Induction of endotoxin tolerance increases expression of Pellino-3, which acts as an inhibitor of TLR-driven MyD88- and TRIF-dependent pathways, while Pellino-3 ablation attenuates TLR4 tolerization.
Keywords: Toll-like receptors, signal transduction, innate immunity, inflammation, lipopolysaccharide
Abstract
Development of endotoxin tolerance in macrophages during sepsis reprograms Toll-like receptor 4 signaling to inhibit proinflammatory cytokines without suppressing anti-inflammatory and antimicrobial mediators and protects the host from excessive inflammation and tissue damage. However, endotoxin tolerance renders septic patients immunocompromised and unable to control secondary infections. Although previous studies have revealed the importance of several negative regulators of Toll-like receptor signaling in endotoxin tolerance, the role of Pellino proteins has not been addressed. The present report shows that the induction of endotoxin tolerance in vivo in mice and in vitro in human monocytes and THP-1 and MonoMac-6 macrophages increases the expression of Pellino-3. Overexpression of Pellino-3 in human embryonic kidney 293/Toll-like receptor 2 or 293/Toll-like receptor 4/myeloid differentiation factor-2 cells inhibited Toll-like receptor 2/4-mediated activation of nuclear factor-κB and induction of CXCL-8 mRNA, and Pellino-3 ablation increased these responses. Pellino-3-deficient THP-1 cells had elevated Toll-like receptor 2/4-driven tumor necrosis factor-α, interleukin-6 mRNA, and Toll-like receptor 4-driven CCL5 gene expression in response to Toll-like receptor agonists and heat-killed Escherichia coli and Staphylococcus aureus, cytokines controlled by the MyD88 and Toll-interleukin-1R domain-containing protein inducing interferon-β-mediated pathways, respectively. In addition, deficiency in Pellino-3 slightly increased phagocytosis of heat-killed bacteria. Transfected Pellino-3 inhibited nuclear factor-κB activation driven by overexpression of MyD88, TIR domain-containing adapter inducing interferon-β, interleukin-1R-associated kinase-1, and tumor necrosis factor receptor activator of nuclear factor-κB-binding kinase-1, TGF-β-activated kinase 1, and tumor necrosis factor receptor-associated factor-6, and inhibited interleukin-1R-associated kinase 1 modifications and tumor necrosis factor receptor activator of nuclear factor-κB-binding kinase 1 phosphorylation. Finally, Pellino-3 ablation in THP-1 decreased the extent of endotoxin tolerization. Thus, Pellino-3 is involved in endotoxin tolerance and functions as a negative regulator of Toll-like receptor 2/4 signaling.
Introduction
Macrophages, dendritic cells, and neutrophils are equipped with PRRs that activate immediate and early innate host defense mechanisms and prime adaptive immune responses to protect the host from microbial infection [1]. PRRs include membrane-associated TLRs and cytosolic sensors such as nucleotide binding and oligomerization domain-containing LRR receptors, retinoic acid-inducible gene I-like receptors, and absent in melanoma 2-like receptors [2]. Thirteen mammalian TLRs share a common structural organization containing ectodomains with multiple LRRs involved in ligand recognition and co-R interactions, transmembrane regions, and intracellular tails with signaling TIR domains [3]. They are expressed on the cell surface (TLR1, TLR5, TLR6), in intracellular endosomes (TLR3, TLR7/8, TLR9, TLR11), or in both compartments (TLR2 and TLR4), and sense bacterial lipoproteins (TLR2-TLR1 or TLR2-TLR6), gram-negative bacterial LPS (TLR4), flagellin (TLR5), or microbial dsRNA (TLR3), ssRNA (TLR7/8), and DNA (TLR9) [4].
Combined with coreceptors (e.g., CD14, MD-2, and CD36), TLRs recognize microbial ligands and endogenous “alarmins” and dimerize, bringing intracellular TIR domains together to create docking platforms in order to enable recruitment of downstream signaling adapters and kinases [4]. All TLRs, except for TLR3, activate a common MyD88-dependent pathway that involves recruitment of MyD88 to TLRs via homotypical TIR-TIR domain interactions, forming a scaffold to recruit IRAK4 proteins that cluster together with MyD88 [2, 3], forming the “myddosome” complex [5]. IRAK4 undergoes auto-transphosphorylation, which activates its kinase activity [6], leading to IRAK4-mediated phosphorylation and activation of IRAK1 and IRAK2 and recruitment and activation of TRAF6 and TAK1 [3]. TAK1 activates MAPK and the inhibitor of NF-kB kinase, IKK-β, leading to activation and nuclear translocation of transcription factors (activator protein-1, NF-κB), which stimulate transcription of inflammatory cytokine genes [2]. TLR3 exclusively uses TRIF to signal expression of proinflammatory cytokines and type I IFNs [7]. TLR4 signals from the cell surface via the MyD88-dependent pathway to induce proinflammatory cytokines and translocates to endosomes, where it engages TRIF to signal type I IFN production via activation of TBK1 and IKK-ε and mediates delayed activation of MAPK and NF-κB via the RIP1-TAK1 module [8]. Collectively, TLR signaling induces expression of cytokines, chemokines, and type I IFNs by macrophages, DCs, and neutrophils and leads to up-regulated expression of MHC and costimulatory molecules (CD80, CD86) on APCs, priming adaptive immune responses [3, 4].
Because of its potential to induce tissue damage and immunopathology, TLR signaling is fine-tuned by a plethora of positive and negative regulators [9]. One class of such regulators, Pellinos, comprise a family of E3 Ub ligases with an N-terminal forkhead-associated domain responsible for interactions with phospho-Thr-expressing targets, and a C-terminal really interesting new gene-like domain that mediates Ub ligase activity [10]. Despite structural similarity, Pellino-1, Pellino-2, and Pellino-3 (with splice variants 3a and 3b in humans) exhibit nonredundant multiple regulatory effects on IL-1R and TLR signaling by exerting degradative, K48-linked polyubiquitination (e.g., c-Rel degradation in CD4+ T cells) or signal-promoting K63-linked ubiquitination (e.g., RIP-1 in myeloid cells) of different members of the TLR signaling intermediates [10]. Recent reports have uncovered regulatory functions of different members of the Pellino family (reviewed in [10]); however, published controversial data and incomplete understanding of Pellinos role in TLR signaling [10] require additional studies.
Sepsis development involves an initial proinflammatory phase characterized by exuberant production of proinflammatory cytokines (systemic inflammatory response syndrome) that often leads to fatal outcomes. Septic patients surviving this “cytokine storm” develop profound immune suppression and become immunocompromised and unable to counteract secondary infections [11–13]. Monocytes from such immunocompromised septic patients show impaired TLR4-induced production of proinflammatory cytokines while retaining or increasing expression of anti-inflammatory and antimicrobial mediators [14, 15], reminiscent of the endotoxin-tolerant phenotype. Endotoxin tolerance has been defined as reprogramming of TLR4 responses to LPS after previous exposure to endotoxin and is thought to protect the host from damage caused by exuberant production of proinflammatory mediators and to impair the abilities of macrophages, neutrophils, and DCs to counteract secondary infection [16]. Studies by us and others have uncovered the mechanisms of reprogramming of TLR4 signaling in LPS-tolerized cells, including deficient LPS-mediated tyrosine phosphorylation of TLR4 and Mal, impaired activation of IRAK4 and TBK1, and increased expression of negative regulators of TLR4 signaling, such as A20, SOCS-1, SH2 inositol phosphatase 1, and IRAK-M [17–28]. However, the involvement of Pellino-3 in endotoxin tolerance has not been studied, and its role in the regulation of TLR signaling is incompletely understood. In the present study, we report new findings indicating that endotoxin tolerance up-regulates expression of Pellino-3 and provide several lines of evidence indicating Pellino-3 is a negative regulator of TLR4 signaling whose ablation attenuates induction of the LPS-tolerant phenotype.
MATERIALS AND METHODS
Reagents and cell culture
Highly purified LPS from Escherichia coli K12, Pam3Cys, and heat-killed Staphylococcus aureus were from InvivoGen (San Diego, CA, USA). pHrodo Red-conjugated, heat-killed E. coli and S. aureus were from Life Technology (Carlsbad, CA, USA), and heat-killed E. coli have been described previously [29]. Abs against p-p38, p-p65, p38, p65, IκB-α, and tubulin were from Cell Signaling Technology (Beverley, MA, USA). Anti-Pellino-1 Ab was a gift from Dr. Peter Cheung (Nanyang Technological University, Singapore), anti-Pellino-3 and anti-actin Abs were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and anti-Flag Ab was from Sigma-Aldrich (St. Louis, MO, USA). Human THP-1 cells were obtained from American Type Culture Collection (Manassas, VA, USA), and the MonoMac6 cells [30] were kindly provided by Dr. Jorge Cervantes (University of Connecticut Health Center, Farmington, CT, USA). HEK293 cells stably expressing YFP-TLR2 (293/TLR2) or YFP-TLR4 and MD2 (293/TLR4/MD2) have been described previously [17, 18, 31, 32]. HEK293 cells were maintained in DMEM supplemented with 10% FBS (HyClone, Logan, UT, USA), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) (cDMEM); 293/TLR2 and 293/TLR4/MD2 cells were cultured in cDMEM containing 5 μg/ml puromycin or 1 mg/ml G418, respectively [31, 32]. THP-1 and MonoMac6 were cultured in RPMI 1640 medium supplemented with 10% FBS (HyClone), 5 × 10−5 M β-mercaptoethanol, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (cRPMI). Human monocytes were provided by Dr. Larry Wahl (National Institute of Dental and Craniofacial Research) as de-identified samples prepared by counter flow elutriation of blood from healthy human volunteers or purchased from Lonza (Walkersville, MD, USA). Studies with human monocytes were approved by the institutional review boards of the University of Connecticut Health Center and University of Maryland School of Medicine. THP-1 cells were differentiated for 72 h with 20 ng/ml PMA to attain macrophage characteristics, as reported previously [33].
