Abstract
Objective
In older patients with cancer, we aimed to investigate associations between a patient-reported outcome measure for sarcopenia (SarcoPRO) and the Short Physical Performance Battery (SPPB), self-reported falls, and limitations in instrumental activities of daily living (IADLs).
Materials and Methods
Assessments were conducted as part of the initial evaluation of older, often frail, patients with cancer seen in the Specialized Oncology Care and Research in the Elderly (SOCARE) clinic. Univariate associations were evaluated using Spearman’s correlation and Wilcoxon sign ranked tests. Logistic regressions were used to identify associations of clinical factors and SarcoPRO scores or SPPB scores with falls and IADL limitations.
Results
In total, 174 older patients with cancer were evaluated. A moderate correlation was found between the SarcoPRO and the SPPB (ρ = 0.62). After adjusting for multiple clinical factors, neither the SarcoPRO nor the SPPB were associated with falls. In contrast, both higher SarcoPRO (i.e., worse) and lower SPPB (i.e., worse) scores were associated with limitations in IADLs (Odds ratio for one unit change in predictor: SarcoPRO: 1.06, p<0.0001; SPPB: 0.71, p = 0.003, respectively). Models using the SarcoPRO and SPPB explained similar amounts of variability in association with IADL limitations (AUC: 0.88 vs 0.87, respectively).
Conclusions
The SarcoPRO was moderately associated with the SPPB, an objective measure of physical performance, and was associated with limitations in IADLs. Thus, older patients with cancer who present with IADL limitations should be screened for sarcopenia. The SarcoPRO shows promise as a measure for screening as well as outcome assessment for research on sarcopenia.
Background
Sarcopenia is the degenerative loss of muscle mass, quality, and strength associated with aging. Sarcopenia is commonly accelerated in patients with cancer as a result of upregulation of the inflammatory response, malnutrition, or cancer treatments (1–8). Low scores on a lower extremity physical performance test, which can indicate muscle weakness, have been identified as a major risk factor for falls in older adults (9, 10). The prevalence of falls is higher for patients with cancer compared with their age-matched counterparts without cancer (11–13). For example, a nationally-representative, population-based study of older Medicare beneficiaries found that over 1 out of 5 cancer survivors reported recent falls, which was higher than in an age-matched cohort without cancer (11). Cancer is more common in the elderly population, in which sarcopenia is more common than in younger adults. Furthermore, patients with cancer are more likely to have sarcopenia than individuals of similar age (14, 15).
In addition to falls, limitations in independent activities of daily living (IADLs) can compromise patient’s ability to live independently and greatly decrease the quality of life of elderly individuals. A longitudinal study in community dwelling older adults showed that baseline limitations in IADLs predicted an increase in the number of physician visits in the subsequent three years (16). Using the Surveillance Epidemiology and End Results data and the Medicare health outcomes survey linked data set (i.e., SEER-MHOS data set), Stafford et al. (17) found that patients with cancer reported interference in IADLs more often than similar patients without cancer. Patients who experienced chemotherapy toxicity reported a larger decrease in physical function and ability to independently perform IADLs than patients with cancer who did not experience chemotherapy toxicity(18).
Several studies have shown that decreased physical function and/or muscle weakness are associated with IADL deficits and can predict future limitations in IADLs (16, 19–22). In these studies, sarcopenia or loss of muscle size and strength is usually measured using physical function tests such as the Short Physical Performance Battery (SPPB) (23). These performance tests, which must be administered in the clinic, include timed short distance walks, repetitive chair stands, and balance tests. These tests require trained professionals with adequate time and space; all of which are often unavailable in busy clinics. Deficits in lower extremity physical function, including muscle weakness, are commonly identified as a risk factor for falls and is associated with interference in IADLs in the elderly and cancer patient populations (24). Therefore, identifying a quick, inexpensive self-report tool that can assess sarcopenia and potentially predict falls or limitations in IADLs could help identify patients for falls prevention and interventions designed to preserve IADLs.
The objective of this study was to investigate the utility of a self-reported measure of sarcopenia and generate hypotheses to test in future studies by assessing its association with an objective measure of lower extremity physical performance (i.e., SPPB) as well as falls and limitations in IADLs in frail, older patients with cancer.
Methods
Patients and assessments
The study cohort consisted of patients aged 65 and over who were referred to the Specialized Oncologic Care and Research in the Elderly (SOCARE) clinic for comprehensive evaluation with geriatric assessment (GA). The older patients referred to the SOCARE clinic are generally newly diagnosed and often frail patients who have recently been started on treatment or in the process of deciding which treatments are appropriate. Because chemotherapy has a high risk of toxicity in frail patients, older patients are referred to the SOCARE clinic for the expert advice of geriatric oncologists who provide assessments and counselling for frail patients regularly to help decide whether to initiate chemotherapy. This study was approved by the University of Rochester’s Research Subjects Review Board. All patients signed a written informed consent. Upon referral to the SOCARE clinic, patients reported if they had fallen in the last 12 months using a standard questionnaire (25). They then completed the SarcoPRO (patient-reported outcome measure for sarcopenia)(26). The SarcoPRO was recently developed using open-ended interviews in which patients with known sarcopenia (n=12) were asked to characterize the functional effects of their reduced muscle strength on their daily lives. A common set of codes was developed to summarize the data and create a preliminary survey. Subsequent cognitive interviews with another cohort of sarcopenia patients (n = 12) were used to finalize the measure (26). The originally published measure consists of 14 items, a series of questions (each ranked on a 0 to 10 numeric rating scale) asking about physical limitations of the lower extremities, including those resulting from muscle weakness. One item, number 10 [“How much difficulty did you have lifting objects that weigh about 10 pounds, for example, a gallon of milk?”], in the original publication was removed from the survey for this study because of close redundancy with number 11 [How much difficulty did you have carrying objects that weigh about 10 pounds, for example, a gallon of milk?”]. The SarcoPRO score was calculated by adding the individual question scores, with the possible composite scores ranging from 0 to 130. The SPPB was also administered during the geriatric assessment. The performance test is based on the time it takes patients to stand from a seated position five times, how long they can balance in various standing positions, and a timed 4 meter walk test. The patients also reported limitations in IADLs including telephone use, travel further than walking distance, grocery shopping, meal preparation, housework, independent medication management, and handling money using Lawton’s measure of IADLs (27). Cognitive function was measured using the validated Blessed Orientation-Memory-Concentration Test (BOMC) (28). Depression was assessed using the Geriatric Depression Scale (GDS), a validated tool for identifying depression in seniors (29).
