Abstract
Nuclear steroid hormone receptors are ubiquitously expressed transcription factors whose activity can be altered by post-translational modifications, such as phosphorylation. The consequences of post-translational modifications have been described for several members of the nuclear steroid hormone receptor superfamily, however little is known about the effects of oestrogen receptor beta (ERβ) phosphorylation in the brain. Moreover, to our knowledge the presence of phosphorylated ERβ has not been detected in the brain of any species to date. Oestrogen receptor β is highly expressed in several regions of the brain and in vitro studies have demonstrated that it can be phosphorylated at two serine residues (S87 and S105) in the N-terminal AF-1 region. The goal of this study was to determine whether phosphorylated ERβ is detectable in the hippocampus of aged female rats, and to determine the functional consequences of ERβ S87 and S105 phosphorylation on transcriptional activity in neuronal cells. First, we used a novel PhosTag™ approach to detect phosphorylated forms of ERβ in the dorsal hippocampus of aged female rats. The data demonstrated several abundant forms of phosphorylated ERβ in the dorsal hippocampus, suggesting that this post translational modification might be an important regulator of ERβ function. To assess the functional consequences of ERβ phosphorylation in neuronal cells, we created phospho-mimetic (S87E, S105E) and phospho-null (S87A, S105A) ERβ receptors that were transiently transfected in a hippocampal-derived cell line. Collectively our results showed that phosphorylation of S87 and S105 altered both ligand-independent and ligand-dependent ERβ transcriptional regulation. Overall these data demonstrate that phosphorylated forms of ERβ are present in the brain of aged female rats and that phosphorylation of ERβ could differentially alter ERβ-mediated gene expression.
Keywords: Oestrogen Receptor β, phosphorylation, hippocampus
INTRODUCTION
Nuclear steroid receptors are master regulators of a broad range of physiological processes through their actions as ligand-activated transcription factors. Oestrogen receptors (ERα and ERβ) are members of this receptor superfamily and their cognate endogenous ligand is 17β-estradiol (E2), which is the major circulating form of oestrogen in premenopausal women. ERα and ERβ are widely expressed in a variety of tissues, although ERα is particularly abundant in breast, uterus and ovary, due to its primary role in mediating the reproductive-related effects of oestrogens. By contrast, ERβ is highly expressed in many non-reproductive tissues such as nervous, cardiovascular, skeletal, gastric, and adipose tissues, and has been implicated in mediating oestrogens effects on anxiety, mood, and memory, as well as numerous other physiological processes [1–6]. Our basic understanding of ERα structure, function, and signalling pathways has significantly advanced in recent years, however similar aspects of ERβ function, especially in non-reproductive tissue/cell types, remain unclear. The overall goal of these studies was to define the functional consequences of post-translational modifications to ERβ, namely site-specific phosphorylation, on its ability to transcriptionally activate gene promoter activity in neuronal cells.
Post-translational modifications (PTMs) such as phosphorylation, ubiquitination, acetylation, and sumoylation have been identified for several nuclear steroid receptors, including ERs [7–11]. These modifications have the potential to alter all aspects of ER function including ligand binding, dimerization, protein:protein interactions, DNA binding and, ultimately, alter ER-mediated transcription. Although several kinase consensus sites have been predicted for ERβ, only a few have been experimentally confirmed [12]. Specifically, the serine residues S87 and S105 located in the N-terminal domain of ERβ are highly conserved among the mouse, rat, and human, suggesting that ERβ phosphorylation at these sites could be a common regulatory mechanism across species [13]. The N-terminal domain of ERβ exhibits greater than 80% homology across species and the specific amino acid residues flanking S87 and S105 are highly conserved [14]. By contrast, the N-terminal domain of ERβ has relatively low homology with ERα, which could contribute to the divergent actions of the two receptors. These S87 and S105 sites are targets of MAPKs (Mitogen Activated Protein Kinases) and the specific MAPKs, P38 and ERK, have been shown to phosphorylate human and mouse ERβ in vitro [13, 15]. Functionally, phosphorylation of these sites increased recruitment of the coregulatory protein SRC-1 (steroid receptor coactivator-1), while coincidentally increasing transcriptional activation at an oestrogen response element (ERE) [15].
Phosphorylation of ERβ has been studied primarily in vitro using breast tumour cell models. The only reports of detection of phosphorylation of ERβ in vivo come from immunohistochemistry analysis of human breast cancer tissue using a human-specific antibody generated against the phosphorylated S105 ERβ [13]. Therefore, our first goal in these studies was to determine whether ERβ is phosphorylated in vivo in the brain of female rats. Using PhosTag™ Acrylamide we were able to detect several phosphorylated species of ERβ in the dorsal hippocampus of aged (18 mo. old) female rats. This, to our knowledge, is the first report of phosphorylated ERβ detection in the brain of any species.
Previous work by our laboratory showed that p38 kinase inhibition altered ERβ-dependent activation of ERE and AP-1 (activator protein-1) promoter activity in neurons [16]. However p38 kinase inhibitors are broad-spectrum inhibitors and can affect multiple signalling pathways in the cell, thereby making it unclear whether ERβ was a direct target of phosphorylation by p38 in those studies. Furthermore ERβ phosphorylation states were not determined. Therefore, in these studies we created phospho-mutants of ERβ to directly assess the consequences of ERβ phosphorylation on its transcriptional activity in neuronal cells.
We hypothesized that phosphorylation of ERβ at specific sites, S87 and S105, would alter ERβ mediated gene regulation in neuronal cells, both directly at a canonical ERE site and also indirectly through protein:protein interactions at an AP-1 site. Collectively, our results demonstrate that phosphorylation of S87 and S105 altered both oestrogen-independent and oestrogen-dependent ERβ mediated transcriptional regulation at ERE and AP-1 sites in neuronal cells. Taken together, these data suggest that altered kinase activity in the brain, as occurs during aging, has the potential to alter the downstream expression of ERβ gene targets resulting in fundamental changes in brain function.
