Abstract
Small-molecule mimetics of the β-hairpin flap of HIV-1 protease (HIV-1 PR) were designed based on a 1,4-benzodiazepine scaffold as a strategy to interfere with the flap–flap protein–protein interaction at the “mouth” of the active site. Michaelis-Menten kinetics suggest our small-molecules are competitive inhibitors, which indicates the mode of inhibition is through binding the active site or sterically blocking access to the active site, as designed. More generally, a new bioactive scaffold for HIV-1PR inhibition has been discovered, with the most potent compound inhibiting the protease with a modest Ki of 10 μM.
Keywords: HIV-1 protease, β-hairpin 1, 4-benzodiazepine, protein–protein interaction, proteomimetic
Graphical abstract

1. Introduction
HIV-1 protease (HIV-1 PR) is a retroviral enzyme that is essential for the life-cycle of HIV, and is, therefore, an important target for AIDS therapy.1–3 Indeed, there are now eleven FDA-approved HIV-1PR inhibitors. HIV-1 PR cleaves newly synthesized gag and gag-pol polyproteins at the appropriate places to create mature protein components of an infectious virion that proceeds to further infect normal host cells.1–3 Owing to the high mutation rate within the active site, however, the enzyme becomes resistant to current drugs that target this region and so novel modes of inhibition are urgently required.4,5 A complementary strategy to targeting the active site is to disrupt the protein–protein interaction (PPI) in the “whiskers” region of the dimeric complex that is comprised of four interdigitating β-strands, two from each monomer (the N-terminal residues 1–4 and the C-terminal residues 96–99). This is a particularly appealing target since this PPI accounts for 75% of the dimerization stabilization energy,6 and the mutation rate here is lower than in the active site, suggesting inhibitors targeting this region may be more refractory to resistance.7 However, there is a considerable entropic challenge here since the isolated monomers are not thermodynamically stable6 and so inhibitors designed to recognize the folded β-strands would need to organize its target peptide sequence(s) into β-strand(s). Nevertheless, Schramm, Chimelewksi and others have made some progress in this area with “interfacial peptides”,8–17 although new drugs with this mechanism of action are yet to be identified.
Extensive X-ray crystal structures have revealed that HIV-1 PR is a C2-symmetric homodimer where the enzyme’s active site is “gated” by two glycine-rich, anti-parallel β-hairpin flaps (residues 43–58) that recognize each other predominantly through hydrophobic interactions.18 These flaps “open” and “close” to allow peptide substrates to enter, and products to exit, the active site of HIV-1 PR; more importantly, they are essential for proper placement of the substrate within the active site. Accordingly, interfering with this PPI in some manner might be expected to compromise enzymatic activity. In fact, Král and Konvalinka have demonstrated that metallacarboranes are capable of competitively inhibiting HIV-1 PR through an unconventional binding mode wherein the metallacarboranes bind the flap-proximal region of the active, approximately the S3 and S3′ substrate binding sites, trapping the enzyme in the open conformation.19
It is noteworthy that this binding mode was unprecedented as all reported crystal structures of ligands bound to HIV-1 PR had shown the enzyme in the closed conformation. It has been speculated that the metallacarboranes inhibit HIV-1 PR through preventing flap closure, and hence proper formation of the active site, as this would still deliver competitive kinetics. Klebe and co-workers have also targeted the open-flap conformation of HIV-1 PR with pyrrolidine-based inhibitors.20 Thus, there exists precedent for trapping the enzyme in the (semi-)open, inactive conformation. We herein describe our efforts to disrupt the enzymatic activity of HIV-1 PR through abrogating the PPI between the β-hairpin flaps with rationally designed synthetic flap mimetics.
2. Results and Discussion
As a novel strategy to inhibit HIV-1 PR, we reasoned that the equilibrium between the “semi-open” and “closed” states of the enzyme could be intercepted by small-molecules that mimic one of the β-hairpin flaps found at the flap–flap PPI. Interaction of one β-hairpin flap with a flap surrogate would result in the incomplete formation of the enzyme’s activity site, providing a novel mechanism for the inhibition of HIV-1 PR. As mimetics of β-turns, β-strands and α-helices, 2-amido-2′-carboxamido-diphenylacetylene derivatives exhibit a privileged role in biomimicry, but their syntheses can be lengthy.21–23 In addition, 1,4-benzodiazepines (BZD) have also been utilized to imitate β-turns.24 Given their simpler synthesis, we selected the BZD scaffold to reproduce the epitope of one of the β-hairpin turns located at the dynamic PPI between the two HIV-1PR monomers.
Residues 47–54 of the β-hairpin flap are shown in Figures 2A and 2B. A corresponding BZD proteomimetic of the flap tip is given in Figure 2C. In order to simplify the synthetic chemistry as well as attempt the mimicry of I47 and I54, the BZD was inverted and then elaborated as shown in Figure 2D. [I50] of the mimetic was intended to interact with I47/I54 of the target β-hairpin flap, whilst [I47] and [I54] were designed to recognize I50 of the flap. R1 represents the side chain of the I50 mimetic and may be readily varied by invoking different amino acids in the construction of the BZD ring. To capture additional hydrophobic interactions with the flap, the side-chain of G51 was emulated by a methyl group, instead of a hydrogen atom. We hypothesized that bulky, hydrophobic R2 groups would putatively mimic I47, and tert-butyl esters at the R3 position were designed to mimic I54. We proposed to also include carboxylic acids here to assist in compound solubility. Accordingly, β-hairpin flap mimetics based on the generic structure in Figure 1D were prepared according to Scheme 1.
Figure 2.

A. Cartoon form of the β-hairpin flap (residues 47–54) from a monomer of HIV-1PR; B. The peptide sequence of HIV-1PR47–54; C. A 1,4-benzodiazepine (BZD)-based mimetic of the four residues at the tip of the β-hairpin flap; D. MM2 energy minimized conformation of C (yellow, coloured by atom type) overlaid on β-hairpin flap tip; E. Flipped BZD from C to simplify the synthetic chemistry, and elaboration towards the additional mimicry of I47 and I54.
Figure 1.

The semi-open and closed forms of HIV-1 PR, with the active site indicated, including the catalytic aspartate residues Asp25 and Asp25′), and the β-hairpin flap regions highlighted (residues 43–58 and 43′–58′). Green and cyan represent the two HIV-1 PR monomers.
Scheme 1.

(a) HNO3, H2SO4; (b) CrO3, H2SO4; (c) tBuOH, DCC, DMAP, CH2Cl2; (d) 10% Pd/C, H2, MeOH; (e) 1. isobutyl chloroformate, NMM, THF; 2. FmocHN(R1)CO2H, NMM; (f) HNEt2, CH3CN; (g) TFA, CH2Cl2; (h) R2CH2Br, K2CO3, DMF.
Regioselective nitration of 1 followed by benzylic oxidation with Jones’s reagent afforded ketone 2. Esterification with tert-butanol and N,N-dicyclohexylcarbodiimide (DCC) then delivered tert-butyl ester 3. Attempted reduction of the nitro group in 3 with stannous chloride (SnCl2.2H2O) yielded a mixture of the desired product 4 along with the corresponding 3-methyl-2,1-benzisoxazole (anthranil) that was presumably generated through heterocyclization of the intermediate hydroxylamine with the ketone. We investigated this more fully, and have published our findings elsewhere.25 Complete transformation of 3 into 4 was instead accomplished via catalytic hydrogenation, which may have occurred through direct reduction of the aryl nitro group to the respective aniline and/or reduction of the weak N-O bond within the 2,1-benzisoxazole generated in situ or a combination thereof. Fmoc-protected amino acids (R1 group) were pre-activated with isobutyl chloroformate then coupled to aniline 4 to deliver amides 5, which were not isolated; subsequent base-mediated cyclization delivered the key BZD nucleus, as in compounds 6. Deprotection of the tert-butyl esters in 6 with TFA furnished acids 7. Alternatively, the anilide nitrogen of 6 was alkylated under standard conditions with R2 bromides to yield target compounds 8, which, upon treatment with TFA, provided the additional target compounds 9. We also prepared two congeners of 8 and 9 in which the methyl imine was replaced by a phenyl imine, as shown in Scheme 2, using similar chemistry to that described previously.
Scheme 2.

(a) tBuOH, DCC, DMAP, CH2Cl2; (b) 1. DMF-dimethyl acetal, 5 h, 140 °C; 2. NaIO4; (c) PhMgBr, 0 °C; (d) CrO3, H2SO4; (e) 10% Pd/C, H2, MeOH; (f) 1. isobutyl chloroformate, NMM, CH2Cl2; 2. FmocHN(R1)CO2H, NMM, THF; (g) HNEt2, CH3CN; (h) 4-cyclohexylbenzyl bromide, K2CO3, DMF; (i) TFA, CH2Cl2.
As shown in Table 1, a small batch of 1,4-benzodiazepines lacking the R2 group was initially prepared to gauge if this novel scaffold could elicit inhibition of HIV-1 PR. Protocols measuring HIV-1 PR activities using chromogenic and fluorogenic substrates have been well-established.26–29 The HIV-1 PR activities were measured spectroscopically allowing inhibition constants, Ki, of the newly synthesized compounds to be determined. Although most of the compounds in Table 1 did not inhibit the enzyme, two of the most hydrophobic compounds, tert-butyl esters 6f and 6g, exhibited appreciable inhibitory activity (Ki = 64 μM and 88 μM, respectively), and so a second set of compounds was prepared in which the anilide nitrogen atom was alkylated with 4-cyclohexylbenzyl (R2) bromide to afford the series of compounds illustrated in Table 2 that were now proposed to be capable of mimicking I47. In general, the bulkier and more hydrophobic the I50-mimicking R1 group, the more potent was the inhibitor. Interestingly, the L-and D-leucine derivatives 9c (Ki = 27 μM) and 9d (Ki = 19 μM) were more potent than the L-isoleucine derivative 9e (Ki = 53 μM). Whilst there appeared little impact of the stereogenic centre on the activity of the leucine derivatives 8c–9d, the L-phenylalanine derivatives 8f and 9f were more potent than their D-counterparts. In contrast to most instances in Table 1, the free acids (R3 = H) were more potent than their corresponding tert-butyl esters, the latter of which were designed to approximate to I54. We surmise this finding may be related to compound solubility. In order to gauge if enhancing the hydrophobicity at the tip of the flap mimetic would confer improved inhibition, the 5-methyl R4 group was replaced with a phenyl ring, although this resulted in reduced inhibition (compare 9d with 16), which is consistent with this group required to imitate only a small glycine side chain. To expand our library further, we selected the most potent compound from Table 2, 9d, and replaced the 4-cyclohexylbenzyl group with a variety of alternative hydrophobic groups as possible mimetics of I47; these compounds are shown in Table 3. Once more, acids were more potent than their corresponding tert-butyl esters. The larger, more hydrophobic R2 groups translated into increasingly improved inhibitors of HIV-1 PR. For example, compare 9h (R2 = Bn) with 9i (R2 = 4-tert-butylbenzyl) and 9j (R2 = 4-phenylbenzyl). For the series of alkyl amides, the larger derivatives 9o and 9p, both with Kis of 11 μM, were more potent than the smaller derivative 9q (Ki = 75 μM), which may be due to better mimicry of I47. Others have also observed improved inhibition of HIV-1 PR through the addition of long chain alkyl groups to moderately active inhibitors.16 An MM2 energy-minimized conformation of 9d overlaid with one of the β-hairpin flaps of HIV-1PR is shown in Figure 3, which reveals good mimicry of I47 and I50.
Table 1.
Inhibition of HIV-1 PR by BZDs unfunctionalized at the anilide nitrogen. Ki is the inhibition constant, the concentration of inhibitor that produces half-maximal inhibition.

| |||
|---|---|---|---|
| Compound | R1 | R3 | Ki (μM) |
| 6a |
|
|
> 200 |
| 7a |
|
|
199 ± 16 |
| 6b |
|
|
> 200 |
| 7b |
|
|
> 200 |
| 6c |

|
|
193 ± 32 |
| 7c |

|
|
>200 |
| 6d |

|
|
>200 |
| 7d |

|
|
>200 |
| 6e |
|
|
> 200 |
| 7e |
|
|
158 ± 5 |
| 6f |

|
|
64 ± 9 |
| 7f |

|
|
> 200 |
| 6g |

|
|
88 ± 14 |
| 7g |

|
|
> 200 |
Table 2.
Inhibition of HIV-1 PR by BZDs alkylated at the anilide nitrogen with a 4-cyclohexylbenzyl moiety. Ki is the inhibition constant, the concentration of inhibitor that produces half-maximal inhibition.

| ||||
|---|---|---|---|---|
| Compound | R1 | R3 | R4 | Ki (μM) |
| 8a |
|
|
|
169 ± 27 |
| 9a |
|
|
|
85 ± 24 |
| 8b |
|
|
|
88 ± 6 |
| 9b |
|
|
|
56 ± 9 |
| 8c |

|
|
|
76 ± 7 |
| 9c |

|
|
|
27 ± 4 |
| 8d |

|
|
|
65 ± 12 |
| 9d |

|
|
|
19 ± 4 |
| 15 |

|
|
|
88 ± 5 |
| 16 |

|
|
|
68 ± 13 |
| 8e |
|
|
|
108 ± 12 |
| 9e |
|
|
|
53 ± 7 |
| 8f |

|
|
|
54 ± 7 |
| 9f |

|
|
|
47 ± 12 |
| 8g |

|
|
|
131 ± 8 |
| 9g |

|
|
|
72 ± 11 |
Table 3.
SAR of the anilide R2 group of the BZD compounds. Ki is the inhibition constant, the concentration of inhibitor that produces half-maximal inhibition.

| |||
|---|---|---|---|
| Compound | R2 | R3 | Ki (μM) |
| 8h |

|
|
> 200 |
| 9h |

|
|
> 200 |
| 8i |

|
|
147 ± 19 |
| 9i |

|
|
46 ± 7 |
| 8j |

|
|
62 ± 5 |
| 9j |

|
|
21 ± 2 |
| 8k |

|
|
89 ± 7 |
| 9k |
|
|
> 200 |
| 8l |

|
|
11 9 ± 40 |
| 9l |

|
|
> 200 |
| 8m |

|
|
> 200 |
| 9m |

|
|
> 200 |
| 8n |

|
|
95 ± 9 |
| 9n |

|
|
75 ± 7 |
| 8o |

|
|
> 200 |
| 9o |

|
|
11 ± 3 |
| 8p |

|
|
165 ± 11 |
| 9p |

|
|
11 ± 3 |
| 8q |

|
|
> 200 |
| 9q |

|
|
75 ± 7 |
| 8r |

|
|
143 ± 9 |
| 9r |

|
|
> 200 |
| 8s |

|
|
> 200 |
| 9s |

|
|
81 ± 28 |
| 8t |

|
|
> 200 |
| 9t |

|
|
49 ± 15 |
Figure 3.

