Abstract
Objectives
To explore the relations of parent-child cardiometabolic risk factors and assess the influence of adiposity on these associations.
Study design
Associations of adiposity, blood pressure, lipids, fasting insulin and glucose, and a risk factor cluster score were evaluated in a cross-sectional study of 179 parents and their children (6–18 years, N=255). Insulin resistance was assessed by euglycemic clamp in parents and children aged 10 or older. Metabolic syndrome in parents was defined by ATPIII criteria. Cluster scores of the risk factors were created based on age-specific z-scores. Analyses included Pearson correlation and linear regression, adjusted for parent and child age, sex, race, and body mass index (BMI), accounting for within-family correlation.
Results
We found positive parent-child correlations for measures of adiposity (BMI, BMI percentile, waist, subcutaneous fat, and visceral fat; r=0.22–0.34, all p≤0.003), systolic blood pressure (SBP) (r=0.20, p=0.002), total cholesterol (r=0.39, p<0.001), low-density lipoprotein cholesterol (r=0.34, p<0.001), high density lipoprotein cholesterol (r=0.26, p<0.001) triglycerides (r=0.19, p=0.01) and insulin sensitivity (r=0.22, p=0.02) as well as cluster scores (r=0.15, p=0.02). After adjustment for BMI all parent-child correlations, except systolic blood pressure, remained significant.
Conclusions
Although adiposity is strongly correlated between parents and children, many cardiometabolic risk factors correlate independent of parent and child BMI. Adverse parental cardiometabolic profiles may identify at-risk children independent of the child’s adiposity status.
Keywords: adiposity, obesity, blood pressure, BMI, cardiovascular, cluster score, correlation, insulin sensitivity, lipid(s)
Cardiovascular (CV) risk factors such as hypertension and hyperlipidemia are well known to be shared traits between parents and children (1–5). Because adiposity is also commonly shared between generations (3–8) and because excess body fat is a known precursor of CV risk (9–12), it is possible that much of the generational transmission of CV risk factors could be mediated by adiposity. The literature is conflicting in this regard. It was recently suggested that associations between parental obesity and individual CV risk factor levels in their children are mediated primarily by obesity in the children (13), and a second study reported no evidence that parent-child correlations were affected by adiposity (3).
It is also known that the risk of CV disease is greatly increased when abnormal levels of multiple risk factors occur in combination, e.g., the metabolic syndrome (14, 15). Although CV risk factor levels are significantly lower in children than adults, children with levels at the upper end of the normal range are thought to be at greater future risk (16). One way to look at this combined risk is to formulate a cluster score based on a simultaneous assessment of multiple risk factor measurements. We know of no studies that have examined how this combined risk associates between parents and children or the role of adiposity in this relation.
The present study was designed to analyze parent-child risk factor correlations, evaluate the influence of body mass index (BMI) on these associations and assess correlation between parent and child risk factor cluster scores.
METHODS
The study was conducted in a cohort of parents (N=179, mean age 39 years) and their children (N=255, age 6–18 years). The parents, then aged 6–9 years, were originally enrolled in a study that began with the blood pressure screening of 10,423 1st–3rd grade children in the Minneapolis Public Schools during the 1977–78 school year. Following this screening a cohort was selected for long-term evaluation of cardiovascular risk factors as follows: all children from the top and bottom five percentiles of the normal systolic blood pressure distribution, fifty percent of the remaining black children, and one out of nine of the remaining white children (17). Because parental participation in the current study was based on their prior childhood enrollment, only one parent per child was eligible. Individuals with chronic diseases including type 2 diabetes, end-stage kidney disease or cancer (n=14, all parents) were excluded. The University of Minnesota Institutional Review Board approved this study. All adult subjects signed informed consent documents for themselves and on behalf of their participating children, who gave signed informed assent.
Parent and child anthropometric measurements were obtained using standardized protocols. Standing height was measured with a stadiometer to the nearest centimeter (cm). A balance scale was used to measure weight in kilograms (kg). Body mass index (BMI) was calculated as weight (kg) divided by height (meters) squared. BMI percentile was calculated for each participant based on age and sex (18). Waist circumference was measured at the umbilicus to the nearest 0.5 cm, taken in duplicate and the mean value reported. Percent body fat, fat mass and lean body mass (LBM) were determined by dual-energy x-ray absorptiometry (DXA) with a Lunar Prodigy scanner (pediatric software version 9.3; General Electric Medical Systems, Madison, WI, USA) in the total body scanning mode. Visceral adipose tissue (VAT) and total abdominal fat were measured by abdominal CT scan at the level of L4–L5 disk as previously described (19). Blood pressure (BP) was measured in duplicate on the right arm after participants were sitting in a quiet room for at least five minutes using a digital BP cuff, and the average of the two values was reported.
Fasting blood samples were collected for lipids (total cholesterol, triglycerides, high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C)), glucose and insulin. Assays were conducted with standard procedures at the Fairview Diagnostic Laboratories, Fairview-University Medical Center (Minneapolis, MN), a Centers for Disease Control and Prevention-certified laboratory.
Euglycemic clamp studies were performed in parents and older children (ages 10–18) as previously described (20). Younger children did not undergo the clamp procedure. Insulin sensitivity, M, was calculated based on the amount of glucose required to maintain euglycemia during the last 40 minutes of the clamp, corrected for lean body mass (LBM) and expressed as MLBM (milligrams of glucose per kilogram of LBM per minute). A higher MLBM represents greater insulin sensitivity (i.e. less insulin resistance).
