Abstract
Objective
To identify patterns of co-existing lesions on MRI in knees free of radiographic osteoarthritis and to examine their relation to incident disease.
Methods
From a prospective cohort study, the Multicenter Osteoarthritis Study, one knee per subject without radiographic osteoarthritis in both tibiofemoral and patellofemoral joints at baseline was selected and followed up to 84-months. We used a novel approach, latent class analysis, to group the constellation of MRI lesions in each joint, i.e., cartilage damage, bone marrow lesion, meniscal tear, meniscal extrusion, synovitis, and effusion, to a manageable number of subgroups. The association of these subgroups with incident radiographic osteoarthritis in the same joint was assessed using logistic regression.
Results
Among 885 eligible knees (mean age 60.5 years, 203 with incident disease in the tibiofemoral joint, 64 in the patellofemoral joint), four latent subgroups were identified in the tibiofemoral joint described briefly as: minimal lesions, mild lesions, moderate lesions (but limited meniscal lesions), and severe lesions. The odds ratios of incident disease in the tibiofemoral joint were 1.0, 5.6, 1.8, and 5.0, respectively. A similar set of four subgroups was identified in the patellofemoral joint, except that the fourth subgroup had limited meniscal lesions. The odds ratios of incident disease in the patellofemoral joint were 1.0, 3.8, 5.1, and 13.7, respectively.
Conclusion
Different patterns of co-existing MRI lesions were identified that have different implications for risk of knee osteoarthritis. Meniscal damage seemed to play a different role in the development of incident disease in the tibiofemoral versus patellofemoral joints.
Keywords: knee osteoarthritis, magnetic resonance imaging, latent class analysis, incidence
Osteoarthritis (OA) is one of the most common causes of disability among older adults and affects millions of persons in the world1,2. The prevention of knee OA and reduction of its burden depend on early diagnosis and fully understanding its risk factors. Even though structural changes on radiographs have long been considered the gold standard in identifying the disease, magnetic resonance imaging (MRI) is more sensitive in identifying tissue-specific lesions, including lesions at earlier stages that may not be evident on radiographs3.
Previous studies reported that bone marrow lesions4, meniscal damage4,5, Hoffa synovitis and effusion6,7 were each individually related to the development of knee radiographic OA (ROA) or cartilage damage among subjects without ROA. Studies to date have primarily focused on individual MRI lesions even though data suggest that a higher number of co-existing MRI lesions is associated with a greater risk of incident cartilage damage4. Injuries such as ACL tears usually affect more than one knee structure8, thus patterns of concurrent structural damage are likely to be important in development of later disease. Focusing on a single structure in the study of MRI lesions is overly simplistic and does not give a comprehensive picture of the relation between MRI lesions and later disease.
However, studying the relation of multiple MRI lesions to later disease onset is challenging. One approach often used is to include all MRI lesions into a multivariable regression model and compare their effects. Unfortunately, the effect estimates obtained from this approach are not directly comparable because some represent the total effect, and others the direct effect, according to the chronology of their occurrence9,10. In the absence of knowledge regarding the temporal sequence, an alternative strategy is to identify the patterns of coexisting MRI lesions among knees without ROA and examine their relation to the development of ROA. Such an approach is attractive because it reduces the otherwise nearly impossible task of model building with many intercorrelated predictors and also because patterns of coexisting MRI lesions may reflect different pathophysiologic pathways leading to the common end-phenotype of ROA, thereby shedding light on biological mechanisms in the development of the disease.
Without a priori potential patterns of interest, identification of discrete, mutually exclusive patterns of the co-existing MRI lesions in knees can be accomplished through statistical clustering approaches, such as latent class analysis (LCA). We applied this approach to data from the Multicenter Osteoarthritis (MOST) Study to identify patterns of the coexisting MRI lesions among knees without ROA at baseline, and then examined the association between the patterns and risk of incident knee ROA.
Patients and Methods
Study design and subjects
Subjects were participants in the MOST study who were recruited from Birmingham, Alabama and Iowa City, Iowa. The study protocol was approved by the Institutional Review Boards at the University of Iowa, University of Alabama, Birmingham, University of California, San Francisco and Boston University. Details about the MOST study are available on the web site of the study (http://most.ucsf.edu) and have been published previously11.
One native (i.e., non-replaced) knee of each MOST subject was randomly selected at baseline. Among them, we excluded knees without MRI examinations and knees with ROA in either the tibiofemoral joint (TFJ) or the patellofemoral joint (PFJ) at baseline. We also excluded knees without radiographs during follow-up or with osteonecrosis on radiographs since their films were not scored for ROA. To study the patterns of MRI lesions, we only used the knees that had baseline MRI reading completed.
Radiographic assessment
At baseline and each follow-up visit (i.e., 30-month, 60-month and 84-month visits), subjects had weightbearing semi-flexed posteroanterior and lateral view knee radiographs obtained using a standard, validated protocol12,13. All longitudinal knee radiographs were read by both a musculoskeletal radiologist and a rheumatologist with knowledge of their time sequence. If there was a disagreement as to whether the knee at any time point had ROA, the reading was adjudicated by a panel of three experienced readers including the two who read the films and a third rheumatologist (DTF). A consensus reading was arrived at when at least two of three readers agreed11.