An MM2 energy minimized conformation of 9d (yellow, coloured by atom type) overlaid with one of the β-hairpin flaps from HIV-1PR (cyan).
To investigate the mode of inhibition of our BZD-based compounds, 9o was studied further. According to Figure 4, 9o is a competitive inhibitor of HIV-1 PR. While these findings indicate that 9o possibly binds the active site of HIV-1 PR, it does not rule out a potential mechanism by which the small-molecule sterically blocks access to the active site, as originally designed, and as reported elsewhere.19,20 Attempts to acquire a co-crystal structure of 9o with HIV-1 PR failed, possibly due to the modest binding affinity of the compound coupled with its poor solubility. We are presently developing some second-generation compounds that might address these drawbacks.
Figure 4.

Compound 9o is a competitive inhibitor of HIV-1 PR.
To examine if our compounds could inhibit HIV virus replication within cells, we first studied the general cytotoxicity of four of our most potent compounds: 9d, 9j, 9o and 9p. For this purpose, MT-4 cells were utilized, which are a lymphocyte-derived cell line used for HIV tissue culture, particularly for assessing anti-retroviral cytotoxicity. The data is shown in Figure 5. All but 9p were tolerated up to 50 μM, but all proved particularly cytotoxic at 100 μM. We then tested the ability of the compounds to inhibit HIV replication in TZM-bl cells, a HeLa-derived cell line expressing CCR5, CXCR4, and CD4 that contains a luciferase gene under the control of a HIV tat-driven promoter. As shown in Figure 5, which is presented as relative luminescence units (RLUs) of a control (HIV infection without any inhibitor), all compounds appeared to inhibit HIV replication within cells at doses of 50 μM or higher. However, according to Figure 5, the HIV infectivity data for 9j and 9p may be attributed in part, or completely, to the general cytotoxicities exhibited by these compounds. Two of our more promising compounds, 9d and 9o, will be subjected to further structure–activity refinement to enhance the inhibition of HIV-1 PR in vitro towards the development of more potent, and less cytotoxic, inhibitors of HIV replication within cells.
Figure 5.

