Abstract
Objective
Based on the hypothesis that neonatal autologous red blood cell (RBC) survival (RCS) is substantially shorter than adult RBC, we concurrently tracked the survival of transfused biotin-labeled autologous neonatal and allogeneic adult RBC into ventilated, very low birth weight (VLBW) infants.
Study design
RBC aliquots from the first clinically ordered, allogeneic adult RBC transfusion and from autologous infant blood were labeled at separate biotin densities (BioRBC) and transfused. Survival of these BioRBCs populations were concurrently followed over weeks by flow cytometric enumeration using leftover blood. Relative tracking of infant autologous and adult allogeneic BioRBC was analyzed by linear mixed modeling of batched weekly data. When possible, Kidd antigen (Jka and Jkb) mismatches between infant and donor RBCs were also used to track these two populations.
Results
Contrary to our hypothesis, concurrent tracking curves of RCS of neonatal and adult BioRBC in 15 study infants did not differ until week 7, after which neonatal RBC survival became shortened to 59% to 79% of adult enumeration values for uncertain reasons. Analysis of mismatched Kidd antigen RBC showed similar results, thus confirming that BioRBC tracking is not perturbed by biotin RBC labeling.
Conclusions
This study illustrates the utility of multi-density BioRBC labeling for concurrent measurement of RCS of multiple RBC populations in vivo. The similar RCS results observed for neonatal and adult BioRBCs transfused into VLBW infants provides strong evidence that the circulatory environment of the newborn infant—not intrinsic infant-adult RBC differences—is the primary determinant of erythrocyte survival.
Trial registration
Clinicaltrials.gov: NCT 00731588.
Keywords: Erythrocyte, kinetics, erythropoiesis
Severe anemia is a common clinical problem among critically ill infants, particularly those born prematurely1. As a result, these patients are among the most commonly transfused of all patient groups2. Cited among contributors to the anemia that characteristically develops during early infancy in both preterm and term infants1, 3–6 is the shorter red blood cell (RBC) lifespan observed in both premature (30–50 d) and full term infants (60–70 d) compared with normal adults (110–130 d).
Red cell survival (RCS) has been most commonly and accurately performed by direct measurement of labeled populations of RBC in the circulation7. In the past, labeling has been done using one of several radioactive isotopes, e.g., 51Cr, 32P and 99mTc7. The major safety problem with this is radiation exposure. Since the 1970s, the use of radioactive compounds in research studies involving vulnerable patient groups such as fetuses, infants, children, and pregnant women has been deemed unacceptable. To overcome this problem and the problem of RBC surface elution of some radioisotopes8, labeling RBC with biotin (BioRBC) has been advocated as a practical, reliable, and accurate method for measuring RCS in vulnerable patient groups9, 10. Furthermore, biotin labeling has the capability of concurrent measurement of RCS of more than one RBC population.
In the present study we employed multi-density biotin RBC labeling to concurrently track survival of neonatal autologous and adult allogeneic RBC in VLBW infants. Based on the preponderance of RCS studies in infants prior to 51Cr use being curtailed in 197011 and RCS in normal adults9, we hypothesized that allogeneic BioRBC from adults would survive about twice as long as autologous newborn BioRBC in very low birth weight (VLBW) infants11. We further hypothesized that measurements based on Kidd antigen mismatches would demonstrate the same difference in VLBW infants.
METHODS
Prior to initiation, this study received approval from the appropriate Institutional Review Boards, with informed written parental consent. Approval included permission to administer Kidd antigen-mismatched RBC.
We enrolled a prospective, convenience sample of newborn infants receiving their first RBC transfusion based on clinical indications. Included were VLBW infants <31-wk gestation requiring mechanical ventilation with survival anticipated. Infants with major congenital anomalies were excluded.
Fresh (<7 d old), AS-3, leukoreduced (Leukotrap RC System; Medsep, Covina, CA), irradiated (3000 cGy) blood bank RBC units were tested for Kidd antigen mismatch between the adult donor and the infant recipient. Once the RBC transfusion was ordered, the designated unit had routine blood bank volume reduction by centrifugation to achieve a packed RBC volume of ~0.80. Standard practice at our institution is to transfuse infants with 15 ml/kg of packed RBC. For this study, the first 14 ml/kg of allogeneic donor RBC was transfused as usual by infusion over 3 to 4 h. The remaining 1 ml/kg of the clinically ordered allogeneic packed RBC transfusion (plus an additional 2.8 ml of allogeneic packed RBC required following biotinylation for culture and hematological analysis) and 0.5 to 3 ml/kg of whole blood from the study infant was biotinylated at two discrete low biotin densities between 6 and 36 μg/ml.