Mice and macrophage isolation
C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were injected intraperitoneally with 3% thioglycollate (REMEL Inc., Lenexa, KS, USA). After 72 h, the mice were administered PBS (control groups) or LPS (20 μg per mouse) for in vivo induction of endotoxin tolerance [21]. At 24 h, peritoneal macrophages were obtained by peritoneal lavage and adherence to plastic. The cells were plated into 6-well plates (4 × 106 cells per well) and cultured in cRPMI 1640 medium. All animal procedures were performed with institutional animal care and use committee approval.
Recombinant plasmids and transfection
pELAM-Luc, p-TK-Renilla-Luc, pEFBOS-Flag-MD2, pcDNA3-AU1-MyD88, pcDNA3-CD14, pcDNA3-TRIF, pRK5-IRAK1, pcDNA3-FLAG-TRAF6, pCMV1-FLAG-TAK1, pcDNA3-hemagglutinin-TAB1, pcDNA3-FLAG-p65 were used as published previously [17–21, 29, 31, 32], and pSuper-TBK1 (plasmid no. 26210) was from Addgene (Cambridge, MA, USA). Flag-Pellino-3b expression vector has been described previously [34] and was kindly provided by Dr. Xiaoxia Li (Lerner Research Institute, Cleveland Clinical Foundation, Cleveland, OH, USA). GIPZ expression vectors encoding scrambled or Pellino-3 shRNA were obtained from GE Health Care/Dharmacon (Lafayette, CO, USA), packaging vectors pSPAX2 (plasmid no. 12260) and pMD2.G (plasmid no. 12259) were from Addgene. HEK293T, 293/TLR2, and 293/TLR4/MD2 cells were plated in 100-mm dishes (for immunoprecipitation), 6-well or 24-well plates (for gene expression studies and reporter assays, respectively), and transiently transfected for 3 h with the respective plasmids, using Lipofectamine 2000 transfection reagent (Life Technologies). To obtain stable transfectants, 293/TLR4/MD2 cells transfected with the plasmids encoding scrambled shRNA or Pellino-3 shRNA were selected in cDMEM with 5 μg/ml puromycin (Sigma-Aldrich), as reported previously [34].
Lentiviral transduction of THP-1 cells
To generate lentiviral particles, HEK293T cells were plated in 6-well plates (2 × 106 cells per well) and transfected with GIPZ shRNA (1 μg/well), pSPAX2 (0.75 μg/well), and pMD2.G (0.25 μg/well) using Lipofectamine 2000, according to the manufacturer’s instruction. After 48 h, medium containing viral particles was collected, and fresh cDMEM was added, the cells were cultured for additional 48 h, and viral particle-containing medium was collected. After filtration through 0.45-μM filters, the medium was centrifuged (16,000g, 2 h), the pellet was resuspended in 0.25 ml of cRPMI with polybrene (2 μg/ml), and the mixture was added to the wells of 24-well plates containing THP-1 cells. The plates were centrifuged for 45 min at 1200g, the medium was replaced with fresh cRPMI containing 5 μg/ml puromycin, and stable transfectants were selected for at least 2 wk.
Isolation of RNA and RT-qPCR
Total RNA was isolated using TRIzol (Life Technologies), residual genomic DNA was digested with DNase, and RNA was repurified, as recommended by the manufacturer. cDNA was prepared from 1 μg total RNA using the Reverse Transcription System (Promega, Madison, WI, USA), and examined by qPCR with primers for the following genes: human hypoxanthine phosphoribosyltransferase (HPRT), 5′-ACCAGTCAACAGGGGACATAAAAG-3′ (forward); 5′-GTCTGCATTGTTTTGCCAGTGTC-3′ (reverse); human CXCL-8, 5′-ACCGGAAGGAACCATCTC ACT-3′ (forward); 5′-TGCACCTTCACACAGAGCTGC-3′ (reverse), human CCL5, forward: 5′-TTTGTCACCCGAAAGAACCG-3′, reverse: 5′-CAAGGACTCTCCATCCTAGCTCAT-3′; human IFN-β, 5′-ACTGCCTCAAGGACAGGATG-3′ (forward), 5′-AGCCAGGAGGTTCTCAACAA-3′ (reverse), human PELLINO-3b, 5′-TCGTCCTGGGCTACAATGGTTG-3′ (forward), 5′-TACGAGATGCTGTGCTGACC-3′ (reverse); mouse Hprt, 5′-ACCAGTCAACAGGGGACATAAAAG-3′ (forward), 5′-GTCTGCATTGTTTTGCCAGTGTC-3′ (reverse); mouse Il-6, 5′-TCAGGAAATTTGCCTATTGAAAATTT-3′ (forward), 5′-GCTTTGTCTTTCTTGTTATCTTTTAAGTTGT-3 (reverse); and mouse Pellino-3, 5′-ACATGCCAACGGAGTGAAGC-3′ (forward), 5′-AGCGGCCAATCTGGAACAT-3′ (reverse), on a MyIQ RT-qPCR machine (Bio-Rad, Hercules, CA, USA). The data were analyzed as reported previously [35].
Nucleofection
Expression vectors encoding scrambled or Pellino-3 shRNA species were introduced into THP-1 cells by nucleofection, using the Nucleofector I device (Lonza) and the cell line nucleofection kit V (Lonza), as recommended by the manufacturer. The cells were differentiated for 48 h with 20 ng/ml PMA, washed, and treated for 3 h with medium or LPS (100 ng/ml). RNA was isolated, reverse transcribed, and analyzed by RT-qPCR, using gene-specific primers.
Coimmunoprecipitation and immunoblotting
Cell lysates were prepared, precleared with protein G-agarose (Roche Applied Science, Indianapolis, IN, USA) according to [21, 31], and incubated overnight at 4°C with the respective Abs in lysis buffer containing 20 mM HEPES (pH 7.4), 0.5% Triton X-100, 150 mM NaCl, 12.5 mM β-glycerophosphate, 50 mM NaF, 1 mM DTT, 1 mM sodium orthovanadate, 2 mM EDTA, 1 mM PMSF, and protease inhibitor cocktail (Roche Applied Science). Immune complexes were pulled down by incubation for 4 h with protein G-agarose (45 μl per sample). Beads were washed 5 times with lysis buffer and resuspended in Laemmli sample buffer containing 50 mM Tris-C, (pH 6.8/10% glycerol), 2% SDS, 0.1% bromophenol blue, and 5% 2-mercaptoethanol. Samples were boiled for 10 min, separated by SDS-PAGE on 4–20% mini-gels (Life Technologies), electrotransferred to Immobilon-P membranes, blocked, and probed with the respective Abs, as previously described [17, 20, 21, 31]. Quantification was performed using the NIH ImageJ software package (Bethesda, MD, USA).
NF-κB reporter assays
293/TLR2 and 293/TLR4/MD2 cells were transfected with plasmids, using Lipofectamine 2000, recovered for 48 h, and treated for 5 h with medium or stimuli, as indicated. The cells were lysed in a passive lysis buffer (Promega), and firefly vs. Renilla luciferase activities were measured using a dual luciferase reporter assay system (Promega) on a Berthold LB 9507 luminometer (Berthold Technologies, Oak Ridge, TN, USA).
Phagocytosis assay and flow cytometry
THP-1 cells were plated in 24-well plates (0.5 × 106 cells per well), incubated with 20 ng/ml PMA for 72 h, washed, and resuspended in cRPMI with or without pHrodo-conjugated heat-killed E.coli or S. aureus bioparticles at bacteria per cell ratio of 50:1. After incubation for 2 h on ice or at 37°C, the cells were detached, washed in ice-cold PBS containing 3% FBS, stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Life Technologies), fixed for 15 min in 3.7% formaldehyde, and analyzed by FACS on an LSRII Flow Cytometer (BD Biosciences, San Diego, CA, USA) to measure pHrodo fluorescence. The data were analyzed using the FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5 program for Windows (GraphPad Software Inc., San Diego, CA, USA). Statistically significant differences were evaluated using Student’s t test with the confidence interval set at the 95% level. The results are expressed as the mean ± sd values.
Online supplemental material
These materials include Supplemental Figures 1 and 2 and their legends.