A knee was defined as having incident ROA in the TFJ if the Kellgren & Lawrence grade was ≥2 at any point during follow-up14, and as having incident ROA in the PFJ if the osteophyte grade was ≥2, or any osteophyte grade ≥1 plus joint space narrowing grade ≥215 in the joint at any point during follow-up.
MRI
The detailed knee MRI protocol has been published previously16. MRIs were read by two musculoskeletal radiologists, with each reading MRIs from some of the participants and ongoing assessments to ensure high inter-reader reliability. The baseline MRI were scored for cartilage morphology (CartM), bone marrow lesion (BML), meniscal tear (MT), meniscal extrusion (MExt), synovitis (SYN) (in Hoffa’s fad pad) and effusion (EFF) (in suprapatellar area combining data on effusion and synovitis there), according to the Whole-Organ MRI Score (WORMS)17. Scores were applied in subregions defined by the WORMS method for each feature in both the TFJ and PFJ. The weighted kappa statistics (95% CI) of inter-reader reliability for the readings were 0.78 (0.76 – 0.81) for CartM; 0.62 (0.57 – 0.68) for BML, 0.80 (0.74 – 0.87) for MT, 0.60 (0.47 – 0.73) for MExt, 0.65 (0.55 – 0.76) for SYN, and 0.65 (0.52 – 0.77) for EFF.
Statistical analysis
We used the worst CartM score from among 10 subregions in the TFJ (medial femur center and posterior; medial tibia anterior, center and posterior; lateral femur center and posterior; lateral tibia anterior, center and posterior) and among 4 subregions in the PFJ (medial femur anterior, medial patella, lateral femur anterior, and lateral patella) to represent the severity of cartilage damage in each of the tibiofemoral and patellofemoral joints, respectively. The worst BML score from among 11 subregions in the TFJ (including tibia subspinus) and among 4 subregions in the PFJ was used to represent the severity of BML in each joint, respectively. The worst MT score from among 6 subregions (medial anterior, body, and posterior; lateral anterior, body, and posterior), the worst MExt score from among 2 subregions (medial and lateral), and the worst SYN score from among 2 subregions (infrapatellar and intercondylar) was used to represent the severity of these lesions in the whole knee.
We performed latent class analysis using SAS procedure PROC LCA18 to identify subgroups representing distinct patterns of coexisting MRI lesions in the TFJ based on the severity of CartM and BML in the TFJ and MT, MExt, SYN and EFF in the whole knee. If there was a small proportion (<3%) of knees with a score level, we collapsed that level to the adjacent severity level with lower score to avoid unstable estimations due to sparse data. We fitted the LCA models with 2–7 subgroups and chose the model which had the lowest Akaike information criterion (AIC) or adjusted Bayesian information criterion (BIC)19 to identify the best model fit, and also had to have sufficient numbers of knees (>5% of the sample) in each subgroup. The posterior probability of subgroup membership for all knees was generated from LCA model and we used the maximum-probability approach to assign each knee to one of the subgroups20. We described the distribution of characteristics at baseline according to subgroups and compared them using analysis of variance for continuous variables and the chi-square test for categorical variables. We examined the relation of subgroups to the risk of incident ROA in the TFJ using logistic regression, adjusting for age, BMI, sex, race, clinic site, history of knee injury and surgery.
The two-step approach described above may be susceptible to possibility of misclassification because it did not account for uncertainty of group membership in assignment21. To circumvent this problem and test the robustness of our results, we applied a one-step model-based LCA approach22, which estimated the probability of subgroups of coexisting MRI lesions as well as their relation with the distal outcome of interest, i.e., incident ROA in our study, from the same model, thus taking the uncertainty of group membership into consideration. Since the one-step approach is unable to adjust for potential confounders, we used it as a sensitivity analysis.
We took the same approach to assess the relation of the patterns of coexisting MRI lesions (i.e., CartM and BML in the PFJ; MT, MExt, SYN, and EFF in the whole knee) at baseline to incident ROA in the PFJ.
All analyses were performed in SAS 9.1 (SAS Institute, Cary NC). Latent class analysis were conducted using PROC LCA18 and LCA distal SAS macro (Version 2.0, 2012)21 download from http:methodology.psu.edu.