Following RBC biotinylation, each BioRBC population was washed and the supernatant fractions discarded. The washed neonatal autologous and adult donor allogeneic packed BioRBC populations (hematocrits ~45% and ~80%, respectively) were each passed through an 18-micron filter (Hemo-Nate; Utah Medical Products, Midvale, UT) to remove microaggregates. Immediately after the 14 ml/kg clinical transfusion, the final 1 ml/kg of adult donor BioRBC and the ~1 ml/kg of neonatal autologous BioRBC were infused separately in less than 10 min.
Beginning at 24-h post-transfusion, leftover anticoagulated whole blood from clinically ordered laboratory testing was salvaged up to four times per week. Samples were analyzed for the proportions (i.e., enrichment) of the two BioRBC populations relative to unlabeled RBC and for the proportion of RBC based on Kidd antigen mismatches as described below.
Biotinylation of RBC
RBC were biotinylated as described previously.10 Briefly, RBC were washed with a carbonate-buffered dextrose sodium phosphate wash solution to remove plasma proteins and resuspended at a hematocrit of 25%. Hematocrit adjustment was verified using the Sysmex XE-2100 (Sysmex, Kobe, Japan). Subsequently, RBC were incubated with the biotinylation reagent, sulfosuccinimidobiotin (sNHS-biotin; Pierce Chemical, Rockford, IL), at two separate concentrations between 6 and 36 μg of sNHS-biotin per ml packed RBC.
Quantitative Flow Cytometric RBC Analysis
BioRBC
The enrichment proportions of the two populations of BioRBC relative to the total number of RBC counted was determined in triplicate on 10 μl of salvaged whole blood. These measurements were made by flow cytometric enumeration of 1×106 total cells using a FACSCalibur or Fortessa flow cytometer (BD Biosciences, San Jose, CA)9. For samples with slightly overlapping fluorescent intensity histograms, mixture modeling was used to calculate the proportion of cells from each RBC population. The lower limit of quantitation for individual BioRBC density peaks is 0.06% of total RBC12.
Kidd RBC Antigen Mismatch
For analysis of Jka or Jkb RBC by flow cytometry, triplicate 3 μl aliquots of the blood samples were washed and incubated with either anti-Jka or anti-Jkb primary antibody (Immucor Inc., Norcross, GA) based on previous antigen typing. This primary antibody incubation was followed by incubation with a secondary goat antibody to human IgG conjugated with the fluorescent dye Alexa Fluor 488 (H10120, Invitrogen, Carlsbad, CA). Jka or Jkb positive RBCs were enumerated by flow cytometry as previously described13.
Data Handling and Statistical Analyses
Statistical analysis of tracking of RCS was performed using linear mixed modeling. The data used for the RCS tracking of the adult and neonate BioRBC populations and the two Kidd antigen-mismatched RBC populations were expressed as ratios relative to the sample closest to 24 hours after the transfusion. Because blood samples for RCS tracking of individual infants were not available at precisely the same time, the ratios at the individual time points were grouped into intervals as follows: 3 d to <7 d, and weekly intervals thereafter (eg, 7 days to <14 days through 49 d to <64 d). Multiple data points for a subject within a given interval were treated statistically as replicates. Because the ratios did not have a normal distribution, a log transformation was applied to the data before linear mixed model analysis.
Two mixed models were fitted. One compared RCS tracking of adult and neonate BioRBC densities. The other compared RCS tracking of Kidd antigens (Jka or Jkb) with the RCS tracking of adult and neonate BioRBC densities. The fixed effects in both models included RBC donor type (Adult, Neonate, or Kidd antigen), time, and donor type x time interaction. In both, the test for donor type x time interaction corresponds to testing whether RCS tracking over time shows nonparallel profiles between adult versus neonate by BioRBC, and between adult versus neonate Kidd antigen differences. In addition to estimating the fixed effects in the mixed model, this analytic method permits selection of the covariance structure that best fits the relationship of the RCS data (i.e., BioRBC donor type and Kidd antigens at different times) measured in the same subject. From the fitted models, tests of mean contrasts were performed to compare adult versus neonate (first model) or adult and neonate versus Kidd antigens (second model) at each time interval. The tracking of RCS for Kidd antigen mismatches used in the second model only included times prior to a potential second RBC transfusion, which prevents following the original populations of Kidd mismatched RBC. The P values for these tests were adjusted using the Bonferroni post hoc method to account for the number of comparisons. The means of the natural logarithmic transformed data were back transformed to obtain the mean ratios for adult, neonate, and for the Kidd antigen at each time interval. Descriptive summary statistics are presented as mean ± SD. A P value <0.05 was considered statistically significant.
