Abstract
Background
Imaging can provide noninvasive neural markers of disease progression in multiple sclerosis that are related to behavioral and cognitive symptoms. Past work suggests that diffusion tensor imaging (DTI) provides a measure of white matter pathology, including demyelination and axonal counts.
Objectives
In the current study, the authors investigate the relationship of DTI measures in the cingulum bundle to common deficits in MS, including episodic memory, working memory, and information processing speed.
Methods
Fifty-seven patients with MS and 17 age- and education-matched controls underwent high-spatial resolution diffusion scans and cognitive testing. Probabilistic tracking was used to generate tracks from the posterior cingulate cortex to the entorhinal cortex.
Results
Radial and axial diffusivity values were significantly different between patients and controls (p<0.031), and in patients bilateral diffusion measures were significantly related to measures of episodic memory and speed of processing (p<0.033).
Conclusions
The tractography-based measures of posterior cingulum integrity reported here support further development of DTI as a viable measure of axonal integrity and cognitive function in patients with MS.
Keywords: Multiple Sclerosis, Cognition, Diffusion Tensor Imaging, Episodic Memory, Disease Progression, Cingulate Gyrus
Introduction
Multiple sclerosis is a neurodegenerative disorder that typically results in white matter lesions, gliosis, and axonal degeneration.1, 2 A common symptom of MS is a decline in cognitive function, estimated to effect as many as 50% of patients.3 Though signs are often subtle, cognitive impairment can have a deleterious effect on employment, daily living skills, and social skills.4 Although no specific pattern of cognitive impairment has been associated with MS, particular domains seem to be preferentially affected. Information processing speed and episodic memory are among the most frequently cited deficits.5
The most obvious marker of MS, evidence of macroscopic lesions on conventional MRI, is not strongly related to cognitive measures.6 It is likely that disability in MS is also related to continual deterioration of white matter pathways through demyelination, axonal degradation, and gliosis.1, 2 In contrast to the global measure of disease provided by lesion load, progressive degeneration of specific axonal pathways can be measured using DTI,7 particularly in highly organized white matter pathways. The current study uses a probabilistic approach to fiber tracking, in which voxel-wise diffusion characteristics are used to calculate probabilistic pathways between two regions. Probabilistic tracking is able to track through lesions, allowing more accurate identification of pathway characteristics and a presenting a fuller picture of white matter integrity.8
The cingulum bundle is a large tract of white matter fibers originating in the cingulate cortex, projecting to the entorhinal cortex (EC) of the temporal lobe.9 Through the cingulum bundle, the posterior cingulate gyrus has multiple reciprocal connections with the hippocampus, a structure involved in episodic memory that shows structural and functional changes in patients with MS.10, 11 Diffusion measures in the posterior cingulum bundle (PCB) have shown relationships to verbal and visual spatial episodic memory, executive function, and working memory in various patient populations and in healthy controls.12, 13
In this study, probabilistic fiber tracking is used to examine the integrity of the PCB between the posterior cingulate cortex (PCC) and the EC. Resultant diffusion measures are compared to performance on measures of verbal and visual spatial episodic memory, information processing speed, and working memory. Radial (RD) and axial diffusivity (AD) are commonly used diffusion tensor-derived scalars. Changes in these diffusivities in white matter have been shown to relate to demyelination and axonal damage,14 both processes know to be involved in MS. The importance of the cingulum bundle in memory and the strong reciprocal connections with the hippocampal formation lead us to hypothesize that measures of RD and AD in the PCB will be increased in patients with MS as compared to controls. Further, we hypothesize that RD and AD will be related to performance on the California Verbal Learning Test-II (CVLT), which measures verbal episodic memory,15 the Brief Visuospatial Memory Test-R (BVMT), measuring visual spatial episodic memory,16 and the Paced Auditory Serial Addition Test (PASAT), which measures speed of processing, attention, calculation ability, and working memory.17 To determine the specificity of our findings, we also assess the relationship of diffusion measures in a portion of the cortiocospinal tract (the posterior limb of the internal capsule; PLIC) to cognitive measures. The corticospinal tract (CST) is not related to memory performance, and we hypothesize that RD and AD in this region will not be related to cognitive function. Mean diffusivity (MD) and fractional anisotropy (FA) are tensor-derived scalars that are commonly reported in the literature. Although these are, in a sense, derived from RD and AD, we report results for these measures for completeness and compatibility with prior literature.