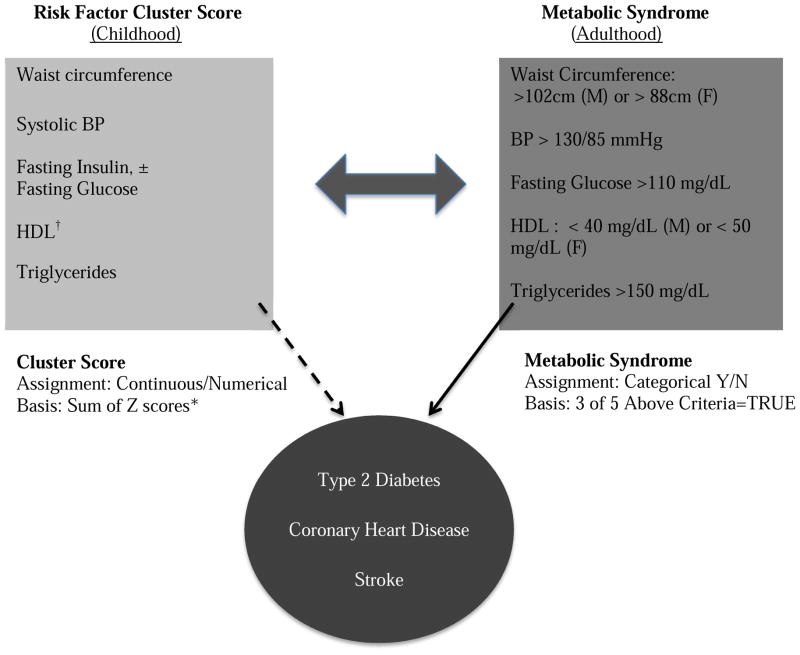
The components of the risk factor cluster scores were chosen to measure health risks similar to those represented in the adult metabolic syndrome (Figure 1; available at www.jpeds.com). First, Z-scores were calculated for waist circumference, SBP, fasting glucose, fasting insulin, HDL-C and triglycerides using age group (6–18, 6–9, 10–18 years and adult)-specific means and standard deviations (Table I). Cluster scores were then created by summing the z-scores for waist, SBP, insulin and triglycerides +/− glucose, subtracting the z-score for HDL-C (which is protective), and dividing by 5 or 6 (depending on whether glucose was included) to create a mean risk score. We created the five-component cluster score (CS5) excluding the fasting glucose component because, although fasting glucose is one of the criteria in the diagnosis of adult metabolic syndrome, it virtually always falls within a narrow normal range in children and adolescents and is therefore less likely to provide useful information about children’s future metabolic risk.
Figure 1.
Children with a high cluster score are at risk for development of the metabolic syndrome as they mature into adulthood. Adults with the metabolic syndrome are at risk for producing offspring with high cluster scores. Youth with high cluster scores and adults with metabolic syndrome are at increased risk of progressing to overt cardiometabolic disease.
*Z scores based on age group-specific norms
†HDL z-scores were assigned a negative value because a higher HDL is associated with lower risk, in contrast to all other components in which the opposite is true.
Table 1.
Subject Characteristics
| Variable | Parents | P | Children Ages 6–9 | P | ICC | Children Ages 10–18 | P | ICC | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n=69) |
Women (n=110) |
Boys (n=44) |
Girls (n=43) |
Boys (n=94) |
Girls (n=74) |
||||||
| Age, y | 39±1.3 | 39±1.4 | 0.02 | 7±1.2 | 7±1.2 | 0.96 | 14±2.5 | 14±2.3 | 0.04 | ||
| Race, %White | 70 | 58 | 0.13 | 73 | 70 | 0.76 | 1.00 | 46 | 43 | 0.75 | 1.00 |
| Adiposity | |||||||||||
| BMI, kg/m2 | 30±6.0 | 29±6.9 | 0.21 | 18±3.7 | 17±2.5 | 0.09 | 0.43 | 23±5.7 | 25±6.2 | 0.02 | 0.37 |
| BMI %tile | N/A | N/A | 64±32.7 | 55±26.7 | 0.20 | 0.18 | 67±29.1 | 71±29.1 | 0.30 | 0.35 | |
| Waist, cm | 105±16.3 | 96±17.8 | 0.001 | 64±11.1 | 59±6.9 | 0.04 | 0.40 | 78±14.1 | 80±14.1 | 0.24 | 0.43 |
| Subcutaneous fat, cm2 | 155.4±81.2 | 183.6±83.8 | 0.03 | 40.3±42 | 35.3±26.2 | 0.52 | 0.00 | 74±72.9 | 130.3±85 | <0.001 | 0.21 |
| Visceral fat, cm2 | 73.4±34.1 | 51.6±26.8 | <0.001 | 12.6±7.3 | 11.3±5.3 | 0.41 | 0.53 | 20.2±13.9 | 22.5±10.2 | 0.21 | 0.29 |
| BP, mmHg | |||||||||||
| Systolic BP | 122±12.9 | 109±15.2 | <0.001 | 101±10.3 | 98±7.6 | 0.19 | 0.55 | 108±10.5 | 104±8.6 | 0.02 | 0.33 |
| Diastolic BP | 78±11.5 | 71±11.6 | <0.001 | 57±6.3 | 58±8.3 | 0.71 | 0.05 | 58±8.1 | 60±7.8 | 0.11 | 0.26 |
| Lipids, mg/dL(mmol/L) | |||||||||||
| Total cholesterol | 195±36.6 (5.04±0.95) | 175±29.0 (4.54±0.75) | <0.001 | 154±26.0 (3.99±0.67) | 164±30.1 (4.23±0.78) | 0.21 | 0.34 | 150±28.9 (3.88±0.75) | 154±26.8 (3.98±0.69) | 0.44 | 0.53 |
| LDL-C | 117±29.0 (3.04±0.75) | 104±25.7 (2.70±0.66) | 0.003 | 89±18.8 (2.30±0.49) | 96±26.1 (2.47±0.68) | 0.32 | 0.40 | 84±24.5 (2.17±0.63) | 87±23.1 (2.24±0.60) | 0.56 | 0.35 |
| HDL-C | 42±11.6 (1.09±0.30) | 53±13.0 (1.36±0.34) | <0.001 | 52±10.3 (1.35±0.27) | 56±11.1 (1.44±0.29) | 0.13 | 0.68 | 51±12.9 (1.31±0.33) | 50±12.0 (1.30±0.31) | 0.88 | 0.47 |
| Triglyceride | 192±163.9 (2.17±1.85) | 92±38.5 (1.04±0.44) | <0.001 | 65±36.9 (0.74±0.42) | 60±19.8 (0.68±0.22) | 0.48 | 0.12 | 77±37.4 (0.87±0.42) | 85±49.3 (0.96±0.56) | 0.30 | 0.42 |