RESULTS
Fifteen premature VLBW infants, including five males and 10 females, were enrolled between November 2009 and May 2014. Birth weight ranged between 494 and 1,042 g, and gestational age at birth ranged between 23.1 and 27.1 wk (Table). The group included 12 singletons (80%) and three twins, but none from the same pregnancy. Body weights at the time of the first transfusion ranged between 564 and 1,102 g; mean birth weight z-score adjusted for sex and singleton or twin status was −0.17 ± 0.9114. The mean (± SD) age at the time of the first transfusion was 3.2 ± 3.4 d, with a range of 0.2 to 14 d. During the nine-week BioRBC tracking period, study infants received a total of 1 to 9 clinically ordered RBC transfusions. Seven of the 15 infants had data for concurrent tracking of mismatched Jka or Jkb, or both, for at least two weeks following their initial transfusion as well as for concurrent tracking of BioRBC.
Table 1.
VLBW study subject demographic and BioRBC transfusion data
| Subject Number | Gestational Age at Birth (wk) | Birth Weight z-Score | Weight at Birth (g) | Post-Natal Age at Study (d) | Post-Natal Weight at Study (g) | Autologous RBC Initial Enrichment† | Allogeneic RBC Initial Enrichment† |
|---|---|---|---|---|---|---|---|
| 1 | 25.7 | −0.86 | 721 | 1.3 | 721 | 0.86% | 2.77% |
| 2 | 26.1 | −1.17 | 708 | 5.4 | 746 | 1.21% | 3.70% |
| 3 | 25.7 | 0.14 | 914 | 2.7 | 775 | 2.24% | 2.01% |
| 4 | 26.0 | 0.01 | 813 | 0.5 | 781 | 3.12% | 4.46% |
| 5 | 26.3 | 0.84 | 1102 | 2.1 | 912 | 2.37% | 3.39% |
| 6 | 24.4 | −0.34 | 590 | 2.9 | 513 | 2.33% | 2.62% |
| 7 | 26.7 | 0.77 | 1026 | 13.6 | 1042 | 1.49% | 3.08% |
| 8 | 24.0 | −0.48 | 657 | 3.4 | 583 | 2.53% | 4.33% |
| 9 | 26.1 | −2.06 | 564 | 5.2 | 494 | 3.27% | 4.73% |
| 10 | 23.4 | 0.73 | 708 | 4.4 | 641 | 2.25% | 2.42% |
| 11 | 27.1 | −0.77 | 875 | 0.7 | 875 | 2.63% | 4.18% |
| 12 | 24.0 | 0.07 | 691 | 0.3 | 691 | 3.27% | 3.75% |
| 13 | 23.1 | 0.13 | 641 | 0.8 | 630 | 2.67% | 4.14% |
| 14 | 26.1 | −0.98 | 785 | 0.2 | 785 | 2.45% | 3.89% |
| 15 | 25.4 | 1.38 | 1019 | 5.2 | 923 | 2.50% | 2.79% |
| Mean | 25.4 | −0.17 | 788 | 3.2 | 741 | 2.35% | 3.48% |
| SD | 1.2 | 0.91 | 166 | 3.4 | 156 | 0.70% | 0.83% |
Enrichment: the percentage of biotin labeled RBC in the circulation
Tracking of post-transfusion RBC survival curves