Results
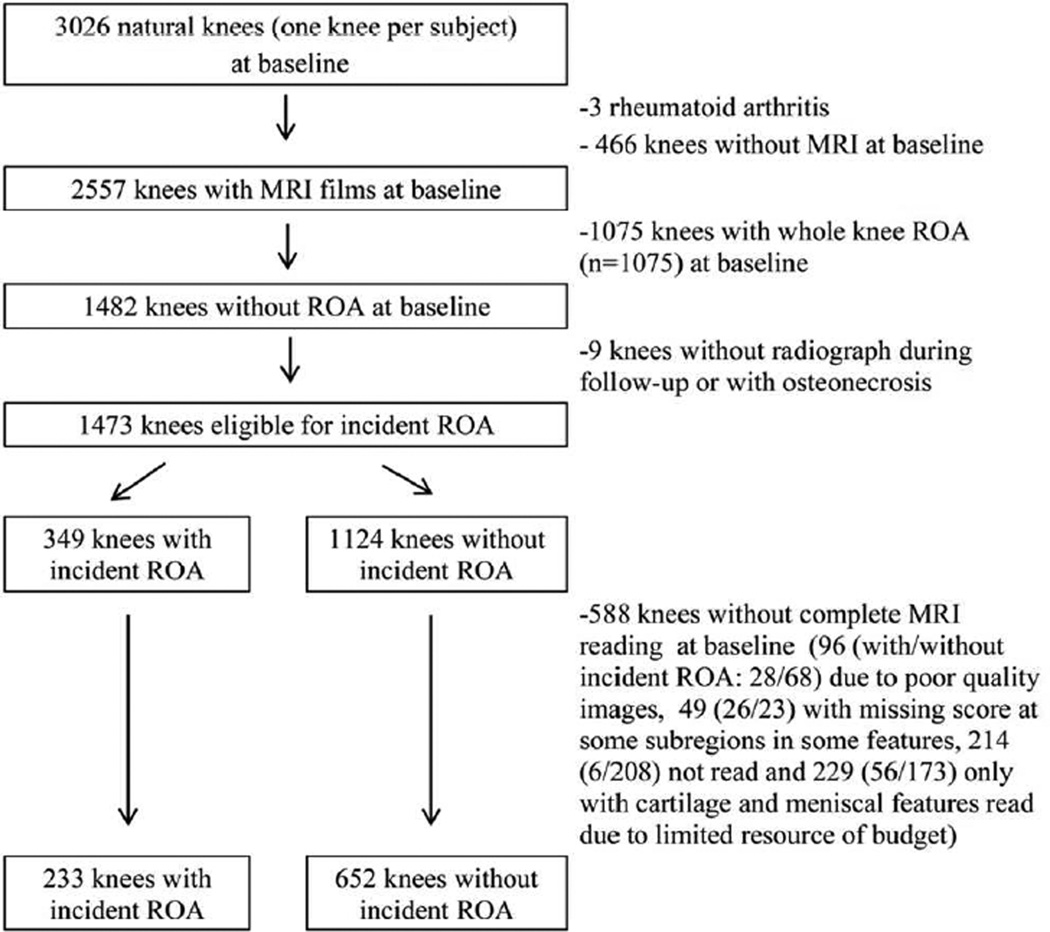
Of the 3026 native knees randomly selected from MOST subjects, 1473 knees with MRI and without ROA in both the TFJ and the PFJ at baseline were eligible for incident ROA. After further excluding 588 (39.9%) knees without complete baseline MRI reading, 885 knees were included in the study (Figure 1). Of these knees, 233 had incident ROA in either the TFJ, PFJ, or both (203 in the TFJ and 64 in the PFJ). Knees with incident ROA had similar baseline characteristics to the ones that were excluded except that the former knees were more likely from UAB clinic site. Knees without incident ROA in the study were from slightly younger subjects than the ones excluded (60.2 vs. 61.6 year) (Table 1).
Figure 1.
Flow chart
Table 1.
Baseline characteristics by incident whole knee ROA and completeness of baseline MRI reading
| characteristics | knees with incident ROA (N=349) |
knees without incident ROA (N=1124) |
||
|---|---|---|---|---|
| baseline MRI reading complete (N=233) |
baseline MRI reading incomplete or missing (N=116) |
baseline MRI reading complete (N=652) |
baseline MRI reading incomplete or missing (N=472) |
|
| age, mean (SD) | 61.4 (7.6) | 61.3 (7.5) | 60.2 (7.3) | 61.6 (8.2) |
| BMI, mean (SD) | 30.7 (5.3) | 30.7 (4.8) | 28.7 (4.5) | 28.6 (4.6) |
| female, % | 159 (68.2) | 72 (62.1) | 361 (55.4) | 281 (59.5) |
| White, % | 195 (83.7) | 100 (86.2) | 568 (87.1) | 413 (87.5) |
| Alabama, % | 117 (50.2) | 40 (34.5) | 325 (49.9) | 213 (45.1) |
| history of knee injury, % | 48 (20.6) | 24 (20.7) | 132 (20.3) | 78 (16.5) |
| history of knee surgery, % | 12 (5.2) | 6 (5.2) | 28 (4.3) | 28 (4.7) |
| Kellgren & Lawrence grade 1, % | 121 (51.9) | 56 (48.3) | 113 (17.3) | 79 (16.7) |
| frequent knee pain at both telephone interview and clinic visit, % | 57 (24.5) | 20 (17.2) | 109 (16.7) | 71 (15.1) |
| frequent knee pain at clinic visit, % | 89 (38.2) | 35 (30.2) | 174 (26.7) | 112 (23.8) |