| Glucose Metabolism | |||||||||||
| Fasting glucose, mg/dL (mmol/L) | 97±36.7 (5.40±2.04) | 88±21.0 (4.89±1.17) | 0.03 | 65±8.5 (3.62±0.47) | 61±7.7 (3.41±0.43) | 0.09 | 0.46 | 86±9.5 (4.78±0.53) | 83±9.7 (4.63±0.54) | 0.04 | 0.39 |
| Fasting insulin, mU/L (pmol/L) | 10.5±8.62 (75.2±61.8) | 7.2±6.12 (51.7±43.9) | 0.004 | 3.2±2.88 (23.1±20.7) | 2.4±1.54 (16.9±11.1) | 0.24 | 0.75 | 9.1±6.53 (65.2±46.9) | 11.2±6.96 (78.4±50.0) | 0.09 | 0.54 |
| Insulin Sensitivity, Mlbm | 9.8±4.15 | 13.9±4.61 | <0.001 | N/A | N/A | -- | -- | 13±4.8 | 11±3.9 | 0.005 | 0.54 |
| Composite | |||||||||||
| Cluster score (CS5) | 0.41±0.70 | −0.26±0.55 | <0.001 | 0.17±0.67 | −0.17±0.48 | 0.03 | 0.39 | −0.02±0.66 | 0.03±0.62 | 0.66 | 0.57 |
| Metabolic syndrome, % | 39 | 15 | <0.001 | N/A | N/A | -- | -- | N/A | N/A | -- | -- |
All data presented as mean ± standard error except for metabolic syndrome and race. Measurements of each variable were obtained for all subjects except for the following: 63 men had LDL-C,108 women had fasting insulin, and 64 men, 90 women, 83 boys (age 10–18) and 66 girls (age 10–18) had insulin sensitivity (Mlbm). Cluster scores were calculated using risk factor Z-scores derived for each age group with males and females pooled. The age group-specific means (standard deviations) for the relevant risk factors are listed here in the same units described in the table; where applicable, metric units are followed by SI units in brackets. Parents: Waist 99.6 (17.68); SBP 114.2 (15.60); HDL-C 48.6 (13.44), [1.26 (0.35)]; triglycerides (TRIG) 130.8 (116.36), [1.48 (1.31)]; fasting glucose (GLUC) 91.6 (23.39), [5.08 (1.58)]; fasting insulin (INS) 8.5 (7.35), [60.8 (52.76)]. Children ages 6–9: Waist 61.4 (9.53); SBP 99.9 (9.17); HDL-C 54.0 (10.77), [1.40 (0.28)]; TRIG 62.7 (29.63), [0.71 (0.33)]; GLUC 63.3 (8.29), [3.51 (0.46)]; INS 2.8 (2.35), [20.0 (16.83)]. Children ages 10–18: Waist 79.0 (14.13); SBP 105.9 (9.92); HDL-C 50.4 (12.49), [1.30 (0.32)]; TRIG 80.3 (43.08), [0.91 (0.49)]; GLUC 84.9 (9.64), [4.71 (0.54)]; INS 9.9 (6.77), [71.0 (48.6)]. Children ages 6–18: Waist 73.0 (15.21); SBP 103.9 (10.06); HDL-C 51.6 (12.03), [1.34 (0.31)]; TRIG 74.3 (39.84), [0.84 (0.45)]; GLUC 77.5 (13.8), [4.3 (0.76)]; INS 7.5 (6.58), [53.6 (47.2)].
Metabolic syndrome in parents was defined by ATP III criteria (21, 22), i.e., meeting at least 3 of the following 5 criteria: 1) fasting glucose ≥110 mg/dl, 2) HDL-C ≤40 mg/dL for men or ≤ 50 mg/dl for women, 3) BP ≥ 130/85 mmHg, 4) triglyceride level ≥150 mg/dl and 5) waist circumference ≥102 cm for men or ≥88 cm for women.
Statistical Analyses
All analyses were conducted using SAS, version 9.3 (SAS Institute Inc., Cary, NC, USA). BMI percentile was calculated for children by age and sex according to the CDC growth charts (18). Due to a skewed distribution, triglyceride levels were log-transformed before analysis, with means exponentiated and reported as geometric means. Multiple regression analyses (PROC MIXED) accounting for within family correlation (interclass correlation, ICC) were used to estimate means (standard errors; SE) for demographic and clinical characteristics by sex for parents and children adjusted for age. Pearson partial correlation coefficients (computed using standardized deviates for both parental and child variables in PROC MIXED to account for within family correlation) were used to evaluate the relations of cardiometabolic risk factors between parents and their children, adjusted for parent and child age, sex, race, and BMI. P-values < 0.05 was considered significant; however, because multiple comparisons were performed, we were cautious in the interpretation of p-values that were close to 0.05.