RESULTS
Endotoxin tolerance leads to increased and sustained expression of Pellino-3
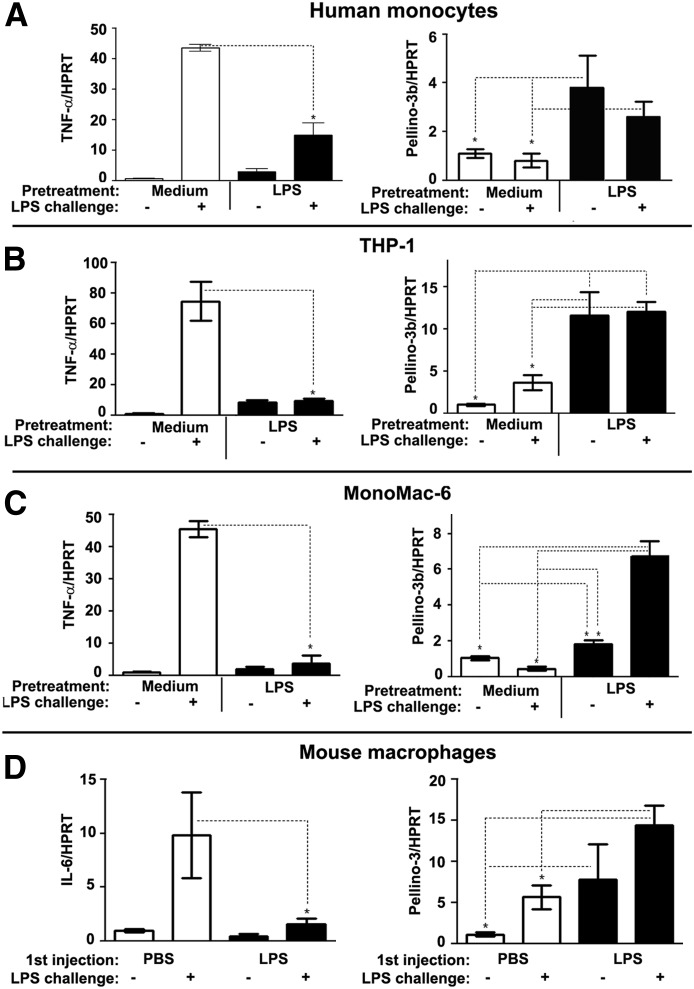
To analyze the role of Pellino-3 in endotoxin tolerance, we first examined Pellino-3b mRNA levels in control and LPS-tolerized human monocytes, THP-1 cells, or MonoMac-6 cells. THP-1 and MonoMac-6 cells were differentiated with PMA to attain macrophage characteristics before treatment (CD14 expression, phagocytosis, adhesion) [33]. Figure 1 shows that previous exposure of monocytes, THP-1 cells, or MonoMac-6 cells to endotoxin reduced LPS-inducible expression of TNF-α mRNA by 71–89% compared with the responses observed in medium-pretreated cells (Fig. 1, left), demonstrating endotoxin tolerance. LPS tolerization increased the levels of Pellino-3b mRNA in monocytes, MonoMac-6 cells, and THP-1 cells by 3.9-, 1.9-, and 10-fold, which remained sustained (monocytes and THP-1 cells; Fig. 1A and B) or increased (from 1.9- to 6.5-fold in MonoMac-6 cells; Fig. 1C) in response to LPS challenge (Fig. 1, right). Macrophages from mice tolerized with endotoxin in vivo [36] had reduced levels of IL-6 mRNA in response to LPS challenge in vitro by 50–75% (Fig. 1D, left) but showed 2–5-fold increases in Pellino-3 mRNA (Fig. 1D, right) compared with the responses in the macrophages from PBS-treated mice.
Figure 1. Induction of endotoxin tolerance in vitro and in vivo leads to increased and maintained expression of Pellino-3 mRNA.
Human monocytes (A), THP-1 cells (B), and MonoMac-6 cells (C) were pretreated for 20 h with medium or 100 ng/ml LPS, washed 3 times, and challenged for 3 h with medium or 100 ng/ml LPS. (D) C57BL/6J mice were intraperitoneal administered thioglycollate. After 72 h, they were intraperitoneal injected with PBS or LPS (20 μg per mouse). After 20 h, peritoneal macrophages were obtained from peritoneal exudate cells by adherence to plastic and treated for 3 h with medium or 100 ng/ml LPS. RNA was isolated, reverse transcribed, and analyzed by RT-qPCR analyses with primers specific for HPRT, TNF-α, and Pellino-3 genes. Shown are the data (mean ± sd) of a representative (n = 3) experiment.
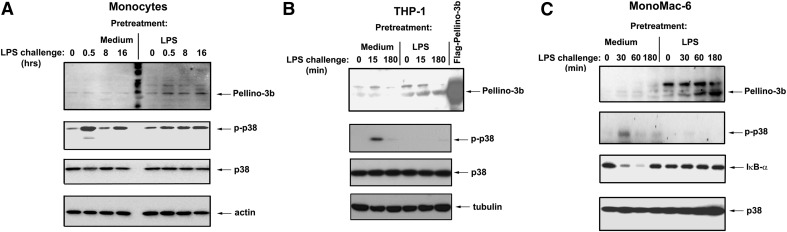
Next, we studied the effect of endotoxin tolerance on Pellino-3b protein expression. Previous exposure to LPS imparted the endotoxin-tolerant phenotype in human monocytes, THP-1 cells, and MonoMac6 macrophages, as evidenced by impaired LPS-inducible phosphorylation of p38 and degradation of IκB-α, in contrast to the robust LPS responses observed in medium-pretreated cells (Fig. 2A–C; Supplemental Fig. 1). Induction of endotoxin tolerance in human monocytes and macrophages led to increased Pellino-3b protein expression by twofold compared with the control, medium-pretreated cells exposed to medium (Fig. 2A-C, top; Supplemental Fig. 1). LPS challenge of endotoxin-tolerized cells further up-regulated Pellino-3b levels compared with those seen in LPS-treated control cells, resulting in 2.6–3.1-, 2.8–5.0-, and 3.5–4.0-fold increases for monocytes, THP-1 cells, and MonoMac-6 cells, respectively (Fig. 2A–C; Supplemental Fig. 1). Collectively, these results indicate that the induction of endotoxin tolerance in vitro and in vivo leads to increased and sustained expression of Pellino-3.
Figure 2. Endotoxin-tolerized THP-1 and MonoMac-6 cells exhibit increased and sustained expression of Pellino-3 proteins.
After 20 h of exposure to medium or 100 ng/ml LPS, monocytes (A), THP-1 cells (B), and MonoMac-6 cells (C) were washed 3 times and restimulated with 100 ng/ml LPS or treated with medium for the indicated times. Cell extracts were prepared and subjected to Western blot analyses to examine expression of p-p38, total p38, IκB-α, Pellino-3, actin, and tubulin proteins. Samples from HEK293T cells transfected with expression vectors encoding Flag-Pellino-3 were examined side-by-side as positive controls for Pellino-3 expression (left).
Pellino-3b negatively regulates NF-κB activation and cytokine gene expression in response to TLR2- or TLR4 agonists
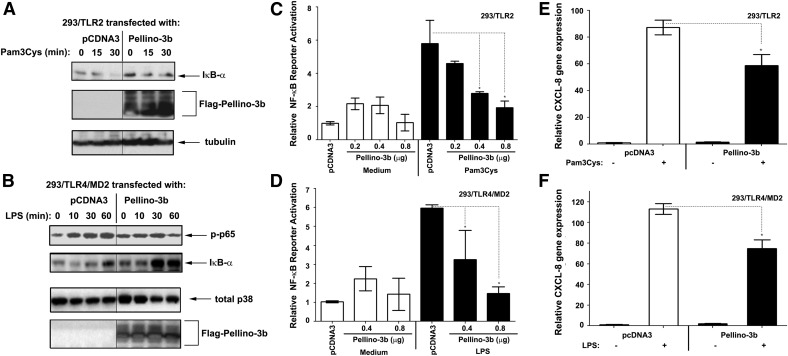
To examine the regulation of TLR2 and TLR4 signaling by Pellino-3b, we first examined the effect of Pellino-3b overexpression in 293/TLR2 and 293/TLR4/MD2 cells on Pam3Cys- or LPS-mediated NF-κB activation and CXCL-8 gene expression. Transfection of Pellino-3b decreased Pam3Cys-and LPS-induced IκB-α degradation (Fig. 3A), p65 phosphorylation (Fig. 3B and data not shown), NF-κB reporter activation (Fig. 3C and D) and expression of the NF-κB-dependent CXCL-8 gene [37] (Fig. 3E and F) compared with the responses seen in pcDNA3-transfected cells (Fig. 3; Supplemental Fig. 2).
Figure 3. Overexpression of Pellino-3 suppresses TLR2- and TLR4-inducible NF-κB activation and CXCL-8 gene expression.