| followed to 60mo clinic visit | 224 (96.6) | 110 (94.8) | 549 (87.6) | 388 (83.4) |
| followed to 84mo clinic visit | 205 (89.1) | 110 (94.5) | 530 (87.2) | 353 (81.5) |
Patterns of coexisting MRI lesions and incident ROA in the TFJ
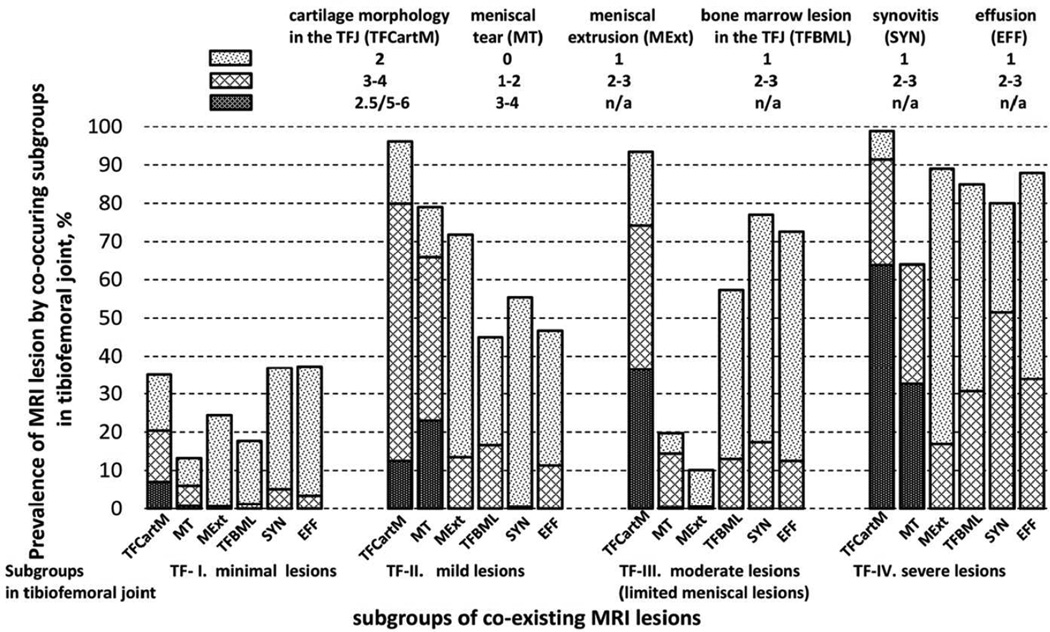
Based on the LCA model, we identified four subgroups: TF-I (51.8%), TF-II (12.7%), TF-III (28.0%), and TF-IV (7.5%). In TF-I, the prevalence of each MRI feature (worst score >0) was below 40% and severe lesions were rare. In each of the three other subgroups, CartM lesions in the TFJ were observed in over 90% of knees, with the severity increasing from TF-II to TF-IV (12.5%, 36.5% and 63.8% full thickness lesions, respectively). The prevalence and severity of BML, SYN and EFF also increased from TF-I to TF-IV (prevalence 80–90%). MT and MExt were rare in TF-III (19.7% and 10.1%, respectively), but common in TF-II (79.0% and 71.8%) and TF-IV (64.0% and 88.9%) (Figure 2). Based on the differences in prevalence and severity of lesions among the subgroups, we labeled them as TF-I: minimal lesions, TF-II: mild lesions, TF-III: moderate lesions (limited meniscal lesions), and TF-IV: severe lesions.
Figure 2.
Estimated prevalence of MRI features in the tibiofemoral joint according to subgroups of coexisting MRI lesions
Using the maximum-probability approach, 465, 102, 253 and 65 knees were assigned to the subgroups TF-I to TF-IV, respectively. The average posterior probability of membership was 0.84, suggesting that subgroup assignment was relatively unambiguous. Compared with those whose knees were in TF-I, subjects in TF-II were older, more likely to be male and White, and more likely to have history of knee surgery. The distribution of baseline characteristics in TF-III was similar to that in TF-I except for being more likely to be male and White. Subjects whose knees were in TF-IV had the oldest age at baseline among these subgroups and were more likely to have a history of knee surgery than subjects in TF-I (Table 2).
Table 2.