RESULTS
Demographic and clinical characteristics of parents and their children are reported by sex in Table I. Adult men had significantly greater CV risk than adult women as evidenced by larger waist circumferences, higher blood pressure, less favorable lipid levels, lower insulin sensitivity, higher fasting blood glucose and insulin levels, and higher prevalence of metabolic syndrome. Of the 255 children, 168 were between the ages of 10 and 18 years and 87 were 6–9 years old. Among the children, both sexes were equally represented. A higher percentage of non-white children were in the older (10–18 year old) age group. There were no statistically significant differences between boys and girls in the 6–9 year old age group, except that girls’ cluster scores tended to be lower. In the 10–18 year old age group, boys had greater insulin sensitivity (higher Mlbm) and tended to have lower fasting insulin, lower BMI and slightly higher SBP.
There was a significant correlation between parents and children for most individual components of the cardiometabolic profile (Table II). Every measure of adiposity (BMI, waist, subcutaneous fat, visceral fat and child BMI percentile/parent BMI) was correlated between parent and child. SBP, total cholesterol, LDL-C, HDL-C, triglycerides and insulin sensitivity (Mlbm) (10–18 year olds only) all correlated. The strength of these associations varied, with the greatest correlations noted between parent and child total cholesterol, parent and child LDL-C, and parent BMI with child BMI percentile. There was no correlation between parent and child fasting glucose or parent and child insulin levels.
Table 2.
Correlations Of Risk Factor Values Within Parent-Child Dyads
| Variable | All Children | Children Age 6–9 | Children Age 10–18 | Age Int | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No BMI Adjustment | Adjusted For BMI | No BMI Adjustment | Adjusted For BMI | No BMI Adjustment | Adjusted For BMI | ||||||||
| r | P | r | P | r | P | r | P | r | P | r | P | P | |
| Adiposity | |||||||||||||
| BMI | 0.28 | <0.001 | -- | -- | 0.25 | 0.045 | -- | -- | 0.34 | <0.001 | -- | -- | 0.10 |
| BMI percentile | 0.34 | <0.001 | -- | -- | 0.30 | 0.03 | -- | -- | 0.34 | <0.001 | -- | -- | 0.57 |
| Waist | 0.22 | <0.001 | -- | -- | 0.14 | 0.19 | -- | -- | 0.31 | <0.001 | -- | -- | 0.05 |
| Subcutaneous Fat | 0.30 | <0.001 | -- | -- | 0.22 | 0.08 | -- | -- | 0.33 | <0.001 | -- | -- | 0.11 |
| Visceral Fat | 0.20 | 0.003 | -- | -- | 0.06 | 0.62 | -- | -- | 0.27 | 0.002 | -- | -- | 0.02 |
| BP | |||||||||||||
| Systolic BP | 0.20 | 0.002 | 0.16 | 0.02 | 0.23 | 0.10 | 0.19 | 0.05 | 0.20 | 0.01 | 0.15 | 0.07 | 0.24 |
| Diastolic BP | 0.14 | 0.05 | 0.12 | 0.10 | 0.01 | 0.93 | 0.02 | 0.91 | 0.18 | 0.04 | 0.18 | 0.06 | 0.72 |
| Lipids | |||||||||||||
| Total cholesterol | 0.39 | <0.001 | 0.39 | <0.001 | 0.45 | 0.007 | 0.46 | 0.009 | 0.37 | <0.001 | 0.37 | <0.001 | 0.78 |
| LDL-C | 0.34 | <0.001 | 0.34 | <0.001 | 0.40 | 0.02 | 0.41 | 0.03 | 0.34 | <0.001 | 0.33 | <0.001 | 0.65 |
| HDL-C | 0.26 | <0.001 | 0.36 | <0.001 | 0.28 | 0.06 | 0.46 | 0.02 | 0.24 | 0.01 | 0.33 | 0.001 | 0.98 |
| Triglycerides | 0.19 | 0.01 | 0.22 | 0.003 | 0.11 | 0.42 | 0.12 | 0.42 | 0.20 | 0.03 | 0.24 | 0.009 | 0.27 |
| Glucose Metabolism | |||||||||||||
| Glucose | −0.01 | 0.90 | −0.02 | 0.68 | −0.06 | 0.65 | −0.17 | 0.26 | 0.02 | 0.77 | 0.04 | 0.66 | 0.36 |
| Insulin | 0.07 | 0.26 | 0.12 | 0.04 | 0.13 | 0.29 | 0.10 | 0.40 | 0.08 | 0.35 | 0.14 | 0.09 | 0.48 |
| Insulin Sensitivity, Mlbm | -- | -- | -- | -- | -- | -- | -- | -- | 0.22 | 0.02 | 0.32 | 0.003 | -- |
| Cluster Score (CS5) | 0.15 | 0.02 | 0.18 | 0.007 | 0.13 | 0.33 | 0.05 | 0.69 | 0.21 | 0.03 | 0.27 | 0.005 | 0.37 |
N=245 for LDL-C, n=149 for Insulin Sensitivity, n=255 for all others. All comparisons are adjusted for parent and child age, race and sex. BMI-adjusted correlations were adjusted for both parent and child BMI. Int = Interaction; r = Pearson Partial correlation coefficient.
After adjustment for parent and child BMI, the parent-child correlation for SBP was attenuated, suggesting that this relation is at least partially mediated by adiposity (Table II). In contrast, other parent and child risk factor associations were maintained (total cholesterol, LDL-C) or increased (HDL-C, triglyceride levels and insulin sensitivity).
