Abstract
The motile and sensory functions of cilia and flagella are indispensable for human health. Cilia assembly requires a dedicated protein shuttle, intraflagellar transport (IFT), a bidirectional motility of multi-megadalton protein arrays along ciliary microtubules. IFT functions as a protein carrier delivering hundreds of distinct proteins into growing cilia. IFT-based protein import and export continue in fully grown cilia and are required for ciliary maintenance and sensing. Large ciliary building blocks might depend on IFT to move through the transition zone, which functions as a ciliary gate. Smaller, freely diffusing proteins such as tubulin depend on IFT to be concentrated or removed from cilia. Recent work provides insights into how IFT interacts with its cargoes and how the transport is regulated.
Keywords: dynein, diffusion, flagella, intraflagellar transport, microtubule, kinesin-2, tubulin
Cilia: conserved cell organelles with multiple functions
Cilia and flagella (interchangeable terms) are thread-like cell extensions of variable length with a diameter of ~200 nm (Fig. 1A, B). The hallmark of all cilia is the axoneme, a microtubular scaffold typically containing nine doublet microtubules and often two central singlet microtubules (referred to as 9+2). The doublet microtubules are continuous with the microtubules of the basal bodies, which anchor and position the cilium within the cell (Fig. 1C-F). Cilia are covered by the ciliary membrane, which is continuous with the plasma membrane but specialized in protein and lipid content [1, 2].
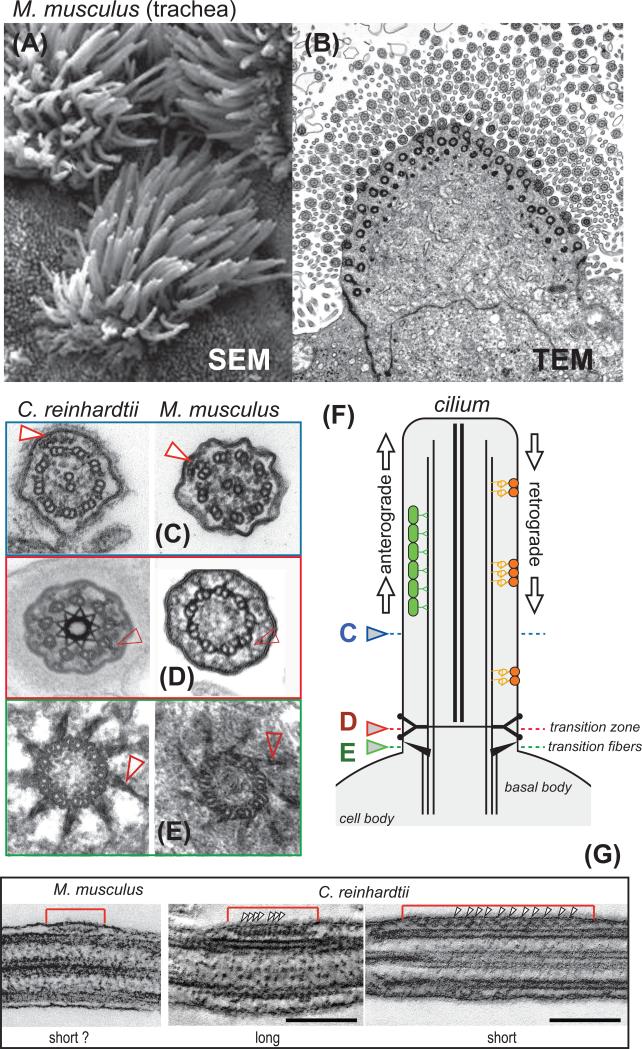
Figure 1. Cilia and intraflagellar transport (IFT).
(A) Scanning electron micrograph and (B) transmission electron micrograph showing multiciliated epithelial cells from mouse airway. (C - E) Cross-sections through cilia of C. reinhardtii (left) and mouse ependymal cilia (right) showing 9+2 axonemes (C), Y-shaped linkers (D), a conserved structural element of the transition zone, and the transitional fibers (E), which link the basal bodies to the plasma membrane and are the putative assembly sites of IFT trains (shown in G). Arrowheads in C mark IFT trains. (F) Schematic presentation of a cilium and IFT trains. The colored arrowheads indicate the level of the cross-sections shown in (C - E). (G) Longitudinal sections through IFT trains in mouse ependymal cilia and C. reinhardtii flagella. Arrowheads mark the periodicities of long and short IFT trains. Bar = 100 nm. The images of C. reinhardtii IFT trains are a curtesy of Dr. Gaia Pagino, MPI Dresden.
Cilia are often structurally adapted to serve diverse functions. Motile cilia possess dynein arms, which are large motor complexes that induce sliding of the axonemal microtubules and thereby bending of the cilium. Ciliary motility propels cells such as protists and spermatozoa or generates fluid flow above the ciliated epithelia lining, such as in the airways. A conserved 9+2 axoneme is characteristic of most motile cilia (Fig. 1C).
Many mammalian cells possess a single non-motile cilium, the primary cilium, which typically retains a 9+0 axoneme but mostly lacks the structures required for ciliary motility. Cilia are rich in signaling proteins [e.g., G-protein coupled receptors (GPCRs), ion channels, protein kinases]. Some cilia are structurally modified for particular sensory functions. The outer segment of rods in the eye, for example, represents a structurally modified cilium functioning in light perception. Similarly, primary cilia in other organs and tissues sense chemical and mechanical cues. Cilia protrude from the cell surface into the environment and have a high surface to volume ratio, features likely fundamental for their role in sensing. Defects in ciliary function lead to a plethora of diseases referred to as ciliopathies (see Box 1). Many of these conditions are caused by cilia of incorrect size or composition, which has fostered a strong interest in understanding ciliary assembly.
IFT: the protein translocation machinery of cilia
Ribosomes are absent from cilia and ~600-1000 distinct polypeptides required to build the organelle need to be imported from the cell body; some of these proteins are concentrated several thousand-fold in cilia [2, 3]. The axoneme elongates by addition of material to its tip, which points away from the cell body [4-6]. Thus, during cilia assembly, large amounts of building materials need to be transferred from the ciliary base to the distal end. These observations indicate the need for a ciliary protein translocation system and intraflagellar transport (IFT) is thought to be the predominant pathway to move proteins into and within cilia [7].
In brief, IFT is the bidirectional movement of supramolecular protein arrays inside cilia. These so-called IFT trains are appressed to the ciliary membrane and move via molecular motors on the axonemal microtubules [8] (Fig. 1C,G). While IFT trains are the primary cargo of the IFT motors, they also function as adaptors allowing other proteins (= IFT cargoes) to be carried along. Cargoes bind to IFT near the basal body and move to the ciliary tip by anterograde IFT. At the tip, many cargoes are released from IFT; the trains reorganize and return to the cell body by retrograde IFT (Fig. 1F). The IFT pathway is well conserved and required for the assembly of most cilia and eukaryotic flagella [9]. This article will review recent progress in the structural analysis of IFT complexes and how they interacts with various cargoes; the regulation of transport frequency and capacity and data obtained by direct imaging of protein transport in cilia will be discussed.