Baseline characteristics of subjects assigned by the maximal-probability approach to subgroups of MRI lesions
| Baseline characteristics | Subgroups in the tibiofemoral joint | Subgroups in the patellofemoral joint | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TF-I (N=465) |
TF-II (N=102) |
TF-III (N=253) |
TF-IV (N=65) |
PF-I (N=325) |
PF-II (N=111) |
PF-III (N=366) |
PF-IV (N=83) |
|||
| age | 60.0 (7.2) | 62.0 (7.9) | 60.3 (7.5) | 62.4 (7.1) | * | 60.0 (7.3) | 62.4 (7.4) | 60.2 (7.5) | 61.3 (7.6) | * |
| baseline BMI | 29.4 (4.9) | 28.6 (4.9) | 29.0 (4.6) | 30.3 (4.9) | 28.3 (4.5) | 29.4 (5.0) | 29.8 (4.9) | 30.0 (5.0) | ǂ | |
| Female, N(%) | 299 (64.3) | 46 (45.1) | 140 (55.3) | 35 (53.8) | † | 183 (56.3) | 52 (46.8) | 247 (67.5) | 38 (45.8) | ǂ |
| White, N(%) | 389 (83.7) | 94 (92.2) | 227 (89.7) | 53 (81.5) | * | 272 (83.7) | 100 (90.1) | 317 (86.6) | 74 (89.2) | † |
| Alabama, N(%) | 283 (60.9) | 46 (45.1) | 87 (34.4) | 26 (40.0) | ǂ | 182 (56.0) | 48 (43.2) | 182 (49.7) | 30 (36.1) | |
| History of knee injury, N(%) | 87 (18.8) | 27 (26.7) | 50 (19.9) | 16 (24.6) | 58 (18.0) | 29 (26.4) | 73 (19.9) | 20 (24.4) | ||
| History of knee surgery, N(%) | 14 (3.0) | 12 (11.8) | 7 (2.8) | 7 (10.8) | ǂ | 14 (4.3) | 7 (6.3) | 14 (3.8) | 5 (6.0) | |
p-value <0.05,
p-value <0.01,
p-value <0.001
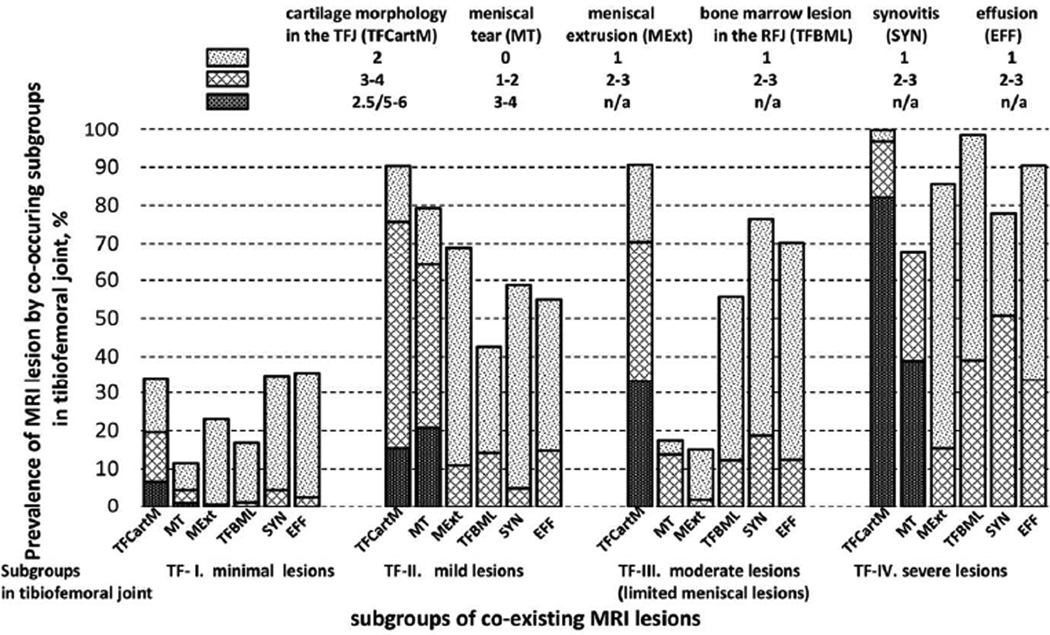
The risk of incident ROA in the TFJ was 15.7% in TF-I. Compared with knees in TF-I, the multivariable adjusted odds ratios were 5.6, 1.8, and 5.0, respectively, in TF-II, TF-III, and TF-IV (Table 3). In the sensitivity analysis using the one-step approach, we also identified four subgroups, with probabilities of membership 47.8%, 16.0%, 30.8%, and 5.3%, respectively. About 90.5% of knees were in the same subgroup as they were assigned based on the two-step approach. These subgroups had similar distribution of MRI lesions to that from the main analysis (Appendix Figure 1). The effect estimates generated from the one-step approach were slightly larger than from the two-step approach, with unadjusted odds ratios (95% CI) being 1.0, 9.3 (4.8, 17.8), 2.0 (0.7, 5.8) and 14.5 (4.7, 44.8), respectively, for TF-I to TF-IV.
Table 3.