Age, gender, use of prescription medications that can cause dizziness (i.e., opioid, benzodiazepine, or sleeping medications / hypnotics, e.g., eszopiclone), cancer stage and treatment history were obtained during in-person interviews and from the medical charts. Opioid, benzodiazepine, or sleeping medication / hypnotic prescription was defined by the patients’ report of taking any of these drugs for any length of time and at any frequency at the time of the assessment. Patients were considered to have had previous chemotherapy, radiation, or surgery for cancer if they had any of these treatments for the cancer for which they were currently being treated at any time in the past.
Statistical analyses
Imputation of missing data: When 1 of 13 data points was missing for the SarcoPRO score, the missing value was replaced with the average of the other 12 answers (imputation of the mean, (30)). If more than 1 data point was missing for the SarcoPRO, the total SarcoPRO score was considered missing. When 1 of 10 data points was missing for the GDS it was replaced with a 0, corresponding to a ‘no’ answer for each yes or no question in the scale (i.e., will bias toward lower estimate of depression). If more than one data point was missing, the data for the GDS was considered missing for that patient. Bivariate analysis: Wilcoxon Rank Sum tests were used to compare the distribution of the SarcoPRO scores between fallers and non-fallers and patients reporting any or no limitations in IADLs. Limitations in IADLs were dichotomized to increase the clinical interpretability of the results. The correlation between the SarcoPRO and the SPPB was evaluated using Spearman’s correlation.
Threshold analysis
Receiver operating characteristic (ROC) curves were used to identify a cut-off SarcoPRO score that could potentially distinguish between groups. Cut-off scores that provided the combination of the highest sensitivity and specificity were identified by the JMP 11 program.
Multivariate analysis
In order to determine if the SarcoPRO or SPPB were associated with falls or limitations in IADLs after adjusting for clinical variables that may confound these associations, independent associations between SarcoPRO or SPPB scores and falling or limitations in IADLs were assessed using separate multivariable logistic regression models including clinical factors. The purpose of creating separate identical models other than the inclusion of the SarcoPRO or the SPPB score was to test the relative utility of similar multivariable models including each measure to assess whether the SarcoPRO has potential to act as a surrogate for the SPPB in future research studies in an older cancer population. Independent variables including age, gender, cancer stage, previous treatment [(radiation, or surgery) (yes vs. no), previous chemotherapy (yes vs. no)], opioids, benzodiazepine, or sleeping medication / hypnotic usage (yes v. no), cognitive impairment (yes (i.e., ≥ 11) v. no), and depression score along with the SarcoPRO or the SPPB were used to create separate logistic regression models. Falls (yes vs. no) or limitations in IADLs (none vs. any) were the dependent variables. Our pre-specified analysis plan indicated that all independent variables would be included in the model simultaneously and retained in the model unless the variance inflation factor (VIF) for any variable is greater than 10. If the VIF of any variable was greater than 10, it would have been removed from the model unless it was the SarcoPRO or SPPB. None of the variables were removed from the models due to VIF. The final regression models with the SarcoPRO and the SPPB were compared using Bayesian information criteria (BIC) and area under the curve (AUC) values.
A p-value < 0.05 was considered significant. Adjustments were not made for multiple testing because of the exploratory nature of the study. Statistical analyses were performed using JMP 11.
Results
Data set
In total, 174 patients provided a SarcoPRO score between May 2011 and August 2013, with 20 of these patients having one answer imputed. Of those 174 patients, 150 reported on falls and 155 completed the IADL assessment. The GDS was completed by 156 participants, with 21 participants having one value imputed. The BOMC was completed by 159 participants and 160 participants provided information regarding medications. The SPPB test was completed by 161 patients.
Participant characteristics
The median participant age was 80 years old and 59% of participants were male. Few participants were identified as having depressive symptoms by the GDS (24% GDS ≥ 5) or cognitive impairment (15% BOMC score ≥ 11). Almost one third (31%) of participants reported having a prescription for either an opioid, benzodiazepine, or sleeping medication / hypnotic. While only 18% of participants had previously received chemotherapy, 70% had previously undergone surgery or radiation therapy for their cancer. Over half (61%) of participants had late stage cancer (i.e., stage 3 or 4). Over one third (36%) reported falling in the previous year and 63% reported at least one limitation in IADLs. The median SPPB score was 7, indicating that limitations in physical function were significant in this sample (Table 1). Between 11% and 37% of patients reported severe difficulty (i.e., 7–10 score) with any individual activity asked about in the SarcoPRO (Table 2).
Table 1.