The building blocks of IFT
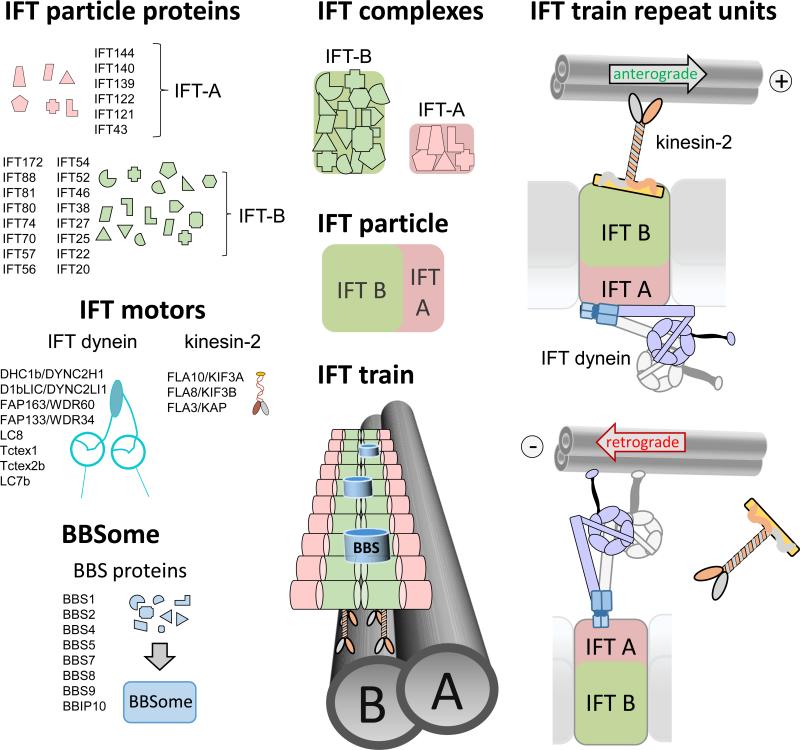
IFT trains are polymers of IFT particles, which move up and down the cilium using motor proteins (Fig. 2). A heterotrimeric kinesin-2 powers anterograde IFT, progressing 0.2 to 2.4 μm/s depending on the species and cilia type. IFT dynein, a complex similar to cytoplasmic dynein, moves retrograde IFT trains at velocities ranging from 0.14 to 5.60 μm/s [10-17]. Additional motors appear to be employed for IFT in certain organisms and cell types, such as the homodimeric kinesin-2 OSM-3/Kif17 in Caenorhabditis elegans [18]. The IFT particles consist of IFT-A and IFT-B subcomplexes composed of at least 6 and 16 distinct polypeptides, respectively [13]. Individual roles in particle assembly and cargo binding are emerging for some IFT proteins (see below). The IFT particles and motors assemble into ~100 – 700 nm long IFT trains, which consist of a double row of particles each presumably representing an IFT A/B complex and its associated motors (Fig. 1F, 2; [19]). The double row appearance could indicate that IFT trains consist of two protofilaments assembled from IFT particles, an architecture which will allow for multiple interactions between the particles and could ensure the relative stability of the trains.
Figure 2. The IFT building blocks.
IFT-A and IFT-B proteins will assemble into IFT-A and IFT-B subcomplexes; the proteins are believed to be represented in equimolar amounts in each complex. IFT complexes assemble into IFT particles and IFT particles associate with the IFT motors into IFT trains. The BBSome, consisting of at least 7 BBS proteins and BBIP10, appears to be a substoichiometric component of the IFT trains. Peptide coverage in the proteome of whole C. reinhardtii cilia suggests a 10:5:1 ratio for IFT-B/IFT motors, IFT-A, and BBSomes, respectively [2, 22]. Anterograde IFT trains are moved by kinesin-2, which is thought to be associated to IFT-B. IFT dynein is moved as a cargo on anterograde IFT trains to the ciliary tip. Retrograde IFT trains are powered by IFT dynein. How IFT dynein, here depicted as being associated with IFT-A, binds to IFT trains is currently unknown.
The BBSome, a complex composed of eight BBS proteins, moves in association with IFT trains through cilia ((Fig. 2)[11, 20-23]). In humans, defects in the BBSome cause Bardet-Biedl syndrome (BBS), a multi-organ disease characterized by blindness, kidney anomalies, and obesity indicative for defects in the sensory functions of cilia (Box 1; [24]). The BBSome is well conserved in many ciliated species, which is indicative of a fundamental role in cilia. In C. elegans, the BBSome is involved in the assembly and stabilization of IFT particles [18, 25]. Bbs mutants often have shorter cilia, but in most cell types BBSomes are not required for general cilia assembly [7, 22, 26]. Nevertheless, BBSomes contribute to protein transport in cilia as indicated by the biochemical defects in the ciliary membrane of Bbs mutants . The BBSome appears to function in the maintenance of ciliary membrane protein content, probably by mediating IFT transport of certain membrane proteins [27].
IFT functions in ciliary assembly, maintenance, and signaling
The best documented function of IFT is its role in ciliary assembly. Defects in IFT-B or IFT kinesin often impair ciliary assembly [28-30]. IFT has been shown to shuttle large amounts of tubulin and other axonemal proteins into growing cilia suggesting that one of the foremost functions of IFT is to deliver proteins for axonemal assembly [31, 32]. When IFT is switched-off, cells will lose the ability to assemble cilia [33] and, in mutants with a reduced frequency of anterograde IFT, cilia assembly is slow suggesting that the number and velocity of anterograde trains determine the ciliary growth rate [34]. It is therefore widely accepted that anterograde IFT is required for the assembly of most eukaryotic cilia. Notable exceptions are the flagella of Plasmodium gametes, whose axonemes form within the cytoplasm of precursor cells and Drosophila gametes, whose axonemes assemble in a short, cilium-like membrane cap requiring transition zone components, but not IFT[35].
IFT continues once cilia are fully assembled. Cilia will slowly shorten after IFT has been switched-off in Chlamydomonas reinhardtii or Tetrahymena thermophila indicating that IFT is required for ciliary maintenance [27, 33, 36]. Similarly, the conditional deletion of IFT88 or Kif3a in kidneys of adult mice will result in ciliary loss or stunting [37]. However, IFT is absent from the flagella of mature spermatozoa indicating that cilia maintenance does not per se require IFT. Sperm flagella are essentially unchanging, but many other cilia adjust their composition and length or need to replace proteins. For example, ~2,000 opsins pass every minute through the connecting cilium into the outer segment to replace protein lost by disc shedding [38]. In addition to providing construction materials for assembly and repair, the role of IFT in ciliary maintenance could also involve the transport of proteins that measure and adjust the length of cilia.
IFT also exports proteins from cilia perhaps to ensure protein homeostasis or to scavenge cell body proteins leaked into the organelle; the BBSome ensures export of certain membrane-associated proteins by mediating transport via retrograde IFT [27]. Many cells disassemble cilia prior to mitosis but the role of IFT in the bulk export of proteins from cilia is unclear [39]. Proteins also exit cilia via ectosomes and vesicle shedding appears to be the preferred exit route for some ciliary transmembrane proteins [40, 41].
Furthermore, IFT is thought to transport proteins during cilia-based signaling [42]. Various signaling pathways (e.g., Hedgehog, Wnt, platelet derived growth factor receptor α α [PDGFRαα]) require that some of the participating proteins are transported in and out of cilia for full pathway activity [43-45]. This could be accomplished by IFT but whether IFT indeed transports proteins as a step in a signaling cascade is not known yet. In summary, IFT is the predominant protein trafficking pathway of cilia.
Cargo binding by IFT
Considering the biochemical complexity of cilia, one wonders how a complex of about two dozen proteins can function as a carrier for potentially hundreds of distinct polypeptides. The isolation or reconstitution of IFT-cargo complexes has not yet been achieved, suggesting that IFT-cargo interactions are weak. IFT-B is thought to be the major protein carrier during anterograde IFT and the specific cargo binding sites so far identified are located on IFT-B (see below). In IFT-A and retrograde IFT motor mutants, IFT-B proteins accumulate near the ciliary tip suggesting that IFT-A functions in returning IFT-B to the cell body [14, 46]. However, certain membrane proteins are depleted from cilia of IFT-A mutants [47, 48]. Also, the IFT-B protein IFT27 is required to export BBSomes from cilia [23, 49]. Thus, both IFT-A and IFT-B appear to participate in protein import and export.