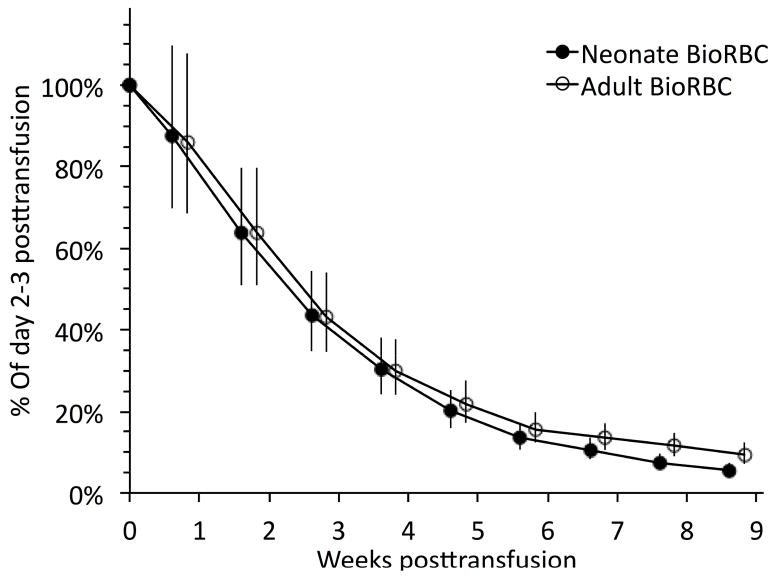
Initial BioRBC enrichments immediately following transfusions of the neonatal autologous BioRBC and the adult allogeneic BioRBC was 3.48 ± 0.83% and 2.35 ± 0.70%, respectively. Group mean survival of autologous BioRBC and adult donor allogeneic were not different based on tracking of their transfused neonatal and adult BioRBC for the first 6 weeks (Figure 1). Only during the last three weeks of RBC tracking, when the proportions of the two BioRBC populations had decreased to < 20% of initial BioRBC enrichment (approximately 0.5% enrichment of circulating RBC) were significant differences detected. For weeks 7, 8, and 9 enrichment values for neonatal RBC relative to adult values were 79% (P = 0.015), 64% (P < 0.0001), and 59% (P = 0.001), respectively.
Figure 1.
Comparison of concurrent red cell survival tracking mean (± 95% CL) of neonatal autologous and adult allogeneic BioRBC including all study subjects (n=15). Weeks on the x-axis are shown relative to the first RBC transfusion. Significant differences were observed for weeks 7 (P<0.05), 8 (P<0.01), and 9 (P<0.01).
To determine if tracking differences from the analysis based on data from all 15 infants differed from those of the seven infants who had BioRBC enrichment determinations throughout the entire nine-week study period, a paired subgroup analysis was performed for the latter group. This analysis differed only slightly from that of the entire 15 infants in that significant differences were detected for weeks 8 (P = 0.002) and 9 (P = 0.001), again with neonatal BioRBC disappearing more rapidly (data not shown).
Tracking of Kidd antigen mismatches in study subjects
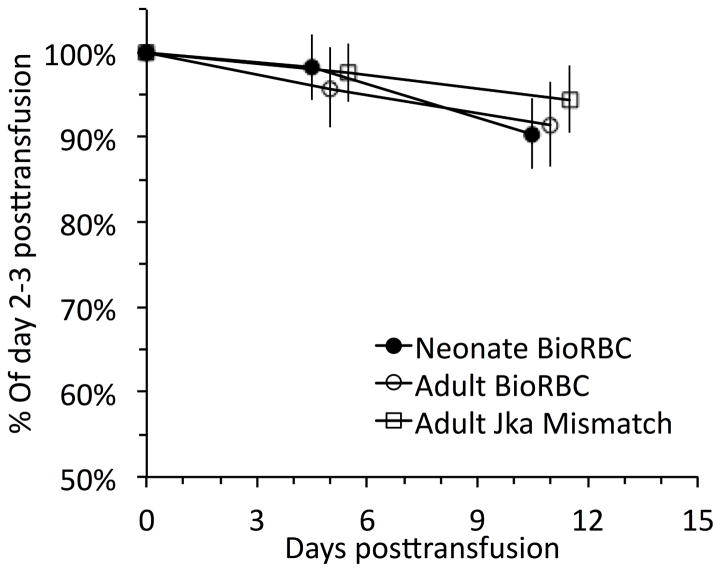
The seven infants who had donor-recipient RBC Jka or Jkb mismatches and who did not receive a second clinical RBC transfusion for two weeks after their initial RBC transfusion were tracked for: 1) neonatal BioRBC; 2) adult BioRBC; and 3) Jka+ and/or Jkb+ RBC. When analyzed in paired fashion, no significant differences were observed among the three adult RBC populations (Figure 2). Because of the large number of study subjects who were treated with clinical RBC transfusions confounding subsequent analysis, it was not possible to follow Kidd antigen mismatched RBC further.