Participant characteristics
| Characteristic | N (%) or Median (IQR) |
|---|---|
|
| |
| Age | 80 (76 – 85) |
|
| |
| Sex | |
| Male | 103 (59%) |
|
| |
| Cancer Type | |
| GI | 53 (30%) |
| Prostate | 35 (20%) |
| Lung | 33 (19%) |
| Bladder | 12 (7%) |
| Breast | 9 (5%) |
| Blood | 3 (2%) |
| Skin | 3 (2%) |
| Other | 26 (15%) |
|
| |
| Depression score | |
| Low (<5) | 108 (76%) |
| High (≥ 5) | 34 (24%) |
|
| |
| Blessed Orientation Memory Concentration Score | |
| Low (<11) | 130 (85%) |
| High (≥ 11) | 23 (15%) |
|
| |
| Opioid / Benzodiazepine / sleeping medication / hypnotic prescription | |
| Yes | 54 (31%) |
| No | 120 (69%) |
|
| |
| Previous Chemotherapy | |
| Yes | 31 (18%) |
| No | 142 (82%) |
|
| |
| Previous Surgery or Radiation therapy | |
| Yes | 122 (70%) |
| No | 52 (30%) |
|
| |
| Stage | |
| 1 | 33 (19%) |
| 2 | 34 (20%) |
| 3 | 65 (37%) |
| 4 | 42 (24%) |
|
| |
| Falls | |
| Yes | 60 (36%) |
| No | 107 (64%) |
|
| |
| Any IADL | |
| Any limitation | 109 (63%) |
| No limitation | 63 (37%) |
|
| |
| SPPB | 7 (4, 10) |
GI – gastrointestinal; IADL – instrumental activities of daily living; SPPB – Short Physical Performance Battery.
Table 2.
Rating of SarcoPRO individual questions asking “how much difficulty” each participant had with the following activities
| Question | 0 – 3 rating N (%) | 4–6 rating N (%) | 7 – 10 rating N (%) | Median (IQR) |
|---|---|---|---|---|
| Walking at your usual speed | 98 (56%) | 33 (19%) | 43 (25%) | 3 (0. 6.25) |
| Walking a distance, for example, walking 100 yards or the length of a football field | 91 (52%) | 18 (10%) | 64 (37%) | 3 (0, 10) |
| Walking in a straight line, for example, down a hallway | 130 (75%) | 16 (9%) | 28 (16%) | 0 (0, 4.25) |
| Walking without stumbling | 137 (79%) | 17 (10%) | 20 (11%) | 0 (0, 3) |
| Going up or down stairs (a flight of stairs or 12 steps) | 101 (58%) | 31 (18%) | 42 (24%) | 2 (0, 6) |
| Standing for 15 minutes without a break | 91 (52%) | 26 (15%) | 57 (33%) | 3 (0, 8) |
| Getting up from a sitting position | 116 (67%) | 25 (14%) | 33 (19%) | 2 (0, 5) |
| Bending to pick up an object off the floor from a standing position | 113 (65%) | 18 (10%) | 43 (25%) | 1.5 (0, 6.25) |
| Opening jars that have never been opened | 109 (63%) | 30 (17%) | 35 (20%) | 2 (0, 5) |
| Lifting objects that weigh about 10 pounds, for example, a bag of potatoes | 128 (74%) | 16 (9%) | 30 (17%) | 0 (0, 4) |
| Doing your usual household activities without resting | 100 (57%) | 36 (21%) | 38 (22%) | 3 (0, 6) |
| Completing a physical activity without resting | 84 (48%) | 44 (25%) | 46 (26%) | 4 (1, 7) |
| Doing social activities outside your home, such as going out to eat with others | 126 (72%) | 22 (13%) | 26 (15%) | 1 (0, 4) |
| Total | --- | --- | --- | 30 (7, 70) |
Univariate associations between SarcoPRO and falls, IADLs, and SPPB
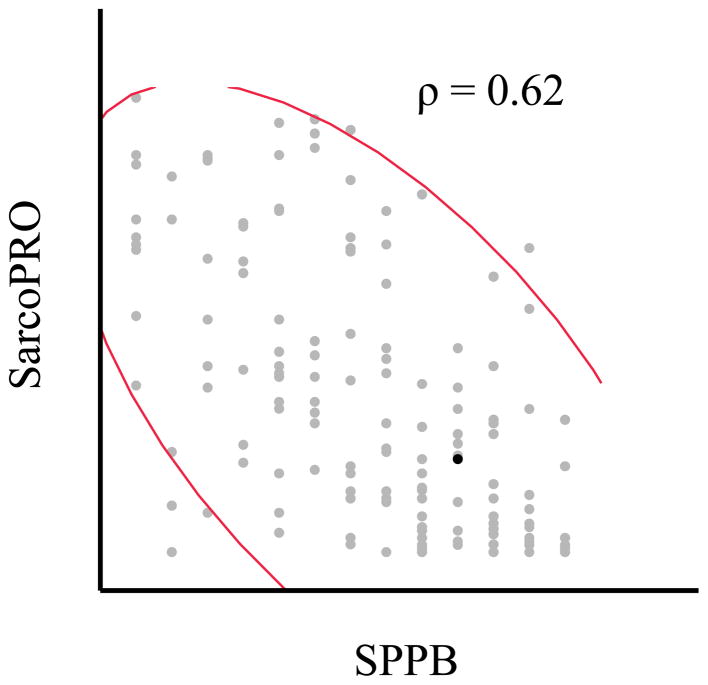
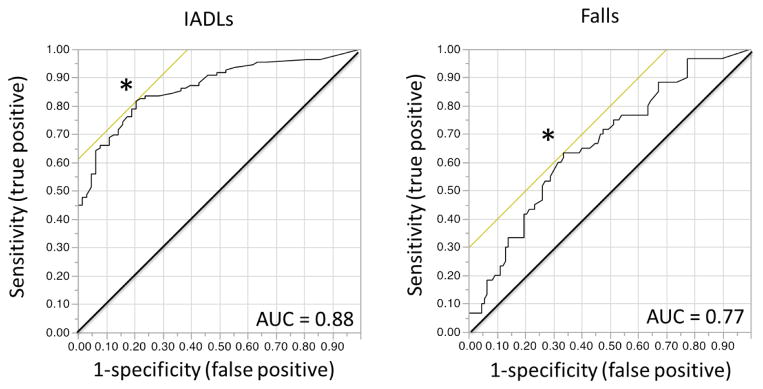
The SarcoPRO score was higher for fallers than non-fallers (mean (SD): 52.8 (38.7) vs. 33.2 (34.6), p = 0.0006) and for patients who reported any IADL impairment vs. patients who reported no IADL impairment (mean (SD): 57.0 (36.5) vs. 12.5 (14.9), p <0.0001). The SarcoPro scores were moderately correlated with the SPPB scores (Spearman’s correlation = 0.62, p < 0.0001) (Figure 1). The ROC curve for any vs. no IADLs indicates the best cut-off in SarcoPRO to identify participants with any limitation in IADLs is 22 (sensitivity: 0.82; specificity 0.80, AUC = 0.88). The ROC curve for fallers vs. non-fallers indicates the best cut-off in SarcoPRO to identify fallers is 39 (sensitivity: 0.63; specificity 0.76, AUC = 0.77) (Figure 2).
