sherman S., Sporn M. B., Gabrilovich D. (2011) Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J. Clin. Invest. 121, 4015–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu X., Li B., Li X., Zhao X., Wan L., Lin G., Yu M., Wang J., Jiang X., Feng W., Qin Z., Yin B., Li Z. (2014) Transmembrane TNF-α promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J. Immunol. 192, 1320–1331. [DOI] [PubMed] [Google Scholar]
- 102.Sade-Feldman M., Kanterman J., Ish-Shalom E., Elnekave M., Horwitz E., Baniyash M. (2013) Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity 38, 541–554. [DOI] [PubMed] [Google Scholar]
- 103.Polz J., Remke A., Weber S., Schmidt D., Weber-Steffens D., Pietryga-Krieger A., Müller N., Ritter U., Mostböck S., Männel D. N. (2014) Myeloid suppressor cells require membrane TNFR2 expression for suppressive activity. Immun. Inflamm. Dis. 2, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao X., Rong L., Zhao X., Li X., Liu X., Deng J., Wu H., Xu X., Erben U., Wu P., Syrbe U., Sieper J., Qin Z. (2012) TNF signaling drives myeloid-derived suppressor cell accumulation. J. Clin. Invest. 122, 4094–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gallina G., Dolcetti L., Serafini P., De Santo C., Marigo I., Colombo M. P., Basso G., Brombacher F., Borrello I., Zanovello P., Bicciato S., Bronte V. (2006) Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest. 116, 2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kusmartsev S., Gabrilovich D. I. (2005) STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J. Immunol. 174, 4880–4891. [DOI] [PubMed] [Google Scholar]
- 107.Schouppe E., Mommer C., Movahedi K., Laoui D., Morias Y., Gysemans C., Luyckx A., De Baetselier P., Van Ginderachter J. A. (2013) Tumor-induced myeloid-derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8+ T-cell activation events. Eur. J. Immunol. 43, 2930–2942. [DOI] [PubMed] [Google Scholar]
- 108.Medina-Echeverz J., Haile L. A., Zhao F., Gamrekelashvili J., Ma C., Métais J. Y., Dunbar C. E., Kapoor V., Manns M. P., Korangy F., Greten T. F. (2014) IFN-γ regulates survival and function of tumor-induced CD11b+ Gr-1high myeloid derived suppressor cells by modulating the anti-apoptotic molecule Bcl2a1. Eur. J. Immunol. 44, 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sinha P., Parker K. H., Horn L., Ostrand-Rosenberg S. (2012) Tumor-induced myeloid-derived suppressor cell function is independent of IFN-γ and IL-4Rα. Eur. J. Immunol. 42, 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bronte V., Serafini P., De Santo C., Marigo I., Tosello V., Mazzoni A., Segal D. M., Staib C., Lowel M., Sutter G., Colombo M. P., Zanovello P. (2003) IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 170, 270–278. [DOI] [PubMed] [Google Scholar]
- 111.Sinha P., Clements V. K., Ostrand-Rosenberg S. (2005) Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J. Immunol. 174, 636–645. [DOI] [PubMed] [Google Scholar]
- 112.Sinha P., Clements V. K., Ostrand-Rosenberg S. (2005) Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 65, 11743–11751. [DOI] [PubMed] [Google Scholar]
- 113.Kohanbash G., McKaveney K., Sakaki M., Ueda R., Mintz A. H., Amankulor N., Fujita M., Ohlfest J. R., Okada H. (2013) GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-α. Cancer Res. 73, 6413–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Terabe M., Matsui S., Park J. M., Mamura M., Noben-Trauth N., Donaldson D. D., Chen W., Wahl S. M., Ledbetter S., Pratt B., Letterio J. J., Paul W. E., Berzofsky J. A. (2003) Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J. Exp. Med. 198, 1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Highfill S. L., Rodriguez P. C., Zhou Q., Goetz C. A., Koehn B. H., Veenstra R., Taylor P. A., Panoskaltsis-Mortari A., Serody J. S., Munn D. H., Tolar J., Ochoa A. C., Blazar B. R. (2010) Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 116, 5738–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Munera V., Popovic P. J., Bryk J., Pribis J., Caba D., Matta B. M., Zenati M., Ochoa J. B. (2010) Stat 6-dependent induction of myeloid derived suppressor cells after physical injury regulates nitric oxide response to endotoxin. Ann. Surg. 251, 120–126. [DOI] [PubMed] [Google Scholar]
- 117.Roth F., De La Fuente A. C., Vella J. L., Zoso A., Inverardi L., Serafini P. (2012) Aptamer-mediated blockade of IL4Rα triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 72, 1373–1383. [DOI] [PubMed] [Google Scholar]
- 118.Rodriguez P. C., Hernandez C. P., Quiceno D., Dubinett S. M., Zabaleta J., Ochoa J. B., Gilbert J., Ochoa A. C. (2005) Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 202, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Donkor M. K., Lahue E., Hoke T. A., Shafer L. R., Coskun U., Solheim J. C., Gulen D., Bishay J., Talmadge J. E. (2009) Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int. Immunopharmacol. 9, 937–948. [DOI] [PubMed] [Google Scholar]