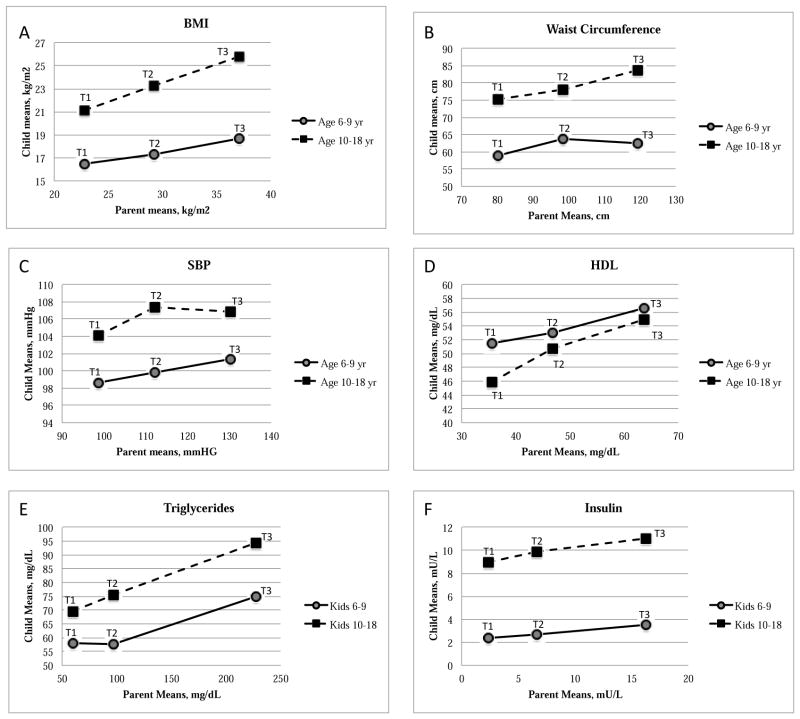
Parent-child correlations were also analyzed separately by child age group (Table II). With the exception of BMI percentile, which correlated with parent BMI in both age groups, measures of adiposity were significantly correlated between parents and 10–18 year old but not 6–9 year old children. The correlation between parent and child triglyceride levels was also higher in the 10–18 year old than younger children, but the age interaction p-value was high. An age interaction was not supported for parent-child correlations of any other risk factor. Parent-child trends for individual risk factors are also presented by comparing child means within parent tertiles for each age group (Figure 2 and Table III; Table III available at www.jpeds.com). Among 10–18 year old children, the tertile means for most risk factors (excluding only DBP and total cholesterol) increased monotonically from one tertile to the next, supporting a linear model of parent-child risk factor associations. This was true even for fasting glucose and insulin levels, in which parent-child correlations were not significant, in that the shallow rise was consistent across tertiles in these cases. Patterns were much more variable in the 6–9 year old age group, and tended to be flatter; clear linear associations were only present for BMI, BMI percentile (within parent BMI tertiles), HDL-C and fasting insulin.
Figure 2.
Parents were grouped into tertiles for each risk factor. Children were assigned to the same tertile as their parents, regardless of their own risk factor values. The mean of the children within each parent tertile was plotted against the corresponding parent tertile mean. Pictured here are the child means within parent tertiles for BMI (A), waist circumference (B), SBP (C), HDL-C (D), Triglycerides (E) and Insulin (F). T1=1st tertile, T2=2nd tertile and T3=3rd tertile.
Table 3.
Risk Factor Means of Children within Parent Tertiles
| Parent Tertile | Children Ages 6–9 yr | Children Ages 10–18 | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Variable | ||||||
| Adiposity | ||||||
| BMI, kg/m2 | 16.5c | 17.3 | 18.7 | 21.a,cc | 23.3b | 25.8 |
| BMI percentile | 49.3c | 61.6 | 73.8 | 55.6aa,cc | 70.7 | 79.3 |
| Waist, cm | 58.9 | 63.8 | 62.4 | 75.2cc | 78.2b | 83.7 |
| Subcutaneous Fat, cm2 | 35c | 29.2b | 57.9 | 63.9a,cc | 98.2 | 125.3 |
| Visceral Fat, cm2 | 11.8 | 11.6 | 12.5 | 16.9cc | 20.5b | 26 |
| BP, mmHg | ||||||
| SBP | 98.6 | 99.8 | 101.4 | 103.4 | 107.4 | 106.9 |
| DBP | 56.8 | 56.6 | 58.2 | 56.9 | 60.1 | 60.2 |
| Lipids, mg/dL (mmol/L) | ||||||
| Total cholesterol | 147.1cc (3.80) | 151.1b (3.91) | 176.4 (4.56) | 140.1cc (3.62) | 148.5bb (3.84) | 167.8 (4.34) |
| LDL-c | 84.4c (2.18) | 84.9b (2.20) | 105.7 (2.73) | 76.8cc (1.99) | 85.1b (2.20) | 95.9 (2.48) |
| HDL-c | 51.5 (1.33) | 53.1 (1.37) | 56.6 (1.46) | 45.9cc (1.19) | 50.7 (1.31) | 54.9 (1.42) |
| Triglycerides | 57.9 (0.65) | 57.7 (0.65) | 74.9 (0.85) | 69.6c (0.79) | 75.6b (0.85) | 94.4 (1.07) |
| Glucose Metabolism | ||||||
| Fasting glucose, mg/dL (mmol/L) | 64.3 (3.57) | 63.9 (3.55) | 62.1 (3.45) | 83.1c (4.61) | 84.2 (4.68) | 87.6 (4.86) |
| Fasting insulin, mU/L (pmol/L) | 2.3 (16.8) | 2.7 (19.5) | 3.5 (25.2) | 8.9 (64.0) | 9.8 (70.7) | 11.1 (79.4) |
| Insulin sensitivity, Mlbm | -- | -- | -- | 11.0c | 12.8 | 13.6 |
| Cluster Score | ||||||
| 6 components | −0.076 | 0.068 | 0.039 | −0.047 | 0.008 | 0.1 |
| 5 components (no glucose) | −0.125 | 0.094 | 0.076 | −0.107 | 0.03 | 0.132 |
Parent tertiles were defined separately for each risk factor. Superscripts: a: P-diff between 1st and 2nd tertile <0.05; aa: P-diff between 1st and 2nd tertile <0.01; b: P-diff between 2nd and 3rd tertile <0.05; bb: P-diff between 2nd and 3rd tertile <0.01; c: P-diff between 1st and 3rd tertile <0.05; cc: P-diff between 1st and 3rd tertile <0.01
Cluster scores were computed for both parents and children to compare a more comprehensive measure of their cardiometabolic risk. Parent and child cluster score correlations were lower than those for adiposity and blood lipids (Table II). Adjustment for parent and child BMI did not change this relation appreciably. However, when we examined the two age groups separately, parent and child cluster scores were more highly correlated in the 10–18 year old age group, especially after adjustment for BMI (Table II). The inclusion of fasting glucose levels in the cluster score calculation reduced this association (data not shown).