METHODS
Animals
18 month old Fisher 344 female rats were obtained from the National Institutes of Aging (NIA) aged rat colony (Charles River Laboratories, Wilmington, MA) and allowed to acclimate for 7 days. Following acclimation, animals were ovariectomized (OVX) as previously reported [17] and allowed to recover for 1 week. Surgeries were performed under vaporized isoflurane anaesthesia. Post-operation, animals were singly housed and provided with acetaminophen analgesic (122.7 mg/kg) in tap water for 3 days. At 1 week post-OVX, animals were administered a subcutaneous injection of 2.5 μg/kg of 17β-estradiol (E2) (Sigma, St. Louis MO) dissolved in sesame oil (N=10) or oil alone (vehicle, N=10) once a day for 3 consecutive days. This dose results in plasma E2 concentrations of 60–80 pg/ml as described previously [17, 18]. Animals were euthanized 24 hours after the last injection, brains rapidly removed, flash frozen and then sectioned at 200 μm using a freezing microtome. The dorsal hippocampus (−2.30 to −4.16 relative to bregma) was microdissected using a Palkovit’s brain punch tool (Stoelting, Inc., Woodale, IL) according to “The Rat Brain in Stereotaxic coordinates” [19]. All measures were taken to minimize pain and suffering and animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Loyola University Chicago, permit number 2009018.
Protein Isolation
Total protein was isolated from the dorsal hippocampus and hypothalamus using T-Per reagent (ThermoFisher Scientific, Rockford IL) containing an added protease and phosphatase inhibitor (ThermoFisher Scientific, Rockford IL). Protein concentration was measured using BCA assay kit according to manufacturer’s instructions (ThermoFisher Scientific, Rockford IL).
PhosTag™ SDS PAGE
50 ug of dorsal hippocampus protein was run on precast PhosTag ™ Acrylamide 12.5% Acrylamide gels, (Wako Pure Chemical Industries, Osaka, Japan). The gel was then transferred on a PVDF membrane (Promega, Madison WI), blocked for 1 hour with 5% BSA, then incubated with the oestrogen receptor β antibody H150 (epitope: 1–150 fragment of hERβ, N-terminal domain) (Santa Cruz, sc-8974, Dallas TX) at a 1:250 dilution in 5% BSA TBST overnight. Blots were washed twice with TBST for 10 minutes prior to application of 1:5000 goat α-rabbit-HRP (Santa Cruz, sc-2004, Dallas TX) in 5% BSA TBST. Blots were washed twice with TBST for 10 minutes and imaged on the Bio-rad Chemidoc XRS+ imager (Bio-rad, Hercules, CA) using ECL Chemiluminescent substrate (Pierce Scientific, Rockford IL). Densitometry was performed using ImageLab software. Antibody specificity was confirmed with parallel Western Blots and PhosTag™ blots using ERα H-184 antibody (Santa Cruz, sc-7207, 1:1000 dilution; data not shown).
Alkaline Phosphatase treatment
Specificity of phosphorylated proteins were confirmed by treating 50 μg of dorsal hippocampus protein (vehicle-treated animals) with 0, 30 or 60 units of alkaline phosphatase (Roche, Basel, Switzerland) for 2 hours at 37°C to dephosphorylate all phosphorylated proteins.
Cell culture
The mouse hippocampal-derived cell line HT-22 (generously provided by Dr. David Schubert, Scripps Institute, San Diego, CA) was maintained in DMEM (Corning, Tewksbury, MA) containing 4.5% glucose and L-glutamine supplemented with 1x nonessential amino acids (Corning, Tewksbury, MA) and 10% foetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA). Cells were used at 70–80% confluency for all experiments.
Hormone treatments
Cells at 70–80% confluency were rinsed with 1x PBS and then media replaced with phenol red-free DMEM plus 10% charcoal-stripped FBS (Atlanta Biologicals, Norcross, GA) at least 36 hours prior to hormone treatments in order to remove all exogenous hormone sources. 17β-estradiol (Sigma, St. Louis, MO) and 4-OH Tamoxifen (Sigma, St. Louis, MO) were diluted in molecular grade ethanol (EtOH) (Sigma, St. Louis, MO) and used at a final concentration of 100 nM as described previously [16].
Expression vectors and reporter constructs
Plasmid expression vector (pcDNA 3.0; Invitrogen, Carlsbad, CA) containing inserts for rER-β1 was provided by Dr. Tom Brown (Pfizer Corp., Cambridge, MA) and has been extensively characterized [20]. The ERE-tk-luciferase reporter construct (generously donated by Dr. Paul Budworth, Case Western Reserve University, Cleveland, OH) contains two repeats of the consensus vitellogenin ERE sequence upstream of the minimal thymidine kinase promoter-firefly luciferase (2xERE-tk-luc) in pGL2-basic plasmid (Promega, Madison, WI). The AP-1-tk-luciferase reporter construct (generously provided by Dr. Colin Clay, Colorado State University, Fort Collins, CO) contains three repeats of the AP-1 sequence into pGL2-basic plasmid. The renilla luciferase pGL4 reporter construct (Promega, Madison, WI) was used as an internal control for transfection efficiency.
Site directed mutagenesis
The pcDNA3.0 plasmid expression vector (Invitrogen, Carlsbad, CA) containing a cDNA insert coding rat ERβ1 was mutated using the Quick Change II XL site-directed mutagenesis kit (Agilent, Santa Clara, CA) to create the phospho-mutants (see Table 1). Primers were designed using the QuickChange primer design available from the Agilent website and point mutations were inserted following manufacturer’s instructions. Vectors were validated by DNA sequencing (ACGT, Inc, Wheeling, IL) to confirm successful site directed mutagenesis.
Table 1.