Figure 2.
Comparison of concurrent mean red cell survival tracking (± 95% CL) of autologous neonatal BioRBC, adult allogeneic BioRBC, and adult allogeneic Jka mismatched RBC (n=6) prior to the subsequent clinically ordered RBC transfusion. Days on the x-axis are shown relative to the first clinically ordered RBC transfusion. Paired differences among the three RBC groups were not significant at either time period shown.
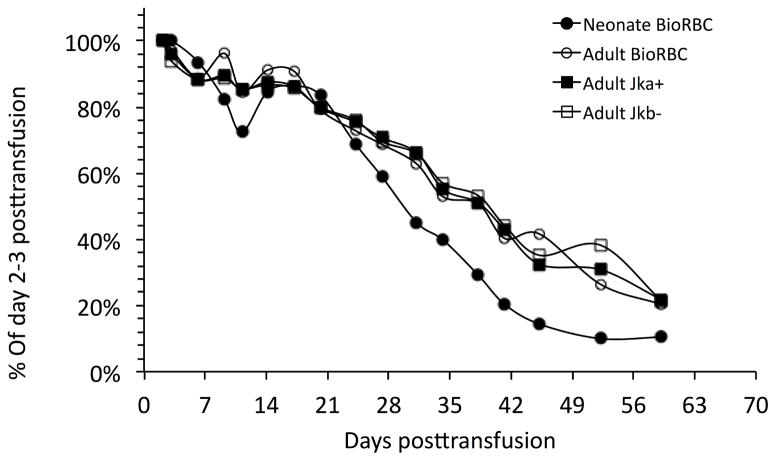
By chance one infant received a double Jka and Jkb mismatch as its first donor RBC transfusion but received no further RBC transfusions. This allowed this infant to be followed for the entire nine-week study period (Figure 3). For this subject there was close agreement in the disappearance of adult donor BioRBC and Jka+ and Jkb− donor RBC. Similar to the neonatal and adult BioRBC tracking data, the neonatal BioRBC data showed more rapid removal, but only during the last half of the study period.
Figure 3.
Close agreement of adult donor BioRBC and adult Kidd antigen RBC tracked in an individual infant study subject following the only RBC transfusion this infant received.
DISCUSSION
The hypothesized and expected two-fold longer survival of adult RBC compared with infant RBC survival was not observed. The similarity in concurrent tracking of these two biotin labeled RBC populations provides evidence that the previously inferred shorter RCS of neonatal RBC is likely due to methodological problems such as the failure to conduct concurrent comparisons in the same subjects. We infer that the biological differences in the circulatory environment of the transfused subject—not intrinsic differences in neonatal RBC and adult RBC— are likely the primary cause of shortened survival of RBC in neonates and infants. We further assert that the modest differences noted in neonatal and adult tracking RCS for the minority of RBC still in circulation beginning after 7 wk is not evidence against this interpretation and speculate that the difference may be attributed to neonatal RBC being produced under non-steady in utero conditions. The tracking of adult RBC based on Kidd antigen mismatch provides evidence that these BioRBC findings are not due to artifactual due to biotin labeling, consistent with prior studies9, 10.
This study’s hypothesis that neonatal RBC would demonstrate two-fold shorter RCS tracking than adult RBC was based on textbooks and reviews indicating that shortened infant RCS is a major contributor to anemia all term and preterm infants experience during the early months of life1, 3–6. Unfortunately, evidence for this conclusion is modest as few studies have concurrently compared newborn and adult RCS. In a report by Mollison in 1951, four preterm newborns were concurrently transfused with adult allogeneic RBC and allogeneic placental RBC from term infants at delivery15. Analysis performed by antigen mismatch using the differential agglutination method—the forerunner of flow cytometric quantification of mismatched Jka and Jkb RBC16—demonstrated “smallness of the difference between the survival of placental and adult blood.” In a prior report by Mollison in which newborn and adult RBC were administered, it was noted “that transfused cells from adult donors are not destroyed more rapidly in the circulation of the newborn infant than in the circulation of an adult”17. This 1943 study of 22 infants, 19 with Rh incompatibility who received both Rh negative and positive transfusions, demonstrated that 16 infants receiving Rh-negative donor blood had RCS similar to the three non-Rh affected infants and to 14 adults. Although clinical information provided by Mollison was limited, a majority of subjects appeared to be non-critically ill term infants sampled for Hb levels and RCS at 1- to 4-week intervals. Mollison’s 1943 infant RCS data are in marked contrast with Pearson’s review of RBC lifespans of 51Cr-labeled RBC18. Pearson reported lifespans of 35–50 days for RBC of preterm infants and 60–70 days for term infants. These RCS differences in prior studies are likely due to methodological and study population differences.