Cytotoxicities of select HIV-1PR inhibitors to MT-4 cells.
In conclusion, a new series of HIV-1 PR inhibitors was developed based on BZD-inspired small-molecule mimicry of one of the β-hairpin turns found at the flap–flap PPI, which is the gated access to the enzyme’s active site. Our molecules possibly mimic I47, I50 and G51. Although the exact binding mode remains unknown at this time, Michaelis-Menten analysis indicates that our compounds function as competitive inhibitors either through (a) binding in the active site, or (b) sterically blocking access to the active site. Efforts towards the determination of the specific binding mode of these new HIV-1 PR inhibitors is underway, and second-generation inhibitors based on lead compounds 9d and 9o are presently a focus of our laboratory.
3. Experimental
3.1 Chemistry
General
Unless otherwise stated, all reaction were performed under an inert atmosphere (N2). Reagents and solvents were ACS grade, and purchased from Sigma-Aldrich, Alfa Aesar, Oakwood and TCI America. Anhydrous solvents were used as provided from Sigma-Aldrich. Reactions were monitored by thin-layer chromatography (TLC), visualizing with a UV lamp and/or KMnO4 stain. Flash column chromatography was performed with silica gel 60 Å (70–230 mesh, Merck). 1H and 13C NMR spectra were recorded on a Varian INOVA 400 MHz NMR spectrometer at 25 °C. Chemical shifts are reported in parts per million (ppm). Data for 1H NMR are reported thus: chemical shift (δ ppm) (multiplicity, coupling constant (Hz), integration), where multiplicities are: s = singlet, d = doublet, t = triplet, m = multiplet. The residual solvent peak was used as an internal reference: CDCl3 (δH 7.26; δC 77.21) and d6-DMSO (δH 2.50; δC 39.51). Mass spectra were obtained on an Electrospray TOF (ESI-TOF) mass spectrometer (Bruker AmaZon X). All final molecules were deemed to be >90% pure by reversed-phased HPLC using a Waters 1525 analytical/preparative HPLC fitted with a C18 reversed-phase column (Atlantis T3 (T3) or Symmetry (S): 4.6 mm × 150 mm) according to the following conditions with solvents (A) H2O/0.1% TFA, (B) CH3CN–H2O, 9:1 with 0.1% TFA at 1 ml min-1: (I) a gradient of 100% A to 100% B over 22 min (T3); (II) a gradient of 100% A to 100% B over 22 min, then maintained for 13 min at 100% B (T3); (III) a gradient of 100% A to 100% B over 10 min (S); (IV) a gradient of 50% A to 100% B over 22 min (S); (V) an isocratic gradient of 100% B over 22 min (S). HPLC data are presented as retention time (tR (min)), purity (%), condition (I, II, II, IV or V).
4-Acetyl-3-nitrobenzoic acid (2).25
To a round bottom flask equipped with a magnetic stirrer bar was charged with HNO3 (25 mL) and 4-ethylbenzoic acid (5 g, 33.3 mmol). The reaction was cooled to −10 °C and H2SO4 (20 mL) was added and the reaction was allowed to stir at room temperature for 1 h. The reaction was poured over ice and the precipitate filtered to give 4-ethyl-3-nitrobenzoic acid as a cream solid (5.9 g, 92 %): δH (DMSO-d6, 500 MHz) 8.36 (s, 1 H, Ar), 8.15 (d, J = 7.5, 1 H, Ar), 7.67 (d, J = 7.5, 1 H, Ar), 2.89 (q, 2 H, CH2), 1.23 (t, 3 H, CH3). To a round bottom flask equipped with a magnetic stirrer bar was charged with NaIO6 (4.54 g, 20.00 mmol) in MeCN (50 mL). 0.1 M CrO3 in MeCN (5 mL, 0.50 mmol) and 4-ethyl-3-nitrobenzoic acid (1.95 g, 10.0 mmol) were added to the reaction mixture, which was allowed to stir at room temperature overnight. The reaction mixture was decanted, washed with EtOAc and reduced in vacuo. The residue was partitioned between EtOAc (100 mL) and H2O (50 mL), washed with 5% Na2S2O3 (50 mL), saturated aqueous NaCl (50 mL), dried over MgSO4, filtered and reduced in vacuo. The residue was purified by column chromatography (CH2Cl2/MeOH) to provide the title compound as a cream solid (1.56 g): δH (DMSO-d6, 500 MHz) 13.90 (br s, 1 H, CO2H), 8.50 (s, 1 H, Ar), 8.34 (d, J = 8.0, 1 H, Ar), 7.88 (d, J = 8.0, 1 H, Ar), 2.60 (s, 3 H, CH3).
tert-Butyl 4-acetyl-3-nitrobenzoate (3)
To a round bottom flask equipped with a magnetic stirrer bar was charged with 2 (209 mg, 1.00 mmol), tBuOH (115 μL, 1.20 mmol) and DMAP (12 mg, 0.10 mmol) in CH2Cl2 (6 mL). N,N-Dicyclohexylcarbodiimide (DCC; 218 mg, 1.20 mmol) was added, and the reaction mixture was stirred at room temperature for 3 h. The reaction mixture was reduced in vacuo, and the residue was purified by column chromatography (hexane/EtOAc) to provide the title compound as a yellow oil (217 mg, 88%): δH (DMSO-d6, 500 MHz) 8.46 (s, 1 H, Ar), 8.31 (d, J = 8.0, 1 H, Ar), 7.89 (d, J = 8.0, 1 H, Ar), 2.60 (s, 3 H, CH3), 1.59 (s, 9 H, 3 × CH3).
tert-Butyl 4-acetyl-3-aminobenzoate (4)
To a round bottom flask equipped with a magnetic stirrer bar was charged with nitro acetophenone (300 mg, 1.44 mmol) in MeOH (10 mL). Pd/C (60 mg, 20 mol%) was added and the reaction put under H2 gas for 1 h. The reaction mixture was filtered through Celite, reduced in vacuo and the residue was purified by silica gel column chromatography (hexane/EtOAc) to provide the title compound as a yellow solid (150 mg): δH (DMSO-d6, 500 MHz) 7.82 (d, J = 8.0, 1 H, Ar), 7.35 (br s, 3 H, Ar, NH2), 6.99 (d, J = 8.0, 1 H, Ar), 2.53 (s, 3 H, CH3), 1.54 (s, 9 H, 3 × CH3).
General Procedure A: Preparation of 1,4-Benzodiazepines 6
To a round bottom flask equipped with a magnetic stirrer bar was charged with N-Fmoc amino acid 4 (1.50 mmol), N-methylmorpholine (1.60 mmol) and THF (10 mL). The reaction was cooled to 0 °C and isobutyl chloroformate (1.60 mmol) was added dropwise. The reaction was stirred at 0 °C for 30 min, aniline (1.00 mmol) was added in one portion and the reaction refluxed for 20 h. The reaction was cooled, quenched with saturated aqueous NH4Cl (1 mL), partitioned between EtOAc (60 mL) and H2O (30 mL). The aqueous phase was extracted with EtOAc (3 × 10 mL) and the organic portions combined, washed with H2O (10 mL), brine (10 mL), dried over MgSO4, filtered and reduced in vacuo. The residue was dissolved in MeCN (10 mL), Et2NH (2 mL) was added at room temperature and stirred for 3 h. The reaction was reduced in vacuo and purified by silica gel column chromatography (hexane/EtOAc) to provide the title compound.
General Procedure B: Preparation of N-alkylated-1,4-benzodiazepines 8
To a round bottom flask equipped with a magnetic stirrer bar was charged with 1,4-benzodiazepine (0.15 mmol), K2CO3 (0.45 mmol) and DMF (3 mL). The reaction was cooled to 0 °C and alkyl halide (0.30 mmol) in DMF (1 mL) was added. The reaction was stirred at room temperature for 3 h. The reaction was partitioned between EtOAc (30 mL) and H2O (10 mL). The organic phase was washed with H2O (3 × 10 mL), brine (10 mL), dried over MgSO4, filtered and reduced in vacuo. The residue was purified by column chromatography (hexane/EtOAc) to provide the title compound.
General Procedure C: Deprotection of 1,4-Benzodiazepines 6 and 8
To a round bottom flask equipped with a magnetic stirrer bar was charged with 1,4-benzodiazepines 6 or 8 (0.10 mmol) and CH2Cl2 (1 mL). TFA (1 mL) was added at room temperature and stirred for 4 h. The reaction was reduced in vacuo, co-evaporated with chloroform and lyophilized from MeCN/H2O (1:1) to provide the title compound 7 or 9 as its TFA salt.
tert-Butyl 5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (6a)
Prepared according to General Procedure A, to give the title compound as a white solid (246 mg, 90%): mp 186–188 °C; δH (DMSO-d6, 400 MHz) 10.53 (s, 1 H, NH), 7.80 (d, J = 8.4, 1 H, Ar), 7.70 (s, 1 H, Ar), 7.64 (d, J = 8.4, 1 H, Ar), 3.90 (s, 2 H, CH2CO), 2.40 (s, 3H, CH3CN), 1.55 (s, 9 H, 3 × CH3); δC (DMSO-d6, 100 MHz) 170.1, 168.5, 163.9, 137.9, 133.3, 131.3, 129.1, 123.1, 121.7, 81.4, 56.2, 27.7, 25.6; LRMS: m/z C15H18N2O3 requires: 274.1, found: 275.3 [M+H]; tR = 1.59 (98.7%, III).
5-Methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (7a)
Prepared according to General Procedure C, to give the title compound as a cream solid (33 mg, 99 %): mp >250 °C; δH (DMSO-d6, 400 MHz) 11.11 (s, 1 H, NH), 8.00 (d, J = 8.0, 1 H, Ar), 7.82 (d, J = 1.6, 1 H, Ar), 7.78 (dd, J = 8.0, 1.6, 1 H, Ar), 4.13 (s, 2 H, CH2CO), 2.69 (s, 3H, CH3CN); δC (DMSO-d6, 100 MHz) 168.3, 166.0, 138.9, 135.5, 131.1 127.6, 123.8, 122.6, 52.6, 24.4; LRMS: m/z C11H10N2O3 requires: 218.1, found: 219.3 [M+H]; tR = 1.27 (98.1%, III).
(S)-tert-Butyl 3,5-dimethyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (6b)
Prepared according to General Procedure A, to give the title compound as a white solid (267 mg, 93%): mp 80–82 °C; δH (DMSO-d6, 400 MHz) 10.53 (s, 1 H, NH), 7.80 (d, J = 8.4, 1 H, Ar), 7.69 (d, J = 1.2, 1 H, Ar), 7.65 (dd, J = 8.4, 1.2, 1 H, Ar), 3.44 (q, J = 6.4, 1 H, CHCO), 2.38 (s, 3H, CH3CN), 1.55 (s, 9 H, 3 × CH3), 1.54 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 171.0, 166.4, 163.9, 137.5, 133.3, 131.6, 128.8, 123.0, 121.7, 81.4, 57.7, 27.7, 25.4, 16.9; LRMS: m/z C16H20N2O3 requires: 288.1, found: 289.2 [M+H]; tR = 1.57 (90.8%, III).
(S)-3,5-Dimethyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (7b)
Prepared according to General Procedure C, to give the title compound as a cream solid (34 mg, 98 %): mp 58–60 °C; δH (DMSO-d6, 400 MHz) 11.25 (s, 1 H, NH), 8.05 (d, J = 8.0, 1 H, Ar), 7.84 (d, J = 1.2, 1 H, Ar), 7.81 (dd, J = 8.0, 1.2, 1 H, Ar), 3.96 (q, J = 6.4, 1 H, CHCO), 2.74 (s, 3H, CH3CN), 1.50 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.4, 166.3, 139.3, 136.4, 131.6, 124.2, 123.1, 56.0, 24.3, 14.1; LRMS: m/z C12H12N2O3 requires: 232.1, found: 233.2 [M+H]; tR = 1.25 (97.6%, III).
(S)-tert-Butyl 3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (6c)
Prepared according to General Procedure A, to give the title compound as a cream solid (280 mg, 85%): mp 84–86 °C; δH (DMSO-d6, 400 MHz) 10.55 (s, 1 H, NH), 7.83 (d, J = 8.4, 1 H, Ar), 7.70 (s, 1 H, Ar), 7.65 (d, J = 8.4, 1 H, Ar), 3.32–3.25 (m, 1 H, CHCO), 2.83 (s, 3H, CH3CN), 1.88–1.81 (m, 1 H, CHMe2), 1.78–1.68 (m, 2 H, CH2iPr), 1.55 (s, 9 H, 3 × CH3), 0.85 (d, J = 5.6, 3 H, CH3), 0.71 (d, J = 5.6, 3 H, CH3); δC (DMSO-d6, 100 MHz) 170.2, 166.7, 163.9, 137.5, 133.3, 131.5, 128.8, 123.1, 121.7, 81.4, 60.5, 27.7, 25.4, 23.9, 23.2, 21.8; LRMS: m/z C19H26N2O3 requires: 330.2, found: 331.3 [M+H]; tR = 5.61 (97.0%, I).
(S)-3-Isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (7c)
Prepared according to General Procedure C, to give the title compound as a cream solid (38 mg, 99 %): mp 113–115 °C; δH (DMSO-d6, 400 MHz) 11.09 (s, 1 H, NH), 7.99 (d, J = 8.4, 1 H, Ar), 7.81 (d, J = 1.2, 1 H, Ar), 7.78 (dd, J = 8.4, 1.2, 1 H, Ar), 3.66 (t, J = 6.4, 1 H, CHCO), 2.66 (s, 3H, CH3CN), 1.92–1.71 (m, 3 H, CHMe2, CH2iPr), 0.89 (d, J = 6.0, 3 H, CH3), 0.77 (d, J = 6.0, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.2, 166.5, 138.9, 135.6, 131.1, 128.7, 124.1, 122.9, 114.8, 59.2, 37.6, 24.9, 24.3, 23.4, 22.1; LRMS: m/z C15H18N2O3 requires: 274.1, found: 275.2 [M+H]; tR = 3.63 (92.6%, I).
(R)-tert-Butyl 3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (6d)