DISCUSSION
Several studies have compared individual cardiovascular risk factors between parents and children (1–5). Our study adds to the current body of literature by evaluating an extensive panel of individual parent-child risk factor associations in both young children and adolescents, and by taking the novel steps of including clustered risk factor associations and assessing the role of adiposity in each of these relations. Because obesity itself is associated with changes in blood pressure, lipid levels and insulin sensitivity within individuals (23–27), and because there is a strong association between parent and child BMI, we hypothesized that parent-child CV risk correlations might be mediated primarily by adiposity. We found significant correlations between parents and children for multiple measures of adiposity, SBP, lipid levels and insulin sensitivity, consistent with other studies that evaluated some of these same components (1–5). Contrary to our hypothesis, however, after adjusting these results for parent and child BMI, every significant correlation except for blood pressure retained statistical significance and, in some cases, even increased. This highlights the importance of shared genetic and/or environmental traits independent of obesity in the transmission of CV risk.
The heritability of blood pressure, independent of adiposity, remains controversial. In this study, parent-child correlations in SBP were at least partially mediated by BMI. This is not surprising, considering that blood pressure has been shown to correlate with BMI in children (28, 29), and the prevalence of pre-hypertension and hypertension has been estimated to be 15 times higher in overweight and obese children and adolescents compared with normal-weight peers (28). Our results contrast with a previous report of a significant genetic effect on blood pressure in Polish twins after adjusting for BMI (30). Closer genetic similarities (about half of the twin dyads were monozygotic) and differences in statistical modeling methods may account for the different findings. Another consideration is that heritability of blood pressure may not be uniform across different populations. For example, in one study of hypertensive children, black children were much more likely to have a hypertensive parent than white children (31). Our study was not powered to explore this issue.
Cholesterol (total, LDL and HDL) levels correlated between parents and children more highly than any other risk factor, except for BMI. Our findings were consistent with other studies associating children’s lipid profiles with those of their parents (1, 3–5). Our findings were also consistent with previous studies documenting a transient decrease in total and LDL cholesterol levels during puberty (32). Although Tanner stages were not assessed, a substantial percentage of the 10–18 year old children (mean age 14) would be expected to be mid-pubertal (Tanner 2–4) based on age. Accordingly, mean total cholesterol and LDL-C levels were lower in the 10–18 year old children than in the 6–9 year old group (Table I). Moreover, cholesterol levels were the only risk factors in which younger children correlated more highly with their parents than the older children (Table II). This is consistent with expectations that the inability to adjust for Tanner stage would reduce the estimated familial correlation in the 10–18. Results were adjusted for age and sex, which may help to account for pubertal changes, but imperfectly because children enter puberty at different ages. The fact that cholesterol levels remained highly correlated even after adjustment for BMI is a novel finding and supports the overall conclusions of the study for the associations of CV risk factors between parents and children independent of adiposity.
Insulin sensitivity in 10–18 year old children correlated with parental values. Interestingly, the correlation between parent and child insulin sensitivity was strengthened after adjustment for child and parent BMI. The lack of Tanner staging also complicates the interpretation of insulin sensitivity data in the older children, as pubertal subjects are known to have transient increases in insulin resistance (33). This relationship might have been interesting to explore in the younger children, but euglycemic clamps were not performed in this age group because of concerns about excessive participant burden.
The risk of developing cardiometabolic disease is higher when multiple risk factors are present. In particular, adults meeting criteria for metabolic syndrome have a demonstrable increase in the risk of future CV disease and type 2 diabetes (34). Attempts to define an analogous pediatric “metabolic syndrome” have been hampered by the fact that few children have cardiometabolic abnormalities in the overtly abnormal range and because the metabolic syndrome construct has been shown to lack stability through childhood (35). Nonetheless, there is a strong body of research demonstrating that higher levels of these risk factors, even within the normal range, tend to track into adulthood, are difficult to reverse, and are associated with increased morbidity over time (16, 36). Standard deviation “cluster scores” have been developed as a means of better defining risk in younger patients by considering each risk factor as a continuous variable that contributes to the total score (37–39). Thus, each subject can be assigned a relative risk rather than being grouped categorically as having or not having the metabolic syndrome. We have previously shown that a cluster score based on metabolic syndrome components predicts cardiovascular risk from adolescence (mean age 13 years) into young adulthood (mean age 22 years) (39). The correlation of parent and child cluster scores and the association of parent metabolic syndrome with higher parent cluster scores (data not shown) in this study support this approach to identification of at-risk children. In this study we also found that the exclusion of the fasting glucose component from the cluster score increased the strength of the parent-child correlation, at least within the older (10–18 year old) subgroup. We were not surprised by this finding because fasting glucose levels did not correlate between parents and children, consistent with previous observations that fasting glucose is rarely abnormal in pediatric subjects and is not a useful predictor of glucose tolerance, even in obese youth (40, 41). We also carried out sensitivity analyses to examine whether findings changed depending of use of different methods of computing the cluster scores: The version that we include here used Z-scores specific to each age group with the intention of creating cluster scores that more accurately reflect deviation from age-specific norms. When we instead used a single set of standards for all age groups so that all cluster scores were calculated using the exact same formula and then compared the relative positions of the parent and child cluster scores on this continuum, our findings were essentially unchanged (data not shown).