Subgroups of MRI lesions and incident ROA in the tibiofemoral and patellofemoral joints
| Subgroups of MRI lesions at baseline |
Risk of ROA n/N(%) |
Crude model | Adjusted model* | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| in the tibiofemoral joint | |||||
| TF-I | 73/465 (15.7) | 1.0 | 1.0 | ||
| TF-II | 43/102 (42.2) | 3.9 (2.5, 6.2) | <0.001 | 5.6 (3.4, 9.4) | <0.001 |
| TF-III | 57/253 (22.5) | 1.6 (1.1, 2.3) | 0.024 | 1.8 (1.2, 2.8) | 0.004 |
| TF-IV | 30/65 (46.2) | 4.6 (2.6, 8.0) | <0.001 | 5.0 (2.8, 9.0) | <.001 |
| in the patellofemoral joint | |||||
| PF-I | 6/325 (1.8) | 1.0 | 1.0 | ||
| PF-II | 7/111 (6.3) | 3.6 (1.2, 10.9) | 0.025 | 3.8 (1.2, 11.9) | 0.020 |
| PF-III | 35/366 (9.6) | 5.6 (2.3, 13.6) | <0.001 | 5.1 (2.1, 12.5) | <0.001 |
| PF-IV | 16/83 (19.3) | 12.7 (4.8, 33.6) | <.001 | 13.7 (5.0, 37.0) | <.001 |
Adjusting for age, BMI, gender, race, clinical site, history of knee injury and surgery
Appendix figure 1.
Estimated prevalence of MRI features in the tibiofemoral joint by subgroups of co-existing MRI lesions from the one-step model
Patterns of coexisting MRI lesions and incident ROA in the PFJ
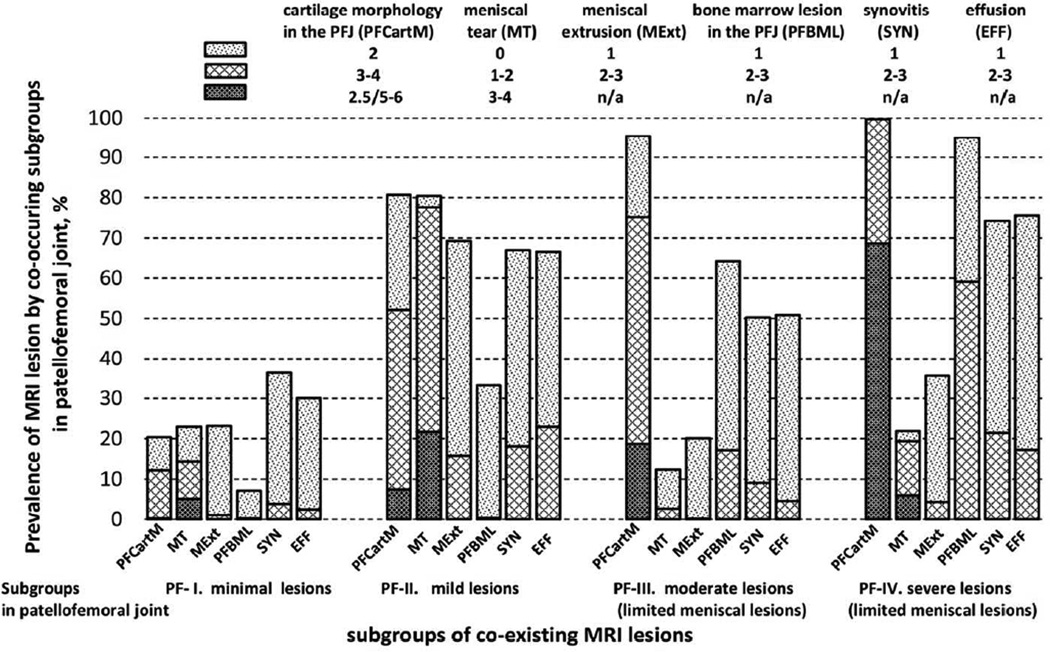
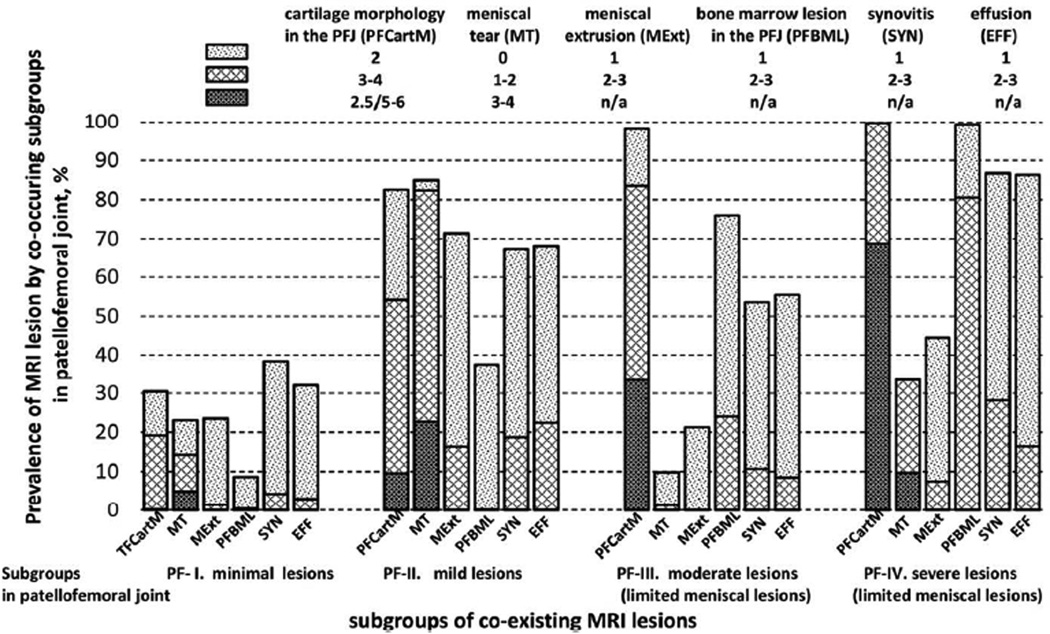
Similarly, we identified four subgroups in the PFJ: PF-I (35.7%), PF-II (13.3%), PF-III (39.9%), and PF-IV (11.1%). The prevalence of MRI lesions in the PFJ subgroups were similar to that observed in the TFJ subgroups with some exceptions: MT and MExt were minimal in PF-IV (33.6% and 44.4%, respectively); and SYN and EFF were more common in PF-II than that in PF-III (67.4% vs. 53.6% and 68.3% vs. 56.5%, respectively). Thus we labelled them as PF-I: minimal lesions, PF-II: mild lesions, PF-III: moderate lesions (limited meniscal lesions); and PF-IV: severe lesions (limited meniscal lesions) (Figure 2).