293/TLR2 (A, C, E) and 293/TLR4 (B, D, F) cells were transiently transfected with pcDNA3-CD14, pEFBOS-MD2, and the Flag-Pellino-3-encoding plasmid. For NF-κB reporter assays (C, D), cells were cotransfected with pELAM-Luc (NF-κB reporter) and pTK-RL (to normalize for transfection efficiency). After recovery for 48 h, cells were treated with 1 μg/ml Pam3Cys (A, C, E) or 100 ng/ml LPS (B, D, F), as indicated (A, B), for 5 h (C, D), or for 3 h (E, F). Cell lysates were subjected to Western blotting with Abs for the analyzed proteins (A, B) or used to examine firefly vs. Renilla luciferase activities (C, D). RNA was isolated, converted to cDNA, and analyzed by RT-qPCR with primers specific for CXCL-8 and HPRT. The results of a representative experiment (n = 3) are shown. *P < 0.05.
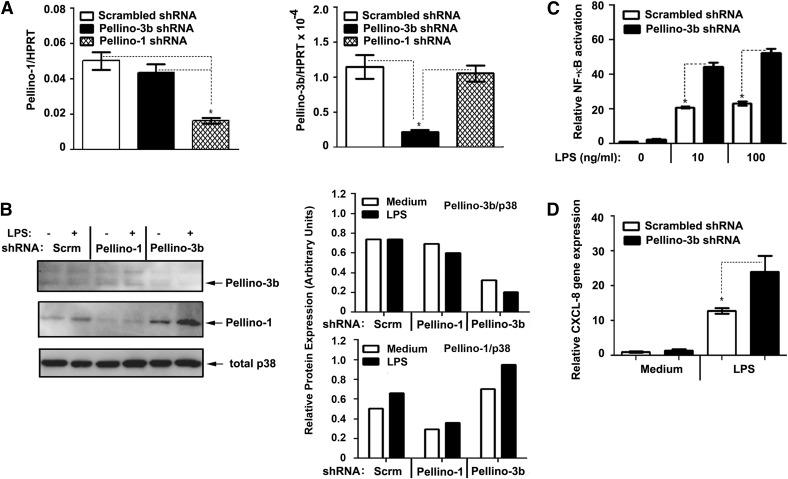
Next, we performed shRNA-based knockdown of Pellino-3 in 293/TLR4/MD2 cells and analyzed LPS-mediated NF-κB reporter activation and CXCL-8 gene expression. 293/TLR4/MD2 cells expressing Pellino-3 shRNA had significantly reduced levels of Pellino-3 mRNA (Fig. 4A) and protein (Fig. 4B) compared with cells expressing scrambled shRNA or unrelated Pellino-1 shRNA. In contrast, Pellino-3b shRNA introduction did not suppress expression of Pellino-1 and p38 proteins (Fig. 4A and B), demonstrating the specificity of Pellino-3 knockdown. 293/TLR4/MD2 cells expressing Pellino-3b shRNA responded to LPS stimulation by 2–2.5-fold increases in the induction of NF-κB reporter (Fig. 4C) and up-regulated CXCL-8 mRNA expression by 2.3-fold more (Fig. 4D) compared with cells transfected with scrambled shRNA. These data show that Pellino-3b negatively regulates TLR2- and TLR4-mediated NF-κB activation and CXCL-8 gene expression.
Figure 4. Pellino-3 knockdown in 293/TLR4/MD2 cells enhances LPS-mediated activation of NF-κB and expression of CXCL-8 mRNA.
293/TLR4 cells stably expressing scrambled, Pellino-3 shRNA (A–D) or Pellino-1 shRNA (A, B) were transiently transfected with pEFBOS-MD2 (A–D) alone or together with pELAM-Luc and pTK-Renilla-Luc (C). The cells were incubated for 3 h with medium (A), medium or 100 ng/ml LPS (B), or treated for 5 h (C) and 3 h (D) with medium, 10 or 100 ng/ml LPS (C, D). A, D) RNA was isolated, converted to cDNA, and subjected to RT-qPCR with primers specific for Pellino-1, Pellino-3, CXCL-8, and HPRT to examine expression of the depicted genes normalized to HPRT levels. B) Cell lysates were examined by Western blot analysis to determine the expression of Pellino-1 and Pellino-3 proteins relative to total p38 levels. (Right panels) Quantification of the immunoblots shown on left. C) Cell lysates were assayed for firefly vs. Renilla luciferase activities. Shown are the results of a representative experiment. Similar data were obtained in at least 3 other independent experiments. *P < 0.05.
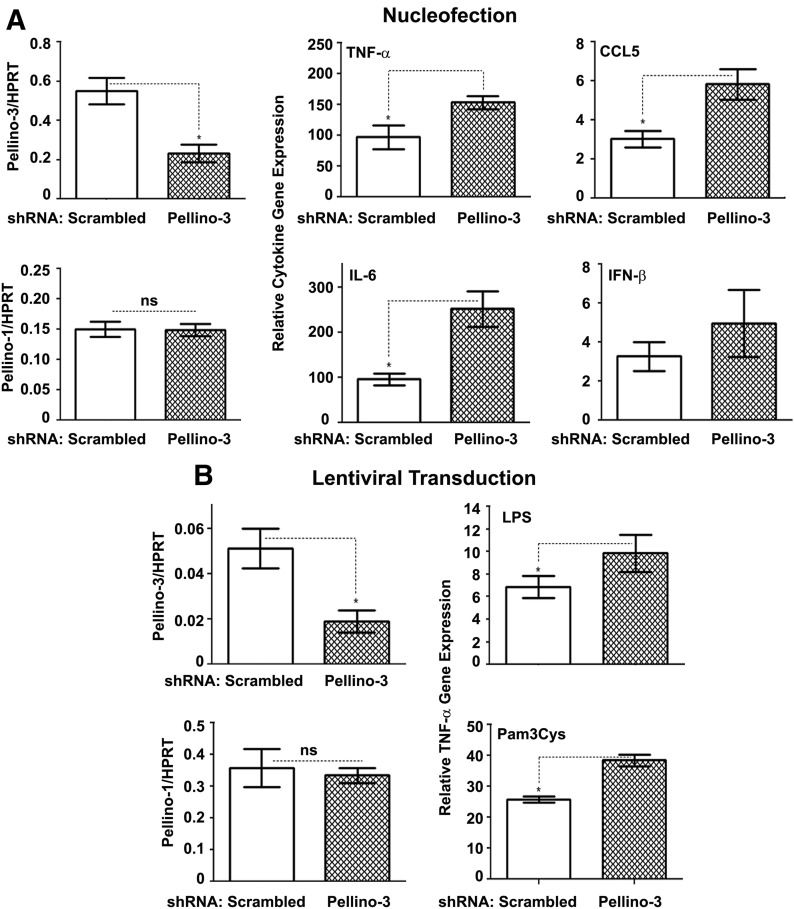
To extend our findings obtained in HEK293 transfectants to cells with macrophage-like phenotype, we used nucleofection- or lentiviral-based introduction of Pellino-3 or scrambled shRNA species into THP-1 cells. We then analyzed the responses of PMA-differentiated THP-1 macrophages to TLR2 and TLR4 agonists. THP-1 cells nucleofected (Fig. 5A, left column) or lentivirally transduced (Fig. 5B, left columns) with Pellino-3 shRNA had decreased Pellino-3b mRNA levels by 61–65% compared with scrambled shRNA-transfected cells but exhibited unaltered Pellino-1 mRNA levels, demonstrating specific ablation of Pellino-3. LPS stimulation of THP-1 cells nucleofected with scrambled shRNA led to ∼100-fold increases in the expression of TNF-α and IL-6 mRNA, and Pellino-3 knockdown further increased their expression to 151- and 225-fold, respectively (Fig. 5A, middle columns). Pellino-3b ablation also increased the LPS-driven induction of TRIF-dependent CCL5 and showed a trend toward augmented expression of IFN-β (Fig. 5A, right columns) compared with the responses exhibited by cells expressing scrambled shRNA. Compared with scrambled shRNA-expressing THP-1 cells, lentiviral transduction of Pellino-3 shRNA led to 40–50% increases in the expression of TNF-α mRNA in response to LPS and Pam3Cys, respectively (Fig. 5B, right). These findings indicate that Pellino-3 acts as an inhibitor of TLR2- and TLR4-mediated activation of NF-κB and induction of cytokine genes.
Figure 5. Pellino-3 ablation in THP-1 cells up-regulates the induction of cytokine genes in response to stimulation with Pam3Cys and LPS.
THP-1 cells were nucleofected (A) or lentivirally transduced (B) with scrambled or Pellino-3 shRNA and either used immediately for differentiation (A) or selected with puromycin (B). Cells were differentiated for 72 h with 20 ng/ml PMA, washed, and treated for 3 h with medium, 100 ng/ml LPS (A, B) or 1 μg/ml Pam3Cys (B). RNA was isolated, reverse transcribed, and examined by RT-qPCR with primers specific for Pellino-1, Pellino-3, HPRT, TNF-α, IL-6, CCL5, and IFN-β. The data of a representative (of 3) experiment is presented. *P < 0.05.