Recently, a nonameric IFT-B core complex and several smaller complexes have been assembled from recombinant IFT proteins (Fig. 4A; [50, 51]). IFT proteins are rich in WD and tetratricopeptide (TPR) repeats (IFT-A and -B) and coiled-coils (IFT-B), most of which are required for the interactions among IFT proteins themselves [52]. However, high resolution structures, interaction studies, and truncation analysis of IFT proteins have identified projection domains that appear to be dispensable for IFT complexes formation and might be involved in cargo binding [53, 54]. The assembly of IFT trains could generate additional cargo binding sites absent from individual IFT particles. Such a model would link train assembly and cargo loading and the remodeling of IFT trains at the ciliary tip to cargo release [55].
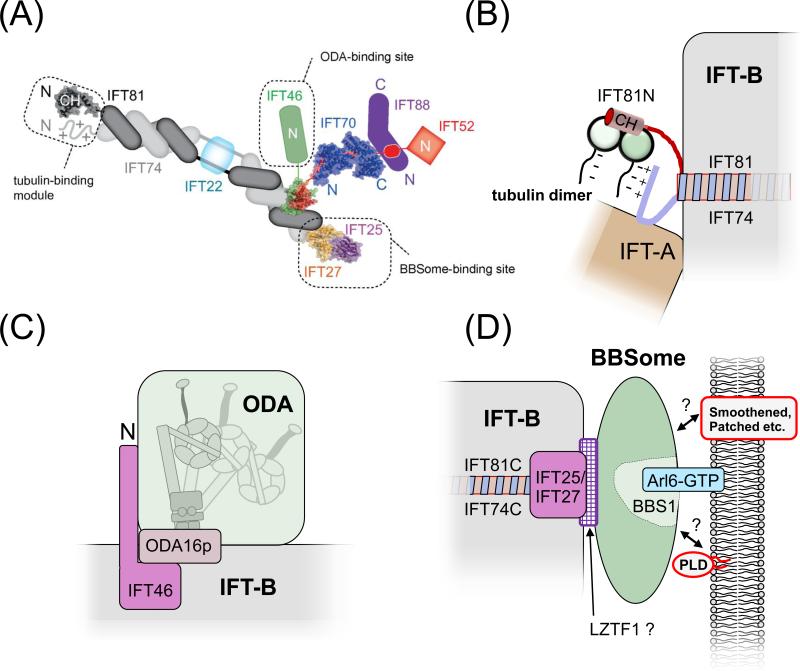
Figure 4. Cargo binding by IFT.
(A) Schematic presentation of the IFT-B core complex. The crystal structures of partial and complete IFT proteins are included where available and the putative cargo binding sites are indicated. Note that the exact positions of IFT27/25 and IFT52/46 on the C-terminal part of IFT74/81 are unknown. Reprinted in modified form from reference [50] with permission of the authors and journal. ©1967 Taschner et al. Journal of Cell Biology. 207:269-282. doi:10.1083/jcb.201408002.
(B) Tubulin binding site. The N-terminal domain of IFT81 forms a CH domain, which binds tubulin dimers. This interaction is stabilized by the N-terminal domain of IFT74, which is positively charged and might interact with the glutamate-rich C-terminal domain of β-tubulin [53].
(C) ODA binding site. Cells expressing an N-terminally truncated IFT46 fail to assemble ODAs onto the axoneme. Mutants in ODA16p have a similar phenotype and ODA16p interacts with IFT46 suggesting that ODA16p stabilizes the interaction of IFT46 and ODAs allowing them to travel on IFT trains [87, 88].
(D) BBSome binding site. Mutant analysis revealed that IFT25/27 are required for the export of BBSomes from cilia suggesting that these IFT proteins form a BBSome binding site. LZTF1 accumulates in Ift27−/− cilia but not those of Bbs−/− mutants and might mediate BBSome binding to IFT27/27. BBS3/Arl6 binds to BBS1 and recruits BBSomes to membranes [69, 103]. The BBSome might function as a cargo adapter allowing various transmembrane and membrane-associated proteins to attach to IFT for ciliary import or export [23, 49].
How many distinct cargo binding sites are present on IFT trains? Abundant proteins such as tubulin appear to have dedicated binding sites [56, 57]. Less abundant proteins may compete for shared or overlapping binding sites on the IFT particles. Many proteins enter cilia as part of multiprotein complexes, such as dynein arms and radial spokes [58-60]. In principle only one protein in such a complex needs to be in direct contact with the IFT train. Other cargoes might interact indirectly with IFT trains via cargo adapters, which mediate the binding of multiple cargoes to IFT trains and thus expand their transport potential. These mechanisms would reduce the number of required cargo binding sites.
Cargo loading and entry into cilia
Protein transport by IFT can be divided into four steps: cargo loading, admission into the cilium, translocation along the shaft, and unloading. Cargo loading likely occurs near the ciliary base and axonemal cargoes have been observed entering cilia already bound to IFT [32, 55]. IFT proteins are concentrated in the basal body region near the transitional fibers, which might function as an assembly platform for IFT particles and trains (Fig. 1E; [61]). Assembling IFT-A and IFT-B into particles appears to require the IFT-B protein IFT74, which might reside at the contact site between the two [54]. After dephosphorylation of its Fla8/Kif3b subunit, Kinesin-2 binds to IFT particles perhaps initiating train formation [62]. Once assembled, trains are likely to be quickly dispatched into the cilium but the chronology of train formation and cargo loading is unknown.
Ciliary transmembrane proteins first enter the plasma membrane in Golgi-derived vesicles and then move laterally into the ciliary membrane potentially involving IFT. Indeed, several transmembrane proteins have been shown to move via IFT in cilia [11, 63, 64]. However, some transmembrane and membrane-associated proteins still enter cilia in the absence of IFT and certain GPCRs essentially move by diffusion once inside cilia [27, 65, 66]. Targeting of acylated proteins such as NPHP3 and arrestin to the ciliary membrane involves the lipid binding chaperone Uncoordinated119 (UNC119); they are released from UNC119 inside the cilium by the GTP-bound form of Arl3 [67, 68]. Whether IFT contributes to the ciliary localization of fatty-acid modified and transmembrane proteins is largely unclear.
Interestingly, IFT and many BBS proteins share their domain organization and phylogenetic ancestry with vesicular coat proteins [9]. Vesicular traffic is widely believed to be absent inside cilia, but IFT trains and BBSomes could be considered planar coats that function in selecting and moving transmembrane proteins from the plasma membrane into the ciliary membrane and vice versa [69]. In mammalian cells, IFT20 is located at the Golgi indicating that some IFT proteins associate to membrane proteins heading toward the cilium well before they reach the basal body [70]. IFT and BBS proteins have been implicated in other intracellular transport pathways such as targeting of Golgi-derived vesicles to cilia, recycling of endosomal proteins, and melanosome transport [71, 72]. It has been also suggested that axonemal proteins reach the ciliary base on the outside of ‘IFT-coated’ vesicles [73].
While moving from the cell body into the cilium proper, IFT trains must pass through the transition zone (TZ) where the axoneme is connected to the overlying plasma membrane via Y-shaped fibers (Fig. 1 E, F). TZ proteins are hotspots for ciliopathy-related mutations that alter the protein composition of cilia and affect ciliogenesis [74-76]. The TZ appears to block the diffusional entry of larger proteins into cilia and might regulate the admission of proteins and IFT trains into the cilium by a mechanism similar to that of the nuclear pore complex [77]. Sequence motifs that are required and sufficient for ciliary entry have been identified in some ciliary transmembrane proteins [78-80]. However, a universal ciliary targeting sequence such as the NLS is absent. An alternative concept suggests that proteins will be selected for ciliary entry based on their ability to bind - directly or indirectly - to IFT trains. Whether an association with IFT trains alone is sufficient for protein admission into cilia or if IFT trains are screened and stripped of unwanted proteins while passing though the TZ is an open question.