Prepared according to General Procedure A, to give the title compound as a yellow solid (273 mg, 83%): mp 85–87 °C; δH (DMSO-d6, 400 MHz) 10.55 (s, 1 H, NH), 7.83 (d, J = 8.4, 1 H, Ar), 7.70 (s, 1 H, Ar), 7.65 (d, J = 8.4, 1 H, Ar), 3.32–3.25 (m, 1 H, CHCO), 2.83 (s, 3 H, CH3CN), 1.88–1.81 (m, 1 H, CHMe2), 1.78–1.68 (m, 2 H, CH2iPr), 1.55 (s, 9 H, 3 × CH3), 0.85 (d, J = 5.6, 3 H, CH3), 0.71 (d, J = 5.6, 3 H, CH3); δC (DMSO-d6, 100 MHz) 170.2, 166.7, 163.9, 137.5, 133.3, 131.5, 128.8, 123.1, 121.7, 81.4, 60.5, 27.7, 25.4, 23.9, 23.2, 21.8; LRMS: m/z C19H26N2O3 requires: 330.2, found: 331.2 [M+H]; tR = 4.48 (99.1%, I).
(R)-3-Isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (7d)
Prepared according to General Procedure C, to give the title compound as a yellow solid (39 mg, 99%): mp 121–123 °C; δH (DMSO-d6, 400 MHz) 11.09 (s, 1 H, NH), 7.99 (d, J = 8.4, 1 H, Ar), 7.81 (d, J = 1.2, 1 H, Ar), 7.78 (dd, J = 8.4, 1.2, 1 H, Ar), 3.66 (t, J = 6.4, 1 H, CHCO), 2.66 (s, 3 H, CH3CN), 1.92–1.71 (m, 3 H, CHMe2, CH2iPr), 0.89 (d, J = 6.0, 3 H, CH3), 0.77 (d, J = 6.0, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.2, 166.5, 138.9, 135.6, 131.1, 128.7, 124.1, 122.9, 114.8, 59.2, 37.6, 24.9, 24.3, 23.4, 22.1; LRMS: m/z C15H18N2O3 requires: 274.1, found: 275.2 [M+H]; tR = 3.68 (92.1%, I).
(S)-tert-Butyl 3-((R)-sec-butyl)-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (6e)
Prepared according to General Procedure A, to give the title compound as a white solid (277 mg, 84 %): mp 81–83 °C; δH (DMSO-d6, 400 MHz) 10.51 (s, 1 H, NH), 7.82 (d, J = 8.4, 1 H, Ar), 7.69 (s, 1 H, Ar), 7.65 (d, J = 8.0, 1 H, Ar), 2.89 (d, J = 9.2, 1 H, CHCO), 2.39 (s, 3H, CH3CN), 2.18–2.12 (m, 1 H, CH), 1.79–1.73 (m, 1 H, CH), 1.55 (s, 9 H, 3 × CH3), 1.09–1.02 (m, 1 H, CH), 0.88 (d, J = 6.5, 3 H, CHCH3), 0.82 (t, J = 7.6, 3 H, CH2CH3); δC (DMSO-d6, 100 MHz) 168.9, 166.3, 163.9, 137.4, 133.3, 131.5, 128.8, 123.1, 121.7, 81.4, 67.2, 34.4, 27.7, 25.4, 24.4, 15.8, 10.8; LRMS: m/z C19H26N2O3 requires: 330.2, found: 331.2 [M+H]; tR = 3.98 (91.2%, III).
(S)-3-((R)-sec-Butyl)-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (7e)
Prepared according to General Procedure C, to give the title compound as a cream solid (38 mg, 99%): mp 73–75 °C; δH (DMSO-d6, 400 MHz) 11.0 (s, 1 H, NH), 7.96 (d, J = 8.0, 1 H, Ar), 7.78–7.75 (m, 2 H, Ar), 3.40–3.23 (m, 1 H, CHCO), 2.61 (s, 3H, CH3CN), 2.22–2.12 (m, 1 H, CH), 1.80–1.66 (m, 1 H, CH), 1.14–1.06 (m, 1 H, CH), 0.94 (d, J = 6.8, 3 H, CHCH3), 0.84 (t, J = 7.6, 3 H, CH2CH3); δC (DMSO-d6, 100 MHz) 168.0, 166.5, 138.6, 130.6, 124.1, 122.7, 114.5, 66.0, 33.7, 25.1, 24.8, 16.0, 11.1; LRMS: m/z C15H18N2O3 requires: 274.1, found: 275.2 [M+H]; tR = 3.70 (92.2%, I).
(S)-tert-Butyl 3-benzyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (6f)
Prepared according to General Procedure A, to give the title compound as a white solid (316 mg, 87%): mp 85–87 °C; δH (DMSO-d6, 400 MHz) 10.59 (s, 1 H, NH), 7.78 (d, J = 8.0, 1 H, Ar), 7.68 (s, 1 H, Ar), 7.63 (d, J = 8.0, 1 H, Ar), 7.24–7.18 (m, 4 H, Ar), 7.14–7.10 (m, 1 H, Ar), 3.54 (t, J = 6.0, 1 H, CHCO), 3.36 (dd, J = 13.6, 6.0, 1 H, CHaHbAr), 3.16 (dd, J = 13.6, 6.0, 1 H, CHaHbAr), 2.37 (s, 3H, CH3CN), 1.53 (s, 9 H, 3 × CH3); δC (DMSO-d6, 100 MHz) 169.6, 166.8, 163.9, 139.2, 137.3, 133.4, 131.6, 129.4, 128.9, 128.0, 125.9, 123.1, 121.7, 81.4, 64.3, 36.9, 27.7, 25.4; LRMS: m/z C22H24N2O3 requires: 364.2, found: 365.2 [M+H]; tR = 5.38 (100%, III).
(S)-3-Benzyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (7f)
Prepared according to General Procedure C, to give the title compound as a cream solid (41 mg, 99 %): mp 78–80 °C; δH (DMSO-d6, 400 MHz) 11.00 (s, 1 H, NH), 7.91 (d, J = 8.4, 1 H, Ar), 7.76 (d, J = 1.6, 1 H, Ar), 7.74 (dd, J = 8.0, 1.6, 1 H, Ar), 7.29–7.21 (m, 4 H, Ar), 7.18–7.13 (m, 1 H, Ar), 3.85 (t, J = 6.4, 1 H, CHCO), 3.40 (dd, J = 14.0, 6.4, 1 H, CHaHbAr), 3.22 (dd, J = 14.0, 6.4, 1 H, CHaHbAr), 2.56 (s, 3H, CH3CN); δC (DMSO-d6, 100 MHz) 169.0, 166.5, 138.5, 138.4, 135.0, 130.6, 129.9, 128.6, 126.7,124.1, 122.7, 63.1, 35.6, 25.2; LRMS: m/z C18H16N2O3 requires: 308.1, found: 309.2 [M+H]; tR = 4.61 (95.5%, I).
(R)-tert-Butyl 3-benzyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (6g)
Prepared according to General Procedure A, to give the title compound as a white solid (291 mg, 80%): mp 90–92 °C; δH (DMSO-d6, 400 MHz) 10.59 (s, 1 H, NH), 7.78 (d, J = 8.0, 1 H, Ar), 7.68 (s, 1 H, Ar), 7.63 (d, J = 8.0, 1 H, Ar), 7.24–7.18 (m, 4 H, Ar), 7.14–7.10 (m, 1 H, Ar), 3.54 (t, J = 6.0, 1 H, CHCO), 3.36 (dd, J = 13.6, 6.0, 1 H, CHaHbAr), 3.16 (dd, J = 13.6, 6.0, 1 H, CHaHbAr), 2.37 (s, 3H, CH3CN), 1.53 (s, 9 H, 3 × CH3); δC (DMSO-d6, 100 MHz) 169.6, 166.8, 163.9, 139.2, 137.3, 133.4, 131.6, 129.4, 128.9, 128.0, 125.9, 123.1, 121.7, 81.4, 64.3, 36.9, 27.7, 25.4; LRMS: m/z C22H24N2O3 requires: 364.2, found: 365.2 [M+H]; tR = 3.53 (96.1%, III).
(R)-3-benzyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (7g)
Prepared according to General Procedure C, to give the title compound as a cream solid (41 mg, 88%): mp 83–85 °C; δH (DMSO-d6, 400 MHz) 11.00 (s, 1 H, NH), 7.91 (d, J = 8.4, 1 H, Ar), 7.76 (d, J = 1.6, 1 H, Ar), 7.74 (dd, J = 8.0, 1.6, 1 H, Ar), 7.29–7.21 (m, 4 H, Ar), 7.18–7.13 (m, 1 H, Ar), 3.85 (t, J = 6.4, 1 H, CHCO), 3.40 (dd, J = 14.0, 6.4, 1 H, CHaHbAr), 3.22 (dd, J = 14.0, 6.4, 1 H, CHaHbAr), 2.56 (s, 3H, CH3CN); δC (DMSO-d6, 100 MHz) 169.0, 166.5, 138.5, 138.4, 135.0, 130.6, 129.9, 128.6, 126.7,124.1, 122.7, 63.1, 35.6, 25.2; LRMS: m/z C18H16N2O3 requires: 308.1, found: 309.2 [M+H]; tR = 3.91 (90.0%, I).
tert-Butyl 1-(4-cyclohexylbenzyl)-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8a)
Prepared according to General Procedure B, to give the title compound as a white solid (52 mg, 78%): mp 171–173 °C; δH (DMSO-d6, 400 MHz) 7.85 (s, 1 H, Ar), 7.75 (d, J = 8.4, 1 H, Ar), 7.68 (d, J = 8.4, 1 H, Ar), 7.12 (d, J = 8.0, 2 H, Ar), 7.00 (d, J = 8.0, 2 H, Ar), 5.05 (d, J = 16.0, 1 H, CHaHbAr), 4.98 (d, J = 16.0, 1 H, CHaHbAr), 4.40 (d, J = 11.2, 1 H, CHaHbCO), 3.69 (d, J = 11.2, 1 H, CHaHbCO), 2.46–2.40 (m, 1 H, Cy), 2.38 (s, 3H, CH3CN), 1.77–1.66 (m, 5 H, Cy), 1.46 (s, 9 H, 3 × CH3), 1.39–1.17 (m, 5 H, Cy); δC (DMSO-d6, 100 MHz) 168.6, 168.2, 163.6, 146.3, 140.8, 134.5, 133.8, 133.4, 128.1, 126.7, 126.4, 124.8, 122.6, 81.3, 79.2, 78.9, 78.6, 55.9, 49.6, 43.3, 33.9, 33.8, 27.6, 26.3, 25.5, 24.9; LRMS: m/z C28H34N2O3 requires: 446.3, found: 447.2 [M+H]; tR = 27.74 (100%, II).
1-(4-Cyclohexylbenzyl)-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9a)
Prepared according to General Procedure C, to gie the title compound as a cream solid (49 mg, 98%): mp 103–105 °C; δH (DMSO-d6, 400 MHz) 7.97 (s, 1 H, Ar), 7.88 (d, J = 8.0, 1 H, Ar), 7.80 (d, J = 8.0, 1 H, Ar), 7.12 (d, J = 8.0, 2 H, Ar), 6.99 (d, J = 8.0, 2 H, Ar), 5.14 (d, J = 16.0, 1 H, CHaHbAr), 4.98 (d, J = 16.0, 1 H, CHaHbAr), 4.42 (d, J = 11.2, 1 H, CHaHbCO), 3.90 (d, J = 11.2, 1 H, CHaHbCO), 2.52 (s, 3H, CH3CN), 2.46–2.38 (m, 1 H, Cy), 1.76–1.65 (m, 5 H, Cy), 1.38–1.16 (m, 5 H, Cy); δC (DMSO-d6, 100 MHz) 167.3, 165.9, 146.4, 141.1, 134.2, 134.1, 129.1, 126.7, 126.4, 125.3, 123.3, 54.1, 49.8, 33.8, 26.3, 25.5, 24.4; LRMS: m/z C24H26N2O3 requires: 390.2, found: 391.2 [M+H]; tR = 4.66 (100%, I).
(S)-tert-Butyl 1-(4-cyclohexylbenzyl)-3,5-dimethyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8b)
Prepared according to General Procedure B, to give the title compound as a white solid (55 mg, 81%): mp 66–68 °C; δH (DMSO-d6, 400 MHz) 7.88 (s, 1 H, Ar), 7.78 (d, J = 8.0, 1 H, Ar), 7.70 (d, J = 8.0, 1 H, Ar), 7.12 (d, J = 7.6, 2 H, Ar), 6.97 (d, J = 7.6, 2 H, Ar), 5.04 (s, 2 H, CH2Ar), 3.65 (q, J = 6.4, 1 H, CHCO), 2.46–2.37 (m, 1 H, Cy), 2.35 (s, 3H, CH3CN), 1.77–1.65 (m, 5 H, Cy), 1.48–1.40 (m, 12 H, 4 × CH3), 1.39–1.17 (m, 5 H, Cy); δC (DMSO-d6, 100 MHz) 169.7, 166.4, 163.7, 146.3, 140.5, 134.7, 134.3, 133.4, 127.9, 126.7, 126.5, 124.7, 122.8; LRMS: m/z C29H36N2O3 requires: 460.3, found: 461.3 [M+H]; tR = 5.97 (96.3%, VI).
(S)-1-(4-cyclohexylbenzyl)-3,5-dimethyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9b)
Prepared according to General Procedure C, to give the title compound as a cream solid (51 mg, 99%): mp 70–72 °C; δH (DMSO-d6, 400 MHz) 8.00 (s, 1 H, Ar), 7.89 (d, J = 8.0, 1 H, Ar), 7.81 (d, J = 8.0, 1 H, Ar), 7.11 (d, J = 8.0, 2 H, Ar), 6.95 (d, J = 8.0, 2 H, Ar), 5.20 (d, J = 16.0, 1 H, CHaHbAr), 4.99 (d, J = 16.0, 1 H, CHaHbAr), 3.89 (q, J = 6.8, 1 H, CHCO), 2.49 (s, 3H, CH3CN), 2.46–2.38 (m, 1 H, Cy), 1.75–1.65 (m, 5 H, Cy), 1.48 (d, J = 6.8, 3 H, CH3), 1.35–1.16 (m, 5 H, Cy); δC (DMSO-d6, 100 MHz) 168.7, 166.0, 146.4, 140.8, 134.4, 134.2, 129.0, 126.7, 126.5, 125.3, 123.6, 56.3, 50.1, 33.9, 33.8, 26.2, 25.5, 24.0, 15.7; LRMS: m/z C25H28N2O3 requires: 404.2, found: 405.2 [M+H]; tR = 4.59 (90.2%, V).
(S)-tert-Butyl 1-(4-cyclohexylbenzyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8c)
Prepared according to General Procedure B, to give the title compound as a white solid (63 mg, 84%): mp 77–79 °C; δH (DMSO-d6, 400 MHz) 7.90 (s, 1 H, Ar), 7.79 (d, J = 8.4, 1 H, Ar), 7.71 (d, J = 8.4, 1 H, Ar), 7.12 (d, J = 8.0, 2 H, Ar), 6.96 (d, J = 8.0, 2 H, Ar), 5.04 (s, 2 H, CH2Ar), 3.49 (t, J = 6.8, 1 H, CHCO), 2.47–2.38 (m, 1 H, Cy), 2.35 (s, 3H, CH3CN), 2.00–1.62 (m, 8 H, CHMe2, CH2iPr, Cy), 1.47 (s, 9 H, 3 × CH3), 1.41–1.10 (m, 5 H, Cy), 0.86 (d, J = 6.4, 3 H, CH3), 0.72 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.9, 166.8, 163.7, 146.4, 140.5, 134.7, 134.2, 133.5, 127.9, 126.8, 126.5, 124.9, 122.8, 81.4, 60.4, 49.8, 43.3, 33.9, 33.8, 27.6, 26.3, 25.5, 24.8, 24.0, 23.2, 21.9; LRMS: m/z C32H42N2O3 requires: 502.3, found: 503.3 [M+H]; tR = 29.28 (90.5%, II).
(S)-1-(4-cyclohexylbenzyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9c)
Prepared according to the general procedure B, to give the title compound as a cream solid (55 mg, 99%): mp 63–65 °C; δH (DMSO-d6, 400 MHz) 8.01 (d, J = 1.2, 1 H, Ar), 7.86 (d, J = 8.0, 1 H, Ar), 7.80 (dd, J = 8.4, 1.2, 1 H, Ar), 7.10 (d, J = 8.4, 2 H, Ar), 6.93 (d, J = 8.4, 2 H, Ar), 5.21 (d, J = 16.0, 1 H, CHaHbAr), 4.98 (d, J = 16.0, 1 H, CHaHbAr), 3.65 (t, J = 7.2, 1 H, CHCO), 2.45 (s, 3H, CH3CN), 2.44–2.37 (m, 1 H, Cy), 2.01–1.62 (m, 8 H, CHMe2, CH2iPr, Cy), 1.38–1.11 (m, 5 H, Cy), 0.87 (d, J = 6.4, 3 H, CH3), 0.74 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.2, 166.1, 146.4, 140.7, 134.3, 134.1, 133.1, 128.6, 126.7, 126.5, 125.7, 123.7, 59.6, 50.0, 43.3, 33.9, 33.8, 26.3, 25.5, 24.4, 23.9, 23.0, 21.8; LRMS: m/z C28H34N2O3 requires: 446.3, found: 447.3 [M+H]; tR = 4.72 (90.2%, V).
(R)-tert-Butyl 1-(4-cyclohexylbenzyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8d)
Prepared according to General Procedure B, to give the title compound as a white solid (64 mg, 85%): mp 75–77 °C; δH (DMSO-d6, 400 MHz) 7.90 (s, 1 H, Ar), 7.79 (d, J = 8.4, 1 H, Ar), 7.71 (d, J = 8.4, 1 H, Ar), 7.12 (d, J = 8.0, 2 H, Ar), 6.96 (d, J = 8.0, 2 H, Ar), 5.04 (s, 2 H, CH2Ar), 3.49 (t, J = 6.8, 1 H, CHCO), 2.47–2.38 (m, 1 H, Cy), 2.35 (s, 3 H, CH3CN), 2.00–1.62 (m, 8 H, CHMe2, CH2iPr, Cy), 1.47 (s, 9 H, 3 × CH3), 1.41–1.10 (m, 5 H, Cy), 0.86 (d, J = 6.4, 3 H, CH3), 0.72 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.9, 166.8, 163.7, 146.4, 140.5, 134.7, 134.2, 133.5, 127.9, 126.8, 126.5, 124.9, 122.8, 81.4, 60.4, 49.8, 43.3, 33.9, 33.8, 27.6, 26.3, 25.5, 24.8, 24.0, 23.2, 21.9; LRMS: m/z C32H42N2O3 requires: 502.3, found: 503.3 [M+H]; tR = 28.86 (96.5%, II).