List of ERβ expression vectors.
| Vector name | Characteristics | |
|---|---|---|
|
| ||
| ERβ | Wild type ERβ | Wild type |
|
|
||
| 87E | Phospho mimetic at S87 | Phospho mutants |
| 87A | Phospho null at S87 | |
| 105E | Phospho mimetic at S105 | |
| 105A | Phospho null at S105 | |
|
|
||
| AA | Phospho null at S87 and S105 | Double mutants |
| EE | Phospho mimetic at S87 and S105 | |
| AE | Phospho null at S87, phospho mimetic at S105 | |
| EA | Phospho mimetic at S87, phospho null at S105 | |
Transient Transfections
HT-22 cells were plated at a density of 20000 cells/well in 96-well plates for 48 h before transfection. Transfections were carried out using Fugene6 (Roche, Basel, Switzerland) or Fugene9 (Roche, Basel, Switzerland) according to manufacturer’s instructions. Twenty-four hours after transfection, cells were washed with 1x PBS and incubated with dextran charcoal-stripped media containing hormone treatment or vehicle (EtOH) for 15 h and then lysed for luciferase assays. Transfection efficiency and expression was verified prior to luciferase experiments using GFP-tagged constructs (data not shown).
Luciferase assays
Following lysis, control reporter (Renilla Luciferase) and reporter (Firefly Luciferase) activities were measured using the Dual-Luciferase Reporter Assay system (DLR; Promega, Madison, WI). Relative light units (RLU) were detected using a Biotek Synergy HT plate reader (Biotek, Winooski, VT) with automatic dual injector system and represented as a ratio of Firefly/Renilla Luciferase RLU. All experiments were conducted with 6 replicates for each condition in each 96 well plate and each assay was repeated in 4 or more independent experiments.
Statistics
Two way ANOVA was performed to determine statistical significance and interaction between the groups followed by Tukey post hoc test for comparisons between mutants and wild type vector. Significance was set at P value < 0.05. All transfection data are represented as the mean percent change in fLUC/rLUC compared to vehicle-treated cells transfected with empty vector ± SEM.
RESULTS
Phosphorylated species of ERβ are present in the dorsal hippocampus of aged female rats
The detection of phosphorylated ERβ in the brain in vivo has not been previously demonstrated, likely due to its relatively low expression and the lack of commercially available phospho-specific antibodies targeting rERβ. A powerful tool that has recently emerged is PhosTag™, a phosphate-binding tag that slows the migration of phosphorylated protein during the electrophoretic run on polyacrylamide gels [21]. Use of PhosTag™ acrylamide results in the detection of several bands when analysed by Western Blot using specific antibodies for the protein of interest, and each band represents the target protein with a different degree of phosphorylation. For instance, higher bands indicate that the target protein contains several phosphate groups, which result in a slower migration through the gel and is visualized as a higher band shift. To verify the specificity of the phosphorylated bands detected, protein samples can be treated with alkaline phosphatase (AP), which will dephosphorylate the protein and result in the absence of detectable phosphorylated bands on the Western blot. The only band detected will be a lower band representing the non-phosphorylated protein of interest.
We tested whether phosphorylated ERβ is present in the dorsal hippocampus of aged female rats to provide rationale for assessing the functional consequences of phosphorylated ERβ in neuronal cells. Oestradiol (E2) is known to have direct effects on the dorsal hippocampus, a brain region involved in cognition and memory formation and a region where ERβ is more highly expressed than ERα [22, 23]. Because E2 therapy is often prescribed to ameliorate the negative cognitive issues that accompany menopause, we used a model of surgically-induced menopause followed by acute E2 treatment to test whether 1) phosphorylated ERβ is present in the brain, and 2) E2 treatment alters ERβ phosphorylation levels. Aged Fisher 344 rats (18 mo. old) underwent ovariectomy (OVX) followed by acute E2 or vehicle administration for 3 days (see Methods). Fig. 1 shows a representative PhosTag™ SDS-PAGE blot probed for ERβ with the ERβ specific antibody. Phosphorylated ERβ was detected in the brains of both the vehicle and E2-treated animals (Fig. 1, arrow). Following AP treatment this same band disappeared, indicating that the band that shifted was specific for phosphorylated ERβ. AP treatment did not diminish the intensity of the lower band, which represent unphosphorylated ERβ, however the higher band is very dark indicating that a large amount of ERβ in the aged female dorsal hippocampus was phosphorylated to some degree (Fig. 1).
Figure 1. Expression of phosphorylated ERβ in the dorsal hippocampus of aged female rats.
Protein isolated from the dorsal hippocampus (50 μg) was resolved using Phos-Tag ™ SDS-PAGE electrophoresis and probed for ERβ with the ERβ specific antibody (SC-150). Phos-Tag™ binds phosphate groups and increased protein phosphorylation retards gel migration. Alkaline phosphatase (AP) treatment removes phosphate groups allowing the protein to migrate further through gel. Aliquots of the same dorsal hippocampus protein sample were treated with different amounts of AP. Arrow indicates phosphorylated ERβ. Samples not treated with AP show upper band, indicating presence of phosphorylated ERβ that is abrogated in samples treated with either 30 or 60 units of AP.
ERβ phosphorylation alters ligand dependent and ligand independent activation of ERE-mediated transcription in neuronal cells
To study the functional effects of phosphorylation of ERβ in neurons, we used site-directed mutagenesis to mutate S87 and S105 into alanine (A), a residue that cannot be phosphorylated, or glutamic acid (E), a residue that resembles a phosphorylated serine because of its similar negative charge and molecular bulk [24]. The expression vector constructs used in the transient transfection analysis are listed in Table 1. ERβ acts as a transcription factor in cis by directly binding to DNA at consensus ERE sequences and activating downstream gene transcription. To test whether phosphorylation of ERβ alters its ability to regulate ERE-mediated transcription, we transiently co-transfected wild type (WT)-ERβ or one of the mutants listed in Table 1 with the reporter construct ERE-tk-luc in a hippocampal-derived cell line (HT-22).