Cargo unloading and the regulation of IFT's transport capacity
To promote axonemal elongation, IFT must pick-up cargoes in the cell body and release them in the cilium raising the question of how unloading is triggered. Axonemal proteins predominately progress directly to the ciliary tip via IFT where they are released (Fig. 3A) [31, 32]. Anterograde IFT trains disassemble at the tip and remodel into retrograde trains presumably aided by numerous local protein kinases [10, 55, 62]. Thus, train remodeling appears to be a mechanism to discharge the cargoes (Fig. 3A, B). After unloading, axonemal proteins diffuse to their docking sites [32]. To ensure that unloaded proteins are not immediately exported from cilia, retrograde trains may have a lower affinity for cargoes, especially those required to remain inside the cilium.
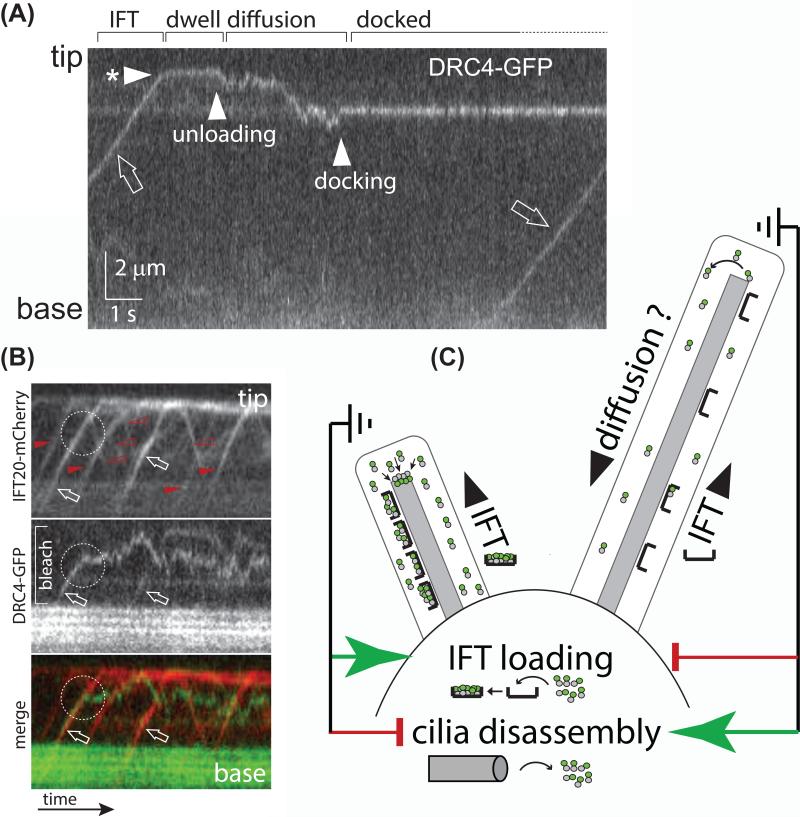
Figure 3. Cargo transport by IFT.
A) Kymogram (time-distance plot) showing the movement of the axonemal protein DRC4 in a Chlamydomonas cilium as recorded by total internal reflection fluorescence (TIRF) microscopy. The position of the ciliary tip and base are indicated. A trajectory running from the bottom left to the top right indicates transport to the ciliary tip; the slope represents transport velocity. The kymogram depicts the phases of cargo delivery into cilia: DRC4-GFP first moves by IFT(open arrow) to the ciliary tip (arrowhead with * marks the arrival at the tip); after a ~ 2-second dwell time the protein begins to diffuse (arrowhead ‘unloading’) until it docks to the axoneme (arrowhead ‘docking’). Reprinted in modified form from reference 32 (doi: 10.1016/j.cub.2013.10.044).
B) Kymograms from two-color TIRF imaging showing DRC4-GFP (middle and green) and IFT20-mCherry (top and red) of a partially bleached cilium. Anterograde trains are marked by filled arrowheads, open arrowheads mark retrograde trains. The two trains marked by arrows carry DRC4-GFP as a cargo. While one cargo is transported to the ciliary tip, the other one is released prematurely along the ciliary shaft (dashed circle) followed by an extended time of diffusion.
C) IFT loading and the regulation of ciliary length. The model suggests that cells are able to measure the length of their cilia. Cilia that fall short of a set length will trigger a two-pronged response of increasing the amount of cargo carried by IFT trains and putatively suppressing ciliary disassembly. Cilia that exceed the set length of cilia will suppress cargo loading onto IFT and activate a ciliary disassembly pathway [102]. Since growing and non-growing cilia can be present on the same cell body, IFT loading and ciliary disassembly appear to be regulated locally within each basal body-cilium entity. Tubulin dimers, as an example of a cargo, are indicated by grey and green dots.
IFT trains also release and rebind cargoes along the ciliary shaft indicating that these events are not spatially restricted (Fig. 3B; [31, 32]). The frequency of these unloading events differs between cargoes, as one might expect given that distinct cargoes likely bind distinct sites on the IFT train and each IFT-cargo pair will have its particular equilibrium and time constants [31, 32]. In Chlamydomonas, the transient interaction of the flagellar membrane glycoprotein-1 (FMG-1) with IFT trains requires extracellular calcium suggesting additional mechanisms regulating IFT-cargo interactions [81, 82].
IFT trains are highly loaded with tubulin and other axonemal proteins during assembly but are mostly devoid of axonemal cargoes in full-length steady-state cilia [31, 32]. The less-loaded IFT trains circulating in fully-assembled cilia could provide a reserve transport capacity that enables cells to quickly change ciliary length and composition. When growing and non-growing cilia are present on the same cell, IFT trains entering the growing cilia are highly loaded with tubulin whereas those entering steady-state cilia are largely devoid of this cargo [31]. This suggests that cells regulate cargo influx independently for each cilium rather than at the cellular level, for example, by increasing cargo supply in the cell body. Cells appear to measure the length of their cilia and use this information to regulate the amount of precursors entering cilia via IFT (Fig. 3C)[31, 83].
How cells adjust the amount of cargo present on an IFT train is unknown. In principle, each cargo binding site could be regulated independently allowing for the fine-tuning of transport of just one or a group of proteins. Phosphorylation of IFT proteins is well documented [84] but its functional role and contribution to cargo binding remains elusive. During ciliary growth, however, many axonemal components are transported with high frequency requiring a simultaneous regulation of numerous distinct IFT-cargo interactions. A simpler scenario would be to adjust the amount of cargo bound to IFT by a general mechanism such as changes in overall train capacity. In C. reinhardtii, at least two structural subclasses of IFT trains have been identified: short IFT trains with a ~16-nm periodicity and long IFT trains with a ~40-nm repeat (Fig. 1F; [19]). Long and short IFT trains could correspond to anterograde and retrograde, moving and stalled, or loaded and unloaded IFT trains.
Cargoes with known IFT binding sites
An IFT cargo can be defined as a protein that moves by IFT inside cilia but is not required for IFT itself. This broad definition encompasses proteins that cycle in almost perpetual association with the IFT machinery through cilia (e.g., the BBSome [21, 22, 49]), proteins that traffic via IFT from the cell body to their ciliary assembly sites (e.g., tubulin [31]), and proteins that only transiently interact with the IFT machinery (e.g., FMG-1 in C. reinhardtii, GPCR olfactory receptor 78 in mouse olfactory cilia [11, 65, 81]). IFT motor proteins also move as cargoes on IFT trains: IFT dynein, for example, is transported by anterograde IFT to the ciliary tip [55, 85]. Although it is widely assumed that the majority of ciliary proteins are trafficked by IFT, the evidence is often indirect. Direct imaging has confirmed IFT transport for a range of proteins from different ciliary subcompartments including many axonemal proteins (Table 2). The following focuses on those IFT cargoes for which the details of their interaction with IFT are emerging.