(R)-1-(4-Cyclohexylbenzyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9d)
Prepared according to General Procedure C, to give the title compound as a yellow solid (55 mg, 99%): mp 87–89 °C; δH (DMSO-d6, 400 MHz) 8.01 (d, J = 1.2, 1 H, Ar), 7.86 (d, J = 8.0, 1 H, Ar), 7.80 (dd, J = 8.4, 1.2, 1 H, Ar), 7.10 (d, J = 8.4, 2 H, Ar), 6.93 (d, J = 8.4, 2 H, Ar), 5.21 (d, J = 16.0, 1 H, CHaHbAr), 4.98 (d, J = 16.0, 1 H, CHaHbAr), 3.65 (t, J = 7.2, 1 H, CHCO), 2.45 (s, 3 H, CH3CN), 2.44–2.37 (m, 1 H, Cy), 2.01–1.62 (m, 8 H, CHMe2, CH2iPr, Cy), 1.38–1.11 (m, 5 H, Cy), 0.87 (d, J = 6.4, 3 H, CH3), 0.74 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.2, 166.1, 146.4, 140.7, 134.3, 134.1, 133.1, 128.6, 126.7, 126.5, 125.7, 123.7, 59.6, 50.0, 43.3, 33.9, 33.8, 26.3, 25.5, 24.4, 23.9, 23.0, 21.8; LRMS: m/z C28H34N2O3 requires: 446.3, found: 447.3 [M+H]; tR = 6.57 (100%, I).
(S)-tert-Butyl 3-((R)-sec-butyl)-1-(4-cyclohexylbenzyl)-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8e)
Prepared according to General Procedure B, to give the title compound as a white solid (70 mg, 93%): mp 75–77 °C; δH (DMSO-d6, 400 MHz) 7.89 (s, 1 H, Ar), 7.79 (d, J = 8.0, 1 H, Ar), 7.70 (d, J = 8.0, 1 H, Ar), 7.12 (d, J = 7.6, 2 H, Ar), 6.95 (d, J = 7.6, 2 H, Ar), 5.07 (d, J = 16.0, 1 H, CHaHbAr), 5.00 (d, J = 16.0, 1 H, CHaHbAr), 3.07 (d, J = 9.6, 1 H, CHCO), 2.46–2.37 (m, 1 H, Cy), 2.34 (s, 3H, CH3CN), 2.31–2.65 (m, 1 H, CH), 1.81–1.62 (m, 6 H, CH, Cy), 1.46 (s, 9 H, 3 × CH3), 1.38–1.13 (m, 5 H, Cy), 1.04–0.96 (m, 1 H, CH), 0.89 (d, J = 6.4, 3 H, CHCH3), 0.81 (t, J = 7.6, 3 H, CH2CH3); δC (DMSO-d6, 100 MHz) 167.8, 166.4, 163.6, 146.4, 140.5, 134.7, 134.0, 133.4, 127.8, 126.8, 126.4, 124.8, 122.7, 81.4, 67.3, 49.7, 43.3, 34.6, 33.9, 33.8, 27.6, 26.3, 25.5, 24.8, 24.3, 15.6, 10.7; LRMS: m/z C32H42N2O3 requires: 502.3, found: 503.3 [M+H]; tR = 25.48 (95.6%, II).
(S)-3-((R)-sec-Butyl)-1-(4-cyclohexylbenzyl)-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9e)
Prepared according to General Procedure C, to give the title compound as a yellow solid (55 mg, 98%): mp 94–96 °C; δH (DMSO-d6, 400 MHz) 7.99 (s, 1 H, Ar), 7.83 (d, J = 8.4, 1 H, Ar), 7.78 (d, J = 8.4, 1 H, Ar), 7.10 (d, J = 8.4, 2 H, Ar), 6.90 (d, J = 8.4, 2 H, Ar), 5.20 (d, J = 16.0, 1 H, CHaHbAr), 4.97 (d, J = 16.0, 1 H, CHaHbAr), 3.21 (d, J = 10.0, 1 H, CHCO), 2.59–2.54 (m, 1 H, Cy), 2.40 (s, 3H, CH3CN), 2.29–2.25 (m, 1 H, CH), 1.82–1.62 (m, 6 H, CH, Cy), 1.43–1.15 (m, 5 H, Cy), 1.07–0.95 (m, 1 H, CH), 0.90 (d, J = 6.4, 3 H, CHCH3), 0.82 (t, J = 7.2, 3 H, CH2CH3); δC (DMSO-d6, 100 MHz) 167.2,166.1, 146.4, 140.5, 134.3, 133.7, 133.5, 128.3, 126.7, 126.4, 125.3, 123.4, 66.6, 49.8, 34.2, 33.9, 26.3, 25.5, 24.4, 24.3, 15.5, 10.6; LRMS: m/z C28H34N2O3 requires: 446.3, found: 447.2 [M+H]; tR = 20.77 (100%, I).
(S)-tert-Butyl 3-benzyl-1-(4-cyclohexylbenzyl)-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8f)
Prepared according to General Procedure B, to give the title compound as a white solid (62 mg, 78%): mp 81–83 °C; δH (DMSO-d6, 400 MHz) 7.85 (s, 1 H, Ar), 7.75 (d, J = 8.4, 1 H, Ar), 7.67 (d, J = 8.4, 1 H, Ar), 7.25–7.19 (m, 4 H, Ar), 7.16–7.13 (m, 1 H, Ar), 7.08 (d, J = 7.6, 2 H, Ar), 6.90 (d, J = 7.6, 2 H, Ar), 5.03 (s, 2 H, CH2Ar), 3.74 (t, J = 6.8, 1 H, CHCO), 3.43 (dd, J = 13.2, 6.8, 1 H, CHaHbAr), 3.22 (dd, J = 13.2, 6.8, 1 H, CHaHbAr), 2.47–2.36 (m, 1 H, Cy), 2.33 (s, 3H, CH3CN), 1.81–1.61 (m, 5 H, Cy), 1.45 (s, 9 H, 3 × CH3), 1.34–1.16 (m, 5 H, Cy); δC (DMSO-d6, 100 MHz) 168.3, 166.9, 163.6, 146.4, 140.3, 138.9, 134.5, 134.1, 133.5, 129.5, 128.0, 127.9, 126.7, 126.5, 126.0, 124.8, 122.7, 81.4, 64.2, 49.8, 43.3, 37.2, 33.9, 33.8, 27.6, 26.2, 25.5, 24.8. LRMS: m/z C35H40N2O3 requires: 536.3, found: 537.3 [M+H]; tR = 27.86 (95.8%, II).
(S)-3-Benzyl-1-(4-cyclohexylbenzyl)-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9f)
Prepared according to General Procedure C, to give the title compound as a cream solid (58 mg, 98%): mp 94–96 °C; δH (DMSO-d6, 400 MHz) 7.97 (s, 1 H, Ar), 7.78 (d, J = 8.4, 1 H, Ar), 7.74 (d, J = 8.4, 1 H, Ar), 7.25–7.19 (m, 4 H, Ar), 7.17–7.13 (m, 1 H, Ar), 7.07 (d, J = 8.0, 2 H, Ar), 6.87 (d, J = 8.0, 2 H, Ar), 5.24 (d, J = 16.0, 1 H, CHaHbAr), 4.94 (d, J = 16.0, 1 H, CHaHbAr), 3.83 (t, J = 6.4, 1 H, CHCO), 3.42 (dd, J = 14.0, 6.4, 1 H, CHaHbAr), 3.24 (dd, J = 14.0, 6.4, 1 H, CHaHbAr), 2.44–2.36 (m, 1 H, Cy), 2.37 (s, 3H, CH3CN), 1.76–1.63 (m, 5 H, Cy), 1.37–1.15 (m, 5 H, Cy); δC (DMSO-d6, 100 MHz) 168.0, 166.0, 146.4, 140.2, 138.5, 134.2, 133.8, 133.6, 129.4, 128.2, 128.0, 126.7, 126.5, 126.0,125.3, 123.4, 63.6, 49.7, 43.2, 36.6, 33.9, 33.8, 26.2, 25.5, 24.6; LRMS: m/z C31H32N2O3 requires: 480.2, found: 481.2 [M+H]; tR = 23.88 (99.0%, II).
(R)-tert-Butyl 3-benzyl-1-(4-cyclohexylbenzyl)-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8g)
Prepared according to General Procedure B, to give the title compound as a white solid (73 mg, 91%): mp 78–80 °C; δH (DMSO-d6, 400 MHz) 7.85 (s, 1 H, Ar), 7.75 (d, J = 8.4, 1 H, Ar), 7.67 (d, J = 8.4, 1 H, Ar), 7.25–7.19 (m, 4 H, Ar), 7.16–7.13 (m, 1 H, Ar), 7.08 (d, J = 7.6, 2 H, Ar), 6.90 (d, J = 7.6, 2 H, Ar), 5.03 (s, 2 H, CH2Ar), 3.74 (t, J = 6.8, 1 H, CHCO), 3.43 (dd, J = 13.2, 6.8, 1 H, CHaHbAr), 3.22 (dd, J = 13.2, 6.8, 1 H, CHaHbAr), 2.47–2.36 (m, 1 H, Cy), 2.33 (s, 3H, CH3CN), 1.81–1.61 (m, 5 H, Cy), 1.45 (s, 9 H, 3 × CH3), 1.34–1.16 (m, 5 H, Cy); δC (DMSO-d6, 100 MHz) 168.3, 166.9, 163.6, 146.4, 140.3, 138.9, 134.5, 134.1, 133.5, 129.5, 128.0, 127.9, 126.7, 126.5, 126.0, 124.8, 122.7, 81.4, 64.2, 49.8, 43.3, 37.2, 33.9, 33.8, 27.6, 26.2, 25.5, 24.8; LRMS: m/z C35H40N2O3 requires: 536.3, found: 537.3 [M+H]; tR = 27.61 (95.6%, II).
(R)-3-Benzyl-1-(4-cyclohexylbenzyl)-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9g)
Prepared according to General Procedure C, to give the title compound as a cream solid (58 mg, 98%): mp 97–99 °C; δH (DMSO-d6, 400 MHz) 7.97 (s, 1 H, Ar), 7.78 (d, J = 8.4, 1 H, Ar), 7.74 (d, J = 8.4, 1 H, Ar), 7.25–7.19 (m, 4 H, Ar), 7.17–7.13 (m, 1 H, Ar), 7.07 (d, J = 8.0, 2 H, Ar), 6.87 (d, J = 8.0, 2 H, Ar), 5.24 (d, J = 16.0, 1 H, CHaHbAr), 4.94 (d, J = 16.0, 1 H, CHaHbAr), 3.83 (t, J = 6.4, 1 H, CHCO), 3.42 (dd, J = 14.0, 6.4, 1 H, CHaHbAr), 3.24 (dd, J = 14.0, 6.4, 1 H, CHaHbAr), 2.44–2.36 (m, 1 H, Cy), 2.37 (s, 3H, CH3CN), 1.76–1.63 (m, 5 H, Cy), 1.37–1.15 (m, 5 H, Cy); δC (DMSO-d6, 100 MHz) 168.0, 166.0, 146.4, 140.2, 138.5, 134.2, 133.8, 133.6, 129.4, 128.2, 128.0, 126.7, 126.5, 126.0,125.3, 123.4, 63.6, 49.7, 43.2, 36.6, 33.9, 33.8, 26.2, 25.5, 24.6; LRMS: m/z C31H32N2O3 requires: 480.2, found: 481.2 [M+H]; tR = 23.84 (100%, II).
(R)-tert-Butyl 1-benzyl-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8h)
Prepared according to General Procedure B, to give the title compound as a yellow solid (55 mg, 88%): mp 94–96 °C; δH (DMSO-d6, 400 MHz) 7.90 (d, J = 1.6, 1 H, Ar), 7.79 (d, J = 8.4, 1 H, Ar), 7.70 (dd, J = 8.4, 1.6, 1 H, Ar), 7.30 (t, J = 7.2, 2 H, Ar), 7.20 (t, J = 7.2, 1 H, Ar), 7.04 (d, J = 7.2, 2 H, Ar), 5.15 (d, J = 16.0, 1 H, CHaHbAr), 5.06 (d, J = 16.0, 1 H, CHaHbAr), 3.66 (t, J = 5.6, 1 H, CHCO), 2.36 (s, 3 H, CH3CN), 1.99–1.91 (m, 1 H, CHMe2), 1.86–1.68 (m, 2 H, CH2iPr), 1.49 (s, 9 H, 3 × CH3), 0.87 (d, J = 6.4, 3 H, CH3), 0.72 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.1, 166.8, 163.7, 140.3, 137.3, 134.3, 133.5, 128.5, 127.9, 127.1, 126.6, 124.9, 122.8, 81.5, 60.4, 49.8, 27.6, 24.8, 24.0, 23.2, 21.9; LRMS: m/z C26H32N2O3 requires: 420.2, found: 421.2 [M+H]; tR = 23.20 (97.6%, II).
(R)-1-Benzyl-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9h)
Prepared according to General Procedure C, to give the title compound as a yellow solid (47 mg, 99%): mp 81–83 °C; δH (DMSO-d6, 400 MHz) 7.99 (d, J = 1.2, 1 H, Ar), 7.83 (d, J = 8.4, 1 H, Ar), 7.77 (dd, J = 8.4, 1.2, 1 H, Ar), 7.26 (t, J = 7.6, 2 H, Ar), 7.19 (t, J = 7.6, 1 H, Ar), 7.02 (d, J = 7.6, 2 H, Ar), 5.26 (d, J = 16.0, 1 H, CHaHbAr), 5.02 (d, J = 16.0, 1 H, CHaHbAr), 3.61 (t, J = 6.8, 1 H, CHCO), 2.43 (s, 3 H, CH3CN), 1.99–1.91 (m, 1 H, CHMe2), 1.88–1.68 (m, 2 H, CH2iPr), 0.88 (d, J = 6.8, 3 H, CH3), 0.73 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.5, 166.1, 140.4, 137.0, 133.7, 128.5, 128.4, 127.1, 126.6, 125.3, 123.4, 59.9, 49.9, 24.6, 24.0, 23.1, 22.2, 21.9; LRMS: m/z C22H24N2O3 requires: 364.2, found: 365.2 [M+H]; tR = 4.63 (98.1%, I).
(R)-tert-Butyl 1-(4-(tert-butyl)benzyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8i)
Prepared according to General Procedure B, to give the title compound as a white solid (65 mg, 92%): mp 82–83 °C; δH (DMSO-d6, 400 MHz) 7.88 (s, 1 H, Ar), 7.80 (d, J = 8.0, 1 H, Ar), 7.71 (d, J = 8.0, 1 H, Ar), 7.30 (d, J = 8.0, 2 H, Ar), 7.00 (d, J = 8.0, 2 H, Ar), 5.08 (d, J = 16.0, 1 H, C HaHbAr), 4.99 (d, J = 16.0, 1 H, CHaHbAr), 3.50 (t, J = 6.8, 1 H, CHCO), 2.36 (s, 3 H, CH3CN), 1.98–1.89 (m, 1 H, CHMe2), 1.86–1.68 (m, 2 H, CH2iPr), 1.46 (s, 9 H, 3 × CH3), 1.23 (s, 9 H, 3 × CH3), 0.86 (d, J = 6.4, 3 H, CH3), 0.72 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.0, 166.8, 163.7, 149.4, 140.6, 134.9, 134.1, 133.5, 127.9, 126.2, 125.3, 124.9, 122.7, 81.5, 60.4, 49.8, 34.1, 27.6, 24.9, 24.0, 23.2, 21.9; LRMS: m/z C30H40N2O3 requires: 476.3, found: 477.3 [M+H]; tR = 6.45 (95.6%, V).
(R)-1-(4-(tert-Butyl)benzyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9i)
Prepared according to General Procedure C, to give the title compound as a cream solid (51 mg, 98%): mp 95–97 °C; δH (DMSO-d6, 400 MHz) 8.00 (s, 1 H, Ar), 7.84 (d, J = 8.4, 1 H, Ar), 7.78 (d, J = 8.4, 1 H, Ar), 7.27 (d, J = 8.4, 2 H, Ar), 6.93 (d, J = 7.6, 2 H, Ar), 5.23 (d, J = 16.0, 1 H, CHaHbAr), 4.96 (d, J = 16.0, 1 H, CHaHbAr), 3.59 (t, J = 6.0, 1 H, CHCO), 2.41 (s, 3 H, CH3CN), 1.98–1.90 (m, 1 H, CHMe2), 1.86–1.67 (m, 2 H, CH2iPr), 1.21 (s, 9 H, 3 × CH3), 0.87 (d, J = 6.0, 3 H, CH3), 0.73 (d, J = 6.0, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.4, 166.1, 149.4, 140.5, 134.0, 133.7, 128.4, 126.2, 125.3, 125.2, 123.4, 60.0, 49.8, 34.1, 31.2, 24.6, 24.0, 23.1, 21.9; LRMS: m/z C26H32N2O3 requires: 420.2, found: 421.2 [M+H]; tR = 5.37 (100%, I).
(R)-tert-Butyl 1-([1,1′-biphenyl]-4-ylmethyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8j)
Prepared according to General Procedure B, to give the title compound as a white solid (61 mg, 82%): mp 76–78 °C; δH (DMSO-d6, 400 MHz) 7.93 (s, 1 H, Ar), 7.82 (d, J = 8.0, 1 H, Ar), 7.72 (d, J = 8.0, 1 H, Ar), 7.63–7.57 (m, 4 H, Ar), 7.44 (t, J = 7.6, 2 H, Ar), 7.34 (t, J = 7.6, 1 H, Ar), 7.15 (d, J = 8.4, 2 H, Ar), 5.18 (d, J = 16.8, 1 H, CHaHbAr), 5.13 (d, J = 16.8, 1 H, CHaHbAr), 3.53 (t, J = 6.8, 1 H, CHCO), 2.39 (s, 3 H, CH3CN), 2.01–1.93 (m, 1 H, CHMe2), 1.88–1.68 (m, 2 H, CH2iPr), 1.47 (s, 9 H, 3 × CH3), 0.88 (d, J = 6.8, 3 H, CH3), 0.73 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.1, 166.9, 163.7, 140.4, 139.6, 138.9, 136.6, 134.3, 133.5, 128.9, 128.0, 127.4, 127.2, 126.8, 126.5, 124.9, 122.8, 81.5, 60.4, 49.6, 27.6, 24.9, 24.0, 23.2, 21.9; LRMS: m/z C32H36N2O3 requires: 496.3, found: 497.3 [M+H]; tR = 6.03 (91.8%, V).
(R)-1-([1,1′-Biphenyl]-4-ylmethyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9j)