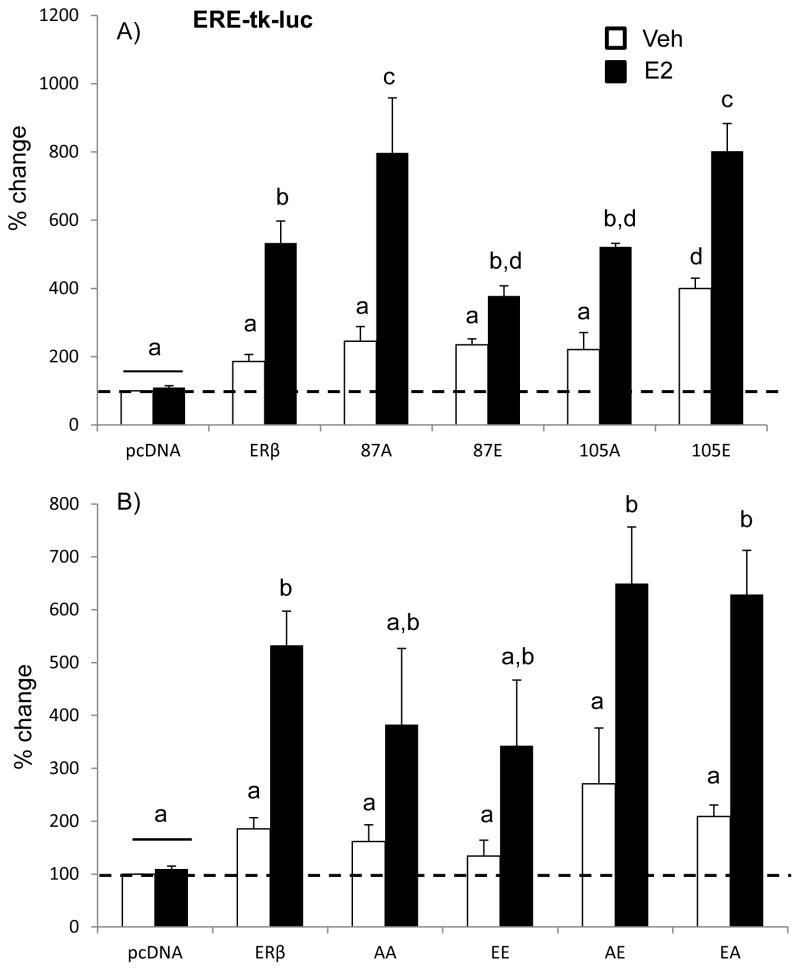
First we analysed the ERE-mediated promoter activity for each of the phospho-mutants with a single site mutated. A two-factor ANOVA analysis revealed that there was a statistically significant interaction between plasmid and treatment, demonstrating that the effect of E2 on ERE-mediated promoter activity depends on whether ERβ is phosphorylated at serine 87 and/or 105 (Fig. 2A; F (5,74)= 9.790, p<0.001). Consistent with previous studies, WT-ERβ tended to increase ERE-dependent transcription in the absence of ligand (approx. 200% increase) and E2 treatment increased it to a much greater statistically significant extent (approx. 550% increase, Fig. 2A) [20]. We then analysed the ERE-mediated promoter activity for each of the phospho-mutant receptors. Mutation of ERβ at serine 87 had differential effects on ERE-mediated promoter activity depending on whether it was phospho-null or phospho-mimetic. First, mutation to alanine (87A, phospho-null) increased ERE-mediated promoter activity to a similar extent as WT-ERβ in the absence of E2, whereas E2 treatment significantly increased ERE-mediated activity to a much greater extent. These results suggest that phosphorylation at this site hinders the E2-dependent activation of the receptor. Interestingly, the opposite effect was observed for mutations at the serine 105 site. In that case, the phospho-null mutation (105A) was not different from WT-ERβ. By stark contrast, the phospho-mimetic (105E) increased not only the E2-independent (approx. 400%), but also the E2-dependent, activation of ERβ.
Figure 2. Effects of ERβ phosphorylation on ERβ-mediated promoter activity at an ERE site.
Hippocampal-derived (HT-22) cell lines were transiently co-transfected with an ERE-tk-Luciferase reporter construct and the wild type ERβ or (A) single phospho-mutant ERβ expression vector (S87A, S87E, S105A, S105E) or (B) double phospho-mutant ERβ expression vector (S87A+S105A; S87E+S105E; S87A+S105E, S87E+S105A) (B). Cells were treated with 100 nM E2 or vehicle (0.01% ethanol) for 15 hours. Data are expressed as the mean percent change compared to empty vector control ± SEM. Different letters denote statistically significant differences as calculated with two-way ANOVA and Tukey post-hoc analysis (p<0.05).
The previous experiments demonstrated the effects of a single amino acid manipulation on ERE- and AP-1-mediated transcription. In those experiments, the phosphorylation status of the opposing site was unknown and entirely dependent on the endogenous kinase activity in the cell. Therefore, in this next series of experiments we tested whether simultaneous phosphorylation (i.e. S87E + S105E (EE)) or complete absence of phosphorylation (i.e. S87A and S105A (AA)) at both serine residues could further alter ERβ regulation. In addition, we also tested the effects on ERE-mediated transcription when one site was phosphorylated, but not the other (i.e. S87A + S105E (AE); S87E + S105A (EA)). Each of the double mutant vectors (see table 1) were transiently co-transfected with an ERE-tk-luc reporter construct in HT-22 cells as described before.
A two-factor ANOVA analysis revealed a statistically significant interaction between plasmid and treatment, once again indicating that E2 regulation of promoter activity depends on the phosphorylation status of both ERβ S87 and S105 residues (Fig. 4, F(5,78)= 5.271, p< 0.001). Figure 2B demonstrates the effects of the double mutants on ERE-mediated promoter activity. All of the double mutants increased ERE-mediated transcription in the presence of E2, although the increase was greater when the two serine residues had an opposite phosphorylation status (AE, EA, Fig. 2B). Moreover, the AE mutation increased ERE-mediated activity by more than 200% in the absence of ligand.