Tubulin
Tubulin is the major structural protein of cilia and in vivo imaging confirmed that tubulin is transported on IFT trains [31, 86]. The N-terminal domains of IFT81 and IFT74 form a module that bind tubulin in vitro (Fig. 4B; [51]). IFT81 possesses a variant calponin homology (CH) domain that binds tubulin dimers with a Kd of 16 μM; binding is increased 18-fold in the presence of IFT74. The positively charged N-terminal region of IFT74 is thought to interact with the acidic C-terminal domain of β-tubulin. The IFT81/74 module also binds to microtubules raising the question of how binding of IFT particles to microtubules is avoided. Notably, cells expressing a truncated IFT74 still assemble cilia, albeit slowly, suggesting ongoing transport of tubulin in the absence of the N-terminal region of IFT74 [54]. A possible explanation is the presence of redundant tubulin-binding sites on IFT particles, which would ensure sufficient delivery during ciliary growth. Supporting this idea, several other IFT-B proteins (IFT54, IFT57, and quilin) possess CH domains, and therefore would be potential alternative tubulin-binding proteins [57].
Outer dynein arms (ODAs)
Cells expressing an N-terminally truncated version of IFT46 assemble cilia devoid of ODAs whereas IFT46 null mutants lack cilia entirely [87]. This suggests that IFT particles assembled using the truncated IFT46 are specifically impaired in transporting ODAs. The N terminus of IFT46 could be a projection domain required for cargo binding but not general IFT. However, ‘truncated IFT46’ mutants regenerate cilia slowly and the ratios between the IFT proteins are altered, indicating additional defects in IFT [87]. The transport of ODAs into cilia also requires ODA16p, which directly binds to IFT46 [88]. ODA16p is not a component of the ODAs themselves and it appears to stabilize ODA binding to IFT trains (Fig. 3). Similarly, the transport of the inner dynein arm I1, one of several distinct inner dynein arms, and of radial spokes appears to require specific IFT-associated factors [89, 90].
BBSomes
The BBSome is dispensable for IFT and therefore can be considered an IFT cargo [22]. Mouse Ift27 mutants still assemble cilia but accumulate BBSomes in their cilia, indicating that the small GTPase IFT27 is required for BBSome export from cilia [23, 49]. Together with IFT25, IFT27 might form a docking site for the BBSome on retrograde IFT trains (Fig. 3D). Since BBSomes continue to enter cilia in Ift25/Ift27 mutants, anterograde IFT trains may possess distinct BBSome-binding sites. BBS mutants display defects in ciliary membrane composition such as the loss or over-abundance of certain GPCRs, ion channels, and membrane-associated proteins [22, 23, 49, 91-93]. Thus, the BBSome might act as an IFT cargo adaptor allowing various membrane proteins to interact with IFT trains [27]. Direct evidence for BBSome-dependent cargo transport on IFT trains, however, is still missing.
These examples reveal heterogeneous mechanisms by which cargoes bind to IFT involving projection domains, cargo adaptors, and factors stabilizing cargoes on the trains. IFT-cargo interactions need to be analyzed on a case-by-case basis, which will be challenging considering the complexity of cilia.
The role of diffusion in ciliary protein transport
Many cytoskeletal structures assemble from precursors recruited from the surrounding cytoplasm by diffusion. Similarly, proteins should rapidly diffuse into cilia, and indeed, rapid diffusional entry of tubulin was observed [31]. Axonemal elongation will remove tubulin and other structural proteins from the ciliary matrix, which should result in a diffusion-driven current and a net influx of these proteins into cilia. Similarly, freely diffusing proteins could rapidly accumulate in or exit from cilia controlled by regulated binding to ciliary substructures (e.g. the axoneme or membrane) [94]. Why then does the assembly of cilia entail a specific transport system, and what are the respective contributions of diffusion and IFT?
First, IFT could be required to move proteins across the TZ, which is believed to function as a barrier limiting protein entry into cilia by diffusion. For soluble proteins, a size exclusion limit of ~50 kD was determined for the TZ suggesting that large cargo complexes such as ODAs (~2 MDa) might depend on IFT to gain access into the cilium [77, 95]. However, much larger proteins have been observed entering cilia apparently by diffusion and the importance of the TZ as a diffusion barrier for endogenous soluble proteins and membrane proteins is still unclear.
Second, IFT could be required to move proteins against a concentration gradient. For example, the concentration of tubulin in the matrix of growing cilia exceeds that of the cell body [31]. High concentrations of tubulin and other axonemal proteins might be necessary for an efficient elongation of the axoneme, which consumes large amounts of precursors (e.g. ~200 tubulin dimers/second) out of the small volume of the ciliary matrix. Just as IFT may concentrate membrane and soluble proteins in cilia to levels above those present in the rest of the cell, it might also remove proteins from cilia that are concentrated in the cell body but can leak into cilia by diffusion [27, 31].
Thus, proteins move into cilia by a mix of diffusion and motor-based transport. It is now of interest to determine whether particular protein features or ciliary conditions can be used to predict the mode of transport.
Concluding remarks
IFT moves a variety of distinct proteins into cilia and some specific cargo-binding sites have been identified. The structural analysis of IFT particles and trains will be the foundation to understand IFT-cargo interactions at the molecular level (see Outstanding Questions Box). The amount of protein transported by IFT is regulated but how cells recognize cilia that need additional building material and the signaling pathway that will adjust the transport capacity of IFT accordingly are still unclear. We will also need a better understanding of how IFT determines, maintains, and adjusts the protein composition of cilia: Is IFT merely a conveyer belt moving proteins or a more sophisticated system that, in conjunction with the ciliary TZ, tailors ciliary protein content? The binding properties of IFT trains are likely to influence the amount and stoichiometry of protein entry into cilia. Similarly, IFT trains might promote the export of proteins from cilia once they exceed a concentration threshold in the matrix; this could be pivotal for ciliary signaling. It will be of interest to determine whether IFT also functions in ciliary quality control by sensing biochemical changes in cilia and providing feed-back to the cell body.
Box 1 IFT and ciliary disease.
Cilia are widely distributed in the mammalian body and malfunctioning cilia have been linked to numerous diseases and developmental disorders. Cilia are ultrastructurally and biochemically specialized and certain mutations will affect only a subset of cilia. For example, defects in axonemal dynein, will affect only processes that depend on ciliary motility while the function of sensory cilia is unaffected. In contrast, mutations affecting general ciliary assembly typically result in multiorgan phenotypes particularly involving the eyes, kidneys, and the skeleton; the complete loss of cilia is embryonic lethal due to severe defects in Hedgehog signaling.
Impaired or abnormal ciliary motility causes primary ciliary dyskinesia (PCD), a genetically heterogenous condition characterized by reoccurring airway infections, male infertility, and an increased risk of hydrocephalus. Establishing the correct left-right body axis requires the motility of embryonic nodal cilia and about half of the PCD patients have situs inversus and other body axis anomalies including heart defects [96]. A recent large-scale study in mice showed that defective cilia are a main cause for congenital heart defects [97]. Notably, motile cilia also possess sensory functions: Receptors for bitter taste are present in the membrane of airway cilia; they detect bacterial quorum-sensing molecules and in response the ciliary beat frequency will increase to remove pathogens [98].