Prepared according to General Procedure C, to give the title compound as a cream solid (53 mg, 97%): mp 108–110 °C; δH (DMSO-d6, 400 MHz) 8.04 (d, J = 1.6. 1 H, Ar), 7.89 (d, J = 8.4, 1 H, Ar), 7.81 (dd, J = 8.4, 1.6, 1 H, Ar), 7.64–7.56 (m, 4 H, Ar), 7.43 (t, J = 8.0, 2 H, Ar), 7.33 (t, J = 7.6, 1 H, Ar), 7.14 (d, J = 8.0, 2 H, Ar), 5.30 (d, J = 16.4, 1 H, CHaHbAr), 5.09 (d, J = 16.4, 1 H, CHaHbAr), 3.70 (t, J = 7.2, 1 H, CHCO), 2.49 (s, 3 H, CH3CN), 2.02–1.93 (m, 1 H, CHMe2), 1.89–1.70 (m, 2 H, CH2iPr), 0.89 (d, J = 6.8, 3 H, CH3), 0.75 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.4, 166.1, 140.6, 139.5, 138.9, 136.3, 134.1, 133.1, 128.9, 128.7, 127.4, 127.2, 126.8, 126.5, 125.4, 123.5, 59.7, 49.9, 24.5, 24.0, 23.1, 21.9; LRMS: m/z C28H28N2O3 requires: 440.2, found: 441.2 [M+H]; tR = 5.08 (99.3%, I).
(R)-tert-Butyl 1,3-diisobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8k)
Prepared according to General Procedure B, to give the title compound as a white solid (44 mg, 76%): mp 116–118 °C; δH (DMSO-d6, 400 MHz) 7.94 (s, 1 H, Ar), 7.83 (d, J = 8.4, 1 H, Ar), 7.75 (d, J = 8.4, 1 H, Ar), 4.14 (dd, J = 14.0, 9.2, 1 H, CHaHbAr), 3.46 (dd, J = 14.0, 9.2, 1 H, CHaHbAr), 3.34–3.32 (m, 1 H, CHCO), 2.40 (s, 3 H, CH3CN), 1.94–1.85 (m, 1 H, CHMe2), 1.76–1.62 (m, 3 H, CHMe2, CH2iPr), 1.56 (s, 9 H, 3 × CH3), 0.83 (d, J = 6.4, 3 H, CH3), 0.71–0.66 (m, 6 H, 2 × CH3) 0.59 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.9, 166.9, 164.3, 140.7, 135.2, 134.0, 128.2, 125.2, 123.6, 82.0, 79.7, 79.4, 79.1, 60.9, 52.9, 28.1, 27.0, 25.2, 24.3, 23.6, 22.2, 20.3, 19.5; LRMS: m/z C23H34N2O3 requires: 386.3, found: 387.2 [M+H]; tR = 4.34 (91.3%, I).
(R)-1,3-Diisobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9k)
Prepared according to General Procedure C, to give the title compound as a cream solid (43 mg, 99%): mp 97–99 °C; δH (DMSO-d6, 400 MHz) 8.02 (d, J = 1.2, 1 H, Ar), 7.91 (d, J = 8.0, 1 H, Ar), 7.84 (dd, J = 8.0, 1.2, 1 H, Ar), 4.14 (dd, J = 14.0, 9.2, 1 H, CHaHbiPr), 3.52 (dd, J = 14.0, 9.2, 1 H, CHaHbiPr), 3.44 (t, J = 5.6, 1 H, CHCO), 2.49 (s, 3 H, CH3CN), 1.95–1.88 (m, 1 H, CHMe2), 1.79–1.52 (m, 3 H, CHMe2, CH2iPr), 0.84 (d, J = 6.8, 3 H, CH3), 0.71–0.68 (m, 6 H, 2 × CH3), 0.57 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.2, 166.3, 140.4, 133.8, 128.5, 125.2, 123.6, 60.0, 52.5, 26.5, 24.5, 23.9, 23.1, 21.8, 19.8, 19.1; LRMS: m/z C19H26N2O3 requires: 330.2, found: 331.2 [M+H]; tR = 1.28 (96.8%, III).
(R)-tert-Butyl 1-(2-(tert-butoxy)-2-oxoethyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8l)
Prepared according to General Procedure B, to give the title compound as a white solid (61 mg, 92%): mp 62–64 °C; δH (DMSO-d6, 400 MHz) 7.86 (d, J = 8.4, 1 H, Ar), 7.79–7.77 (m, 2 H, Ar), 4.48 (d, J = 17.2, 1 H, CHaHbCO2), 4.39 (d, J = 17.2, 1 H, CHaHbCO2), 3.37 (t, J = 6.4, 1 H, CHCO), 2.42 (s, 3 H, CH3CN), 1.91–1.62 (m, 3 H, CHMe2, CH2iPr), 1.55 (s, 9 H, 3 × CH3), 1.40 (s, 9 H, 3 × CH3), 0.84 (d, J = 6.8, 3 H, CH3), 0.71 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.4, 167.8, 167.0, 163.8, 140.5, 133.6, 133.5, 127.9, 124.9, 122.1, 81.6, 81.4, 60.1, 50.3, 27.7, 27.6, 25.1, 24.0, 23.0, 21.9; LRMS: m/z C25H36N2O5 requires: 444.3, found: 445.2 [M+H]; tR = 21.17 (99.5%, II).
(R)-1-(Carboxymethyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9l)
Prepared according to General Procedure C, to give the title compound as a yellow solid (43 mg, 98%): mp 83–85 °C; δH (DMSO-d6, 400 MHz) 7.93 (d, J = 8.4, 1 H, Ar), 7.90 (s, 1 H, Ar), 7.86 (d, J = 8.4, 1 H, Ar), 4.51 (s, 2 H, CH2CO), 3.56 (t, J = 6.8, 1 H, CHCO), 2.52 (s, 3 H, CH3CN), 1.94–1.64 (m, 3 H, CHMe2, CH2iPr), 0.85 (d, J = 6.8, 3 H, CH3), 0.72 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 170.2, 168.6, 166.1, 140.9, 134.1, 132.7, 128.6, 125.4, 123.1, 59.4, 49.7, 24.6, 24.0, 23.0, 21.8; LRMS: m/z C17H20N2O5 requires: 332.2, found: 333.3 [M+H]; tR = 1.29 (100%, III).
(R)-tert-Butyl 3-isobutyl-5-methyl-2-oxo-1-(pyridin-4-ylmethyl)- 2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8m)
Prepared according to General Procedure B, to give the title compound as a white solid (56 mg, 89%): mp 64–66 °C; δH (DMSO-d6, 400 MHz) 8.49 (d, J = 5.2, 2 H, Py), 7.85 (d, J = 8.0, 1 H, Ar), 7.78 (s, 1 H, Ar), 7.73 (d, J = 8.0, 1 H, Ar), 7.10 (d, J = 5.2, 2 H, Py), 5.16 (d, J = 17.2, 1 H, CHaHbPy), 5.06 (d, J = 17.2, 1 H, CHaHbPy), 3.56 (t, J = 6.4, 1 H, CHCO), 2.42 (s, 3 H, CH3CN), 1.99–1.91 (m, 1 H, CHMe2), 1.82–1.68 (m, 2 H, CH2iPr), 1.47 (s, 9 H, 3 × CH3), 0.88 (d, J = 6.8, 3 H, CH3), 0.72 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.4, 167.0, 163.6, 149.7, 146.6, 140.3, 134.0, 133.5, 128.1, 125.0, 122.5, 121.6, 81.5, 60.3, 49.4, 27.6, 25.0, 23.9, 23.2, 21.8; LRMS: m/z C25H31N3O3 requires: 421.2, found: 422.2 [M+H]; tR = 3.94 (96.3%, I).
(R)-3-Isobutyl-5-methyl-2-oxo-1-(pyridin-4-ylmethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9m)
Prepared according to General Procedure C to give the title compound as a yellow solid (47 mg, 99%): mp 98–100 °C; δH (DMSO-d6, 400 MHz) 8.79 (d, J = 6.4, 2 H, Py), 7.93 (d, J = 8.8, 1 H, Ar), 7.86–7.83 (m, 2 H, Ar), 7.62 (d, J = 6.4, 2 H, Py), 5.32 (s, 2 H, CH2Py), 3.69–3.65 (m, 1 H, CHCO), 2.52 (s, 3 H, CH3CN), 1.98–1.90 (m, 1 H, CHMe2), 1.78–1.69 (m, 2 H, CH2iPr), 0.88 (d, J = 6.8, 3 H, CH3), 0.72 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.3, 168.8, 166.1, 155.2, 144.0, 140.3, 133.8, 133.5, 128.5, 125.6, 123.6, 122.9, 59.9, 50.3, 24.9, 23.9, 23.3, 21.7; LRMS: m/z C21H23N3O3 requires: 365.2, found: 366.2 [M+H]; tR = 3.48 (94.6%, I).
(R)-tert-Butyl 3-isobutyl-5-methyl-2-oxo-1-(2-oxo-2-(phenethylamino)ethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8n)
Prepared according to General Procedure B, to give the title compound as a white solid (70 mg, 95%): mp 80–82 °C; δH (DMSO-d6, 400 MHz) 8.27 (t, J = 5.6, 1 H, NH), 7.88 (s, 1 H, Ar), 7.84 (d, J = 8.0, 1 H, Ar), 7.76 (d, J = 8.0, 1 H, Ar), 7.28 (t, J = 7.2, 2 H, Ar), 7.22–7.17 (m, 3 H, Ar), 4.48 (d, J = 17.6, 1 H, CHaHbCONH), 4.21 (d, J = 17.6, 1 H, CHaHbCONH), 3.40 (t, J = 6.4, 1 H, CHCO), 3.33–3.26 (m, 2 H, CH2NH), 2.72 (t, J = 6.4, 2 H, CH2Ar), 2.43 (s, 3 H, CH3CN), 1.95–1.88 (m, 1 H, CHMe2), 1.77–1.65 (m, 2 H, CH2iPr), 1.54 (s, 9 H, 3 × CH3), 0.85 (d, J = 6.4, 3 H, CH3), 0.70 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.1, 167.4, 163.3, 141.2, 139.3, 133.6, 133.5, 128.6, 128.3, 127.8, 126.1, 124.7, 122.8, 81.5, 60.1, 50.5, 40.5, 40.1, 38.9, 35.1, 27.6, 25.0, 23.9, 23.2, 21.7; LRMS: m/z C29H37N3O4 requires: 491.3, found: 492.2 [M+H]; tR = 5.54 (100%, I).
(R)-3-Isobutyl-5-methyl-2-oxo-1-(2-oxo-2-(phenethylamino)ethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9n)
Prepared according to General Procedure C, to give the title compound as a cream solid (54 mg, 99%): mp 69–71 °C; δH (DMSO-d6, 400 MHz) 8.25 (t, J = 5.6, 1 H, NH), 7.95 (d, J = 1.6, 1 H, Ar), 7.92 (d, J = 8.0, 1 H, Ar), 7.85 (dd, J = 8.0, 1.6, 1 H, Ar), 7.24 (t, J = 7.2, 2 H, Ar), 7.17–7.13 (m, 3 H, Ar), 4.46 (d, J = 16.4, 1 H, CHaHbCONH), 4.32 (d, J = 16.4, 1 H, CHaHbCONH), 3.67 (t, J = 6.8, 1 H, CHCO), 3.31–3.26 (m, 2 H, CH2NH), 2.70 (t, J = 7.2, 2 H, CH2Ar), 2.60 (s, 3 H, CH3CN), 1.97–1.89 (m, 1 H, CHMe2), 1.84–1.68 (m, 2 H, CH2iPr), 0.87 (d, J = 6.8, 3 H, CH3), 0.74 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.0, 167.3, 166.1, 141.6, 139.3, 134.6, 131.8, 129.0, 128.6, 128.3, 126.1, 125.3, 123.7, 58.9, 50.9, 40.4, 38.1, 35.1, 24.4, 23.9, 23.0, 21.8; LRMS: m/z C25H29N3O4 requires: 435.2, found: 436.2 [M+H]; tR = 4.16 (97.3%, I).
(R)-tert-Butyl 3-isobutyl-5-methyl-2-oxo-1-(2-oxo-2-(decylamino)ethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8o)
Prepared according to General Procedure B, to give the title compound as a white solid (68 mg, 86%): mp 64–66 °C; δH (DMSO-d6, 400 MHz) 8.10 (t, J = 5.2, 1 H, NH), 7.87–7.83 (m, 2 H, Ar), 7.76 (d, J = 8.4, 1 H, Ar), 4.46 (d, J = 16.4, 1 H, CHaHbCONH), 4.21 (d, J = 16.4, 1 H, CHaHbCONH), 3.45 (t, J = 6.8, 1 H, CHCO), 3.08–3.03 (m, 2 H, CH2NH), 2.44 (s, 3 H, CH3CN), 1.93–1.86 (m, 1 H, CHMe2), 1.77–1.64 (m, 2 H, CH2iPr), 1.54 (s, 9 H, 3 × CH3), 1.42–1.36 (m, 2 H, Alk), 1.30–1.18 (m, 15 H, Alk), 0.86–0.82 (m, 5 H, CH3, Alk), 0.70 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.9, 167.1, 163.8, 141.3, 133.7, 133.2, 128.0, 124.6, 122.8, 81.5, 59.9, 50.5, 38.6, 31.3, 29.1, 29.0, 28.8, 28.7, 27.6, 26.3, 24.9, 23.9, 23.2, 22.1, 21.7, 13.9; LRMS: m/z C31H49N3O4 requires: 527.4, found: 528.4 [M+H]; tR = 27.36 (99.2%, II).
(R)-3-Isobutyl-5-methyl-2-oxo-1-(2-oxo-2-(decylamino)ethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9o)
Prepared according to General Procedure C, to give the title compound as a cream solid (58 mg, 99%): mp 139–141 °C; δH (DMSO-d6, 400 MHz) 8.11 (t, J = 5.6, 1 H, NH), 7.96 (d, J = 1.6, 1 H, Ar), 7.92 (d, J = 8.4, 1 H, Ar), 7.85 (dd, J = 8.4, 1.6, 1 H, Ar), 4.44 (d, J = 16.8, 1 H, CHaHbCONH), 4.28 (d, J = 16.8, 1 H, CHaHbCONH), 3.60 (t, J = 6.8, 1 H, CHCO), 3.08–3.03 (m, 2 H, CH2NH), 2.55 (s, 3 H, CH3CN), 1.95–1.88 (m, 1 H, CHMe2), 1.81–1.66 (m, 2 H, CH2iPr), 1.42–1.35 (m, 2 H, Alk), 1.29–1.16 (m, 15 H, Alk), 0.87 (d, J = 6.4, 3 H, CH3), 0.85 (t, J = 7.2, 3 H, Alk), 0.73 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.3, 167.1, 166.1, 141.5, 134.2, 132.2, 128.7, 125.2, 123.5, 59.3, 50.9, 38.6,38.5, 31.3, 29.1, 29.0, 28.9, 28.8,28.7, 26.3, 24.6, 23.9, 23.1, 22.1, 21.8, 14.0; LRMS: m/z C27H41N3O4 requires: 471.3, found: 472.3 [M+H]; tR = 5.93 (100%, I).
(R)-tert-Butyl 3-isobutyl-5-methyl-2-oxo-1-(2-oxo-2-(hexadecylamino)ethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8p)
Prepared according to General Procedure B, to give the title compound as a yellow oil (82 mg, 90%): δH (DMSO-d6, 400 MHz) 8.06 (t, J = 5.6, 1 H, NH), 7.85 (s, 1 H, Ar), 7.78 (d, J = 8.0, 1 H, Ar), 7.74 (d, J = 8.0, 1 H, Ar), 4.47 (d, J = 16.4, 1 H, CHaHbCONH), 4.17 (d, J = 16.4, 1 H, CHaHbCONH), 3.42–3.37 (m, 1 H, CHCO), 3.09–3.03 (m, 2 H, CH2NH), 2.41 (s, 3 H, CH3CN), 1.93–1.86 (m, 1 H, CHMe2), 1.77–1.64 (m, 2 H, CH2iPr), 1.54 (s, 9 H, 3 × CH3), 1.42–1.36 (m, 2 H, Alk), 1.30–1.15 (m, 27 H, Alk), 0.86–0.82 (m, 5 H, CH3, Alk), 0.70 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.1, 167.1, 163.7, 141.1, 133.4, 127.6, 124.5, 122.7, 81.2, 79.2, 78.9, 78.6, 60.1, 50.5, 38.7, 31.3, 29.2, 29.1, 29.0, 28.8, 28.7, 27.6, 26.3, 25.0, 23.9, 23.2, 22.1, 21.7, 13.9; LRMS: m/z C37H61N3O4 requires: 611.5, found: 612.4 [M+H]; tR = 34.07 (100%, II).
(R)-3-isobutyl-5-methyl-2-oxo-1-(2-oxo-2-(hexadecylamino)ethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9p)
Prepared according to General Procedure C, to give the title compound as a cream solid (66 mg, 99%): mp 96–98 °C; δH (DMSO-d6, 400 MHz) 8.11 (t, J = 5.6, 1 H, NH), 7.95 (d, J = 1.2, 1 H, Ar), 7.91 (d, J = 8.0, 1 H, Ar), 7.84 (dd, J = 8.0, 1.2, 1 H, Ar), 4.44 (d, J = 16.4, 1 H, CHaHbCONH), 4.27 (d, J = 16.4, 1 H, CHaHbCONH), 3.58 (t, J = 6.8, 1 H, CHCO), 3.08–3.02 (m, 2 H, CH2NH), 2.54 (s, 3 H, CH3CN), 1.95–1.87 (m, 1 H, CHMe2), 1.81–1.66 (m, 2 H, CH2iPr), 1.38–1.35 (m, 2 H, Alk), 1.27–1.17 (m, 27 H, Alk), 0.86 (d, J = 6.4, 3 H, CH3), 0.84 (t, J = 7.2, 3 H, Alk), 0.72 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 173.5, 172.3, 171.3, 146.7, 133.7, 130.3, 128.6, 122.3, 64.5, 56.1, 43.7, 36.5, 34.2, 34.1, 34.0, 33.9, 33.8, 31.4, 29.8, 29.1, 28.2, 27.3, 26.9, 19.1; LRMS: m/z C33H53N3O4 requires: 555.4, found: 556.4 [M+H]; tR = 29.83 (95.4%, II).