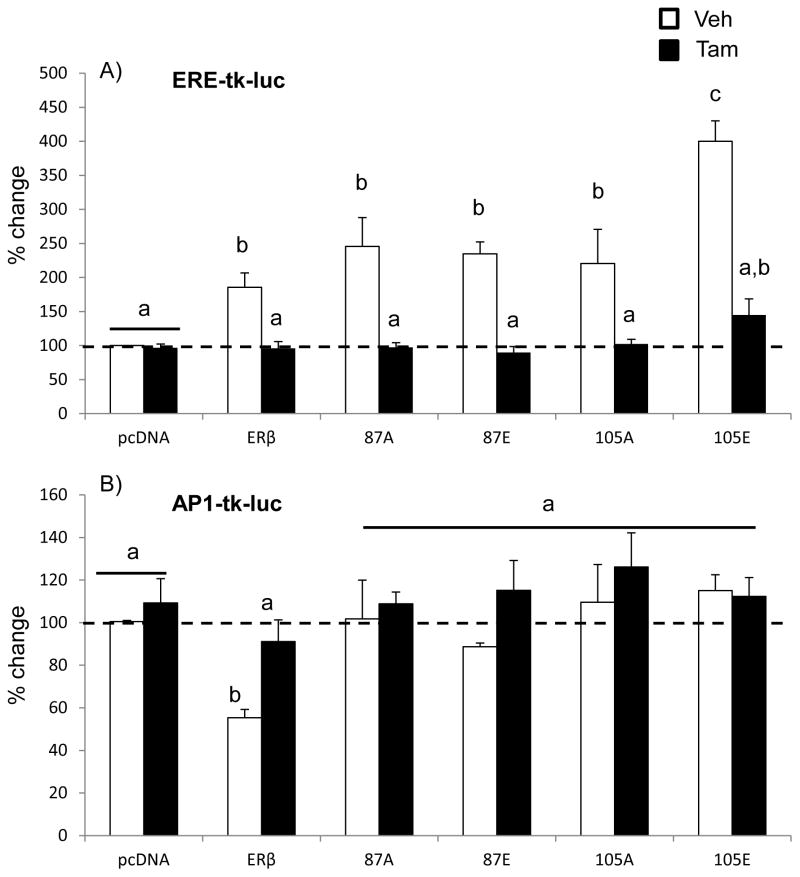
Figure 4. Effects of tamoxifen treatment on ERβ or ERβ mutant-mediated promoter activity at an ERE or AP-1 site.
Hippocampal-derived (HT-22) cell lines were transiently co-transfected with an (A) ERE-tk-Luciferase or (B) AP-1-tk-Luciferase reporter construct and the wild type ERβ or phospho-mutant ERβ expression vector (S87A, S87E, S105A, S105E). 24 hours following transfection, cells were treated with 100 nM 4-OH-tamoxifen (TAM) or vehicle (0.01% ethanol) for 15 hours. Transfection efficiency was normalized using a second renilla luciferase reporter construct (rLUC) in all experiments. Data are expressed as the mean percent change in fLUC/rLUC compared to empty vector control ± SEM taken from 4 independent experiments with 6 replicates/experiment. Different letters denote statistically significant differences as calculated with two-way ANOVA and Tukey post-hoc analysis (p<0.05).
Overall, these data demonstrate that phosphorylation of ERβ at both S87 and S105 has important functional consequences on its ability to transcriptionally activate ERE-mediated promoters in neurons.
ERβ phosphorylation alters ligand dependent and ligand independent activation of AP-1 dependent transcription in neuronal cells
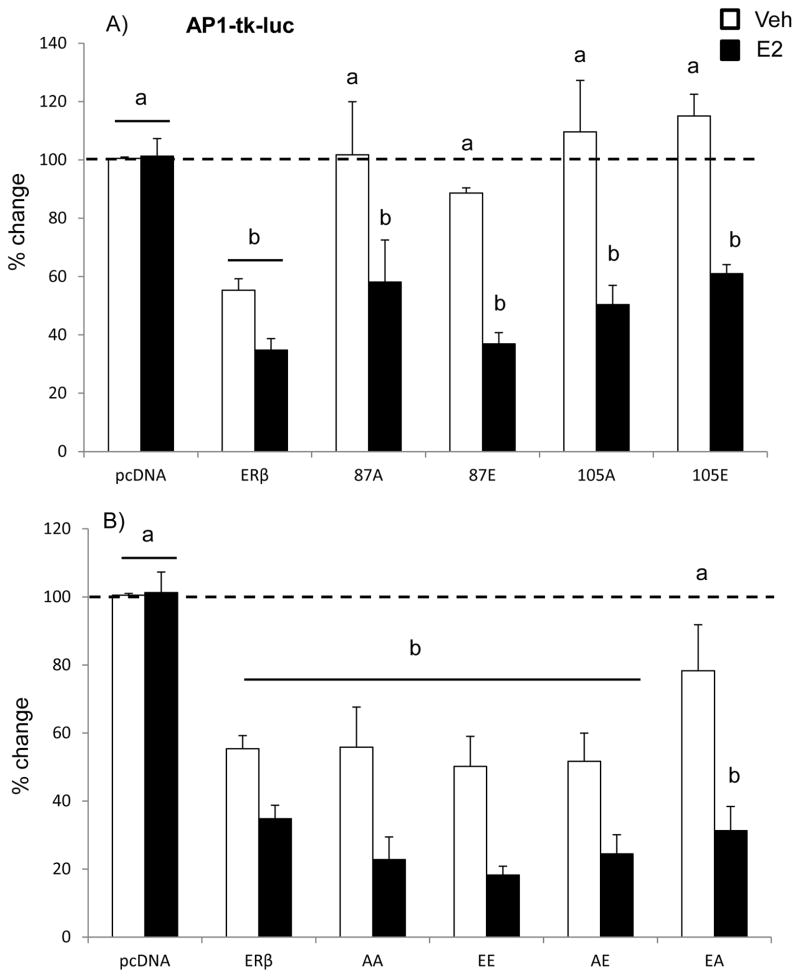
Estrogen receptor β can also act as a transcription factor in trans by tethering other transcription factors, thereby regulating a larger subset of genes whose promoters might lack a consensus ERE. For instance, ERβ-mediated regulation at AP-1 sites requires ERβ interaction with transcription factors of the Fos and Jun families. Similar to our observed results on ERE-mediated promoter activity, a two-factor ANOVA analysis revealed that there was a statistically significant interaction between plasmid and treatment (Fig. 3A; F (5, 59)= 6.046, p< 0.001). First, WT-ERβ had a constitutive (ligand independent) inhibition of AP-1-mediated promoter activity (approx. 50% decrease, Fig. 3A), which showed a trend towards a decrease following E2 treatment. These results are consistent with our previous reports of ERβ repression of AP-1-mediated promoter activity in neuronal cells [16, 20]. Surprisingly, however, mutation of S87 or S105 to any form (phospho-null or phospho-mimetic) completely abolished the ligand independent inhibition of AP-1-mediated transcriptional regulation, yet the E2-induced reduction was preserved. These results suggest that S87 and S105 are critical residues mediating the ligand independent actions of ERβ at an AP-1 site.