Methods
Sample
The original dataset was composed of 64 patients with MS and 20 controls with complete DTI scans. Prior to data analysis, the sub-sample used in this research was selected with the goal of creating an age- and education-matched sample that included as many participants as possible. From this original dataset, seven patients with MS were excluded, all the oldest participants with the lowest levels of education (two males; mean (standard deviation) age: 52.0 (6.1) and education: 12.0 (0.8)). Three control participants were excluded, all the youngest with the highest levels of education (three males; mean (standard deviation) age: 34.3 (2.5) and education: 20.0 (0.0)). The final sample included 57 patients with MS and 17 age- and education-matched controls.
MR Imaging
All data was acquired under a Cleveland Clinic Institutional Review Board-approved protocol. All participants were fitted for a bite bar to restrict head motion during scanning and were then scanned using a 12-channel receive-only head array on a Siemens TIM Trio 3 tesla scanner (Siemens Medical Solutions, Erlangen, Germany). The following scans were performed:
Scan 1: Whole-brain T1. T1-weighted inversion recovery turboflash (MPRAGE): 120 axial slices; slice thickness=1.2 mm; field-of-view (FOV) 256×256 mm2; inversion time (TI)/echo time (TE)/repetition time (TR)/flip angle (FA)=900/1.71/1900 ms/8°; matrix 256×128; receiver bandwidth (BW)=62 kHz.
Scan 2: SPACE 3D T2. 144 sagittal slices; slice thickness=1.2 mm; FOV=256×224 mm2; matrix=256×224; 6/8 partial Fourier acquisition; TE/TR=528/3200 ms; FA mode; GRAPPA factor=2; 24 reference lines; BW=434 Hz/pixel.
Scan 3: SPACE 3D FLAIR. 144 sagittal slices; slice thickness=1.2 mm; FOV=256×224 mm2; matrix=256×224; 6/8 partial Fourier acquisition; TI/TE/TR=2000/395/6500 ms; GRAPPA factor=2; 24 reference lines; BW=698 Hz/pixel.
Scan 4: Whole-brain fieldmap. Axial gradient recalled echo: 32 axial slices; slice thickness=4 mm; FOV=256×256 mm2; matrix=64×64; TE1/TE2/TR/Flip 4.89/7.35/388ms/60°; BW=260 Hz/pixel.
Scan 5: High angular resolution diffusion imaging (HARDI). Single-shot echo-planar imaging readout; FOV=192×192 mm2; matrix=192×192; 45 1mm thick slices; TE/TR=90/7700 ms; 6/8 partial Fourier factor with GRAPPA acceleration factor=2; readout BW=930 Hz/pixel; 71 directions; 2 averages; 8 b=0 acquisitions per average. Diffusion weighting was achieved with a Stejskal-Tanner scheme at high angular resolution and with multiple diffusion weightings (72, 32, 8, and 9 image volumes acquired at b = 750, 333, 83, and 0 s/mm2, respectively).
HARDI postprocessing
Motion correction was performed with an iterative algorithm and the diffusion tensor was calculated using a standard log-linear fit.18, 19 Eigenvalues of each tensor were then used to calculate AD, RD, FA, and MD. The fiber orientation distribution (FOD) was calculated from the b = 750 s/mm2 images, using regularized spherical deconvolution as the basis for probabilistic tractography.20
Regions of Interest (ROIs)
For each participant, the MPRAGE was transformed to Talairach space using AFNI.21 EC ROIs were drawn manually on the individual subject MPRAGE in Talairach space and were restricted to coronal slices 10P-30P. PCC ROIs consisted of a 6mm sphere placed on the Talairach-transformed MPRAGE, according to the coordinates [−12 −42 36] given in Grecius et al.22 The PLIC was drawn bilaterally in Talairach space to ensure similar coverage for all subjects. All ROIs were transformed to individual subject space and checked for accuracy. PCC and EC ROIs (Figure 1) and PLIC ROIs (Figure 2) are shown in representative subjects. Because of the high level of atrophy in some of the patient sample, multiple steps were taken to ensure accurate registration between anatomical and diffusion scans. First, the mean b=0 image from the HARDI scan was spatially unwarped using the FSL tool FLIRT,23, 24 allowing for more accurate registration between the DTI and anatomic images. For each subject, the spatial transformation matrix between anatomic images and DTI was calculated using the AFNI routine align_epi_anat.py and the resulting registration was visually inspected for accuracy. All ROIs were spatially registered to DTI space and again checked for placement accuracy. PLIC ROIs were also inspected on color FA maps to verify accurate placement.