Compared with those in PF-I, subjects in the other subgroups had higher BMI. Subjects in PF-III were more likely to be female, and those in PF-IV were older (Table 2). The risk of incident ROA in the PFJ was lower in PF-I than those in other subgroups and the multivariable adjusted odds ratios were 3.8, 5.1 and 13.7 for PF-II, PF-III and PF-IV, respectively (Table 3). Similar probability of subgroups membership (29.9%, 14.2%, 34.7%, and 21.3%) and distribution of MRI lesions in each subgroup were observed from the one-step approach (Appendix figure 2). About 80.3% of knees were in the same subgroup as they were assigned based on the two-step approach. Compared with those in PF-I, the risk of incident ROA in the PFJ from the one-step approach was higher in PF-II (crude odds ratio (95% CI) = 5.7 (0.6, 51.3)), and probably higher in PF-IV (crude odds ratios (95% CI) = 33.5 (5.30, 212.4)), but not in PF-III (crude odds ratios 0.8 (0, 56.2)).
Appendix figure 2.
Estimated prevalence of MRI features in the patellofemoral joint by subgroups of co-existing MRI lesions from the one-step model
Discussion
Among knees without ROA we identified four distinct patterns of coexisting MRI lesions at baseline in the TFJ. Except for meniscal damage, the severity of all lesions increased across the four subgroups. Incident ROA in the TFJ markedly increased in TF-II and TF-IV, while it was only mildly increased in TF-III which had limited meniscal damage and moderate defects of all other lesions. On the other hand, the patterns of coexisting MRI lesions in the PFJ were somewhat different with limited meniscal lesions in both PF-III and PF-IV. The risk of incident ROA in the PFJ was higher in subgroups with more severe lesions such as cartilage damage, regardless of severity of meniscal lesion.
As one of the best approaches to detect structural lesions in the knee, MRI is time-consuming and expensive, allowing only infrequent repetition. It is therefore difficult to capture the first occurrence of a specific lesion and determine the time sequence of structural lesions in the natural history of OA. Latent class analysis and other clustering approaches allow insights into the naturally occurring patterns of coexisting lesions, thus providing a way to circumvent the dilemma of whether we should control for other lesions or not when studying a specific MRI lesion. This is pertinent because one may inadvertently control for a mediator (i.e., a lesion which is on the pathway from the specific lesion of interest to the outcome), resulting in missing the target effect10. On the other hand, not controlling for a potential confounder lesion biases the effect estimate obtained.
Our results highlight the patterns of coexisting MRI lesions among knees without ROA and shed insights into potential distinct pathologic pathways leading to incident knee ROA. For example, for incident ROA in the TFJ, meniscal damage appears to play a critical role since TF-III was at lower risk of ROA than TF-II in which all lesions were less severe than the former subgroup except for meniscal lesions. Subjects in TF-II reported more previous knee surgery and were slightly thinner; thus representing a group of people who were likely physically active and prone to injury of knees. In contrast to the results in the TFJ, even though the coexisting patterns of MRI lesions in subgroups PF-II and PF-III were similar to the corresponding subgroups in TFJ, the incidence of ROA in subgroup PF-III was similar to or might be slightly higher than the incidence in PF-II. This suggests that meniscal damage is not as important in the development of ROA in the PFJ as in the TFJ, which is not surprising given the weight-absorbing role of the meniscus in the TFJ.
While previous studies have shown that BMLs, synovitis, and effusion were each individually associated with risk of knee ROA4–7, we found that the three lesions often coexisted, especially with cartilage damage. In addition, the severity of a specific lesion correlated well with the severity of other lesions in the subgroups. While not definitive, these MRI lesions may be markers of disease severity and may not themselves be pathogenic for incident radiographic disease. Synovitis and effusion in OA is thought to be elicited by cartilage and/or meniscal damage as a response to release of degradation products into the joint23. BMLs reflect increased focal load across the joint that could be triggered locally by either cartilage or meniscal damage.