In the absence of alloantibodies, post-transfusion RCS is reliably determined using mismatched RBC antigens19, 20. Thus, tracking of RCS based on mismatched Kidd RBC Jka and Jkb in the present study reinforces the conclusion that low density BioRBC determinations accurately reflect RCS, i.e., that ex vivo biotinylation does not artifactually shorten RCS9, 10. An important advantage of BioRBC over mismatched RBC antigens is that RCS studies of both autologous and allogeneic RBC are possible.
RCS of adult RBC in the circulation of fetuses and infants also has been indirectly estimated. In 19 VLBW infants, Bard and Widness reported that the interval between the last transfusion and when the percentage of HbF returned to the anticipated post-menstrual HbF levels for non-transfused infants was approximately 60 d21. In a study of fetuses transfused with adult RBC as treatment for Rh-D maternal alloimmune disease, Egberts et al.22 estimated that adult donor transfused RBC was also approximately 60 d.
Population RBC labeling with biotin has important advantages relative to radiolabeling. First, when measured by flow cytometry, the biotin method—unlike radiolabeling RBC—permits simultaneous, concurrent determination of multiple, discrete RBC biotin densities. RCS studies utilizing multiple concurrently administered BioRBC densities are more cost effective because paired analysis eliminates intra-subject variability and the need to perform sequential studies in the same subject. Second, biotin is securely attached to the RBC surface, permitting highly reliable determinations when analyzed by flow cytometry. In contrast, elution of 51Cr has resulted in the recommendation that RCS by 51Cr be determined for no more than 30 d8. In contrast, BioRBC (or mismatched RBC surface antigens) can be reliably followed to the limit of detection, i.e., 0.06% enrichment9, 10, 23. Characteristically, this encompasses >95% of the RBC lifespan. Third, subject radiation exposure is avoided with biotin labeling. Thus for the first time since the 1970s, RCS studies can be performed on infants and other vulnerable groups.
The biotin method has shortcomings and limitations. Because of the limited clinical experience and safety data for BioRBC, utilizing BioRBC requires a Food and Drug Administration investigational new drug application. We have reported that 15 to 20% of adults receiving autologous BioRBC transfusions develop anti-BioRBC antibodies24, 25. However, perhaps because of their immunological immaturity, newborn infants have not been observed to develop anti-BioRBC antibodies26.
In addition to these safety concerns, this study has several additional limitations. First, determination of RCS variables such as mean potential lifespan or half-life (T50) was not possible with the tracking approach used. Deriving such RCS variables requires modeling to adjust for confounding cofactors noted above. Second, direct comparison of RCS of neonatal and adult RBC might be minimally biased because neonatal RBC were produced under a non-steady state whereas adult donor blood RBC were produced under steady-state conditions. Third, the population of biotin-labeled neonatal RBC were not irradiated but adult blood donor RBC were, which theoretically might have shortened survival of adult RBC in fetuses and infants27. Fourth, the study population consisted of a small convenience sample of 15 VLBW subjects that may not be representative of all VLBW infants. Finally, the duration of Kidd antigen mismatch tracking was limited to only 2 weeks in all but one infant.