Figure 1.
Correlation between the SarcoPRO and Short Physical Performance Battery (SPPB) scores (n = 160).
Figure 2.
Received operating characteristic curves for SarcoPRO scores predicting patients with any limitation vs. no limitation in instrumental activities of daily living (IADLs) (n = 167) and fallers vs. non-fallers. * represents the cut-off value for each curve that maximizes sensitivity and specificity. Area under the curve (AUC).
Multivariable regressions evaluating associations between SarcoPRO or SPPB and falls and limitations in IADLs
The association between SarcoPRO or SPPB scores and falls was not maintained in multivariable logistic regression analyses (Table 3). In both models, previous surgery or radiation therapy was highly associated with falls. Patients who had previous radiation or surgery were approximately 11 times more likely to report falling than those who had not had previous surgery or radiation.
Table 3.
Logistic regression for Falls vs. No Falls (n = 132)
| SarcoPRO Model | SPPB Model | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Independent variable | ODDs ratio | 95% Confidence limits | p-value | ODDs ratio | 95% Confidence limits | p-value |
|
| ||||||
| Sex (M v F) | 0.75 | 0.29, 1.90 | 0.54 | 0.81 | 0.31, 2.10 | 0.66 |
|
| ||||||
| Age | 1.03 | 0.96, 1.11 | 0.36 | 1.02 | 0.94, 1.10 | 0.65 |
|
| ||||||
| Previous chemo (Y v N) | 0.97 | 0.32, 3.16 | 0.96 | 1.06 | 0.32, 3.31 | 0.92 |
|
| ||||||
| Previous surgery or radiation (Y v N) | 10.90 | 3.10, 54.86 | <0.0001 | 11.8 | 3.31, 60.40 | <0.0001 |
|
| ||||||
| Sedating med (Y v N) | 1.30 | 0.49, 3.38 | 0.59 | 1.53 | 0.58, 4.04 | 0.39 |
|
| ||||||
| Cancer stage | 0.47 | 0.34 | ||||
| 1 vs 2 | 0.59 | 0.13, 2.51 | 0.69 | 0.14, 3.08 | ||
| 1 vs 3 | 0.45 | 0.11, 1.61 | 0.45 | 0.11, 1.62 | ||
| 1 vs 4 | 0.31 | 0.06, 1.39 | 0.27 | 0.05, 1.20 | ||
|
| ||||||
| BOMC | 1.00 | 0.99, 1.02 | 0.90 | 0.98 | 0.91, 1.06 | 0.72 |
|
| ||||||
| GDS | 1.06 | 0.92, 1.21 | 0.43 | 1.04 | 0.91, 1.19 | 0.53 |
|
| ||||||
| SarcoPRO | 1.01 | 0.99, 1.02 | 0.41 | --- | --- | --- |
|
| ||||||
| SPPB | --- | --- | --- | 0.87 | 0.75, 1.00 | 0.055 |
|
| ||||||
| Goodness of Fit | AUC: 0.77, BIC: 192.40 | AUC: 0.78, BIC: 190.70 | ||||
BOMC – Blessed Orientation Memory Concentration Test, GDS – Geriatric Depression Scale, SPPB – Short Physical Performance Battery.
SarcoPRO and SPPB scores were both still significantly associated with IADLs in multivariable logistic regression models (Table 4). For every 1-unit increase in SarcoPRO (range: 0–130), the odds of reporting a limitation in IADLs were 6% higher (Table 4). For every 1-standard deviation (i.e., 36.95) change in SarcoPRO, the odds of reporting a limitation in IADLs were 735% (i.e., 7.35 times) higher (31). BOMC score was also highly associated with IADLs in the SarcoPRO model; patients who scored higher on the BOMC score (i.e., higher degree of cognitive impairment) were more likely to report a limitation in IADLs. For every 1-unit increase in SPPB score (range: 0–12, lower score indicating poorer physical function), the odds of reporting a limitation in IADLs were 30% higher (Table 4). For every 1-standard deviation (i.e., 3.46) change in SPPB, the odds of reporting a limitation in IADLs were 69% higher. In the SPPB model a BOMC score was also associated with limitations in IADLs. In the SPPB model, a higher GDS score was associated with reporting any limitation in IADLs but not in the SarcoPRO model. All variance inflation factors were under 2.0, suggesting no correlation between independent variables was too high. The measures of model fit (i.e., AUC and BIC) were similar for the models used to predict limitations in IADLs with SarcoPRO and SPPB scores with AUCs 0.88 and 0.87, respectively and BICs of 172.4 and 173.3, respectively.