Figure 1.
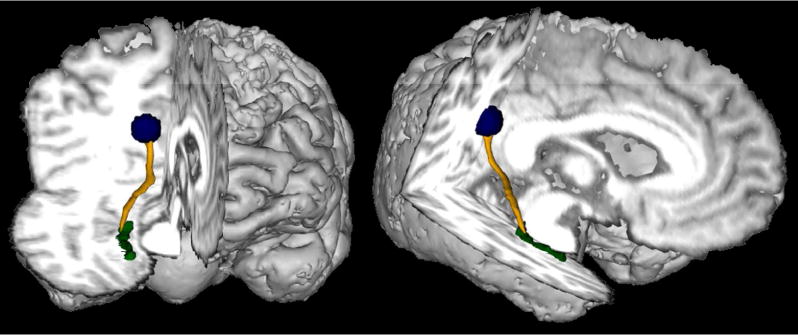
A representative PCB pathway (yellow), and ROIs for the EC (green) and PCC (blue).
Figure 2.
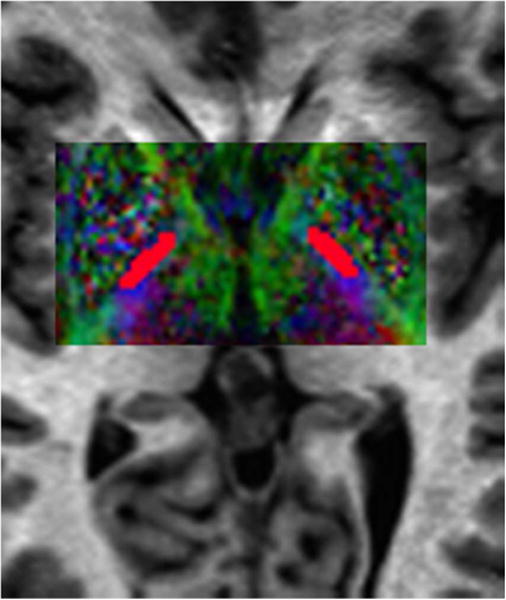
Representative ROIs for the PLIC (red) overlaid on a color FA map.
White Matter Mask and Volumetric Analysis
To ensure that DTI measures were from white matter, FSL was used to derive a white matter (WM) mask from the MPRAGE, T2, and FLAIR. The WM mask, w(v), was spatially registered to DTI space and visually inspected for accurate registration. For our analysis, w(v)=1 for all voxels (v) that were determined with high probability to contain white matter and w(v)=0 otherwise. For group comparisons, whole-brain measures of WM and grey matter (GM) and a scaling factor used as a correction for head size were estimated using FSL.
Probabalistic tracking
More than 1 million tracks were generated from seed regions placed in the bilateral PCC. Of the generated tracks, only those that terminated in a target region placed in the EC were saved. The number of saved tracks intersecting each voxel was then used to generate track density maps. Typically, approximately 4,000 tracks passed through the target ROI; this number was determined to be sufficient to produce track density maps with dynamic range such that stable pathway-dependent measures could be obtained.
Pathway-based DTI measures were produced by using a weighted mean:
in which D(v) is the particular tensor-based value of interest (eg, RD) at voxel (v), and w(v) is the value of the white matter mask at voxel v. The inner summation is over all voxels (v) on track (T); the outer summation is over all PCB pathway tracks (T). Note that the effect of the above equation is to weight the tensor value of a voxel by the number of times a generated track passes through it. Thus, voxels with only a few tracks passing through will count very little toward the pathway tensor estimate, whereas tracks more central to the path will be counted very highly. The result is AD, RD, FA, and MD for every track. Within subjects, left and right PCB track values showed high agreement (intraclass correlation coefficient > 0.77). Accordingly, the mean of each measure across the right and left tracks was taken for each subject.
PLIC analysis
For each subject, mean and standard deviation values for RD, AD, FA, and MD were calculated for voxels that were included in the PLIC ROIs and the WM mask. To ensure PLIC measures were as comparable to PCB measures as possible, the mean of the right and left PLIC diffusion values were taken as the final measure.