The most common ciliopathy is polycystic kidney disease (PKD) which occurs in ~1:1,500 adults. PKD is mostly caused by mutations in PC1 and PC2, which form a ciliary TRP calcium channel. Defects in ciliary signaling will then trigger cell proliferation in the kidney and the formation of kidney cysts ultimately causing kidney failure [99]. Kidney anomalies often also result from defects in IFT, the BBSome, and TZ proteins. Such mutations will affect cilia length and/or composition interfering with the fidelity of cilia-based signaling. Not surprisingly, mutations affecting ciliary assembly and signaling result is a plethora of additional features such as blindness, anosmia, neuronal malformations, and polydactyly (Fig. I). Mutations in IFT-A particularly promote severe and often lethal skeletal malformations such as short rib asphyxiating thoracic dystrophy [100]. The features of Bardet-Biedl syndrome (BBS) and a few other ciliopathies encompass obesity due to overeating suggesting that cilia might be involved in satiety control [37, 101]. To which extend mutations in IFT and BBS proteins contribute to common human diseases such as diabetes and obesity needs to be explored. In summary, cilia have an important role in human health and disease.
Fig. I) Cast of a hand with polydactyly from a display in the Museum of Science, Boston.

Outstanding Questions.
Which ciliary proteins depend on IFT to enter cilia and which move by diffusion?
How are IFT proteins organized into IFT particles and the supramolecular IFT trains?
When and where in the cell do cargoes bind to the IFT particles and where are they located on the trains?
How does IFT interact with numerous distinct proteins that need to be transported into cilia ?
How do IFT trains get through the transition zone which excludes even mid-sized proteins from cilia?
How is the amount of cargo transported by IFT regulated to ensure that cilia of a right length and composition are assembled and maintained?
Trends.
Defects in intraflagellar transport (IFT), a process that moves proteins into cilia, cause disease in humans.
IFT functions in ciliary assembly, maintenance, protein export, and probably ciliary signaling.
Direct imaging of protein transport on IFT trains revealed that the amount of cargo attached to each trains is regulated in a ciliary-length dependent manner.
Structural analysis of IFT proteins and complexes revealed how tubulin, the major protein of cilia and flagella, binds to IFT.
Table 1.
Cargo proteins observed to move with IFT velocities inside cilia.
| Protein | type | Species/cell type | reference | |
|---|---|---|---|---|
| Axoneme | TBB-4, TBA-5 | microtubule | C. elegans | [86] |
| a-tubulin | microtubule | C. reinhardtii | [31] | |
| DRC4, DRC2 | nexin-dynein regulatory complex | C. reinhardtii | [32] | |
| RSP3, RSP4 | radial spoke | C. reinhardtii | (a) | |
| IC2 | outer dynein arm | C. reinhardtii | (b) | |
| PF16 | central pair | C. reinhardtii | [32] | |
| Transmembrane* | OSM-9 | TRPV | C. elegans | [64] |
| OCR-2 | TRPV | C. elegans | [64] | |
| PKD2-like | TRP calcium channel | C. reinhardtii | [63] | |
| Olfr78 | olfactory receptor | M. musculus OSN | [11] | |
| ACIII | adenylate cyclase | M. musculus OSN | [11] | |
| Cnga2 | cNTP-gated olfactory channel | M. musculus OSN | [11] | |
| Membrane-associated | Arl13b | Small GTPase | M. musculus OSN | [11] |
| BBS3/Arl6 | Small GTPase | M. musculus OSN | [11] | |
| IFT-associated | XBX-1/ CHE-3# | IFT dynein | C. elegans | [85] |
| BBS7, BBS8 | BBSome | C. elegans, IMCD-3 cells | [21, 104] | |
| BBS4 | BBSome | C. reinhardtii | [22] | |
| BBS1 | BBSome | IMCD3 cells | [23] | |
| BBS3, BBS4 | BBSome | M. musculus OSN | [11] | |
| Kif17 | IFT motor | IMCD-3 cells | [104] | |
| Soluble | ICK | protein kinase | IMCD-3 cells | [104] |
| MOK | protein kinase | IMCD-3 cells | [104] |
XBX-1 moves by kin-2 but independently of IFT and BBS proteins; OSM, olfactory sensory neurons: (a), B. Okivie & K. Lechtreck, unpublished; (b), D. Mitchell & K. Lechtreck, unpublished.
IFT transport of various transmembrane proteins was observed upon overexpression of the proteins.
Acknowledgement
I apologize to my colleagues whose work I could not discuss due to space limitations. I am grateful to my students J. Aaron Harris and Julie M. Craft for helpful comments on the manuscript. This work was supported by a grant from the National Institutes of Health (GM110413).
Glossary
- Axoneme
A nine-fold symmetric microtubular scaffold in cilia and flagella. Primary cilia typically possess a 9+0 axoneme. In motile cilia the microtubules carry numerous protein complexes such as the outer dynein arms (ODAs), radial spokes (RS), and the dynein regulatory complexes (DRC); most motile cilia have two additional central pair microtubules forming a 9+2.
- BBSome
A complex of at least eight BBS proteins that moves in association with IFT through cilia. Defects in this complex cause Bardet-Biedel syndrome (BBS), a rare disorder primarily characterized by blindness and obesity.
- Chlamydomonas reinhardtii
a unicellular green alga with two 9+2 flagella widely used as a model to studying flagella.
- Cilia
Thread-like cell protrusions surrounded by the plasma membrane and stabilized by a microtubular scaffold, the axoneme. The terms cilia and flagella refer to the structure.
- Ciliopathy
Diseases caused by defects in ciliary assembly and function.
- Dynein
A protein complex consisting of heavy chains and associated regulatory, intermediate, light-intermediate, and light chains. Dyneins move toward the minus-end of microtubules; axonemal ODAs and IDAs power ciliary beating, IFT dynein transports trains to the ciliary base.
- Kinesin-2
a class of plus-end directed molecular motor which includes the heterotrimeric kinesin-2 that moves IFT trains to the ciliary tip.
- Intraflagellar transport (IFT)
a bidirectional motility of IFT trains along cilia. Anterograde IFT moves toward the ciliary tip, retrograde IFT moves toward the ciliary base.
- IFT trains
Array of multiple IFT particles, each consisting of IFT-A and IFT-B subcomplexes.
- Primary ciliary dyskinesia (PCD)
A genetically heterogeneous condition caused by immotile cilia; features of PCD are reoccurring airway infections, male infertility, and often situs anomalies.
- Polycystic kidney disease (PKD)
an inherited disorder affecting about 1 in 1,500 adults; most cases of PKD are caused by mutations in polycystin-1 and polycystin-2, which form a ciliary
- TRP calcium channel
Defective ciliary signaling results in the abnormal proliferation of kidney cells and cyst formation. PKD patients depend on dialysis or require kidney transplants.
- Transition zone
A structurally and biochemically specialized region located between the basal body and the cilium that functions as a diffusion barrier and ciliary gate to control protein influx into cilia.