Table 4.
Logistic regression for any limitation in IADL vs. no limitation in IADL (n = 134)
| SarcoPRO Model | SPPB Model | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Independent variable | ODDs ratio | 95% Confidence limits | p-value | ODDs ratio | 95% Confidence limits | p-value |
|
| ||||||
| Sex (M v F) | 0.74 | 0.26, 2.18 | 0.58 | 0.57 | 0.20, 1.60 | 0.29 |
|
| ||||||
| Age | 1.01 | 0.92, 1.11 | 0.79 | 1.06 | 0.97, 1.16 | 0.20 |
|
| ||||||
| Previous chemo (Y v N) | 1.51 | 0.36, 6.48 | 0.57 | 2.98 | 0.77, 12.62 | 0.11 |
|
| ||||||
| Previous surgery or radiation (Y v N) | 1.72 | 0.53, 5.93 | 0.37 | 2.02 | 0.69, 6.21 | 0.20 |
|
| ||||||
| Sedating med (Y v N) | 1.04 | 0.34, 3.19 | 0.94 | 1.49 | 0.53, 4.29 | 0.45 |
|
| ||||||
| Cancer stage | 0.90 | 0.44 | ||||
| 1 vs 2 | 1.14 | 0.24, 4.01 | 1.69 | 0.37, 8.10 | ||
| 1 vs 3 | 0.99 | 0.22, 3.34 | 0.98 | 0.25, 3.93 | ||
| 1 vs 4 | 0.62 | 0.11, 2.81 | 0.48 | 0.09, 2.34 | ||
|
| ||||||
| BOMC | 1.17 | 1.07, 1.33 | 0.0006 | 1.14 | 1.04, 1.29 | 0.005 |
|
| ||||||
| GD | 1.15 | 0.94, 1.45 | 0.19 | 1.32 | 1.10, 1.65 | <0.0001 |
|
| ||||||
| SarcoPRO | 1.06 | 1.03, 1.09 | <0.0001 | --- | --- | --- |
|
| ||||||
| SPPB | --- | --- | --- | 0.71 | 0.59, 0.84 | 0.003 |
|
| ||||||
| Goodness of Fit | AUC: 0.88, BIC: 172.4 | AUC: 0.87, BIC: 173.3 | ||||
BOMC – Blessed Orientation Memory Concentration Test, GDS – Geriatric Depression Scale, SPPB – Short Physical Performance Battery.
Discussion
This study investigated the relationship between a self-report measure of sarcopenia (SarcoPRO) and an objective measure of lower extremity physical function (SPPB) as well as falls and limitations in IADLs. The SarcoPRO and the SPPB were moderately correlated (ρ = 0.62) and both were associated with limitations in IADLs, even after adjusting for multiple clinical factors using multivariable regressions. These results suggest that the SarcoPRO could be used as a screening tool to alert clinicians to a possible problem with lower extremity physical function in the oncology clinic where it may not be feasible to perform the performance assessment of lower extremity physical function with SPPB on all patients. The data also support the construct validity (32) of the SarcoPRO as an outcome measure for research studies. However, more research is necessary to evaluate the reliability and discriminant validity of the measure (32).
Both the SarcoPRO and the SPPB were highly associated with any limitation in IADLs. The SarcoPRO could identify patients who reported any limitation with IADLs with both a sensitivity and specificity of at least 80%. Furthermore, even after adjusting for clinical factors in multivariable models, both the SarcoPRO and SPPB scores remained associated with limitations in IADLs. When considering the odds ratios for a standard deviation change in the SarcoPRO and SPPB, the SarcoPRO is more strongly associated with limitations in IADLs than the SPPB. Interestingly, depression was highly associated with limitations in IADLs in the model that contained the SPPB scores, but not the model that included the SarcoPRO. Depression has been linked to IADLs in previous studies (33–35), and in our study depression was associated with IADLs, but not falls. The models demonstrated very similar goodness of fit statistic (i.e., BIC and AUC values), and the standardized odds ratio (i.e., OR for a standard deviation change) is larger for the SarcoPRO than the SPPB suggesting that, in this case, the subjective, easily administered, PRO measure is likely just as effective as the more intensive objective measure. Consistent with previous studies (36–41), cognitive abilities were also associated with limitations in IADLs in both models.
Other studies have demonstrated an association between limitations in IADLs and decreased physical function and/or muscle weakness. For example, a meta-analysis of intervention trials showed that lower scores on various tests of physical function and muscle strength, including gait speed, balance, grip strength, and chair raise time were significantly associated with limitations in IADLs (22). Our results are consistent with this and other previous studies that have found associations between lower extremity physical function and IADLs (21, 22, 42–44), but our study is the first to show this association in older patients with cancer, using an easy to administer PRO measure. Two previous longitudinal studies have shown that muscle weakness or lower extremity physical performance at baseline can predict limitations with IADLs in the future in community dwelling elders (21, 22). Although prospective studies should be conducted in older patients with cancer, these data suggest that interventions targeted at improving lower extremity physical function and muscle strength could decrease limitations in IADLs in this population.