Behavioral data
All participants completed a neuropsychological assessment and several measures of cognitive function. A neurologist specializing in MS rated all patients on the Expanded Disability Status Scale (EDSS) and a composite Multiple Sclerosis Functional Composite (MSFC) score was calculated for each participant. Cognitive tests included:
CVLT - verbal episodic memory (sum of trials 1–5)
BVMT - visual spatial episodic memory (sum of trials 1–3)
Symbol Digit Modalities Test (SDMT) – processing speed, attention, and working memory (Total score)25
PASAT - working memory, calculation, and speed of processing (Total score, 3-second administration)
Results
Fifty-seven patients with MS (44 relapse-remitting, 13 secondary progressive) and 17 healthy controls were scanned under the above imaging protocol. All subjects were right handed. Demographic information and disease characteristics are detailed in Table 1. Unpaired Student’s t-tests showed no differences in age or level of education between patients and controls. Scaled WM and GM volumes are reported in Table 1. Both WM (p=0.0063) and GM (p=2×10−4) volumes were smaller in patients with MS.
Table 1.
Mean (standard deviation) of demographics and neuropsychological performance
| MS n = 57 |
Control n = 17 |
pa | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Sex (% male) | 18 (31.5) | 4 (23.5) | – |
| Age (years) | 44.6 (8.4) | 42.7 (10.1) | 0.449 |
| Education (years) | 15.1 (2.4) | 15.6 (1.5) | 0.454 |
| Disease characteristics | |||
| MSFC | 0.15 (0.64) | 0.73 (0.24) | 4×10−4 |
| Median EDSS (range) | 2.5 (1–6.5) | – | – |
| Median disease duration | 11 (1–33) | – | – |
| Behavioral measures | |||
| CVLT | 46.7 (11.2) | 57.7 (9.4) | 4×10−4 |
| BVMT | 45.9 (13.3) | 56.6 (7.9) | 0.002 |
| SDMT | 51.5 (13.6) | 67.4 (11.6) | 1×10−4 |
| PASAT | 45.6 (12.3) | 53.5 (6.2) | 0.019 |
| Volumetric measures | |||
| WM voxels, scaled | 651,875 (55,528) | 692,857 (41,212) | 0.0063 |
| GM voxels, scaled | 759,762 (42,178) | 804,744 (39,493) | 2×10−4 |
P-values for behavioral measures are based on demographically-corrected scores (see text for details);
P-values for unpaired Student’s t-tests, all values survive FDR correction;
BVMT=Brief Visuospatial Memory Test-Revised; CVLT=California Verbal Learning Test-II; PASAT=Paced Auditory Serial Addition Test; SDMT=Symbol Digit Modalities Test; WM=white matter; GM=grey matter.
Behavioral results
Uncorrected results of cognitive measures for both groups are shown in Table 1. Published norms were used to correct raw scores for demographic variables. The sum of trials 1–5 on the CVLT and total score on the SDMT were both corrected for age and education.15–25 The sum of trials 1–3 on the BVMT was corrected for age,16 and total score on the PASAT was corrected for education.26 Unpaired Student’s t-tests were used to compare patient and control performance. Patients scored significantly lower than controls on all measures (p<0.014). All comparisons survived the False Discovery Rate (FDR) correction.27
Diffusion results
Table 2 summarizes group diffusion measures for the PCB and PLIC, and Figure 1 shows PCB tracks in a representative subject. Unpaired Student’s t-tests showed that bilateral PCB RD (p=0.0001) and AD (p=0.0103) were significantly higher in patients with MS than in controls. All comparisons survived the FDR correction. There were no differences between patient and control PLIC measures (Table 2). There were no sex differences in diffusion measures in either patients or controls.
Table 2.