- Total internal reflection fluorescence microscopy (TIRF)
a microscopy technique especially useful to image fluorescent objects within a range of 30-300 nm off the cover glass. TIRF microscopy allows for the imaging of single proteins moving inside cilia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gealt MA, Adler JH, Nes WR. The sterol and fatty acids from purified flagella of Chlamydomonas reinhardtii. Lipids. 1981;16:133–136. [Google Scholar]
- 2.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. The Journal of cell biology. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A, Boucher RC. A proteomic analysis of human cilia: identification of novel components. Molecular & cellular proteomics : MCP. 2002;1:451–465. doi: 10.1074/mcp.m200037-mcp200. [DOI] [PubMed] [Google Scholar]
- 4.Johnson KA, Rosenbaum JL. Polarity of flagellar assembly in Chlamydomonas. The Journal of cell biology. 1992;119:1605–1611. doi: 10.1083/jcb.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenbaum JL, Child FM. Flagellar regeneration in protozoan flagellates. The Journal of cell biology. 1967;34:345–364. doi: 10.1083/jcb.34.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witman GB. The site of in vivo assembly of flagellar microtubules. Annals of the New York Academy of Sciences. 1975;253:178–191. doi: 10.1111/j.1749-6632.1975.tb19199.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum JL, Witman GB. Intraflagellar transport. Nature reviews. Molecular cell biology. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 8.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dam TJ, Townsend MJ, Turk M, Schlessinger A, Sali A, Field MC, Huynen MA. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6943–6948. doi: 10.1073/pnas.1221011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buisson J, Chenouard N, Lagache T, Blisnick T, Olivo-Marin JC, Bastin P. Intraflagellar transport proteins cycle between the flagellum and its base. Journal of cell science. 2012 doi: 10.1242/jcs.117069. [DOI] [PubMed] [Google Scholar]
- 11.Williams CL, McIntyre JC, Norris SR, Jenkins PM, Zhang L, Pei Q, Verhey K, Martens JR. Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nature communications. 2014;5:5813. doi: 10.1038/ncomms6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. The Journal of cell biology. 2001;153:13–24. doi: 10.1083/jcb.153.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. The Journal of cell biology. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. The Journal of cell biology. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Molecular biology of the cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, Rose LS, Scholey JM. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. The Journal of cell biology. 1999;147:519–530. doi: 10.1083/jcb.147.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Yi P, Ou G. Somatic CRISPR-Cas9-induced mutations reveal roles of embryonically essential dynein chains in Caenorhabditis elegans cilia. The Journal of cell biology. 2015;208:683–692. doi: 10.1083/jcb.201411041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 19.Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener DR, Rosenbaum JL, Lupetti P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. The Journal of cell biology. 2009;187:135–148. doi: 10.1083/jcb.200905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 21.Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, et al. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes & development. 2004;18:1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. The Journal of cell biology. 2009;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liew GM, Ye F, Nager AR, Murphy JP, Lee JS, Aguiar M, Breslow DK, Gygi SP, Nachury MV. The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Developmental cell. 2014;31:265–278. doi: 10.1016/j.devcel.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheffield VC. The blind leading the obese: the molecular pathophysiology of a human obesity syndrome. Transactions of the American Clinical and Climatological Association. 2010;121:172–181. discussion 181-172. [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Q, Zhang Y, Li Y, Zhang Q, Ling K, Hu J. The BBSome controls IFT assembly and turnaround in cilia. Nature cell biology. 2012;14:950–957. doi: 10.1038/ncb2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mykytyn K, Sheffield VC. Establishing a connection between cilia and Bardet-Biedl Syndrome. Trends in molecular medicine. 2004;10:106–109. doi: 10.1016/j.molmed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Lechtreck KF, Brown JM, Sampaio JL, Craft JM, Shevchenko A, Evans JE, Witman GB. Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. The Journal of cell biology. 2013;201:249–261. doi: 10.1083/jcb.201207139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonassen JA, San Agustin J, Follit JA, Pazour GJ. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. The Journal of cell biology. 2008;183:377–384. doi: 10.1083/jcb.200808137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brazelton WJ, Amundsen CD, Silflow CD, Lefebvre PA. The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Current biology : CB. 2001;11:1591–1594. doi: 10.1016/s0960-9822(01)00485-7. [DOI] [PubMed] [Google Scholar]
- 30.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. The Journal of cell biology. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craft JM, Harris JA, Hyman S, Kner P, Lechtreck KF. Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. The Journal of cell biology. 2015;208:223–237. doi: 10.1083/jcb.201409036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wren KN, Craft JM, Tritschler D, Schauer A, Patel DK, Smith EF, Porter ME, Kner P, Lechtreck KF. A Differential Cargo-Loading Model of Ciliary Length Regulation by IFT. Current biology : CB. 2013;23:2463–2471. doi: 10.1016/j.cub.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. The Journal of cell biology. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller J, Perrone CA, Bower R, Cole DG, Porter ME. The FLA3 KAP subunit is required for localization of kinesin-2 to the site of flagellar assembly and processive anterograde intraflagellar transport. Molecular biology of the cell. 2005;16:1341–1354. doi: 10.1091/mbc.E04-10-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basiri ML, Ha A, Chadha A, Clark NM, Polyanovsky A, Cook B, Avidor-Reiss T. A migrating ciliary gate compartmentalizes the site of axoneme assembly in Drosophila spermatids. Current biology : CB. 2014;24:2622–2631. doi: 10.1016/j.cub.2014.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JM, Marsala C, Kosoy R, Gaertig J. Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Molecular biology of the cell. 1999;10:3081–3096. doi: 10.1091/mbc.10.10.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Current biology : CB. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young RW. The renewal of photoreceptor cell outer segments. The Journal of cell biology. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engel BD, Ishikawa H, Wemmer KA, Geimer S, Wakabayashi K, Hirono M, Craige B, Pazour GJ, Witman GB, Kamiya R, et al. The role of retrograde intraflagellar transport in flagellar assembly, maintenance, and function. The Journal of cell biology. 2012;199:151–167. doi: 10.1083/jcb.201206068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood CR, Huang K, Diener DR, Rosenbaum JL. The cilium secretes bioactive ectosomes. Current biology : CB. 2013;23:906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao M, Ning J, Hernandez-Lara CI, Belzile O, Wang Q, Dutcher SK, Liu Y, Snell WJ. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. eLife. 2015;4 doi: 10.7554/eLife.05242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Q, Pan J, Snell WJ. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125:549–562. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 43.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Current biology : CB. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nature reviews. Genetics. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes & development. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pazour GJ, Wilkerson CG, Witman GB. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). The Journal of cell biology. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liem KF, Jr., Ashe A, He M, Satir P, Moran J, Beier D, Wicking C, Anderson KV. The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. The Journal of cell biology. 2012;197:789–800. doi: 10.1083/jcb.201110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes & development. 2010;24:2180–2193. doi: 10.1101/gad.1966210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eguether T, San Agustin JT, Keady BT, Jonassen JA, Liang Y, Francis R, Tobita K, Johnson CA, Abdelhamed ZA, Lo CW, et al. IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Developmental cell. 2014;31:279–290. doi: 10.1016/j.devcel.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taschner M, Kotsis F, Braeuer P, Kuehn EW, Lorentzen E. Crystal structures of IFT70/52 and IFT52/46 provide insight into intraflagellar transport B core complex assembly. The Journal of cell biology. 2014;207:269–282. doi: 10.1083/jcb.201408002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhogaraju S, Taschner M, Morawetz M, Basquin C, Lorentzen E. Crystal structure of the intraflagellar transport complex 25/27. The EMBO journal. 2011;30:1907–1918. doi: 10.1038/emboj.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taschner M, Bhogaraju S, Lorentzen E. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation; research in biological diversity. 2012;83:S12–22. [Google Scholar]