(R)-tert-Butyl 3-isobutyl-5-methyl-2-oxo-1-(2-oxo-2-(hexylamino)ethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8q)
Prepared according to General Procedure B, to give the title compound as a white solid (61 mg, 87%): mp 66–68 °C; δH (DMSO-d6, 400 MHz) 8.11 (t, J = 5.6, 1 H, NH), 7.87–7.83 (m, 2 H, Ar), 7.76 (d, J = 8.4, 1 H, Ar), 4.47 (d, J = 17.2, 1 H, CHaHbCONH), 4.22 (d, J = 17.2, 1 H, CHaHbCONH), 3.45 (t, J = 6.8, 1 H, CHCO), 3.08–3.04 (m, 2 H, CH2NH), 2.44 (s, 3 H, CH3CN), 1.93–1.86 (m, 1 H, CHMe2), 1.78–1.66 (m, 2 H, CH2iPr), 1.54 (s, 9 H, 3 × CH3), 1.40–1.36 (m, 2 H, Alk), 1.27–1.19 (m, 7 H, Alk), 0.86–0.84 (m, 5 H, CH3, Alk), 0.70 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.9, 167.1, 163.8, 141.3, 133.7, 133.2, 128.0, 124.6, 122.8, 81.5, 59.9, 50.5, 38.6, 30.9, 29.1, 27.6, 26.0, 24.9, 23.9, 23.2, 22.0, 21.8, 13.9; LRMS: m/z C27H41N3O4 requires: 471.3, found: 472.3 [M+H]; tR = 6.05 (91.8%, II).
(R)-3-Isobutyl-5-methyl-2-oxo-1-(2-oxo-2-(hexylamino)ethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9q)
Prepared according to General Procedure C, to give the title compound as a cream solid (52 mg, 99%): mp 130–132 °C; δH (DMSO-d6, 400 MHz) 8.10 (t, J = 5.6, 1 H, NH), 7.93 (d, J = 1.2, 1 H, Ar), 7.91 (d, J = 8.4, 1 H, Ar), 7.82 (dd, J = 8.4, 1.2, 1 H, Ar), 4.42 (d, J = 16.4, 1 H, CHaHbCONH), 4.26 (d, J = 16.4, 1 H, CHaHbCONH), 3.61 (t, J = 6.4, 1 H, CHCO), 3.04–3.00 (m, 2 H, CH2NH), 2.55 (s, 3 H, CH3CN), 1.92–1.84 (m, 1 H, CHMe2), 1.79–1.62 (m, 2 H, CH2iPr), 1.35–1.30 (m, 2 H, Alk), 1.25–1.16 (m, 7 H, Alk), 0.83 (d, J = 6.4, 3 H, CH3), 0.82 (t, J = 7.2, 3 H, Alk), 0.71 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.1, 167.1, 166.1, 141.2, 134.5, 131.9, 128.9, 125.2, 123.6, 117.0, 114.1, 59.1, 50.9, 38.2, 31.0, 29.0, 25.9, 24.5, 23.9, 23.0, 22.0, 21.8, 13.9; LRMS: m/z C23H33N3O4 requires: 415.2, found: 416.3 [M+H]; tR = 4.23 (98.3%, I).
(R)-tert-Butyl 1-(2-(4-(tert-butoxycarbonyl)piperazin-1-yl)-2-oxoethyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8r)
Prepared according to General Procedure B, to give the title compound as a white solid (55 mg, 81%): mp 128–130; δH (DMSO-d6, 400 MHz) 7.84 (s, 1 H, Ar), 7.78–7.71 (m, 2 H, Ar), 4.82 (d, J = 16.4, 1 H, CHaHbCO), 4.63 (d, J = 16.4, 1 H, CHaHbCO), 3.55–3.20 (m, 9 H, piperazine, CHCO), 2.41 (s, 3 H, CH3CN), 1.92–1.83 (m, 1 H, CHMe2), 1.82–1.61 (m, 2 H, CH2iPr), 1.54 (s, 9 H, 3 × CH3), 1.41 (s, 9 H, 3 × CH3), 0.84 (d, J = 6.4, 3 H, CH3), 0.71 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.1, 165.8, 163.8, 153.8, 140.9, 133.8, 133.4, 127.8, 124.6, 122.5, 81.5, 79.2, 60.1, 49.0, 44.0, 41.3, 28.0, 27.7, 25.0, 24.0, 23.1, 21.9; LRMS: m/z C30H44N4O6 requires: 556.3, found: 557.3 [M+H]; tR = 4.65 (97.7%, IV).
(R)-3-Isobutyl-5-methyl-2-oxo-1-(2-oxo-2-(piperazin-1-yl)ethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9r)
Prepared according to General Procedure C, to give the title compound as a cream solid (52 mg, 99%): mp 69–71; δH (DMSO-d6, 400 MHz) 8.88 (br s, 1 H, NH), 7.93–7.81 (m, 3 H, Ar), 4.86–4.72 (m, 2 H, CH2CO), 3.78–3.50 (m, 5 H, piperazine, CHCO), 3.22–3.00 (m, 4 H, piperazine), 2.50 (s, 3 H, CH3CN), 1.96–1.85 (m, 1 H, CHMe2), 1.80–1.62 (m, 2 H, CH2iPr), 0.85 (d, J = 6.4, 3 H, CH3), 0.71 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.7, 166.3, 166.2, 141.0, 133.6, 128.1, 125.3, 123.5, 117.3, 114.4, 59.6, 48.9, 42.7, 24.7, 24.0, 23.1, 21.9; LRMS: m/z C21H28N4O4 requires: 400.2, found: 401.2 [M+H]; tR = 3.36 (94.0%, I).
(R)-tert-Butyl 1-(2-(4-((benzyloxy)carbonyl)piperazin-1-yl)-2-oxoethyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8s)
Prepared according to General Procedure B, to give the title compound as a white solid (82 mg, 77%): mp 109–111; δH (DMSO-d6, 400 MHz) 7.81 (d, J = 8.8, 1 H, Ar), 7.75 (d, J = 8.8, 1 H, Ar), 7.74 (s, 1 H, Ar), 7.42–7.29 (m, 5 H, Cbz), 5.11 (s, 2 H, Cbz), 4.83 (d, J = 17.2, 1 H, CHaHbCO), 4.64 (d, J = 17.2, 1 H, CHaHbCO), 3.57–3.34 (m, 9 H, piperazine, CHCO), 2.41 (s, 3 H, CH3CN), 1.90–1.83 (m, 1 H, CHMe2), 1.80–1.64 (m, 2 H, CH2iPr), 1.53 (s, 9 H, 3 × CH3), 0.84 (d, J = 6.4, 3 H, CH3), 0.71 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.5, 166.3, 164.3, 154.9, 141.4, 137.2, 134.3, 133.8, 128.9, 128.3, 128.2, 128.1, 125.1, 123.0, 81.9, 66.9, 60.6,49.5, 44.3, 41.7, 28.1, 25.4, 24.4, 23.5, 22.3; LRMS: m/z C33H42N4O6 requires: 590.3, found: 591.3 [M+H]; tR = 4.74 (98.4%, IV).
(R)-1-(2-(4-((Benzyloxy)carbonyl)piperazin-1-yl)-2-oxoethyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9s)
Prepared according to General Procedure C, to give the title compound as a white solid (62 mg, 98%): mp 88–90; δH (DMSO-d6, 400 MHz) 7.95–7.82 (m, 3 H, Ar), 7.42–7.29 (m, 5 H, Ar), 5.10 (s, 2 H, CH2Ph), 4.87–4.68 (m, 2 H, CH2CO), 3.64–3.26 (m, 9 H, piperazine, CHCO), 2.54 (s, 3 H, CH3CN), 1.96–1.85 (m, 1 H, CHMe2), 1.80–1.62 (m, 2 H, CH2iPr), 0.85 (d, J = 6.4, 3 H, CH3), 0.73 (d, J = 6.4, 3 H, CH3); δC (DMSO-d6, 100 MHz) 166.2, 165.8, 154.4, 141.2, 136.7, 128.5, 127.9, 127.6, 125.3, 117.5, 66.4, 59.3, 49.2, 43.9, 41.3, 24.5, 23.9, 23.0, 21.9; LRMS: m/z C29H34N4O6 requires: 534.2, found: 535.1 [M+H]; tR = 5.25 (90.1%, I).
(R)-tert-Butyl 1-(2-(4-(((9H-fluoren-9-yl)methoxy)carbonyl)piperazin-1-yl)-2-oxoethyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (8t)
Prepared according to General Procedure B, to give the title compound as a white solid (82 mg, 77%): mp 124–126; δH (DMSO-d6, 400 MHz) 7.90 (d, J = 7.4, 2 H, Fmoc), 7.84 (d, J = 8.0, 1 H, Ar), 7.77–7.72 (m, 2 H, Ar), 7.64 (d, J = 7.4, 2 H, Fmoc), 7.42 (t, J = 7.4, 2 H, Fmoc), 7.34 (t, J = 7.4, 2 H, Fmoc), 4.82 (d, J = 17.2, 1 H, CHaHbCO), 4.63 (d, J = 17.2, 1 H, CHaHbCO), 4.42 (d, J = 6.0, 2 H, Fmoc), 4.29 (t, J = 6.0, 1 H, Fmoc), 3.55–3.20 (m, 9 H, piperazine, CHCO), 2.41 (d, J = 0.8, 3 H, CH3CN), 1.92–1.83 (m, 1 H, CHMe2), 1.82–1.61 (m, 2 H, CH2iPr), 1.54 (s, 9 H, 3 × CH3), 0.84 (d, J = 6.8, 3 H, CH3), 0.71 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.1, 167.1, 165.9, 163.9, 154.3, 143.8, 140.9, 140.8, 133.9, 133.4, 128.9, 127.8, 127.7, 127.2, 125.0, 124.6, 122.6, 120.2, 81.5, 66.6, 60.2, 49.0, 46.8, 27.7, 25.0, 24.0, 23.1, 21.9; LRMS: m/z C40H46N4O6 requires: 678.3, found: 679.2 [M+H]; tR = 4.67 (98.5%, IV).
(R)-1-(2-(4-(((9H-Fluoren-9-yl)methoxy)carbonyl)piperazin-1-yl)-2-oxoethyl)-3-isobutyl-5-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (9t)
Prepared according to General Procedure C, to give the title compound as a white solid (37 mg, 97%): mp 80–82; δH (DMSO-d6, 400 MHz) 7.98–7.77 (m, 5 H, Ar, Fmoc), 7.64 (d, J = 7.2, 2 H, Fmoc), 7.42 (t, J = 7.2, 2 H, Fmoc), 7.34 (t, J = 7.2, 2 H, Fmoc), 4.82 (d, J = 17.2, 1 H, CHaHbCO), 4.73 (d, J = 17.2, 1 H, CHaHbCO), 4.41 (s, 2 H, Fmoc), 4.29 (s, 1 H, Fmoc), 3.64–3.58 (m, 1 H, CHCO), 3.51–3.20 (m, 8 H, piperazine), 2.55 (s, 3 H, CH3CN), 1.97–1.87 (m, 1 H, CHMe2), 1.82–1.64 (m, 2 H, CH2iPr), 0.86 (d, J = 6.0, 3 H, CH3), 0.74 (d, J = 6.8, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.3, 166.2, 165.8, 162.3, 154.3, 143.8, 141.2, 140.8, 128.5, 127.7, 127.2, 125.3, 125.0, 123.7, 120.1, 66.6, 59.3, 49.2, 46.8, 41.2, 35.8, 24.5, 23.9, 23.0, 21.9; LRMS: m/z C36H38N4O6 requires: 622.3, found: 401.2 [(M+H) – Fmoc]; tR = 5.04 (91.0%, I).
tert-Butyl 4-methyl-3-nitrobenzoate (11)
To a round bottom flask equipped with a magnetic stirrer bar was charged with 4-methyl-3-nitobenzoic acid (2.30 mg, 12.70 mmol), tBuOH (1.45 mL, 15.24 mmol) and DMAP (50 mg, 0.03 mmol) in CH2Cl2 (60 mL). DCC (3.14 g, 15.24 mmol) was added and then the reaction was stirred at room temperature for 3 h. The reaction mixture was reduced in vacuo and then the residue was purified by column chromatography (hexane/EtOAc) to provide the title compound as a white solid (1.21 g, 40%): δH (DMSO-d6, 500 MHz) 8.50 (d, J = 1.6, 1 H, Ar), 8.07 (dd, J = 8.0, 1.6, 1 H, Ar), 7.39 (d, J = 8.0, 1 H, Ar), 2.63 (s, 3 H, CH3), 1.59 (s, 9 H, 3 × CH3).
tert-Butyl 4-formyl-3-nitrobenzoate (12).24
To a round bottom flask equipped with a magnetic stirrer bar was charged with tert-butyl 4-methyl-3-nitrobenzoate (11; 237 mg, 1.00 mmol) in DMF (2 mL). N,N-Dimethylformamide dimethylacetal (172 μL, 1.30 mmol) was added and the reaction mixture was heated to 140 °C for 5 h, then cooled and reduced in vacuo. The residue was dissolved in 50 % THF in H2O (6 mL), NaIO4 (642 mg, 3.00 mmol) was added and allowed to stir at room temperature for 2 h. The reaction mixture was decanted with CH2Cl2, washed with H2O, brine (10 mL), dried over MgSO4, filtered and reduced in vacuo. The residue was purified by silica gel column chromatography (hexane/EtOAc) to provide the title compound as a cream solid (100 mg, 40%): δH (CDCl3, 400 MHz) 10.46 (s, 1 H, CHO), 8.67 (s, 1 H, Ar), 8.36 (d, J = 8.0, 1 H, Ar), 7.99 (d, J = 8.0, 1 H, Ar), 1.64 (s, 9 H, 3 × CH3).
tert-Butyl 4-benzoyl-3-nitrobenzoate (13)
To a round bottom flask equipped with a magnetic stirrer bar was charged with tert-butyl 4-benzoyl-3-nitrobenzoate (277 mg, 1.10 mmol) in THF (20 mL). The reaction was cooled to 0 °C and 1 M phenylmagnesium bromide (1.32 mL, 1.32 mmol) was added and stirred at 0 °C for 3 h. The reaction was quenched with saturated aqueous NH4Cl (1 mL), partitioned between EtOAc (60 mL) and H2O (30 mL). The aqueous phase was extracted with EtOAc (3 × 10 mL) and the organic portions combined, washed with H2O (10 mL), brine (10 mL), dried over MgSO4, filtered and reduced in vacuo. The residue was purified by silica gel column chromatography (hexane/EtOAc) to provide a yellow oil that was subsequently dissolved in acetone (20 mL), 2.6 M Jones Reagent (634 μL, 1.65 mmol) was added at 0 °C and the mixture was stirred for 2 h. The reaction was filtered through Celite, reduced in vacuo, and then purified by column chromatography (hexane/EtOAc) to provide the title compound as a yellow solid (292 mg, 81 % (two steps)): δH (CDCl3, 400 MHz) 8.79 (d, J = 1.2, 1 H, Ar), 8.37 (dd, J = 8.0, 1.2, 1 H, Ar), 7.23 (d, J = 7.6, 2 H, Ar), 7.61 (t, J = 7.6, 1 H, Ar), 7.55 (d, J = 8.0, 1 H, Ar), 7.46 (t, J = 7.6, 2 H, Ar), 1.65 (s, 9 H, 3 × CH3).
tert-Butyl 3-amino-4-benzoylbenzoate (14)
To a round bottom flask equipped with a magnetic stirrer bar was charged with nitro benzophenone 12 (47 mg, 0.14 mmol) in MeOH (3 mL). Pd/C (10 mg) was added and the reaction placed under 1 atm of H2 gas (balloon) for 1 h. The reaction mixture was filtered through Celite, reduced in vacuo and the residue was purified by silica gel column chromatography (hexane/EtOAc) to provide the title compound as a yellow oil (40 mg, 95 %): δH (CDCl3, 400 MHz) 7.63 (d, J = 7.2, 2 H, Ar), 7.53 (t, J = 7.2, 1 H, Ar), 7.49–7.41 (m, 3 H, Ar), 7.35 (s, 1 H, Ar), 7.16 (d, J = 8.0, 1 H, Ar), 6.04 (br s, 2 H, NH2), 1.58 (s, 9 H, 3 × CH3).
(R)-tert-Butyl 1-(4-cyclohexylbenzyl)-3-isobutyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylate (15)