Mean (standard deviation) diffusion values (mm2 s−1) for the PCB and PLIC
| MS | Controls | pa | |
|---|---|---|---|
|
| |||
| PCB | |||
| RD | l496.8 (50.6) | 443.1 (38.8) | 0.0001 |
| AD | 1028.7 (67.5) | 981.0 (58.4) | 0.0103 |
| FA | 460.3 (30.8) | 491.3 (25.3) | 0.0003 |
| MD | 674.1 (53.3) | 622.4 (42.5) | 0.0004 |
| PLIC | |||
| RD | 316.9 (33.2) | 308.1 (32.1) | 0.3353 |
| AD | 1037.6 (81.2) | 1056.7 (78.7) | 0.3940 |
| FA | 649.0 (42.8) | 664.5 (48.6) | 0.2091 |
| MD | 557.1 (37.2) | 557.6 (30.6) | 0.9618 |
Results of unpaired Student’s t-tests;
PCB=posterior cingulum bundle; PLIC=posterior limb of the internal capsule; FA=fractional anisotropy; MD=mean diffusivity; RD=radial diffusivity; AD=axial diffusivity
In patients, age-adjusted partial correlations were used to assess the relationship of diffusion measures to disease characteristics. PCB measures were not related to the MSFC. PCB RD was related to EDSS (r=0.347, p=0.009) and AD was related to disease duration (r=0.342, p=0.010), but these results did not survive the FDR procedure. PLIC AD and MD were related to the MSFC (r=−0.293, p=0.029 and r=−0.346, p=0.009, respectively), but this result also did not survive the FDR procedure.
Table 3 shows age-adjusted partial correlations between diffusion measures and cognitive performance. BVMT and SDMT performance were significantly related to RD in the PCB (p<0.001), and SDMT performance was significantly related to RD in the PLIC (p=0.007). Neither the CVLT nor the PASAT were related to diffusion measures. Multiple linear regression was used to further investigate the relationship between cognitive performance and PCB RD. Predictor variables included age and performance on the BVMT and SDMT. Only the BVMT had significant (p = 0.035) partial effects in the full model. This model accounted for 25% of the variance in PCB RD, F = 5.85, 53 degrees of freedom, R2 = .249, p = 0.0016.
Table 3.
Linear partial correlation coefficient between cognition and bilateral PCB pathway diffusion measures, controlled for age, in 57 patients with MS.
| RD | AD | FA | MD | |
|---|---|---|---|---|
|
| ||||
| PCB | ||||
| CVLT | −0.288 | −0.203 | 0.231 | −0.267 |
| BVMT | −0.461d | −0.311 | 0.358a | −0.422b |
| SDMT | −0.418c | −0.285 | 0.342a | −0.384b |
| PASAT 3 | −0.222 | −0.147 | 0.173 | −0.202 |
| PLIC | ||||
| CVLT | −0.233 | −0.202 | 0.078 | −0.284 |
| BVMT | −0.081 | −0.077 | 0.023 | −0.104 |
| SDMT | −0.357a | −0.247 | 0.181 | −0.392b |
| PASAT 3 | −0.072 | −0.203 | −0.037 | −0.189 |
Results in bold survive the FDR correction.
p<0.01;
p<0.005;
p<0.001;
p<0.0005;
PCB=posterior cingulum bundle; PLIC=posterior limb of the internal capsule
Discussion
We found significant differences between patients with MS and controls on all measures of diffusion in the PCB. In addition, RD was significantly related to performance on the BVMT and SDMT, and BVMT performance was a significant predictor of RD. PLIC measures did not show between-group differences, but RD was significantly related to performance on the SDMT.
Group differences in PCB diffusion measures are consistent with findings of axonal degradation and demylination in MS damage.14 Our findings are in agreement with previously published reports of diffusion differences in ROI-based measures of the cingulum bundle in MS,28–30 and concur with a previous report of relationships between PCB diffusion and measures of both disease duration and EDSS.30 Lesions identified with conventional MR methods are not well-correlated with clinic measures, and pathway-specific diffusion measures may be more sensitive to subtle changes in normal appearing white matter (NAWM).1 DTI has been shown to detect focal lesions and lesion burden in NAWM, with both showing an increase in MD and a reduction in FA.31 Probabilistic tracking allows tracking in NAWM and through focal lesions, and our results suggest that track-based measures provide a fuller picture of white matter damage compared to measures such as T2 lesion burden.
Our ROI-based PLIC measures did not show group differences, in contrast to previously published investigations of the CST.32–34 In a tract-based study of the CST, 59 patients with MS showed decreased FA compared to healthy controls.34 The PLIC FA reported in Table 2 is slightly higher than in that study and another tract-based CST analysis, though the standard deviations are similar.32, 34 Our PLIC ROIs were carefully inspected to ensure anatomical accuracy, but it is possible that our ROI-based measure does not fully capture diffusion along the CST. Both tract-based CST studies showed a relationship between diffusion and EDSS.32, 34 Though we did not find a relationship with EDSS, we did find relationships between PLIC diffusion and another measure of disability, MSFC score. Our sample appears to have a slightly truncated EDSS range compared to those used in the tract-based analyses, which may have influenced this result.