Limitations of our study need to be acknowledged. First, we used a two-step approach, i.e., identifying latent subgroups and then assessing their relation to the outcome in separate models. This approach did not take into account uncertainty in subgroup membership assignment; thus it might lead to potentially biased effect estimate. When using the one-step approach we observed a stronger association between the subgroups and incident ROA which supported what we found using the two-step approach. Second, because the number of knees with incident ROA in the PFJ was small, the effect estimates were not stable. This may explain the difference in the effect size in subgroup PF-III observed in the one-step and two-step approaches. Third, while we identified patterns of coexisting structural lesions on MRI among knees without evidence of ROA, we still can’t tell the temporal sequence of these MRI lesions. Nonetheless, even among knees with KL=0, there was no subgroup in which only a single MRI lesion existed. One reason might be that knees included in the current study consisted of those at high risk of developing knee OA, albeit without evidence of ROA at baseline. Thus, the prevalence of each specific MRI feature may be higher in our study sample than the prevalence among knees without OA in the general population. As the result, it might affect the pattern of co-existing MRI features and limit the generalizability of our study findings. An alternative interpretation may be that it may be unusual for a solitary lesion to exist on its own for a substantial amount of time and act as the major driving force behind ROA development.
In conclusion, we identified distinct patterns of coexisting MRI lesions that had differential risk for incident ROA. The magnitude of lesions such as cartilage damage and co-existing meniscal damage appear to be the main distinction between the subgroups. Further, meniscal damage might play a prominent role in the development of incident ROA in the TFJ but not the PFJ. Assessment of patterns of coexisting structural lesions provides novel and unique insights into the pathogenesis of OA.
Figure 3.
Estimated prevalence of MRI features in the patellofemoral joint according to subgroups of coexisting MRI lesions
Acknowledgments
Supported by MOST grants: NIA U01 AG18820; U01 AG18832; U01 AG18947; U01 AG19079
NIAMS P60 AR47785
References
- 1.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60(2):91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 3.Roemer FW, Crema MD, Trattnig S, Guermazi A. Advances in imaging of osteoarthritis and cartilage. Radiology. 2011;260(2):332–354. doi: 10.1148/radiol.11101359. [DOI] [PubMed] [Google Scholar]
- 4.Sharma L, Chmiel JS, Almagor O, Dunlop D, Guermazi A, Bathon J, et al. Significance of pre-radiographic MRI lesions in persons at higher risk for knee osteoarthritis. Arthritis Rheumatol. 2014 [Google Scholar]
- 5.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60(3):831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804–1809. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atukorala I, Kwoh CK, Guermazi A, Roemer FW, Boudreau RM, Hannon MJ, et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: A 10-year study. Knee. 2006;13(3):184–188. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Neogi T, Hunter D, Roemer F, Niu J. What effect is really being measured? An alternative explanation of paradoxical phenomena in studies of osteoarthritis progression. Arthritis Care Res (Hoboken) 2014;66(5):658–661. doi: 10.1002/acr.22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol. 2008;35(10):2047–2054. [PMC free article] [PubMed] [Google Scholar]
- 12.Peterfy CG, Li J, Duryea J, Lynch JA, Miaux Y, Genant HK. Nonfluoroscopic method for flexed radiography of the knee that allows reproducible joint-space width measurement. Abstract Arthritis Rheum. 1998;41(Suppl 89):S361. [Google Scholar]
- 13.Kothari M, Guermazi A, von Ingersleben G, Miaux Y, Sieffert M, Block JE, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14(9):1568–1573. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 14.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felson DT, McAlindon TE, Anderson JJ, Naimark A, Weissman BW, Aliabadi P, et al. Defining radiographic osteoarthritis for the whole knee. Osteoarthritis Cartilage. 1997;5(4):241–250. doi: 10.1016/s1063-4584(97)80020-9. [DOI] [PubMed] [Google Scholar]
- 16.Roemer FW, Guermazi A, Javaid MK, Lynch JA, Niu J, Zhang Y, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009;68(9):1461–1465. doi: 10.1136/ard.2008.096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: a SAS procedure for latent class analysis. Struct Equ Modeling. 2007;14(4):671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sclove S. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;52:333–343. [Google Scholar]
- 20.Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 21.Lanza ST, Tan X, Bray BC. A technical introduction: a model-based approach to latent class analysis with distal outcomes. University Park: The Methodology Center, Penn State; 2012. Retrieved from http://methodology.psu.edu. [Google Scholar]
- 22.Yang J, Tan X, Lanza ST, Wagner AT. Lca distal SAS macro users' guide (Version 2.0) 2012 retrieved from http://methodology.psu.edu. [Google Scholar]
- 23.Roemer FW, Guermazi A, Hunter DJ, Niu J, Zhang Y, Englund M, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis Cartilage. 2009;17(6):748–753. doi: 10.1016/j.joca.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]