In summary, contrary to our hypothesis that adult allogeneic RBC survive about twice as long as the neonatal autologous RBC, survival of both adult and neonatal RBC were similar. We infer that the environment of the newborn VLBW infant’s circulation rather than intrinsic differences between infant and adult RBC, primarily determines RCS. The present study corroborates and extends our previous infant RCS study10 in documenting that multi-density BioRBC can also be used to concurrently track autologous and allogeneic RCS without concern regarding label elution. Furthermore, close agreement of the Kidd antigen and BioRBC tracking data provides evidence that long-term survival of allogeneic RBC labeled at low biotin BioRBC density accurately reflects true RCS9. Our group continues to investigate the extrinsic environmental factors potentially responsible for the present findings. We are also working to develop a mechanistic modeling approach that adjusts for important confounding and extrinsic factors in infants to predict discrete RCS parameters, e.g., T50 and life span. Clinical implications of the present finding are that autologous RBC from delayed umbilical cord clamping or milking performed at birth is likely to have similar RCS as transfused adult allogeneic RBC. Moreover, this study lays the groundwork for, and demonstrates the feasibility of, accurately and safely performing BioRBC survival studies in critically ill infants using the exquisitely small volumes of leftover blood usually available. Such studies can potentially shed new light on mechanisms of and therapies for the prevention and treatment of neonatal anemia and its sequelae.
Acknowledgments
The authors acknowledge helpful discussions with Robert S. Franco, Ph.D. (University of Cincinnati), and Guohua An, Ph.D. (University of Iowa), regarding methodological and theoretical aspects of the present study. We also appreciate the many contributions of the University of Iowa clinical laboratory staff led by Mitchell J. Owen, MT (ASCP), and Mary Capper, MT (ASCP)SH, and overseen by Matthew D. Krasowski, M.D., Ph.D. This work would not have been possible without the outstanding clinical research contributions of Iowa’s neonatal research nurse team that included Karen Johnson, R.N., Gin Zhou, R.N., and Ruthann Schrock, R.N. Mark Hart provided valuable editorial and secretarial assistance. Finally, we are grateful to the families of study subjects in allowing their infants to participate.
Supported by US Public Health Service National Institutes of Health (P01 HL046925), the Thrasher Research Fund (0285-3), the National Center for Research Resources (UL1RR024979, UL1TR000039, and 1S10 RR027219). The Sysmex XE-2100 automatic hematology analyzer used in this study was provided on an on-loan basis from Sysmex Corporation (Kobe, Japan). J.W. serves as a paid consultant for HemoGenix and holds a loan agreement with Sysmex Corporation (Kobe, Japan) for the use of the XE-2100 hematology analyzer in his research lab. D.M. serves as a consultant for MedDay Pharmaceutical.
ABBREVIATIONS
- BioRBC
biotin labeled red blood cell(s)
- RCS
red blood cell survival
- RBC
red blood cell(s)
- VLBW
very low birth weight
Footnotes
The other authors declare no conflicts of interest.
Portions of this study were presented at the American Association of Blood Banks meeting, Denver, CO, October <day(s)>, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohls RK. Evaluation and treatment of anemia in the neonate. In: Christensen RD, editor. Hematologic Problems of the Neonate. Philadelphia, PA: W.B. Saunders Company; 2000. pp. 137–69. [Google Scholar]
- 2.Levy GJ, Strauss RG, Hume H, Schloz L, Albanese MA, Blazina J, et al. National survey of neonatal transfusion practices: I. Red blood cell therapy. Pediatrics. 1993;91:523–9. [PubMed] [Google Scholar]
- 3.Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. 10. Philadelphia, PA: Elsevier/Saunders; 2015. [Google Scholar]
- 4.Maisels MJ. Jaundice. In: Avery GB, MacDonald MG, Seshia MMK, Mullett MD, editors. Avery’s Neonatology: Pathophysiology & Management of the Newborn. 6. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 768–846. [Google Scholar]
- 5.Ohls RK. Developmental erythropoiesis. In: Polin RA, Fox WW, editors. Fetal and Neonatal Physiology. 2. Philadelphia: Saunders; 1998. pp. 1762–86. [Google Scholar]
- 6.Roberts IAG, Murray NA. Hematology. In: Rennie JM, editor. Textbook of neonatology. 5. Edinburgh: Churchill Livingstone; 2012. pp. 755–90. [Google Scholar]