Using the SarcoPRO as an outcome measure in future intervention trials for IADL limitations could help determine if interventions improving IADLs could be mediated through treatments that decrease sarcopenia. Future research should also examine sarcopenia prospectively in combination with functional issues and other clinical outcomes associated with cancer treatment. Studies should investigate whether the SarcoPRO measure can identify which patients are at the highest risk of toxicity from treatments (i.e., chemotherapy, surgery, or radiation). Ultimately, this could lead to development of interventions targeted directly at the patients with the highest risk of toxicity from treatment.
In addition to a potential role in research, the SarcoPRO could be useful in clinical practice. Muscle strength is not routinely measured in cancer clinics. These results indicate that patients who have limitations in IADLs should be screened for lower extremity physical function and considered for interventions targeted at improve limitations in physical function, including those caused by muscle weakness. These results suggest the SarcoPRO could be used as an initial screen of lower extremity functional limitations to determine if more extensive evaluation and treatment is needed. However, future research is necessary to confirm this hypothesis.
Although univariate analyses suggested a relationship between both the SarcoPRO and the SPPB with self-reported falls, this relationship was not maintained in the multivariable model when the variance was adjusted for multiple clinical factors. This result is inconsistent with previous studies that have found relationships between muscle weakness and falls (9, 10, 24). For example, Tofthagen et al.(24) reported that falls were associated with increased muscle weakness, as measured by an item in a self-report chemotherapy-induced peripheral neuropathy measure, loss of balance, chemotherapy cycle number, and neuropathic symptoms. This lack of association could be due to the long recall time for the falls (i.e., one year).
Clinical factors, such as the use of medications with sedative properties, depression, and cognitive status have also been linked to falling in previous research (25, 45, 46). For example, Stone et al. (46) found that the following factors significantly predicted time to fall in a multivariate model: primary brain cancer or brain metastases, the number of falls in the three months prior to enrollment in the study, severity of depression, and daily benzodiazepine dose. Primary brain cancer or brain metastases may have increased falls by causing cognitive impairment. None of these clinical factors were found to be associated with falls in this study. It is important to note, however, that the population in the paper published by Stone et al. (46) included only patients receiving palliative care, which is likely a very different population than patients being treated at the SOCARE clinic. These differences could explain the difference in the associations between clinical factors and falls found between the two studies. Alternatively, the fact that patients were asked to recall whether they had fallen in the past year was a limitation of the study and could explain these results. Furthermore, the relatively few numbers of patients who reported a fall (i.e., 60) could be have led to overfitting of the model, which could produce a model that is too specific for the data set and not reproducible externally. Future research that assesses falls prospectively with a larger sample of fallers should be used to examine the relationship between the SarcoPRO and falling should be performed in older patients with cancer before any causal conclusions are drawn about this relationship.
The strengths of this study include the varied statistical approaches to investigate the associations between the SarcoPRO and falls and limitations in IADLs. The fact that the data were collected in a geriatric cancer clinic and not as part of a clinical trial suggests that the subjects are representative of a broad range of geriatric patients with cancer. The study has some limitations. These limitations include the cross-sectional design, and therefore, can only identify associations and not causation. The analyses include ROC curves and multivariable models, which are tools used to assess a variable(s) predictive value for an outcome, but in this case the results can only be interpreted as hypothesis-generating for future prospective studies. Patients were asked to recall falling in the year prior to assessment at the clinic. While this is a standard question for falls (25), this long recall time may underestimate the actual number of falls, and could explain why we did not find a strong association between falls and the SarcoPRO or SPPB. IADLs were assessed at the same time as sarcopenia, thus, we cannot make any conclusions about whether the SarcoPRO is useful as an early predictor of IADL limitations in the older patient population with cancer. This hypothesis should be tested in future studies to determine if the SarcoPRO can be used to help target interventions. Patients reported their current medications. We cannot be sure how consistently the patients took the medications or how long they were taking them prior to the assessment date and some of the patients may have difficulty remembering the names of all the medications that they are taking, which could lead to under reporting of opioids, benzodiazepine, or sleeping-pill use. Furthermore, other medications that we did not include, such as antidepressants, can also cause dizziness. These issues could explain why we did not find a relationship between prescription of a sedating medication and falls as has been identified in other studies (25, 46). However, other studies have also failed to find an association of falls with benzodiazepines and opiates in older cancer populations (47, 48).
Although the time interval between diagnosis and/or treatment and evaluation in the SOCARE clinic could be related to falls or limitations in IADLs, we did not include this variable in the model to avoid overfitting for the available sample size and heterogenous nature in terms of previous treatments, which were included in the model. Future studies of larger and less heterogenous samples should be used to investigate this possible association.
In conclusion, this study demonstrated a strong association between both measures of lower extremity physical function, the SarcoPRO and the SPPB, and limitations in IADLs. These findings in combination with results from two longitudinal studies in community dwelling elders suggest that interventions targeted at improving lower extremity physical function, including functional limitations caused by muscle weakness, could improve limitations in IADLs. These results also suggest that the SarcoPRO may be a valuable tool to assess sarcopenia related physical function limitations for research purposes and in the clinic when objective physical performance measures are not feasible. However, future studies are needed to further characterize the utility of the SarcoPRO in these settings. Furthermore, prospective studies are needed to assess the longitudinal relationship between physical function limitations identified by the SarcoPRO and IADL limitations in older patients with cancer prospectively.
Acknowledgments
We’d like to thank the staff (Terri Lloyd) that administers the geriatric assessments at the SOCARE clinic for generously allowing me to use their data for my thesis work. This work was funded by the National Institute on Aging, the National Cancer Institute and the Patient Centered Outcomes Research Institute (R03 AG042342, U10CA37420 and R01 CA177592). The work was also funded by the Susan H Green Memorial Grant and Hartford Foundation pilot grant (to Magnuson) and by the philanthropic donation of Sandy Lloyd to the Geriatric Oncology Program at the James Wilmot Cancer Institute, as well as by grant 1 K24 RR024198-02 from the National Heart Lung and Blood Institute (J. Dolan’s time). We would also like to thank the patients whose data was used for this study.