- 120.Obermajer N., Muthuswamy R., Lesnock J., Edwards R. P., Kalinski P. (2011) Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 118, 5498–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Obermajer N., Kalinski P. (2012) Generation of myeloid-derived suppressor cells using prostaglandin E2. Transplant. Res. 1, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mao Y., Poschke I., Wennerberg E., Pico de Coaña Y., Egyhazi Brage S., Schultz I., Hansson J., Masucci G., Lundqvist A., Kiessling R. (2013) Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 73, 3877–3887. [DOI] [PubMed] [Google Scholar]
- 123.Mao Y., Sarhan D., Steven A., Seliger B., Kiessling R., Lundqvist A. (2014) Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res. 20, 4096–4106. [DOI] [PubMed] [Google Scholar]
- 124.Fujita M., Kohanbash G., Fellows-Mayle W., Hamilton R. L., Komohara Y., Decker S. A., Ohlfest J. R., Okada H. (2011) COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 71, 2664–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Veltman J. D., Lambers M. E., van Nimwegen M., Hendriks R. W., Hoogsteden H. C., Aerts J. G., Hegmans J. P. (2010) COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer 10, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sinha P., Clements V. K., Fulton A. M., Ostrand-Rosenberg S. (2007) Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 67, 4507–4513. [DOI] [PubMed] [Google Scholar]
- 127.Obermajer N., Muthuswamy R., Odunsi K., Edwards R. P., Kalinski P. (2011) PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 71, 7463–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Obermajer N., Wong J. L., Edwards R. P., Odunsi K., Moysich K., Kalinski P. (2012) PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol. Invest. 41, 635–657. [DOI] [PubMed] [Google Scholar]
- 129.Holcik M., Sonenberg N. (2005) Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 6, 318–327. [DOI] [PubMed] [Google Scholar]
- 130.Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529. [DOI] [PubMed] [Google Scholar]
- 131.Cláudio N., Dalet A., Gatti E., Pierre P. (2013) Mapping the crossroads of immune activation and cellular stress response pathways. EMBO J. 32, 1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bettigole S. E., Glimcher L. H. (2015) Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 33, 107–138. [DOI] [PubMed] [Google Scholar]
- 133.Zhang K., Kaufman R. J. (2008) From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mahadevan N. R., Anufreichik V., Rodvold J. J., Chiu K. T., Sepulveda H., Zanetti M. (2012) Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8⁺ T cell priming. PLoS One 7, e51845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mahadevan N. R., Rodvold J., Sepulveda H., Rossi S., Drew A. F., Zanetti M. (2011) Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc. Natl. Acad. Sci. USA 108, 6561–6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mahadevan N. R., Zanetti M. (2011) Tumor stress inside out: cell-extrinsic effects of the unfolded protein response in tumor cells modulate the immunological landscape of the tumor microenvironment. J. Immunol. 187, 4403–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Condamine T., Kumar V., Ramachandran I. R., Youn J. I., Celis E., Finnberg N., El-Deiry W. S., Winograd R., Vonderheide R. H., English N. R., Knight S. C., Yagita H., McCaffrey J. C., Antonia S., Hockstein N., Witt R., Masters G., Bauer T., Gabrilovich D. I. (2014) ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J. Clin. Invest. 124, 2626–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee B. R., Chang S. Y., Hong E. H., Kwon B. E., Kim H. M., Kim Y. J., Lee J., Cho H. J., Cheon J. H., Ko H. J. (2014) Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells. Oncotarget 5, 12331–12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thevenot P. T., Sierra R. A., Raber P. L., Al-Khami A. A., Trillo-Tinoco J., Zarreii P., Ochoa A. C., Cui Y., Del Valle L., Rodriguez P. C. (2014) The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity 41, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Condamine T., Gabrilovich D. I. (2014) Can the suppressive activity of myeloid-derived suppressor cells be “chop”ped? Immunity 41, 341–342. [DOI] [PubMed] [Google Scholar]
- 141.Zeng L., Liu Y. P., Sha H., Chen H., Qi L., Smith J. A. (2010) XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J. Immunol. 185, 2324–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goodall J. C., Wu C., Zhang Y., McNeill L., Ellis L., Saudek V., Gaston J. S. (2010) Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl. Acad. Sci. USA 107, 17698–17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Y. P., Zeng L., Tian A., Bomkamp A., Rivera D., Gutman D., Barber G. N., Olson J. K., Smith J. A. (2012) Endoplasmic reticulum stress regulates the innate immunity critical transcription factor IRF3. J. Immunol. 189, 4630–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao C., Pavicic P. G. Jr., Datta S., Sun D., Novotny M., Hamilton T. A. (2014) Cellular stress amplifies TLR3/4-induced CXCL1/2 gene transcription in mononuclear phagocytes via RIPK1. J. Immunol. 193, 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]