The relationships between diffusion values and cognitive performance in patients were only partially in line with our hypothesis. As predicted, performance on the BVMT, a measure of visual spatial episodic memory, was correlated with diffusion measures, particularly RD. BVMT was also the only significant predictor of RD in a regression using significant cognitive measures. The EC is known to play a role in visual spatial episodic memory, and a recent study of mild cognitive impairment (MCI) and early Alzheimer’s disease shows a relationship between diffusion measures in the PCB and visual memory.12 Verbal memory has also been related to diffusion measures in the posterior region of the cingulum bundle and entorhinal cortex in patients with MCI and Alzheimer’s disease.35 We anticipated a relationship with our measure of verbal episodic memory, the CVLT, but the correlation with RD did not survive a correction for multiple comparisons. Surprisingly, we did not find a relationship between performance on the PASAT and diffusion measures. Previous studies have shown relationships between cingulum diffusion and performance on the PASAT in MS patients, though it should be noted that in both works the region of correlation was superior to the cingulum region analyzed in the current study.29, 30 One limitation of this study is that of low disease burden in the patient sample. It is possible that the relationship of CVLT and PASAT performance to diffusion measures would strengthen with a greater range of disease burden, though work in healthy controls has shown a relationship between diffusion measures in the PCB and working memory and executive function, leading to expectations of a relationship to a measure such as the PASAT.13
Finally, we found a significant relationship between performance on the SDMT and RD in both the PCB and the PLIC. The SDMT is very sensitive to cognitive deterioration in MS and showed the greatest difference between patients and controls in this sample. We previously found a strong relationship between SDMT performance and diffusion measures,11 and it is possible that a change in information processing speed is an indicator of overall cognitive decline in MS, unrelated to track-specific changes.
In this study we observed a significant increase in PCB RD and AD in patients with MS. Further, patients showed a significant relationship between measures of visual spatial memory and information processing speed and diffusion of the PCB. Though this work includes only 57 patients, it does support continued development of DTI measures as a marker of cognitive function in patients with MS. It suggests that changes in specific abilities are associated with dysfunction in related tracts, and that information processing speed might be related to overall cognitive and neurofunctional decline. Future refinement and validation will require larger sample sizes, longitudinal follow-up, and the inclusion of validation cohorts.
Acknowledgments
The authors would like to thank John Cowan, Tami Gaebelein, Sarah Gallucci, Blessy Mathews, Katie Murphy, Christine Reece, and Derrek Tew for their contributions.
Funding
This work was supported by the National Multiple Sclerosis Society [grant number RG 4110-A2]; and the National Institutes of Health [grant number NS035058].
References
- 1.Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165–71. doi: 10.1016/s0022-510x(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 2.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 3.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–91. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 4.Hakim EA, Bakheit AM, Bryant TN, et al. The social impact of multiple sclerosis – a study of 305 patients and their relatives. Disability and Rehabilitation. 2000;22:288–93. doi: 10.1080/096382800296755. [DOI] [PubMed] [Google Scholar]
- 5.Nocentini U, Pasqualetti P, Bonavita S, et al. Cognitive dysfunction in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2006;12:77–87. doi: 10.1191/135248506ms1227oa. [DOI] [PubMed] [Google Scholar]
- 6.Rovaris M, Judica E, Gallo A, et al. Grey matter damage predicts the evolution of primary progressive multiple sclerosis at 5 years. Brain. 2006;129:2628–34. doi: 10.1093/brain/awl222. [DOI] [PubMed] [Google Scholar]
- 7.Henry R, Oh J, Nelson S, Pelletier D. Directional diffusion in relapsing-remitting multiple sclerosis: a possible in vivo signature of Wallerian degeneration. J Magn Reson Imaging. 2003;18:420–6. doi: 10.1002/jmri.10379. [DOI] [PubMed] [Google Scholar]