Footnotes
Disclosures and Conflict of Interest Statements
The authors have no conflicts of interest to disclose.
Author Contributions
Study Concept: J Gewandter, W Dale, S Mohile, J Dolan, K Noyes, A Magnuson
Study Design: J Gewandter, S Mohile, J Dolan, K Noyes, C Pandya, C Heckler
Data Acquisition: S Mohile, A Magnuson, T Lemelman, B Roussel, R Ifthikhar
Quality Control of Data and Algorithms: C Pandya
Data Analysis and Interpretation: J Gewandter, S Mohile, C Heckler
Statistical Analysis: J Gewandter, C Heckler
Manuscript Preparation: Jennifer Gewandter
Manuscript Editing: J Gewandter
Manuscript Review: W Dale, A Magnuson, C Pandya, C Heckler, T Lemelman, B Roussel, R Ifthikhar, J Dolan, K Noyes, S Mohile
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114(3):370–8. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol. 2010;28(6):1054–60. doi: 10.1200/JCO.2009.24.9730. [DOI] [PubMed] [Google Scholar]
- 3.Aslani A, Smith RC, Allen BJ, Levi JA. Changes in body composition during adjuvant chemotherapy for breast cancer. Appl Radiat Isot. 1998;49(5–6):637–8. doi: 10.1016/s0969-8043(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 4.Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, et al. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9(4):369–83. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen RD, Raja C, Allen BJ. Total body protein in chronic diseases and in aging. Ann N Y Acad Sci. 2000;904:345–52. doi: 10.1111/j.1749-6632.2000.tb06480.x. [DOI] [PubMed] [Google Scholar]
- 6.Preston T, Slater C, McMillan DC, Falconer JS, Shenkin A, Fearon KC. Fibrinogen synthesis is elevated in fasting cancer patients with an acute phase response. J Nutr. 1998;128(8):1355–60. doi: 10.1093/jn/128.8.1355. [DOI] [PubMed] [Google Scholar]
- 7.Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TL, Ke C, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2012;30(26):3271–6. doi: 10.1200/JCO.2011.38.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tisdale MJ. Pathogenesis of cancer cachexia. J Support Oncol. 2003;1(3):159–68. [PubMed] [Google Scholar]
- 9.Delbaere K, Van den Noortgate N, Bourgois J, Vanderstraeten G, Tine W, Cambier D. The Physical Performance Test as a predictor of frequent fallers: a prospective community-based cohort study. Clin Rehabil. 2006;20(1):83–90. doi: 10.1191/0269215506cr885oa. [DOI] [PubMed] [Google Scholar]
- 10.Stalenhoef PA, Diederiks JP, Knottnerus JA, Kester AD, Crebolder HF. A risk model for the prediction of recurrent falls in community-dwelling elderly: a prospective cohort study. J Clin Epidemiol. 2002;55(11):1088–94. doi: 10.1016/s0895-4356(02)00502-4. [DOI] [PubMed] [Google Scholar]
- 11.Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011;29(11):1458–64. doi: 10.1200/JCO.2010.31.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohile SG, Xian Y, Dale W, Fisher SG, Rodin M, Morrow GR, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101(17):1206–15. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisa Sprod SGM, Fan Lin, Janelsins Michelle Christine, Peppone Luke Joseph, Chandwani Kavita Dayal, Morrow Gary R, Mustian Karen Michelle. Physical activity participation and functional limitations in geriatric cancer survivors. J Clin Oncol. 2012;30(suppl):abstr 9009. [Google Scholar]
- 14.SEER Cancer Statistics. National Cancer Institute; 2011. Available from: http://seer.cancer.gov/statfacts/html/all.html. [Google Scholar]
- 15.Balducci L. Epidemiology of cancer and aging. J Oncol Manag. 2005;14(2):47–50. [PubMed] [Google Scholar]
- 16.Wolinsky FD, Miller DK, Andresen EM, Malmstrom TK, Miller JP, Miller TR. Effect of subclinical status in functional limitation and disability on adverse health outcomes 3 years later. J Gerontol A Biol Sci Med Sci. 2007;62(1):101–6. doi: 10.1093/gerona/62.1.101. [DOI] [PubMed] [Google Scholar]
- 17.Stafford RS, Cyr PL. The impact of cancer on the physical function of the elderly and their utilization of health care. Cancer. 1997;80(10):1973–80. doi: 10.1002/(sici)1097-0142(19971115)80:10<1973::aid-cncr15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Cantor A, Meyer J, Beth Corcoran M, Grendys E, Cavanaugh D, et al. Can older cancer patients tolerate chemotherapy? A prospective pilot study. Cancer. 2003;97(4):1107–14. doi: 10.1002/cncr.11110. [DOI] [PubMed] [Google Scholar]
- 19.Cesari M, Onder G, Russo A, Zamboni V, Barillaro C, Ferrucci L, et al. Comorbidity and physical function: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study) Gerontology. 2006;52(1):24–32. doi: 10.1159/000089822. [DOI] [PubMed] [Google Scholar]
- 20.Chan BK, Marshall LM, Winters KM, Faulkner KA, Schwartz AV, Orwoll ES. Incident fall risk and physical activity and physical performance among older men: the Osteoporotic Fractures in Men Study. Am J Epidemiol. 2007;165(6):696–703. doi: 10.1093/aje/kwk050. [DOI] [PubMed] [Google Scholar]
- 21.Giampaoli S, Ferrucci L, Cecchi F, Lo Noce C, Poce A, Dima F, et al. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing. 1999;28(3):283–8. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- 22.Judge JO, Schechtman K, Cress E. The relationship between physical performance measures and independence in instrumental activities of daily living. The FICSIT Group. Frailty and Injury: Cooperative Studies of Intervention Trials. J Am Geriatr Soc. 1996;44(11):1332–41. doi: 10.1111/j.1532-5415.1996.tb01404.x. [DOI] [PubMed] [Google Scholar]