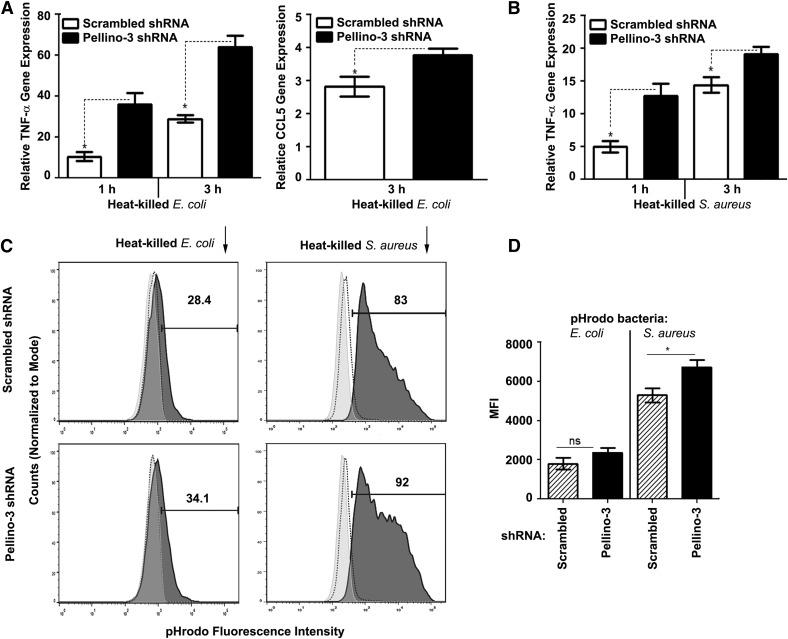
Pellino-3b ablation in THP-1 cells increases cytokine gene expression in response to heat-killed E. coli or S. aureus but only moderately affects bacterial phagocytosis
Next, we studied the effect of Pellino-3 deficiency in THP-1 macrophages on the expression of MyD88- and TRIF-dependent cytokines upon stimulation with heat-inactivated E. coli and S. aureus. Transduction of Pellino-3 shRNA in THP-1 cells resulted in the augmented expression of TNF-α mRNA in response to stimulation with E. coli by 2.8–2.9-fold and increased the levels of CCL5 mRNA compared with the responses of nontargeting shRNA-expressing cells (Fig. 6A). Likewise, THP-1 cells lacking Pellino-3 exhibited up to a 2.4-fold increase in the expression of TNF-α upon stimulation with heat-killed S. aureus (Fig. 6B).
Figure 6. Pellino-3 deficiency enhances cytokine gene expression in response to heat-killed E. coli or S. aureus but only moderately affects bacterial phagocytosis.
THP-1 cells stably expressing scrambled or Pellino-3 shRNA after lentiviral transduction and puromycin selection were differentiated with PMA and incubated for 3 h with heat-killed E. coli (A) or S. aureus (B) or were treated for 2 h with pHrodo-conjugated E. coli or S. aureus bioparticles (C, D) (bacteria per macrophage ratio, 10:1). (A, B) RNA was isolated, reverse transcribed, and subjected to RT-qPCR analyses of the indicated cytokine genes. (C) After incubation with pHrodo-labeled heat-killed bacteria, the cells were washed, fixed, and examined by FACS to detect changes in pHrodo fluorescence. (D) Quantification of the results from triplicate wells of a representative (n = 3) experiment based on MFI values and percentages of pHrodo-positive cells. The data of a representative experiment (n = 3) are shown (A–D).
Because TLR signaling modulates phagocytosis [38–40] and Pellinos regulate TLR signal transduction [10], we examined how Pellino-3 deficiency affects phagocytosis of E. coli and S. aureus in THP-1 cells. To measure phagocytosis, we used pHrodo Red-conjugated heat-killed bacteria that exhibit fluorescence of pH-sensitive pHrodo Red on its localization in phagolysosomes undergoing acidification but do not emit fluorescence under basal conditions [41]. Incubation of pHrodo-conjugated E. coli and S. aureus with THP-1 cells expressing scrambled shRNA for 2 h at 37°C markedly increased MFI values compared with the values observed in cultures incubated on ice. Pellino-3 knockdown further augmented MFI values by 22–33% and increased the number of pHrodo-positive cells by 11–20% (Fig. 6C and D). Thus, Pellino-3 positively regulates the expression of MyD88- and TRIF-dependent cytokine genes in response to heat-killed E. coli and S. aureus and moderately enhances bacterial phagocytosis.
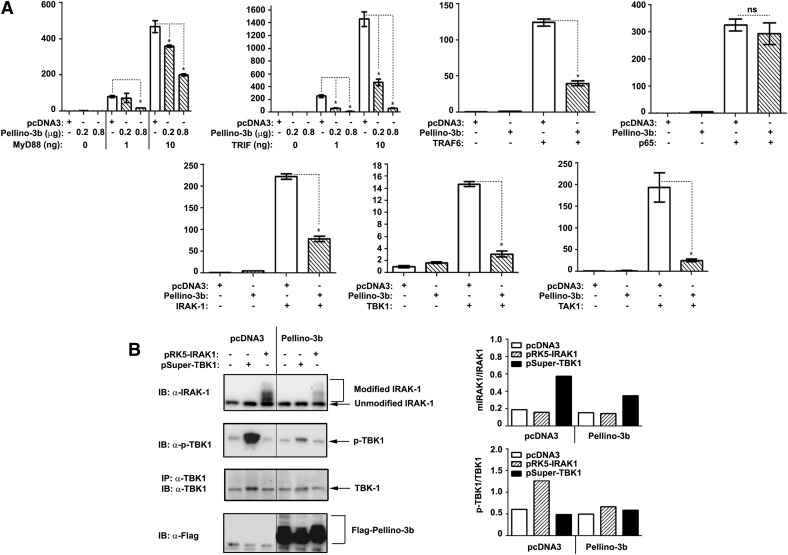
Pellino-3b inhibits NF-κB reporter activation induced by overexpression of MyD88, TRIF, IRAK1, TBK1, or TAK1, suppresses IRAK1 modifications and TBK1 phosphorylation but does not affect p65-inducible responses
To position Pellino-3 within the MyD88- and TRIF-dependent cascades, we transfected HEK293T cells with a series of adapter proteins or kinases to activate a co-transfected NF-κB luciferase reporter in HEK293T cells and examined the effect of overexpressed Pellino-3b. Overexpressed TLR pathway components initiate signaling downstream in a ligand-independent fashion due to their ability to dimerize, bypassing the requirement for upstream activators [34, 42, 43]. Ectopic expression of Pellino-3b markedly down-regulated MyD88- and TRIF-driven NF-κB reporter activation by 2.5–35-fold (Fig. 7A, top 2 left panels) and blunted NF-κB transactivation driven by IRAK1, TAK1, and TBK1 (Fig. 7A, bottom panels). Transfected Pellino-3b also markedly mitigated the ability of overexpressed TRAF6 to mediate activation of the pELAM-Luc NF-κB luciferase reporter but did not affect p65-driven NF-κB activation (Fig. 7A, top 2 right panels). In line with constitutive activation of overexpressed kinases [18, 19, 42, 44, 45], transfected IRAK1 and TBK1 showed post-translational modifications (IRAK1) and phosphorylation (TBK1), and coexpressed Pellino-3b decreased the abundance of modified species of IRAK1 and phosphorylated TBK1 (Fig. 7B). These results indicate that Pellino-3b inhibits NF-κB activation driven by overexpression of MyD88, TRIF, IRAK1, TBK1, TAK1, and TRAF6 and mitigates modifications of IRAK1 and phosphorylation of TBK1 but does not affect p65-induced NF-κB transactivation.
Figure 7. Pellino-3b acts within the MyD88-IRAK1-TRAF6-TAK1 and TRIF-TBK1 signaling axes but does not affect p65-induced NF-κB reporter activation.
(A) HEK293T cells were transfected with pELAM-Luc (0.4 μg/well) and pTK-RL (20 ng/well) plus the indicated amounts of pcDNA3-AU1-MyD88 or pcDNA3-TRIF or expression vectors encoding IRAK1, TBK1, TRAF6, TAK1, or p65 (10 ng/well each), with or without p-Super-Flag-Pellino-3b (0.2 and 0.8 μg/well; 2 left panels in the first row of A; 0.8 μg/well for all other panels of A). After recovery for 48 h, cell lysates were assayed for firefly vs. Renilla luciferase activities. Relative luciferase units of firefly Luc were normalized to those for Renilla Luc, and the data were presented as the fold induction compared with the values in cells transfected with pcDNA3, pELAM-Luc, and pTK-RL taken as 1. (B) HEK293T cells were plated in 6-well plates and transfected with pcDNA3, pRK5-IRAK1, or pSuper-TBK1 (1 μg/well), along with pcDNA3 or Flag-Pellino-3b (1 μg/well). After recovery for 48 h, cell lysates were analyzed by Western blotting with anti-IRAK1, anti-p-TBK1, anti-TBK1 and anti-Flag Abs. The data of a representative (n = 3) experiment are shown.