Figure 3. Effects of ERβ phosphorylation on ERβ-mediated promoter activity at an AP-1 site.
Hippocampal-derived (HT-22) cell lines were transiently co-transfected with an AP-1-tk-Luciferase reporter construct and the wild type ERβ or (A) single phospho-mutant ERβ expression vector (S87A, S87E, S105A, S105E) or (B) double phospho-mutant ERβ expression vector (S87A+S105A; S87E+S105E; S87A+S105E, S87E+S105A) (B). Cells were treated with 100 nM E2 or vehicle (0.01% ethanol) for 15 hours. Data are expressed as the mean percent change compared to empty vector control ± SEM. Different letters denote statistically significant differences as calculated with two-way ANOVA and Tukey post-hoc analysis (p<0.05).
We next tested whether simultaneous phosphorylation (i.e. S87E + S105E (EE)) or complete absence of phosphorylation (i.e. S87A and S105A (AA)) at both serine residues could further alter ERβ regulation at an AP-1 site. The regulation of AP-1-mediated transcription by double ERβ mutants is shown in Figure 3B. Surprisingly, the ligand independent inhibition of AP-1 was restored when both S87 and S105 were concurrently mutated, while the single mutation of those same sites abolished the constitutive inhibition of AP-1 regulation by WT-ERβ (Fig. 3B). Moreover, E2 significantly inhibited the constitutive repression to a greater extent when S105 was mutated to a phospho-mimetic (E) regardless of the phosphorylation status of S87 (Fig. 3B; F (5,70)= 3.741, p< 0.005).
Overall, we show that ERβ regulation of AP-1 dependent transcription is fundamentally altered by single, but not double, mutation of S87 and S105.
Phosphorylation of ERβ alters Tamoxifen effects on ERE and AP-1 transcription in neuronal cells
Next, we tested whether phosphorylation of ERβ alters the effects of tamoxifen (TAM), a known selective ER modulator (SERM). TAM can be both agonistic and antagonistic, depending on cell type, ER subtype, and promoter response element [25–29]. We have previously shown than TAM abrogates the constitutive activation of ERE in HT-22 neuronal cells, and this was also true for some of the phospho-mutants tested (Fig. 4) [20]. Similar to the previously described experimental results, a two-factor ANOVA revealed a significant interaction between the two factors, plasmid type and TAM treatment (F (5, 71) = 12.978, p< 0.001). Specifically, TAM treatment completely eliminated the constitutive ligand-independent activation (i.e. vehicle-treated) of ERE-mediated transcription (Fig. 4A). Further, phosphorylation of S105 (105E) increased constitutive activity to the greatest extent (>400%), and also increased TAM dependent activation of ERE-mediated transcription compared to wild type ERβ (Fig. 4A). These results suggest that there would be differential activation of ERE regulated genes following TAM treatment when S105 is phosphorylated.
Our previous studies showed that TAM abolished the observed ligand independent inhibition of AP-1 activity by ERβ. The current studies confirm that TAM does abolish the ligand independent inhibition of AP-1 activity for the WT-ERβ (Fig. 4B). However, mutation of S87 or S105 alone abolished the ligand independent repression of AP-1-mediated transcription (see Figs. 3, 4B vehicle), and treatment with TAM did not have any additional effects.
DISCUSSION
The overall objective of these studies was to determine the consequences of ERβ phosphorylation on its functional capacity to act as a transcription factor at known promoter enhancer sites in neurons. Further, we hypothesized that phosphorylated ERβ would be detectable in the brain of aged animals, due to the potential alterations in kinase activity that can occur with aging [30–34]. Our data demonstrated the novel finding that phosphorylated ERβ is not only present in the brain of aged females, it is likely the major form of ERβ expressed in the dorsal hippocampus and phosphorylation tended to increase following E2 treatment. These data provide strong evidence for the physiological relevance of our functional in vitro data, which together demonstrated that phosphorylation of ERβ at specific serine residues altered its ability to activate and/or repress promoter activity in neurons. Collectively, these data suggest that age-related changes in hormonal milieu and cellular kinase activity could impact the expression of ERβ-regulated genes, such as those mediating stress, anxiety, and cognitive function.
Post-translational modifications of nuclear steroid receptors have been widely investigated and these modifications are known to regulate their signalling abilities [7, 10–12]. Structurally, ERβ is similar to other members of the nuclear steroid receptor superfamily. The N-terminal A and B domains are collectively defined as the AF-1 (Activation Function-1, or N-terminal transactivation) domain, which is a highly variable domain amongst the steroid receptor family and is fundamental for binding coregulatory proteins that assist in transcriptional activation or repression [16, 20]. Phosphorylation of mouse ERβ at the N-terminal serine 106 and 124 has previously been shown to mediate ligand independent recruitment of the coregulatory protein SRC-1 (steroid receptor coactivator 1) and alter its subsequent transcriptional activity at an ERE in COS-1 cells [15]. In those studies, overexpression of SRC-1 significantly increased ligand independent activation at an ERE site. This effect was dependent on the MAPK phosphorylation of S106 and S124, as the double phospho-null mutation (S106A/S124A) completely abolished the SRC-1-induced activation at an ERE. However, that study did not evaluate the effects of S106A or S124A in the presence of ligand (E2), or the transcriptional activity at an ERE resulting from a S106/S124 phospho-mimetic (i.e. mutation to glutamic acid). In our studies the single mutation of S87A significantly increased E2-induced transcription at an ERE, but there was no effect in the absence of E2. Moreover, there was no difference in ERE-mediated transcription when S87 and S105 were both phospho-null (87A/105A) compared to WT-ERβ. The discrepancies between our results and the previously reported could be due to the overexpression of SRC-1 in those studies. In addition, it is possible that there is a differential endogenous expression of SRC-1 in neurons or that ERs interact with different set of proteins depending on cell-type, resulting in altered signalling.