- 7.Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109–14. doi: 10.1002/ajh.21298. [DOI] [PubMed] [Google Scholar]
- 8.Recommended method for radioisotope red-cell survival studies. International Committee for Standardization in Haematology. Br J Haematol. 1980;45:659–66. doi: 10.1111/j.1365-2141.1980.tb07189.x. [DOI] [PubMed] [Google Scholar]
- 9.Mock DM, Matthews NI, Zhu S, Strauss RG, Schmidt RL, Nalbant D, et al. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion. 2011;51:1047–57. doi: 10.1111/j.1537-2995.2010.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widness JA, Nalbant D, Matthews NI, Strauss RG, Schmidt RL, Cress GA, et al. Tracking donor RBC survival in premature infants: agreement of multiple populations of biotin-labeled RBCs with Kidd antigen-mismatched RBCs. Pediatr Res. 2013;74:689–97. doi: 10.1038/pr.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson HA. Life-span of the fetal red blood cell. J Pediatr. 1967;70:166–71. doi: 10.1016/s0022-3476(67)80410-4. [DOI] [PubMed] [Google Scholar]
- 12.Mock DM, Matthews NI, Zhu S, Burmeister LF, Zimmerman MB, Strauss RG, et al. Red blood cell (RBC) volume can be independently determined in vivo in humans using RBCs labeled at different densities of biotin. Transfusion. 2011;51:148–57. doi: 10.1111/j.1537-2995.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nalbant D, Bhandary P, Matthews NI, Schmidt RL, Bogusiewicz A, Cress GA, et al. Comparison of multiple red cell volume methods performed concurrently in premature infants following allogeneic transfusion. Pediatr Res. 2013;74:592–600. doi: 10.1038/pr.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbuckle TE, Wilkins R, Sherman GJ. Birth weight percentiles by gestational age in Canada. Obstet Gynecol. 1993;81:39–48. [PubMed] [Google Scholar]
- 15.Mollison PL. Blood Transfusion in Clinical Medicine. 1. Oxford: Blackwell Scientific Publications; 1951. Life-span of red cells; pp. 108–9. [Google Scholar]
- 16.Ashby W. The determination of the length of life of transfused blood corpuscles in man. J Exp Med. 1919;29:267–81. doi: 10.1084/jem.29.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mollison PL. The survival of transfused erythrocytes in haemolytic disease of the newborn. Arch Dis Child. 1943;18:161–72. doi: 10.1136/adc.18.96.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson HA. Life-span of the fetal red blood cell. J Pediatr. 1967;70:166–71. doi: 10.1016/s0022-3476(67)80410-4. [DOI] [PubMed] [Google Scholar]
- 19.Arndt PA, Garratty G. A critical review of published methods for analysis of red cell antigen-antibody reactions by flow cytometry, and approaches for resolving problems with red cell agglutination. Transfus Med Rev. 2010;24:172–94. doi: 10.1016/j.tmrv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–85. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 21.Bard H, Widness JA. The life span of erythrocytes transfused to preterm infants. Pediatr Res. 1997;42:9–11. doi: 10.1203/00006450-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Egberts J, van Kamp IL, Kanhai HH, Meerman RH, Giordano PC, Gravenhorst JB. The disappearance of fetal and donor red blood cells in alloimmunised pregnancies: a reappraisal. Br J Obstet Gynaecol. 1997;104:818–24. doi: 10.1111/j.1471-0528.1997.tb12026.x. [DOI] [PubMed] [Google Scholar]
- 23.Garratty G, Arndt PA. Applications of flow cytofluorometry to red blood cell immunology. Cytometry. 1999;38:259–67. doi: 10.1002/(sici)1097-0320(19991215)38:6<259::aid-cyto1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Cordle DG, Strauss RG, Lankford G, Mock DM. Antibodies provoked by the transfusion of biotin-labeled red cells. Transfusion. 1999;39:1065–9. doi: 10.1046/j.1537-2995.1999.39101065.x. [DOI] [PubMed] [Google Scholar]
- 25.Mock DM, Widness JA, Strauss RG, Franco RS. Posttransfusion red blood cell (RBC) survival determined using biotin-labeled RBCs has distinct advantages over labeling with (51) Cr. Transfusion. 2012;52:1596–8. doi: 10.1111/j.1537-2995.2012.03588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss RG, Schmidt RL, Mock DM, Nalbant D, Kiosseva S, Cress GA, et al. Transfusion of premature infants with biotinylated red blood cells (BioRBCs) does not elicit antibody response. Transfusion. 2014;54:133A. [Google Scholar]
- 27.Egberts J, Hardeman MR, Luykx LM. Decreased deformability of donor red blood cells after intrauterine transfusion in the human fetus: possible reason for their reduced life span? Transfusion. 2004;44:1231–7. doi: 10.1111/j.1537-2995.2004.04014.x. [DOI] [PubMed] [Google Scholar]