The relation between parent and child cluster scores and parent-child correlations for measures of adiposity were stronger in older (10–18 year old) children compared with younger (6–9 year old) children. It is not surprising that these parent-child relations strengthen with age. Changes likely coincide with the transition to a more adult body habitus and physiology during puberty. We previously demonstrated a continuation of this trend into young adulthood, by describing the correlations of CV risk factors between parents and older children during the transition from adolescence (mean age 15 years) to young adulthood (mean age 22 years); parent-child correlations become stronger over this interval (42). Thus, there appears to be a natural progression where the correlations between shared CV risk factors become more significant as young children transition through adolescence, and into adulthood.
An important limitation of this study is that parent factors were not fully represented because only one parent per child was included. This also limited sex-specific parental contribution analysis because we were not able to show differences in parent-child correlations between mothers and fathers (data not shown); our numbers were likely too small to allow accurate assessment. Strengths of the study include the large number of parent-child dyads representing a wide range of child ages, direct measurement of insulin sensitivity by euglycemic clamp, the multiple measurements used to assess adiposity, contemporaneous and state-of-the-art measurements for the entire study population, and the use of a cluster score applicable to both children and parents as a measure of CV risk burden.
Although there are many public health reasons to tackle the problem of childhood overweight/obesity, these data support a role for early monitoring and more intensive intervention in children of parents with less favorable risk factors, even in normal weight children. Furthermore, similar to the concept of metabolic syndrome in adults, the development and use of risk factor cluster scores may help to target children with relatively higher cardiometabolic risk prior to the development of overt disease.
Acknowledgments
Funded by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072124 [to J.S.]), the National Center for Research Resources (1UL1RR033183 to the University of Minnesota Clinical and Translational Science Institute), and the General Clinical Research Center Program (M01-RR00400 and T32DK65519).
LIST OF ABBREVIATIONS
- BMI
body mass index
- cm
centimeters
- kg
kilogram
- m
meter
- mm
millimeter
- Hg
mercury
- BP
blood pressure
- CS5
cluster score 5
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- LDL-C
low density lipoprotein cholesterol
- HDL-C
high density lipoprotein cholesterol
- lbm
lean body mass
- P
p-value
- ICC
interclass correlation
Footnotes
The authors declare no conflicts of interest.
Portions of the study were presented as a platform at the meeting of the Pediatric Academic Societies, Vanouver, BC, Canada, May 3–6, 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harrap SB, Stebbing M, Hopper JL, Hoang HN, Giles GG. Familial patterns of covariation for cardiovascular risk factors in adults: The Victorian Family Heart Study. Am J Epidemiol. 2000;152:704–15. doi: 10.1093/aje/152.8.704. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen-Torvik LJ, Pankow JS, Jacobs DR, Steffen LM, Moran AM, Steinberger J, et al. Heritability and genetic correlations of insulin sensitivity measured by the euglycaemic clamp. Diabet Med. 2007;24:1286–9. doi: 10.1111/j.1464-5491.2007.02271.x. [DOI] [PubMed] [Google Scholar]
- 3.Reis EC, Kip KE, Marroquin OC, Kiesau M, Hipps L, Jr, Peters RE, et al. Screening children to identify families at increased risk for cardiovascular disease. Pediatrics. 2006;118:e1789–97. doi: 10.1542/peds.2006-0680. [DOI] [PubMed] [Google Scholar]
- 4.Schwandt P, Bischoff-Ferrari HA, Staehelin HB, Haas GM. Cardiovascular risk screening in school children predicts risk in parents. Atherosclerosis. 2009;205:626–31. doi: 10.1016/j.atherosclerosis.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Vik KL, Romundstad P, Nilsen TI. Tracking of cardiovascular risk factors across generations: family linkage within the population-based HUNT study, Norway. J Epidemiol Community Health. 2013;67:564–70. doi: 10.1136/jech-2012-201634. [DOI] [PubMed] [Google Scholar]
- 6.Danielzik S, Langnase K, Mast M, Spethmann C, Muller MJ. Impact of parental BMI on the manifestation of overweight 5–7 year old children. Eur J Nutr. 2002;41:132–8. doi: 10.1007/s00394-002-0367-1. [DOI] [PubMed] [Google Scholar]
- 7.Dev DA, McBride BA, Fiese BH, Jones BL, Cho H. Risk factors for overweight/obesity in preschool children: an ecological approach. Child Obes. 2013;9:399–408. doi: 10.1089/chi.2012.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafaghi K, Shariff Z, Taib MNM, Rahman H, Mobarhan M, Jabbari H. Parental body mass index is associated with adolescent overweight and obesity in Mashhad, Iran. Asia Pacific Journal of Clinical Nutrition. 2014;23:225–31. doi: 10.6133/apjcn.2014.23.2.11. [DOI] [PubMed] [Google Scholar]
- 9.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 10.Van Itallie TB. Health implications of overweight and obesity in the United States. Ann Intern Med. 1985;103:983–8. doi: 10.7326/0003-4819-103-6-983. [DOI] [PubMed] [Google Scholar]
- 11.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 12.Plourde G. Impact of obesity on glucose and lipid profiles in adolescents at different age groups in relation to adulthood. BMC family practice. 2002;3:18. doi: 10.1186/1471-2296-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper R, Pinto Pereira SM, Power C, Hypponen E. Parental obesity and risk factors for cardiovascular disease among their offspring in mid-life: findings from the 1958 British Birth Cohort Study. Int J Obes (Lond) 2013;37:1590–6. doi: 10.1038/ijo.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendall DM, Harmel AP. The metabolic syndrome, type 2 diabetes, and cardiovascular disease: understanding the role of insulin resistance. Am J Manag Care. 2002;8:S635–53. quiz S54–7. [PubMed] [Google Scholar]
- 15.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. 2005;47:201–10. [PubMed] [Google Scholar]
- 16.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Changes in risk variables of metabolic syndrome since childhood in pre-diabetic and type 2 diabetic subjects: the Bogalusa Heart Study. Diabetes Care. 2008;31:2044–9. doi: 10.2337/dc08-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prineas RJ, Gillum RF, Horibe H, Hannan PJ. The Minneapolis children’s blood pressure study. Part 1: standards of measurement for children’s blood pressure. Hypertension. 1980;2:I18–24. doi: 10.1161/01.hyp.2.4_pt_2.i18. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health S. CDC Growth Charts: United States 2000. [updated 5/30/2000; cited 2014 2/21]. Available from: http://www.cdc.gov/growthcharts.