A similar study evaluated the effects of phosphorylated human ERβ at S105 in cancer cell lines [13]. In that study, phosphorylation of hERβ at S105 inhibited breast cancer cell invasion and migration in vitro, suggesting that phosphorylation of ERβ at S105 mediates the anti-proliferative action of ERβ in the breast. [13, 15]. Moreover, phosphorylated S105 ERβ has been detected both in benign and invasive breast cancer tissue samples using a human-specific phosphoS105 antibody; however no studies to date have detected the presence of phosphorylated ERβ in the brain. Our results using neuronal cells showed that the phospho-mimetic S105E significantly increased both ligand independent and ligand dependent transcriptional activation at an ERE compared to WT-ERβ, whereas conversely, the opposite phospho-null mutation of S105A had no effect. The LBD of ERβ lies between residues 223–457, suggesting that phosphorylation of S87 or S105 is unlikely to alter ligand binding. However, cross talk between the N-terminal and C-terminal domain has been demonstrated, suggesting that altered ligand binding is a possible mechanism for phosphorylation-mediated changes in transcriptional activity [35].
It is well accepted that ERβ has lower transactivation ability at an ERE site compared to ERα and our data suggest that this discrepancy could be partly explained by the phosphorylation status of ERβ in the N-terminal AF-1 domain [25]. For instance, phosphorylation of the N-terminal domain could alter the ability of ERβ to bind consensus ERE sites, thereby allowing preferential binding of ERα to those same sites. This concept is supported by the observation that various types of post translational modifications have been shown to alter DNA binding in other contexts. For example, acetylation of ERα at two lysine residues in the DBD enhanced its ability to bind to an ERE [36]. By contrast, sumoylation of oestrogen-related receptor alpha (ERRα) at two sites in the N- terminal domain did not affect its DNA binding ability, yet still altered its transcriptional activity [37]. Our results add to existing evidence supporting the idea that post translational modifications in nuclear receptors at regions distant to the DBD alters their ability to modulate transcription, and possible alterations in DNA binding affinity cannot be ruled out as a potential explanation for the observed increase in ERE-mediated promoter activity.
Perhaps the most interesting results from this study were the effects of ERβ phosphorylation at an AP-1 site. We have previously shown that ERβ exerts strong transcriptional repression at an AP-1 site in neuronal cells in the absence of ligand and the results presented here indicate that this might depend on phosphorylation of the N-terminal domain [16, 20]. The single mutation of either S87 or S105 completely abolished ligand independent inhibition of AP-1 mediated transcription compared to WT-ERβ. However, AP-1 mediated transcription was equally repressed with WT-ERβ as it was with double mutations of AA, EE, or AE. Notably, only the 87E/105A mutation showed a significant difference from the other mutants, suggesting that S87 phosphorylation status is the critical serine residue for mediating ERβ-induced repression at an AP-1 site. The precise dynamics of p38 and ERK phosphorylation of ERβ are unknown and the folding of singly phosphorylated ERβ could render the other site inaccessible to the kinase. Nevertheless, our data suggest that a single phosphorylation change in either S87 or S105 abrogates the ligand independent inhibition of AP-1, whereas no effect is observed due to concurrent phosphorylation of both sites. This could be explained by altered ERβ protein-protein interactions with Jun/Fos proteins or with other co-regulatory proteins recruited to the AP-1 complex, such as p160, based on evidence that phosphorylation alters coregulatory protein recruitment to ERβ [15]. The next step will be to determine which of these sites are phosphorylated in vivo and the precise molecular environment that facilitates those phosphorylation changes.
Recently, Vivar and colleagues identified 3 classes of ERβ target genes and discovered that the majority of genes (453) fell into the Class I category, which were genes regulated by unliganded ERβ [38]. Further, the Class I genes showed a high enrichment for AP-1 binding sites, demonstrating that ligand independent repression of AP-1-mediated promoter activity is a common mechanism of ERβ signalling. Similarly, Zhao et al. showed that over 60% of ERβ interacting regions in MCF-7 breast cancer cells contain an AP-1 site [39]. Together these studies underscore the significance of our findings that ERβ ligand independent activity at an AP-1 site is abolished when the N-terminal domain is phosphorylated. Aging in women results in dramatic declines in circulating oestrogens raising the possibility that the ligand-independent actions of ERβ could play a prominent role in regulating gene transcription in the aging brain.
Our studies were limited to the investigation of just one type of post-translational modification (phosphorylation) at specific serine residues. However, there is evidence that other post-translational modifications of ERβ could also be present, such as acetylation and sumoylation, and these modifications can create cross talk between different residues of the receptor. For example, phosphorylation at S305 on ERα inhibits subsequent acetylation at K303 leading to enhanced transcriptional regulation [40]. Moreover, phosphorylation of S94 and S106 on mERβ leads to recruitment of ubiquinating enzymes to the AF-1 domain resulting in enhanced degradation of the receptor [41]. Future studies are required to determine the precise mechanisms and consequences resulting from post-translational modifications acting in concert to modulate overall ERβ function. Our in vitro studies were conducted using rat ERβ expression vectors in a mouse-derived neuronal cell line. Given the high degree of homology between rat and mouse ERβ, we expect that similar results would be obtained using rat neuronal cell lines or primary neurons derived from either species [14]. However, it is important to consider these results in this context as both brain-region and species-specific effects of ERβ have been observed [42, 43].