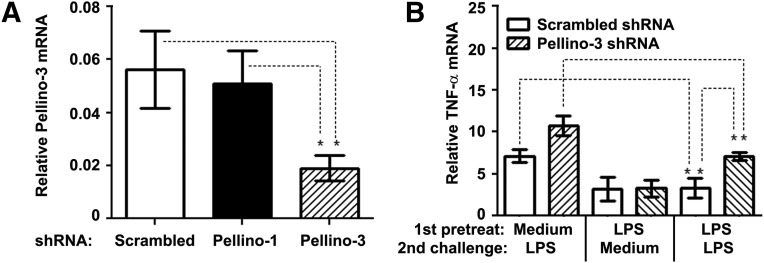
Pellino-3 ablation attenuates the extent of endotoxin tolerance
Having established Pellino-3b as a TLR2/4 negative regulator, it was important to delineate the functional significance of increased Pellino-3b expression in endotoxin-tolerized cells. Thus, we sought to mechanistically determine the effect of Pellino-3 deficiency on the capacity of macrophages to develop the LPS-tolerant phenotype. THP-1 cells stably expressing Pellino-3 shRNA had a 68% reduction in the expression of Pellino-3 mRNA compared with cells transduced with scrambled shRNA (Fig. 8A). Pellino-3 ablation did not affect expression of Pellino-1, and the introduction of Pellino-1 shRNA had no effect on Pellino-3 mRNA levels (Fig. 8A), showing the specificity of Pellino-3 targeting. Endotoxin pretreatment of THP-1 cells expressing scrambled shRNA mitigated the induction of TNF-α mRNA upon subsequent LPS challenge by 62% compared with medium-exposed macrophages, while LPS tolerization of THP-1 cells harboring Pellino-3 shRNA led to a 31% reduction (Fig. 8B). Thus, Pellino-3 knockdown ameliorates the extent of endotoxin tolerization, suggesting Pellino-3 as a critical mediator of endotoxin tolerance and regulator of TLR signaling.
Figure 8. Ablation of Pellino-3 significantly attenuates endotoxin tolerance induction.
THP-1 cells stably expressing scrambled, Pellino-1 or Pellino-3 shRNA were differentiated for 72 h with PMA, washed, and pretreated for 20 h with medium or 100 ng/ml LPS. After washing, the cells were treated for 3 h with medium or 100 ng/ml LPS, RNA was prepared, reverse-transcribed, and subjected to RT-qPCR analyses to examine the expression of Pellino-3 (A), TNF-α (B), and HPRT mRNA, using the corresponding gene-specific primers. The data of a representative experiment (n = 3) are depicted. *P < 0.05.
DISCUSSION
The development of sepsis-associated immunosuppression and the TLR-tolerant phenotype in macrophages of septic patients represents a serious challenge to therapeutic interventions [11, 12, 14, 15]. Recent findings have revealed the altered balance of several positive and negative regulators of TLR signaling as an important molecular mechanism of reprogramming of TLR4 signaling in endotoxin tolerance [16, 19, 20, 22, 24, 26–28]. Although these results uncovered A20 and SOCS-1 as important E3 Ub ligases that negatively regulate K63-linked polyubiquitination of IRAK1, TRAF6, and IKK-γ (A20) [20, 46–48] or induce K48-linked ubiquitination and degradation of MyD88-adapter-like (SOCS-1) [49], the role of the Pellino family of E3 Ub ligases has not been addressed. The present study has uncovered the critical role of Pellino-3b as a negative regulator of TLR2 and TLR4 signaling acting within the MyD88-IRAK1-TAK1 and TRIF-TBK1 signaling axis and indicates Pellino-3b as an important mediator of endotoxin tolerance.
The present report has demonstrated that the induction of endotoxin tolerance in vitro in primary human monocytes, THP-1 and MonoMac-6 macrophages leads to increased and sustained expression of Pellino-3b mRNA and protein. Likewise, endotoxin tolerization of mice in vivo after intraperitoneal endotoxin administration also increased Pellino-3 mRNA basal and LPS-inducible expression in peritoneal macrophages. Pellino-3 ablation in THP-1 cells led to a marked reduction in the extent of endotoxin tolerization, indicating its important role in TLR4 tolerance. To the best of our knowledge, this is the first demonstration of the role of Pellino-3 in endotoxin tolerance, adding Pellino-3b to A20 [20, 21, 26] and SOCS-1 [28] as critical E3 Ub ligases mediating TLR4 tolerance. The mechanism responsible for increased expression of Pellino-3b in endotoxin-tolerized macrophages is yet to be determined and very little is known about the transcriptional regulation of Pellino-3b expression. It is tempting to speculate that the preponderance of p50, p100, and RelB NF-κB subunits in the nucleus of endotoxin-tolerized macrophages [50–54] and the selective recruitment of other transcription factors (e.g., heat shock factor-1 [55]) to the promoter of the Pellino-3 gene could promote increased expression of Pellino-3.
Negative regulators of TLR signaling, including E3 Ub ligases A20 and SOCS-1 [20, 21, 25, 26, 28, 47], exhibit increased and sustained expression in LPS-tolerant macrophages, similar to Pellino-3b, and Pellino-3b exerts suppression of IL-1R signaling [34]. Therefore, we sought to determine a possible role of Pellino-3b in the regulation of TLR4-driven MyD88 and TRIF signaling cascades. We found that Pellino-3b overexpression decreased and Pellino-3b knockdown increased TLR2- and TLR4-mediated degradation of IκB-α, NF-κB reporter activation, and induction of CXCL-8 gene expression in HEK293 cells expressing the corresponding TLRs. These data extend previous results on the negative regulatory role of Pellino-3b in IL-1R signaling [34]. Ablation of Pellino-3b in THP-1 macrophages revealed its ability to affect both MyD88-dependent (TNF-α, IL-6) [56] and TRIF-dependent (IFN-β, CCL5) [7] cytokine gene expression. Our overexpression studies support this conclusion, showing Pellino-3b-mediated suppression of NF-κB activation caused by overexpression of MyD88, IRAK1, TAK1 or TRIF, and TBK1. Consistent with these results, we report that transfected Pellino-3b markedly decreased post-translational modifications of IRAK1 and phosphorylation of TBK1 in HEK293 cells overexpressing IRAK1 or TBK1 and blocked the ability of overexpressed IRAK1 and TBK1 to drive NF-κB reporter activation. To the best of our knowledge, this is the first demonstration of the ability of Pellino-3b to inhibit TBK1 phosphorylation and suppress TRIF-mediated signaling outcomes in the context of TLR4 signaling. Our data on Pellino-3b-mediated suppression of IRAK1 modifications and functions correspond with a similar effect of Pellino-3b on IL-1β-mediated IRAK1 modifications and activation [34] and the capacity of Pellino-3 to mitigate TLR3-mediated IRF7-mediated activation of type I IFN expression by ubiquitination of TRAF6 [57].
The mechanisms by which Pellino-3b deactivates IRAK1, TBK1, and MyD88- and TRIF-dependent signaling cascades in endotoxin tolerance are unknown. Pellino-3b could directly interact with IRAK1 [10, 58, 59] and, possibly, TBK1 [57] and mediate attachment of K48- or K63-linked polyUb moieties. In vitro ubiquitination assays showed the capacity of Pellinos to activate both types of ubiquitination [10, 34, 44]. However, experiments in cells demonstrated primarily K63-linked ubiquitination of Pellino substrates [34, 44], with a notable exception of Pellino-1-mediated K48-linked polyubiquitination of c-Rel in T cells [60]. Similar to a suggested role for Pellino-3b in IL-1R signaling [34], K63-linked polyubiquitination of IRAK1 by Pellino-3b might prevent IRAK1 interactions with the Skp1-Cullin1-F-box-β-TrCP1 E3 Ub ligase complex mediating K48-linked ubiquitination [61]. This has been proposed to stabilize IRAK1 expression and prevent dissociation of TRAF6 and TAK1 from the TLR/MyD88 signaling complex (resulting from IRAK1 degradation) to activate downstream signaling [34]. However, we consider this unlikely, because endotoxin-tolerant macrophages exhibit impaired TLR-mediated K63-linked polyubiquitination of IRAK1, TRAF6, and TAK1 [20, 21], and because K63-linked polyubiquitination has been generally accepted to promote protein-protein interactions and signaling [9, 62]. It is tempting to speculate that Pellino-3b mediates its effects via recruitment of other negative regulators of TLR signaling (e.g., A20, IRAK-M, and SOCS-1). In this scenario, activated Pellino-3b could interact and mediate K63-linked ubiquitination of such negative regulators, co-opting them into the TLR signaling complexes and inducing their activity (e.g., activating the ability of A20 to remove K63-linked polyUb moieties from IRAK1 or attach K48-linked polyUb to its targets). Additional studies are required to delineate the exact molecular mechanisms by which Pellino-3 suppresses MyD88- and TRIF-dependent signaling.