- 53.Bhogaraju S, Cajanek L, Fort C, Blisnick T, Weber K, Taschner M, Mizuno N, Lamla S, Bastin P, Nigg EA, et al. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science. 2013;341:1009–1012. doi: 10.1126/science.1240985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown JM, Cochran DA, Craige B, Kubo T, Witman GB. Assembly of IFT Trains at the Ciliary Base Depends on IFT74. Current biology : CB. 2015;25:1583–1593. doi: 10.1016/j.cub.2015.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedersen LB, Geimer S, Rosenbaum JL. Dissecting the molecular mechanisms of intraflagellar transport in chlamydomonas. Current biology : CB. 2006;16:450–459. doi: 10.1016/j.cub.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 56.Bhogaraju S, Engel BD, Lorentzen E. Intraflagellar transport complex structure and cargo interactions. Cilia. 2013;2:10. doi: 10.1186/2046-2530-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhogaraju S, Weber K, Engel BD, Lechtreck KF, Lorentzen E. Getting tubulin to the tip of the cilium: One IFT train, many different tubulin cargo-binding sites? BioEssays : news and reviews in molecular, cellular and developmental biology. 2014 doi: 10.1002/bies.201400007. [DOI] [PubMed] [Google Scholar]
- 58.Diener DR, Yang P, Geimer S, Cole DG, Sale WS, Rosenbaum JL. Sequential assembly of flagellar radial spokes. Cytoskeleton (Hoboken) 2011;68:389–400. doi: 10.1002/cm.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fok AK, Wang H, Katayama A, Aihara MS, Allen RD. 22S axonemal dynein is preassembled and functional prior to being transported to and attached on the axonemes. Cell motility and the cytoskeleton. 1994;29:215–224. doi: 10.1002/cm.970290304. [DOI] [PubMed] [Google Scholar]
- 60.Fowkes ME, Mitchell DR. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Molecular biology of the cell. 1998;9:2337–2347. doi: 10.1091/mbc.9.9.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Current biology : CB. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 62.Liang Y, Pang Y, Wu Q, Hu Z, Han X, Xu Y, Deng H, Pan J. FLA8/KIF3B phosphorylation regulates kinesin-II interaction with IFT-B to control IFT entry and turnaround. Developmental cell. 2014;30:585–597. doi: 10.1016/j.devcel.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 63.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. The Journal of cell biology. 2007;179:501–514. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Current biology : CB. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 65.Ye F, Breslow DK, Koslover EF, Spakowitz AJ, Nelson WJ, Nachury MV. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife. 2013;2:e00654. doi: 10.7554/eLife.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belzile O, Hernandez-Lara CI, Wang Q, Snell WJ. Regulated Membrane Protein Entry into Flagella Is Facilitated by Cytoplasmic Microtubules and Does Not Require IFT. Current biology : CB. 2013;23:1460–1465. doi: 10.1016/j.cub.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, Wang W, Pretorius PR, Sheffield VC, Sengupta P, et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes & development. 2011;25:2347–2360. doi: 10.1101/gad.173443.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Constantine R, Zhang H, Gerstner CD, Frederick JM, Baehr W. Uncoordinated (UNC)119: coordinating the trafficking of myristoylated proteins. Vision research. 2012;75:26–32. doi: 10.1016/j.visres.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Molecular biology of the cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finetti F, Patrussi L, Masi G, Onnis A, Galgano D, Lucherini OM, Pazour GJ, Baldari CT. Specific recycling receptors are targeted to the immune synapse by the intraflagellar transport system. Journal of cell science. 2014;127:1924–1937. doi: 10.1242/jcs.139337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yen HJ, Tayeh MK, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Human molecular genetics. 2006;15:667–677. doi: 10.1093/hmg/ddi468. [DOI] [PubMed] [Google Scholar]
- 73.Wood CR, Rosenbaum JL. Proteins of the ciliary axoneme are found on cytoplasmic membrane vesicles during growth of cilia. Current biology : CB. 2014;24:1114–1120. doi: 10.1016/j.cub.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. The Journal of cell biology. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Omran H. NPHP proteins: gatekeepers of the ciliary compartment. The Journal of cell biology. 2010;190:715–717. doi: 10.1083/jcb.201008080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. The Journal of cell biology. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kee HL, Dishinger JF, Blasius TL, Liu CJ, Margolis B, Verhey KJ. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nature cell biology. 2012;14:431–437. doi: 10.1038/ncb2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. The Journal of cell biology. 2000;151:1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Current topics in developmental biology. 2008;85:115–149. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- 80.Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Molecular biology of the cell. 2008;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shih SM, Engel BD, Kocabas F, Bilyard T, Gennerich A, Marshall WF, Yildiz A. Intraflagellar transport drives flagellar surface motility. eLife. 2013;2:e00744. doi: 10.7554/eLife.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collingridge P, Brownlee C, Wheeler GL. Compartmentalized calcium signaling in cilia regulates intraflagellar transport. Current biology : CB. 2013;23:2311–2318. doi: 10.1016/j.cub.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 83.Lefebvre PA, Flagellar length control . In: The Chlamydomonas sourcebook Vol. 3 Cell motility and behavior. 2.ed. Edition Harris EH, Witmann GB, editors. Elsevier; Amsterdam [u.a.]: 2009. [Google Scholar]
- 84.Wang H, Gau B, Slade WO, Juergens M, Li P, Hicks LM. The global phosphoproteome of Chlamydomonas reinhardtii reveals complex organellar phosphorylation in the flagella and thylakoid membrane. Molecular & cellular proteomics : MCP. 2014;13:2337–2353. doi: 10.1074/mcp.M114.038281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hao L, Efimenko E, Swoboda P, Scholey JM. The retrograde IFT machinery of C. elegans cilia: two IFT dynein complexes? PloS one. 2011;6:e20995. doi: 10.1371/journal.pone.0020995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nature cell biology. 2011;13:790–798. doi: 10.1038/ncb2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hou Y, Qin H, Follit JA, Pazour GJ, Rosenbaum JL, Witman GB. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. The Journal of cell biology. 2007;176:653–665. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahmed NT, Gao C, Lucker BF, Cole DG, Mitchell DR. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. The Journal of cell biology. 2008;183:313–322. doi: 10.1083/jcb.200802025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alford LM, Mattheyses AL, Hunter EL, Lin H, Dutcher SK, Sale WS. The Chlamydomonas mutant pf27 reveals novel features of ciliary radial spoke assembly. Cytoskeleton (Hoboken) 2013;70:804–818. doi: 10.1002/cm.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Viswanadha R, Hunter EL, Yamamoto R, Wirschell M, Alford LM, Dutcher SK, Sale WS. The ciliary inner dynein arm, I1 dynein, is assembled in the cytoplasm and transported by IFT before axonemal docking. Cytoskeleton (Hoboken) 2014;71:573–586. doi: 10.1002/cm.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valentine MS, Rajendran A, Yano J, Weeraratne SD, Beisson J, Cohen J, Koll F, Van Houten J. Paramecium BBS genes are key to presence of channels in Cilia. Cilia. 2012;1:16. doi: 10.1186/2046-2530-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cellular and molecular life sciences : CMLS. 2011;68:2951–2960. doi: 10.1007/s00018-010-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr., Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends in cell biology. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 95.Breslow DK, Koslover EF, Seydel F, Spakowitz AJ, Nachury MV. An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. The Journal of cell biology. 2013;203:129–147. doi: 10.1083/jcb.201212024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Klena NT, Gabriel GC, Liu X, Kim AJ, Lemke K, Chen Y, Chatterjee B, Devine W, Damerla RR, et al. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature. 2015 doi: 10.1038/nature14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee RJ, Cohen NA. Bitter and sweet taste receptors in the respiratory epithelium in health and disease. Journal of molecular medicine. 2014;92:1235–1244. doi: 10.1007/s00109-014-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. Journal of the American Society of Nephrology : JASN. 2007;18:1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- 100.Huber C, Cormier-Daire V. Ciliary disorder of the skeleton. American journal of medical genetics. Part C, Seminars in medical genetics. 2012;160C:165–174. doi: 10.1002/ajmg.c.31336. [DOI] [PubMed] [Google Scholar]
- 101.Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr., Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nature medicine. 2009;15:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hilton LK, Gunawardane K, Kim JW, Schwarz MC, Quarmby LM. The kinases LF4 and CNK2 control ciliary length by feedback regulation of assembly and disassembly rates. Current biology : CB. 2013;23:2208–2214. doi: 10.1016/j.cub.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 103.Mourao A, Nager AR, Nachury MV, Lorentzen E. Structural basis for membrane targeting of the BBSome by ARL6. Nature structural & molecular biology. 2014;21:1035–1041. doi: 10.1038/nsmb.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Broekhuis JR, Verhey KJ, Jansen G. Regulation of cilium length and intraflagellar transport by the RCK-kinases ICK and MOK in renal epithelial cells. PloS one. 2014;9:e108470. doi: 10.1371/journal.pone.0108470. [DOI] [PMC free article] [PubMed] [Google Scholar]