Prepared according to General Procedure A and B, to give the title compound as a yellow solid (43 mg, 99%): mp 93–95 °C; δH (DMSO-d6, 400 MHz) 8.07 (s, 1 H, Ar), 7.69 (d, J = 7.6, 1 H, Ar), 7.51 (t, J = 7.6, 1 H, Ar), 7.41 (t, J = 7.6, 2 H, Ar), 7.33–7.26 (m, 3 H, Ar), 6.99 (d, J = 7.6, 2 H, Ar), 6.91 (d, J = 7.6, 2 H, Ar), 5.30 (d, J = 15.6, 1 H, CHaHbAr), 4.99 (d, J = 15.6, 1 H, CHaHbAr), 3.69–3.65 (m, 1 H, CHCO), 2.44–2.34 (m, 1 H, Cy), 2.17–2.11 (m, 1 H, CHMe2), 1.95–1.60 (m, 7 H, CH2iPr, Cy), 1.52 (s, 9 H, 3 × CH3), 1.38–1.12 (m, 5 H, Cy), 0.95 (d, J = 5.6, 3 H, CH3), 0.78 (d, J = 5.6, 3 H, CH3); δC (DMSO-d6, 100 MHz) 169.4, 167.6, 164.2, 146.9, 142.4, 138.3, 135.0, 134.3, 133.5, 131.0, 130.3, 129.5, 128.7, 127.3, 127.2, 125.1,123.8, 82.1, 61.7, 49.8, 43.7, 34.4, 34.2, 28.1, 26.8, 26.7, 26.0,24.7, 23.8, 22.4; LRMS: m/z C37H44N2O3 requires: 564.3, found: 565.3 [M+H]; tR = 19.01 (100%, V).
(R)-1-(4-Cyclohexylbenzyl)-3-isobutyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepine-8-carboxylic acid (16)
Prepared according to General Procedure C to give the title compound as a yellow solid (43 mg, 99%): mp 104–105 °C; δH (DMSO-d6, 400 MHz) 8.17 (s, 1 H, Ar), 7.73 (d, J = 7.6, 1 H, Ar), 7.51 (t, J = 7.2, 1 H, Ar), 7.40 (t, J = 7.2, 2 H, Ar), 7.30–7.21 (m, 3 H, Ar), 6.94 (d, J = 7.6, 2 H, Ar), 6.84 (d, J = 7.6, 2 H, Ar), 5.47 (d, J = 15.6, 1 H, CHaHbAr), 4.90 (d, J = 15.6, 1 H, CHaHbAr), 3.67–3.64 (m, 1 H, CHCO), 2.40–2.32 (m, 1 H, Cy), 2.19–2.05 (m, 1 H, CHMe2), 1.95–1.60 (m, 7 H, CH2iPr, Cy), 1.38–1.09 (m, 5 H, Cy), 0.94 (d, J = 5.6, 3 H, CH3), 0.77 (d, J = 5.6, 3 H, CH3); δC (DMSO-d6, 100 MHz) 168.8, 167.3, 166.3, 146.4, 141.7, 137.9, 134.3, 133.5, 133.3, 130.5, 129.8, 129.0, 128.2, 127.0, 126.7, 125.1, 123.9, 61.2, 48.9, 43.2, 34.0, 33.7, 26.3, 25.5, 24.3, 23.3, 22.0; LRMS: m/z C33H36N2O3 requires: 508.3, found: 509.3 [M+H]; tR = 27.29 (100%, II).
3.2 HIV-1PR Assay
Materials
Recombinant HIV-1PR was prepared from Escherichia coli inclusion bodies and purified according to previously published procedures.26–29 Stock solutions (20 mM) of inhibitors were prepared in 100% DMSO. The catalytic activities of the HIV-1PR were monitored by the hydrolysis of the chromogenic substrate Lys-Ala-Arg-Val-Nle-nPhe-Glu-Ala-Nle-NH2, where Nle stands for norleucine and nPhe stands for 4-nitrophenylalanine (California Peptide Research, Napa, CA), and the fluorogenic substrate Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg (Molecular Probes).
Determination of Kinetic Parameters
Inhibition constants, Ki, for the inhibitors were obtained at 25 °C by measuring the rate of fluorogenic substrate hydrolysis using final concentrations of 30–40 nM HIV-1 protease in 10 mM sodium acetate, 1 M sodium chloride, 2% DMSO, pH 5.0, and 16.7 μM substrate at different inhibitor concentrations in the range of 0–200 μM. Inhibition constants were obtained by fitting the data to standard equations for tight-binding competitive inhibitors.
Kinetic parameters, Km, kcat, and Vmax were determined by initial rate measurements at 25 °C. HIV-1 protease was added to a 120-μl microcuvette containing substrate at 25°C. Final concentrations in the assay were: 30–40 nM active HIV-1 protease, 0–255 μM substrate, 10 mM sodium acetate, 1 M sodium chloride, and 2% DMSO, pH 5.0. In the spectrophotometric assay, the absorbance was monitored at 300 nm by using a Cary 100 spectrophotometer (Varian Instruments, Palo Alto, CA, USA). An extinction coefficient for the difference in absorbance upon hydrolysis (1,800 M−1·cm−1 at 300 nm) was used to convert absorbance change to reaction rates.27 Hydrolysis rates were obtained from the initial portion of the data, where at least 80% of the substrate remains unhydrolyzed. In the spectrofluorometric assay, the fluorescence was monitored by using a Cary Eclipse fluorescence spectrophotometer (Instruments, Palo Alto, CA, USA) with excitation and emission wavelengths of 340 nm and 490 nm, respectively. The concentration of active protease was determined by performing active site titrations with KNI-727, a very potent inhibitor (at pH 5.0, Ki ≈ 1.4 nM), using protease concentrations much higher (≈2 μM) than the corresponding Ki.
3.3 Cell assays
Cells and viruses
TZM-bl cells were a gift from Dr. Eric O. Freed (National Cancer Institute, Frederick, MD) and MT-4 cells were obtained from the NIH AIDS Reagent Program (Germantown, MD). Infected and uninfected cells were maintained in DMEM or RPMI, respectively, containing 10% heat-inactivated fetal bovine serum and 1× penicillin-streptomycin, at 37 °C with 5% CO2. Replication-competent HIV was generated by transfection of 293T cells using the pNL4-3 proviral sequence. The inhibitor compounds were initially resuspended in DMSO then diluted in the appropriate media, resulting in a final carrier DMSO concentration of 0.25%.
Endpoint cytotoxicity assays
Cytotoxicity assays were performed in triplicate on MT-4 cells, which were seeded at 2 × 105 cells/ml into 24 well plates in media containing the indicated concentration of inhibitor (carrier only, 10 μM, 50 μM, or 100 μM). After three days, the number of viable cells in each well was determined by trypan-blue dye exclusion.
Viral replication inhibition assays
TZM-bl cells were seeded onto 96 well plates at 1×104 cells/mL in 200μl and incubated overnight. After 24 h, the media was removed and diluted virus (MOI = 0.01) or mock was added to the cells in media containing either the carrier or inhibitor (final concentrations of 25 μM, 50 μM, or 100 μM). All conditions were done in triplicate.
At the indicated days post infection, the cells were washed once with 1× phosphate-buffered saline and then luciferase production was measured with the Britelite™plus Reporter Gene Assay System (Perkin-Elmer), according to manufacturer’s instructions. Plates were read on the Centro XS3 LB 960 Microplate Luminometer from Berthold Technologies and recorded with the MikroWin 2000 software.
Supplementary Material
Figure 6.
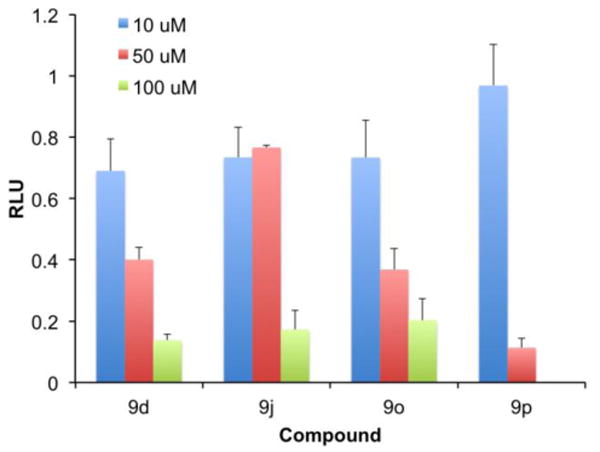
HIV-1 virus replication inhibition by select HIV-1PR inhibitors in HeLa CD4+ TZM-bl cells. RLU = relative luminescence units, relative to untreated control.
Acknowledgments
We thank the University of Maryland School of Pharmacy (start-up funds to SF) and the University of Maryland Seed Grant (SF and JJD) for financial support of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Brik A, Wong C-H. Org Biomol Chem. 2002;1:5–14. doi: 10.1039/b208248a. [DOI] [PubMed] [Google Scholar]
- 2.Pokorná J, Machala L, Řezáčová P, Konvalinka J. Viruses. 2009;1:1209–1239. doi: 10.3390/v1031209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potempa M, Lee SK, Wolfenden R, Swanstrom R. Curr Top Microbiol Immunol. 2015;389:203–241. doi: 10.1007/82_2015_438. [DOI] [PubMed] [Google Scholar]
- 4.Little S, Holte S, Routy J-P, Daar E, Markowitz M, Collier A, Koup R, Mellors J, Connick E, Conway B, Kilby M, Wang L, Whitmomb J, Hellmann N, Richman D. New Eng J Med. 2002;347:385–394. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 5.Flexner C. N Engl J Med. 1998;338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 6.Todd MJ, Semo N, Freire E. J Mol Biol. 1998;283:475–488. doi: 10.1006/jmbi.1998.2090. [DOI] [PubMed] [Google Scholar]
- 7.Bannwarth L, Reboud-Ravaux M. Biochem Soc Trans. 2007;35:551–554. doi: 10.1042/BST0350551. [DOI] [PubMed] [Google Scholar]
- 8.Schramm HJ, Bilich A, Jaeger E, Rücknagel KP, Arnold G, Schramm W. Biochem Biophys Res Commun. 1993;194:595–600. doi: 10.1006/bbrc.1993.1863. [DOI] [PubMed] [Google Scholar]
- 9.Schramm HJ, Boetzel J, Buttner J, Fritsche E, Gohring W, Jaeger E, Konig S, Thumfart O, Wenger T, Nagel NE, Schramm W. Antiviral Res. 1996;30:155–170. doi: 10.1016/0166-3542(96)00940-0. [DOI] [PubMed] [Google Scholar]
- 10.Zutshi R, Franciskovich J, Shultz M, Schweitzer B, Bishop P, Wilson M, Chmielewski J. J Am Chem Soc. 1997;119:4841–4845. [Google Scholar]
- 11.Schramm HJ, de Rosny E, Reboud-Ravaux M, Buttner J, Dick A, Schramm W. Lipopeptides as dimerization inhibitors of HIV-1 protease. Biol Chem. 1999;380:593–596. doi: 10.1515/BC.1999.076. [DOI] [PubMed] [Google Scholar]
- 12.Bouras A, Boggetto N, Benatalah Z, de Rosny E, Sicsic S, Reboud-Ravaux M. J Med Chem. 1999;42:957–962. doi: 10.1021/jm9803976. [DOI] [PubMed] [Google Scholar]
- 13.Shultz MD, Bowman MJ, Ham YW, Zhao X, Tora G, Chmielewski J. Angew Chem Int Ed Engl. 2000;39:2710–2713. doi: 10.1002/1521-3773(20000804)39:15<2710::aid-anie2710>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Bannwarth L, Kessler A, Pethe S, Collinet B, Merabet N, Boggetto N, Sicsic S, Reboud-Ravaux M, Ongeri S. J Med Chem. 2006;49:4657–4664. doi: 10.1021/jm060576k. [DOI] [PubMed] [Google Scholar]
- 15.Bowman MJ, Chmielewski J. Sidechain-linked inhibitors of HIV-1 protease dimerization. Bioorg Med Chem. 2009;17:967–976. doi: 10.1016/j.bmc.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 16.Dumond J, Boggetto N, Schramm HJ, Schramm W, Takahashi M, Reboud-Ravaux M, Bannwarth L, Rose T, Dufau L, Vanderesse R, Dumond J, Jamart-Gregoire B, Pannecouque C, de Clercq E, Reboud-Ravaux M. Biochemistry. 2009;48:379–387. doi: 10.1021/bi801422u. [DOI] [PubMed] [Google Scholar]
- 17.Lee SG, Chmielewski J. Chem Bio Chem. 2010;11:1513–1516. doi: 10.1002/cbic.201000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.B-flaps Ishima R, Freedberg D, Wang Y-X, Louis JM, Torchi DA. Structure. 1999;7:1047–1055. doi: 10.1016/s0969-2126(99)80172-5. [DOI] [PubMed] [Google Scholar]
- 19.Cígler P, Kozísek M, Rezácová P, Brynda J, Otwinowski Z, Pokorná J, Plesek J, Grüner B, Dolecková-Maresová L, Mása M, Sedlácek J, Bodem J, Kräusslich HG, Král V, Konvalinka J. Proc Natl Acad Sci U S A. 2005;25:15394–15399. doi: 10.1073/pnas.0507577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Böttcher J, Blum A, Dörr S, Heine A, Diederich WE, Klebe G. ChemBioChem. 2008;3:1337–1344. doi: 10.1002/cmdc.200800113. [DOI] [PubMed] [Google Scholar]
- 21.Kemp DS, Li Z. Q Tetrahedron Lett. 1995;36:4175–4178. [Google Scholar]
- 22.Wyrembak PN, Hamilton AD. J Am Chem Soc. 2009;131:4566–4567. doi: 10.1021/ja809245t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung K-Y, Vanommeslaeghe K, Lanning ME, Yap JL, Gordon C, MacKerell AD, Jr, Fletcher S. Org Lett. 2013;15:3234–3237. doi: 10.1021/ol401197n. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan J, Fletcher S. Tetrahedron Lett. 2012;53:4951–4954. [Google Scholar]
- 25.Salerno CP, Cleaves HJ. Synth Commun. 2004;34:2379–2386. [Google Scholar]
- 26.Mildner AM, Rothrock DJ, Leone JW, Lull JM, Reardon IM, Bannow CA, Sarcich JL, Howe WJ, Tomich CC, Smith CW, Heinrickson RL, Tomasselli AG. Biochemistry. 1994;33:9405–9413. doi: 10.1021/bi00198a005. [DOI] [PubMed] [Google Scholar]
- 27.Todd MJ, Semo N, Freire E. J Mol Biol. 1998;283:475–488. doi: 10.1006/jmbi.1998.2090. [DOI] [PubMed] [Google Scholar]
- 28.Velazquez-Campoy A, Luque I, Todd MJ, Milutinovich M, Kiso Y, Freire E. Protein Science. 2000;9:1801–1809. doi: 10.1110/ps.9.9.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawasaki Y, Chufan EE, Lafont V, Hidaka K, Kiso Y, Amzel LM, Freire E. Chem Biol Drug Des. 2010;75:143–151. doi: 10.1111/j.1747-0285.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