- 19.Steffen LM, Sinaiko AR, Zhou X, Moran A, Jacobs DR, Jr, Korenfeld Y, et al. Relation of adiposity, television and screen time in offspring to their parents. BMC Pediatr. 2013;13:133. doi: 10.1186/1471-2431-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran A, Jacobs DR, Jr, Steinberger J, Steffen LM, Pankow JS, Hong CP, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation. 2008;117:2361–8. doi: 10.1161/CIRCULATIONAHA.107.704569. [DOI] [PubMed] [Google Scholar]
- 21.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 22.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 23.Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension. 2005;45:1035–41. doi: 10.1161/01.HYP.0000165680.59733.d4. [DOI] [PubMed] [Google Scholar]
- 24.Poobalan A, Aucott L, Smith WC, Avenell A, Jung R, Broom J, et al. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes--a systematic review. Obes Rev. 2004;5:43–50. doi: 10.1111/j.1467-789x.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 25.Staessen J, Fagard R, Amery A. Obesity and hypertension. Acta Cardiol Suppl. 1988;29:37–44. [PubMed] [Google Scholar]
- 26.Staessen J, Fagard R, Amery A. The relationship between body weight and blood pressure. Journal of human hypertension. 1988;2:207–17. [PubMed] [Google Scholar]
- 27.Karter AJ, D’Agostino RB, Jr, Mayer-Davis EJ, Wagenknecht LE, Hanley AJ, Hamman RF, et al. Abdominal obesity predicts declining insulin sensitivity in non-obese normoglycaemics: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Obes Metab. 2005;7:230–8. doi: 10.1111/j.1463-1326.2004.00441.x. [DOI] [PubMed] [Google Scholar]
- 28.Mazor-Aronovitch K, Lotan D, Modan-Moses D, Fradkin A, Pinhas-Hamiel O. Blood pressure in obese and overweight children and adolescents. Isr Med Assoc J. 2014;16:157–61. [PubMed] [Google Scholar]
- 29.Schiel R, Beltschikow W, Kramer G, Stein G. Overweight, obesity and elevated blood pressure in children and adolescents. Eur J Med Res. 2006;11:97–101. [PubMed] [Google Scholar]
- 30.Jedrusik P, Januszewicz A, Busjahn A, Zawadzki B, Wocial B, Ignatowska-Switalska H, et al. Genetic influence on blood pressure and lipid parameters in a sample of Polish twins. Blood Press. 2003;12:7–11. [PubMed] [Google Scholar]
- 31.Johnson CC, Nicklas TA, Arbeit ML, Franklin FA, Cresanta JL, Harsha DW, et al. Cardiovascular risk in parents of children with elevated blood pressure. “Heart Smart”--family health promotion. J Clin Hypertens. 1987;3:559–66. [PubMed] [Google Scholar]
- 32.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–S56. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran A, Jacobs DR, Jr, Steinberger J, Hong CP, Prineas R, Luepker R, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 34.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–12. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 35.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 36.Balakrishnan PL. Identification of obesity and cardiovascular risk factors in childhood and adolescence. Pediatr Clin North Am. 2014;61:153–71. doi: 10.1016/j.pcl.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Batey LS, Goff DC, Tortolero SR, Nichaman MZ, Chan W, Chan FA, et al. Summary Measures of the Insulin Resistance Syndrome Are Adverse Among Mexican-American Versus Non-Hispanic White Children : The Corpus Christi Child Heart Study. Circulation. 1997;96:4319–25. doi: 10.1161/01.cir.96.12.4319. [DOI] [PubMed] [Google Scholar]
- 38.Brambilla P, Lissau I, Flodmark CE, Moreno LA, Widhalm K, Wabitsch M, et al. Metabolic risk-factor clustering estimation in children: to draw a line across pediatric metabolic syndrome. Int J Obes (Lond) 2007;31:591–600. doi: 10.1038/sj.ijo.0803581. [DOI] [PubMed] [Google Scholar]
- 39.Kelly AS, Steinberger J, Jacobs DR, Hong CP, Moran A, Sinaiko AR. Predicting cardiovascular risk in young adulthood from the metabolic syndrome, its component risk factors, and a cluster score in childhood. Int J Pediatr Obes. 2011;6:e283–9. doi: 10.3109/17477166.2010.528765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsay J, Pomeranz C, Hassoun A, Zandieh SO, Rutledge J, Vogiatzi MG, et al. Screening markers of impaired glucose tolerance in the obese pediatric population. Horm Res Paediatr. 2010;73:102–7. doi: 10.1159/000277625. [DOI] [PubMed] [Google Scholar]
- 41.Yesiltepe Mutlu G, Ozsu E, Cizmecioglu FM, Hatun S. Can HbA1c and one-hour glucose concentration in standard OGTT be used for evaluation of glucose homeostasis in childhood? J Clin Res Pediatr Endocrinol. 2013;5:80–4. doi: 10.4274/Jcrpe.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frohnert BI, Jacobs DR, Jr, Steinberger J, Moran A, Steffen LM, Sinaiko AR. Relation between serum free fatty acids and adiposity, insulin resistance, and cardiovascular risk factors from adolescence to adulthood. Diabetes. 2013;62:3163–9. doi: 10.2337/db12-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]