- 8.Pine A, Jones S, Lowe M, Sakaie K, Phillips M. Fiber-Tracking Through Multiple Sclerosis Lesions Using Probabilistic Tracking. 17th Annual Meeting of the International Society for Magnetic Resonance in Medicine; Honolulu. 2009. [Google Scholar]
- 9.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 10.Hulst H, Schoonheim M, Roosendaal S, et al. Functional Adaptive Changes Within the Hippocampal Memory System of Patients With Multiple Sclerosis. Human Brain Mapping. 2012;33:2268–80. doi: 10.1002/hbm.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenig KA, Sakaie KE, Lowe MJ, et al. Hippocampal Volume Is Related to Cognitive Decline and Fornicial Diffusion Measures in Multiple Sclerosis. MRI. 2013;32:354–8. doi: 10.1016/j.mri.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y-C, Shih Y-C, Tseng W-YI, et al. Cingulum Correlates of Cognitive Functions in Patients with Mild Cognitive Impairment and Early Alzheimer’s Disease: A Diffusion Spectrum Imaging Study. Brain Topography. 2014;27:393–402. doi: 10.1007/s10548-013-0346-2. [DOI] [PubMed] [Google Scholar]
- 13.Koch K, Wagner G, Dahnke R, et al. Structure-function relationships in the context of reinforcement-related learning: a combined diffusion tensor imaging-functional magnetic resonance imaging study. Neuroscience. 2010;168:190–9. doi: 10.1016/j.neuroscience.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–22. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test – Second Edition, Adult Version. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 16.Benedict RH. Brief Visuospatial Memory Test-Revised: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 17.Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT) Arch Clin Neuropsychol. 2006;21:53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Sakaie K, Lowe M. Quantitative assessment of motion correction for high angular resolution diffusion imaging. Magn Reson Imaging. 2010;28:290–6. doi: 10.1016/j.mri.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basser P, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 20.Sakaie KE, Lowe MJ. An objective method for regularization of fiber orientation distributions derived from diffusion-weighted MRI. Neuroimage. 2007;34:169–76. doi: 10.1016/j.neuroimage.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 22.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith S, Jenkinson M, Woolrich M, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 25.Smith A. Symbol Digit Modalities Test: Manual. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 26.Rao S. A manual for the brief, repeatable battery of neuropsychological tests in multiple sclerosis. Milwaukee, WI: Medical College of Wisconsin; 1990. [Google Scholar]
- 27.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. [Google Scholar]
- 28.Pardini M, Bergamino M, Bommarito G, Bonzano L, Luigi Mancardi G, Roccatagliata L. Structural correlates of subjective and objective memory performance in multiple sclerosis. Hippocampus. 2013;24:436–45. doi: 10.1002/hipo.22237. [DOI] [PubMed] [Google Scholar]
- 29.Van Hecke W, Nagels G, Leemans A, Vandervliet E, Sijbers J, Parizel PM. Correlation of cognitive dysfunction and diffusion tensor MRI measures in patients with mild and moderate multiple sclerosis. J Magn Reson Imaging. 2010;31:1492–8. doi: 10.1002/jmri.22198. [DOI] [PubMed] [Google Scholar]
- 30.Syc S, Harrison D, Saidha S, Seigo M, Calabresi P, Reich D. Quantitative MRI Demonstrates Abnormality of the Fornix and Cingulum in Multiple Sclerosis. Multiple Sclerosis International. 2013;2013:1–9. doi: 10.1155/2013/838719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werring D, Clark C, Barker G, Thompson A, Miller D. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–32. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- 32.Tovar-Moll F, Evangelou IE, Chiu AW, et al. Diffuse and Focal Corticospinal Tract Disease and Its Impact on Patient Disability in Multiple Sclerosis. J Neuroimaging. 2014 doi: 10.1111/jon.12171. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inal M, Unal B, Kala I, Turkel Y, Bilgili YK. ADC evaluation of the corticospinal tract in multiple sclerosis. Acta Neurologica Belgica. 2014 doi: 10.1007/s13760-014-0311-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Tortorella P, Laganà MM, Saresella M, et al. Determinants of disability in multiple sclerosis: an immunological and MRI study. Biomed Res Int. 2014;2014:1–8. doi: 10.1155/2014/875768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grambaite R, Reinvang I, Selnes P, et al. Pre-dementia Memory Impairment is Associated with White Matter Tract Affection. Journal of the International Neuropsychological Society. 2011;17:143–57. doi: 10.1017/S1355617710001360. [DOI] [PubMed] [Google Scholar]