- 23.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women's Health and Aging Study. J Clin Epidemiol. 2002;55(9):916–21. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 24.Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2012;20(3):583–9. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–7. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 26.Evans CJ, Chiou CF, Fitzgerald KA, Evans WJ, Ferrell BR, Dale W, et al. Development of a new patient-reported outcome measure in sarcopenia. Journal of the American Medical Directors Association. 2011;12(3):226–33. doi: 10.1016/j.jamda.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- 28.Davous P, Lamour Y, Debrand E, Rondot P. A comparative evaluation of the short orientation memory concentration test of cognitive impairment. J Neurol Neurosurg Psychiatry. 1987;50(10):1312–7. doi: 10.1136/jnnp.50.10.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 30.Little RJ, Rubin D. Statistical Analysis with Missing Data. 2. New York: John Wiley & Sons; 2002. [Google Scholar]
- 31.DWH, SL . Applied logistic regression. 2. Vol. 200 New York: Wiley; [Google Scholar]
- 32.LJC, PEM Construct validity in psychological tests. Psychol Bull. 1955;52:281–302. doi: 10.1037/h0040957. [DOI] [PubMed] [Google Scholar]
- 33.Pierluissi E, Mehta KM, Kirby KA, Boscardin WJ, Fortinsky RH, Palmer RM, et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. J Am Geriatr Soc. 2012;60(12):2254–62. doi: 10.1111/jgs.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawaskar MD, Anderson RT, Balkrishnan R. Self-reported predictors of depressive symptomatology in an elderly population with type 2 diabetes mellitus: a prospective cohort study. Health Qual Life Outcomes. 2007;5:50. doi: 10.1186/1477-7525-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiosses DN, Klimstra S, Murphy C, Alexopoulos GS. Executive dysfunction and disability in elderly patients with major depression. Am J Geriatr Psychiatry. 2001;9(3):269–74. [PubMed] [Google Scholar]
- 36.Anstey KJ, Cherbuin N, Eramudugolla R, Sargent-Cox K, Easteal S, Kumar R, et al. Characterizing mild cognitive disorders in the young-old over 8 years: prevalence, estimated incidence, stability of diagnosis, and impact on IADLs. Alzheimers Dement. 2013;9(6):640–8. doi: 10.1016/j.jalz.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Puente AN, Terry DP, Faraco CC, Brown CL, Miller LS. Functional Impairment in Mild Cognitive Impairment Evidenced Using Performance-Based Measurement. J Geriatr Psychiatry Neurol. 2014 doi: 10.1177/0891988714532016. [DOI] [PubMed] [Google Scholar]
- 38.Pirogovsky E, Schiehser DM, Obtera KM, Burke MM, Lessig SL, Song DD, et al. Instrumental activities of daily living are impaired in Parkinson's disease patients with mild cognitive impairment. Neuropsychology. 2014;28(2):229–37. doi: 10.1037/neu0000045. [DOI] [PubMed] [Google Scholar]
- 39.Brown PJ, Devanand DP, Liu X, Caccappolo E. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68(6):617–26. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn IS, Kim JH, Kim S, Chung JW, Kim H, Kang HS, et al. Impairment of instrumental activities of daily living in patients with mild cognitive impairment. Psychiatry Investig. 2009;6(3):180–4. doi: 10.4306/pi.2009.6.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burton CL, Strauss E, Bunce D, Hunter MA, Hultsch DF. Functional abilities in older adults with mild cognitive impairment. Gerontology. 2009;55(5):570–81. doi: 10.1159/000228918. [DOI] [PubMed] [Google Scholar]
- 42.Bylow K, Dale W, Mustian K, Stadler WM, Rodin M, Hall W, et al. Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology. 2008;72(2):422–7. doi: 10.1016/j.urology.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58(11):2055–62. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 44.Landi F, Russo A, Cesari M, Barillaro C, Onder G, Zamboni V, et al. The ilSIRENTE study: a prospective cohort study on persons aged 80 years and older living in a mountain community of Central Italy. Aging Clin Exp Res. 2005;17(6):486–93. doi: 10.1007/BF03327416. [DOI] [PubMed] [Google Scholar]
- 45.Spoelstra S, Given B, von Eye A, Given C. Fall risk in community-dwelling elderly cancer survivors: a predictive model for gerontological nurses. J Gerontol Nurs. 2010;36(2):52–60. doi: 10.3928/00989134-20100108-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128–33. doi: 10.1200/JCO.2011.40.7791. [DOI] [PubMed] [Google Scholar]
- 47.Puts MT, Monette J, Girre V, Wolfson C, Monette M, Batist G, et al. The fall rate of older community-dwelling cancer patients. Support Care Cancer. 2013;21(3):775–83. doi: 10.1007/s00520-012-1579-4. [DOI] [PubMed] [Google Scholar]
- 48.Spoelstra SL, Given BA, Schutte DL, Sikorskii A, You M, Given CW. Do older adults with cancer fall more often? A comparative analysis of falls in those with and without cancer. Oncology nursing forum. 2013;40(2):E69–78. doi: 10.1188/13.ONF.E69-E78. [DOI] [PubMed] [Google Scholar]