Because Pellino-3 inhibits TLR2/4-mediated functional outcomes and because TLR signaling regulates phagocytosis [38, 39], we were interested in studying the relationships between Pellino-3 and phagocytosis of bacteria engaging TLR2 (S. aureus [56, 63]) and TLR4 (E. coli [64]). We have demonstrated that Pellino-3 ablation moderately potentiates phagocytosis of pHrodo-conjugated inactive S. aureus and E. coli by THP-1 cells, judged by the fluorescence of bacteria-conjugated, pH-sensitive fluorophore in acidified phagolysosomes. Thus, the ability of Pellino-3 to potently inhibit TLR2- and TLR4-induced NF-κB activation and expression of MyD88- and TRIF-dependent cytokines does not translate into its equal capacity to influence phagocytosis of heat-killed bacteria. These results might suggest that the primary regulatory effect of Pellino-3 during bacterial infection is to regulate the production of MyD88- and TRIF-dependent inflammatory cytokines, with a secondary possible effect on macrophage phagocytosis.
In conclusion, we have uncovered a previously unappreciated role for Pellino-3b as an important mediator of endotoxin tolerance and a critical negative regulator of TLR2 and TLR4 signaling operating within the MyD88-IRAK1-TAK1-TRAF6 and TRIF-TBK1 signaling axes. Despite its ability to suppress MyD88- and TRIF-dependent signaling in response to defined TLR2 and TLR4 agonists and whole heat-killed E. coli and S. aureus, Pellino-3b was found to only moderately affect the phagocytosis of inactivated bacteria. From these results, it is plausible that cell-permeating peptide or small molecule inhibitors of Pellino-3b could represent a novel modality for the treatment of sepsis-associated immune suppression by targeting Pellino-3 in macrophages and other myeloid cells exhibiting the endotoxin-tolerant phenotype.
AUTHORSHIP
M.B.M., Y.X., G.P., F.Q., and T.T.M. performed experiments to determine the basal and LPS-inducible expression of Pellino-3 mRNA and proteins in control and endotoxin-tolerized human monocytes, THP-1 cells, and MonoMac6 cells (Figs. 1 and 2). M.B.M. analyzed the effect of Pellino-3 ablation on LPS-induced cytokine gene expression (Figs. 4 and 5) and the induction of endotoxin tolerance (Fig. 8) and determined how the overexpression of Pellino-3 influences post-translational modifications of co-expressed IRAK1 or TBK1 (Fig. 7B). Y.X. examined whether Pellino-3b overexpression affects TLR2/4-mediated NF-κB activation and cytokine gene expression (Fig. 3). G.P. analyzed the uptake and phagocytosis of pHrodo-conjugated E. coli and S. aureus by THP-1 cells expressing scrambled or Pellino-3 shRNA. A.E.M. conceived the idea, designed the experiments, oversaw the entire project, conducted experiments to determine the effect of Pellino-3b on NF-κB activation driven by TLR signaling intermediates (Fig. 7A), and prepared the manuscript.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grant R01AI059524 (to A.E.M.). We are grateful to Dr. Xiaoxia Li (Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH) for kindly providing us with the Pellino-3b expression vectors and shRNA constructs.
Glossary
- cDMEM
complete DMEM
- cRPMI 1640
complete RPMI 1640
- DCs
dendritic cells
- HEK
human embryonic kidney
- HPRT
hypoxanthine-guanine phosphoribosyl transferase
- IKK
inhibitor of NF-κB kinase
- IP
immunoprecipitation
- IRAK
IL-1R-associated kinase
- LRR
leucine-rich repeats
- Luc
luciferase
- MD-2
myeloid differentiation factor 2
- MFI
mean fluorescence intensity
- Pam3Cys
S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys-Ser-Lys4-OH
- pcDNA
plasmid construct DNA
- PRRs
pattern recognition receptors
- RIP
receptor interacting protein
- RT-qPCR
quantitative real-time polymerase chain reaction
- shRNA
short hairpin RNA
- SOCS
suppressor of cytokine signaling
- TAK
transforming growth factor-activated kinase
- TANK
TNFR activator of NF-κB
- TBK
TANK-binding kinase
- TIR
Toll/IL-1R
- TK
thymidine kinase
- TRAF
TNFR-associated factor
- TRIF
TIR domain-containing adapter inducing IFN-β
- Ub
ubiquitin
- YFP
yellow fluorescent protein
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Medzhitov R., Janeway C. A. Jr (1998) An ancient system of host defense. Curr. Opin. Immunol. 10, 12–15. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B. (2009) Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol. Rev. 227, 248–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T., Akira S. (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill L. A., Golenbock D., Bowie A. G. (2013) The history of Toll-like receptors—redefining innate immunity. Nat. Rev. Immunol. 13, 453–460. [DOI] [PubMed] [Google Scholar]
- 5.Lin S. C., Lo Y. C., Wu H. (2010) Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrao R., Zhou H., Shan Y., Liu Q., Li Q., Shaw D. E., Li X., Wu H. (2014) IRAK4 dimerization and trans-autophosphorylation are induced by myddosome assembly. Mol. Cell 55, 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oshiumi H., Matsumoto M., Funami K., Akazawa T., Seya T. (2003) TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4, 161–167. [DOI] [PubMed] [Google Scholar]
- 8.Kagan J. C., Su T., Horng T., Chow A., Akira S., Medzhitov R. (2008) TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat. Immunol. 9, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coll R. C., O’Neill L. A. (2010) New insights into the regulation of signalling by Toll-like receptors and nod-like receptors. J. Innate Immun. 2, 406–421. [DOI] [PubMed] [Google Scholar]
- 10.Moynagh P. N. (2014) The roles of Pellino E3 ubiquitin ligases in immunity. Nat. Rev. Immunol. 14, 122–131. [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss R. S., Monneret G., Payen D. (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotchkiss R. S., Monneret G., Payen D. (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 13, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adib-Conquy M., Cavaillon J. M. (2009) Compensatory anti-inflammatory response syndrome. Thromb. Haemost. 101, 36–47. [PubMed] [Google Scholar]
- 14.Cavaillon J. M., Adib-Conquy M. (2006) Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit. Care 10, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavaillon J. M., Adrie C., Fitting C., Adib-Conquy M. (2005) Reprogramming of circulatory cells in sepsis and SIRS. J. Endotoxin Res. 11, 311–320. [DOI] [PubMed] [Google Scholar]
- 16.Biswas S. K., Lopez-Collazo E. (2009) Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487. [DOI] [PubMed] [Google Scholar]
- 17.Medvedev A. E., Piao W., Shoenfelt J., Rhee S. H., Chen H., Basu S., Wahl L. M., Fenton M. J., Vogel S. N. (2007) Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J. Biol. Chem. 282, 16042–16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piao W., Song C., Chen H., Wahl L. M., Fitzgerald K. A., O’Neill L. A., Medvedev A. E. (2008) Tyrosine phosphorylation of MyD88 adapter-like (Mal) is critical for signal transduction and blocked in endotoxin tolerance. J. Biol. Chem. 283, 3109–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piao W., Song C., Chen H., Diaz M. A., Wahl L. M., Fitzgerald K. A., Li L., Medvedev A. E. (2009) Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. J. Leukoc. Biol. 86, 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong Y., Qiu F., Piao W., Song C., Wahl L. M., Medvedev A. E. (2011) Endotoxin tolerance impairs IL-1 receptor-associated kinase (IRAK) 4 and TGF-beta-activated kinase 1 activation, K63-linked polyubiquitination and assembly of IRAK1, TNF receptor-associated factor 6, and IkappaB kinase gamma and increases A20 expression. J. Biol. Chem. 286, 7905–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong Y., Medvedev A. E. (2011) Induction of endotoxin tolerance in vivo inhibits activation of IRAK4 and increases negative regulators IRAK-M, SHIP-1, and A20. J. Leukoc. Biol. 90, 1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi K., Hernandez L. D., Galán J. E., Janeway C. A. Jr., Medzhitov R., Flavell R. A. (2002) IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110, 191–202. [DOI] [PubMed] [Google Scholar]
- 23.Escoll P., del Fresno C., García L., Vallés G., Lendínez M. J., Arnalich F., López-Collazo E. (2003) Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem. Biophys. Res. Commun. 311, 465–472. [DOI] [PubMed] [Google Scholar]
- 24.van ’t Veer C., van den Pangaart P. S., van Zoelen M. A., de Kruif M., Birjmohun R. S., Stroes E. S., de Vos A. F., van der Poll T. (2007) Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J. Immunol. 179, 7110–7120. [DOI] [PubMed] [Google Scholar]
- 25.Li Y., Zhang P., Wang C., Han C., Meng J., Liu X., Xu S., Li N., Wang Q., Shi X., Cao X. (2013) Immune responsive gene 1 (IRG1) promotes endotoxin tolerance by increasing A20 expression in macrophages through reactive oxygen species. J. Biol. Chem. 288, 16225–16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Ouyang Y., Guner Y., Ford H. R., Grishin A. V. (2009) Ubiquitin-editing enzyme A20 promotes tolerance to lipopolysaccharide in enterocytes. J. Immunol. 183, 1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sly L. M., Rauh M. J., Kalesnikoff J., Song C. H., Krystal G. (2004) LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity 21, 227–239. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa R., Naka T., Tsutsui H., Fujimoto M., Kimura A., Abe T., Seki E., Sato S., Takeuchi O., Takeda K., Akira S., Yamanishi K., Kawase I., Nakanishi K., Kishimoto T. (2002) SOCS-1 participates in negative regulation of LPS responses. Immunity 17, 677–687. [DOI] [PubMed] [Google Scholar]
- 29.Quevedo-Diaz M. A., Song C., Xiong Y., Chen H., Wahl L. M., Radulovic S., Medvedev A. E. (2010) Involvement of TLR2 and TLR4 in cell responses to Rickettsia akari. J. Leukoc. Biol. 88, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegler-Heitbrock H. W., Thiel E., Fütterer A., Herzog V., Wirtz A., Riethmüller G. (1988) Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int. J. Cancer 41, 456–461. [DOI] [PubMed] [Google Scholar]
- 31.Xiong Y., Song C., Snyder G. A., Sundberg E. J., Medvedev A. E. (2012) R753Q polymorphism inhibits Toll-like receptor (TLR) 2 tyrosine phosphorylation, dimerization with TLR6, and recruitment of myeloid differentiation primary response protein 88. J. Biol. Chem. 287, 38327–38337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figueroa L., Xiong Y., Song C., Piao W., Vogel S. N., Medvedev A. E. (2012) The Asp299Gly polymorphism alters TLR4 signaling by interfering with recruitment of MyD88 and TRIF. J. Immunol. 188, 4506–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwende H., Fitzke E., Ambs P., Dieter P. (1996) Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 59, 555–561. [PubMed] [Google Scholar]
- 34.Xiao H., Qian W., Staschke K., Qian Y., Cui G., Deng L., Ehsani M., Wang X., Qian Y. W., Chen Z. J., Gilmour R., Jiang Z., Li X. (2008) Pellino 3b negatively regulates interleukin-1-induced TAK1-dependent NF kappaB activation. J. Biol. Chem. 283, 14654–14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- 36.Wysocka M., Robertson S., Riemann H., Caamano J., Hunter C., Mackiewicz A., Montaner L. J., Trinchieri G., Karp C. L. (2001) IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J. Immunol. 166, 7504–7513. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann E., Dittrich-Breiholz O., Holtmann H., Kracht M. (2002) Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72, 847–855. [PubMed] [Google Scholar]
- 38.Blander J. M., Medzhitov R. (2004) Regulation of phagosome maturation by signals from Toll-like receptors. Science 304, 1014–1018. [DOI] [PubMed] [Google Scholar]
- 39.Redlich S., Ribes S., Schütze S., Eiffert H., Nau R. (2013) Toll-like receptor stimulation increases phagocytosis of Cryptococcus neoformans by microglial cells. J. Neuroinflammation 10, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cervantes J. L., Dunham-Ems S. M., La Vake C. J., Petzke M. M., Sahay B., Sellati T. J., Radolf J. D., Salazar J. C. (2011) Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-beta. Proc. Natl. Acad. Sci. USA 108, 3683–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miksa M., Komura H., Wu R., Shah K. G., Wang P. (2009) A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J. Immunol. Methods 342, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Z., Johnson H. J., Nie H., Qin J., Bird T. A., Li X. (2003) Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J. Biol. Chem. 278, 10952–10956. [DOI] [PubMed] [Google Scholar]
- 43.Kim T. W., Yu M., Zhou H., Cui W., Wang J., DiCorleto P., Fox P., Xiao H., Li X. (2012) Pellino 2 is critical for Toll-like receptor/interleukin-1 receptor (TLR/IL-1R)-mediated post-transcriptional control. J. Biol. Chem. 287, 25686–25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ordureau A., Smith H., Windheim M., Peggie M., Carrick E., Morrice N., Cohen P. (2008) The IRAK-catalysed activation of the E3 ligase function of Pellino isoforms induces the Lys63-linked polyubiquitination of IRAK1. Biochem. J. 409, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kishore N., Huynh Q. K., Mathialagan S., Hall T., Rouw S., Creely D., Lange G., Caroll J., Reitz B., Donnelly A., Boddupalli H., Combs R. G., Kretzmer K., Tripp C. S. (2002) IKK-i and TBK-1 are enzymatically distinct from the homologous enzyme IKK-2: comparative analysis of recombinant human IKK-i, TBK-1, and IKK-2. J. Biol. Chem. 277, 13840–13847. [DOI] [PubMed] [Google Scholar]
- 46.Shembade N., Ma A., Harhaj E. W. (2010) Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boone D. L., Turer E. E., Lee E. G., Ahmad R. C., Wheeler M. T., Tsui C., Hurley P., Chien M., Chai S., Hitotsumatsu O., McNally E., Pickart C., Ma A. (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060. [DOI] [PubMed] [Google Scholar]
- 48.Skaug B., Chen J., Du F., He J., Ma A., Chen Z. J. (2011) Direct, noncatalytic mechanism of IKK inhibition by A20. Mol. Cell 44, 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mansell A., Smith R., Doyle S. L., Gray P., Fenner J. E., Crack P. J., Nicholson S. E., Hilton D. J., O’Neill L. A., Hertzog P. J. (2006) Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 7, 148–155. [DOI] [PubMed] [Google Scholar]
- 50.Chen X., El Gazzar M., Yoza B. K., McCall C. E. (2009) The NF-kappaB factor RelB and histone H3 lysine methyltransferase G9a directly interact to generate epigenetic silencing in endotoxin tolerance. J. Biol. Chem. 284, 27857–27865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoza B. K., Hu J. Y., Cousart S. L., Forrest L. M., McCall C. E. (2006) Induction of RelB participates in endotoxin tolerance. J. Immunol. 177, 4080–4085. [DOI] [PubMed] [Google Scholar]
- 52.Yan Q., Carmody R. J., Qu Z., Ruan Q., Jager J., Mullican S. E., Lazar M. A., Chen Y. H. (2012) Nuclear factor-κB binding motifs specify Toll-like receptor-induced gene repression through an inducible repressosome. Proc. Natl. Acad. Sci. USA 109, 14140–14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porta C., Rimoldi M., Raes G., Brys L., Ghezzi P., Di Liberto D., Dieli F., Ghisletti S., Natoli G., De Baetselier P., Mantovani A., Sica A. (2009) Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc. Natl. Acad. Sci. USA 106, 14978–14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cubillos-Zapata C., Hernández-Jiménez E., Toledano V., Esteban-Burgos L., Fernández-Ruíz I., Gómez-Piña V., Del Fresno C., Siliceo M., Prieto-Chinchiña P., Pérez de Diego R., Boscá L., Fresno M., Arnalich F., López-Collazo E. (2014) NFκB2/p100 is a key factor for endotoxin tolerance in human monocytes: a demonstration using primary human monocytes from patients with sepsis. J. Immunol. 193, 4195–4202. [DOI] [PubMed] [Google Scholar]
- 55.Muralidharan S., Ambade A., Fulham M. A., Deshpande J., Catalano D., Mandrekar P. (2014) Moderate alcohol induces stress proteins HSF1 and hsp70 and inhibits proinflammatory cytokines resulting in endotoxin tolerance. J. Immunol. 193, 1975–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeuchi O., Hoshino K., Akira S. (2000) Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165, 5392–5396. [DOI] [PubMed] [Google Scholar]
- 57.Siednienko J., Jackson R., Mellett M., Delagic N., Yang S., Wang B., Tang L. S., Callanan J. J., Mahon B. P., Moynagh P. N. (2012) Pellino3 targets the IRF7 pathway and facilitates autoregulation of TLR3- and viral-induced expression of type I interferons. Nat. Immunol. 13, 1055–1062. [DOI] [PubMed] [Google Scholar]
- 58.Butler M. P., Hanly J. A., Moynagh P. N. (2005) Pellino3 is a novel upstream regulator of p38 MAPK and activates CREB in a p38-dependent manner. J. Biol. Chem. 280, 27759–27768. [DOI] [PubMed] [Google Scholar]
- 59.Jensen L. E., Whitehead A. S. (2003) Pellino3, a novel member of the Pellino protein family, promotes activation of c-Jun and Elk-1 and may act as a scaffolding protein. J. Immunol. 171, 1500–1506. [DOI] [PubMed] [Google Scholar]
- 60.Chang M., Jin W., Chang J. H., Xiao Y., Brittain G. C., Yu J., Zhou X., Wang Y. H., Cheng X., Li P., Rabinovich B. A., Hwu P., Sun S. C. (2011) The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat. Immunol. 12, 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui W., Xiao N., Xiao H., Zhou H., Yu M., Gu J., Li X. (2012) β-TrCP-mediated IRAK1 degradation releases TAK1-TRAF6 from the membrane to the cytosol for TAK1-dependent NF-κB activation. Mol. Cell. Biol. 32, 3990–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang X., Chen Z. J. (2012) The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 12, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., Akira S. (1999) Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11, 443–451. [DOI] [PubMed] [Google Scholar]
- 64.Fischer H., Yamamoto M., Akira S., Beutler B., Svanborg C. (2006) Mechanism of pathogen-specific TLR4 activation in the mucosa: fimbriae, recognition receptors and adaptor protein selection. Eur. J. Immunol. 36, 267–277. [DOI] [PubMed] [Google Scholar]