Tamoxifen (TAM) has been described as a partial antagonist due to its cell-type specific effects [27, 29, 44–47]. Relevant to these studies, TAM action in the brain has been shown to block the neuroprotective effects of E2, confirming that the beneficial effects of E2 are mediated by classical oestrogen receptors [1]. In addition, TAM can activate numerous second messenger signalling pathways, including MAPK, thereby potentially phosphorylating and altering ERβ transcriptional activity [48]. Our results demonstrated that TAM worked as an antagonist of both the WT and phosphorylated forms of ERβ by preventing the ligand independent increase in ERE-mediated transcription. Consistent with our previous studies, TAM also blocked the ligand independent repression of WT-ERβ at an AP-1 site. The ligand binding domain (LBD) of ERα and ERβ is more conserved than other domains and X-Ray crystallography studies have shown that both receptors bind several types of selective estrogen receptor modulators (SERMs), including TAM [49]. The structure of TAM complexed with the ERα or ERβ LBD have been resolved and TAM:LBD binding resulted in conformational changes that inhibited subsequent binding of coactivator proteins, pointing to a clear mechanism for TAM antagonism [50, 51].
In summary, our results show that phosphorylation of ERβ alters its function in neuronal cells both in a ligand dependent and independent manner, and that ERβ is phosphorylated in vivo in the hippocampus. Our work highlights the importance of further understanding the effects of post-translational modifications on nuclear steroid receptors, as these modifications fundamentally alter their function as transcription factors and could result in clinically important physiological changes.
Table 2.
Schematic summary of reporter assay results. Arrows indicate direction of change in relation to wild type ERβ. In grey are highlighted the instances of differences between each mutant compared to wild type ERβ.
| ERE (DNA binding) | AP1 (Prot-Prot interaction) | |||
|---|---|---|---|---|
| constitutive | E2 dependent | constitutive | E2 dependent | |
| wtERβ | ↑ | ↑↑ | ↓ | ↓↓ |
| S87A | ↑ | ↑↑↑ | – | ↓↓ |
| S87E | ↑ | ↑↑ | – | ↓↓ |
| S105A | ↑ | ↑↑ | – | ↓↓ |
| S105E | ↑↑ | ↑↑↑ | – | ↓ |
Acknowledgments
Support for this work was provided by NIH R01AG033605.
References
- 1.Lund TDR, Chung T, Handa WC, RJ Novel Actions of Estrogen Receptor-beta on Anxiety-Related Behaviors. Endocrinology. 2004;146(2):797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 2.Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22(4):656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, et al. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nature Neuroscience. 2008;11(3):334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 4.Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–146. doi: 10.1016/j.neuroscience.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böttner M, Thelen P, Jarry H. Estrogen receptor beta: Tissue distribution and the still largely enigmatic physiological function. The Journal of Steroid Biochemistry and Molecular Biology. 2014;139:245–251. doi: 10.1016/j.jsbmb.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Ward RD, Weigel NL. Steroid receptor phosphorylation: Assigning function to site-specific phosphorylation. Biofactors. 2009;35(6):528–36. doi: 10.1002/biof.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigel NL, Moore NL. Steroid Receptor Phosphorylation: A Key Modulator of Multiple Receptor Functions. Molecular Endocrinology. 2007;21(10):2311–2319. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- 9.Weigel NL, Moore NL. Kinases and protein phosphorylation as regulators of steroid hormone action. Nuclear Receptor Signaling. 2007;5:e005. doi: 10.1621/nrs.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muralidharan Anbalagan BH, Murphy Leigh, Rowan Brian G. Post-translational modifications of nuclear receptors and human disease. Nuclear Receptor Signaling. 2012;10(1):1–13. doi: 10.1621/nrs.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Romancer M, Poulard C, Cohen P, Sentis S, Renoir JM, Corbo L. Cracking the estrogen receptor's posttranslational code in breast tumors. Endocr Rev. 2011;32(5):597–622. doi: 10.1210/er.2010-0016. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez M, Picard N, Sauvé K, Tremblay A. Challenging estrogen receptor β with phosphorylation. Trends in Endocrinology & Metabolism. 2010;21(2):104–110. doi: 10.1016/j.tem.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Lam H-M, Suresh Babu CV, Wang J, Yuan Y, Lam Y-W, Ho S-M, Leung Y-K. Phosphorylation of human estrogen receptor-beta at serine 105 inhibits breast cancer cell migration and invasion. Molecular and Cellular Endocrinology. 2012;358(1):27–35. doi: 10.1016/j.mce.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders PT. Oestrogen receptor beta (ER beta) Rev Reprod. 1998;3(3):164–71. doi: 10.1530/ror.0.0030164. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay AT, Labrie GB, Giguere FV. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell. 1999;3(4):513–9. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 16.Mott NN, Pak TR. Characterisation of human oestrogen receptor beta (ERbeta) splice variants in neuronal cells. J Neuroendocrinol. 2012;24(10):1311–21. doi: 10.1111/j.1365-2826.2012.02337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mott NN, Pinceti E, Rao YS, Przybycien-Szymanska MM, Prins SA, Shults CL, Yang X, Glucksman MJ, Roberts JL, Pak TR. Age-dependent Effects of 17beta-estradiol on the dynamics of estrogen receptor beta (ERbeta) protein-protein interactions in the Ventral Hippocampus. Mol Cell Proteomics. 2014;13(3):760–79. doi: 10.1074/mcp.M113.031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao YS, Mott NN, Wang Y, Chung WC, Pak TR. MicroRNAs in the aging female brain: A putative mechanism for age-specific estrogen effects. Endocrinology. 2013 doi: 10.1210/en.2013-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxinos G, WC . The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; Waltham, MA: 1998. [Google Scholar]
- 20.Pak TRC, Lund WC, Hinds TD, Clay LR, Handa CM, RJ The Androgen Metabolite, 5alpha -Androstane-3beta, 17beta -Diol, Is a Potent Modulator of Estrogen Receptor-beta 1-Mediated Gene Transcription in Neuronal Cells. Endocrinology. 2004;146(1):147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita E, K-KE, Takiyama K, Koike T. Phosphate-binding Tag, a New Tool to Visualize Phosphorylated Proteins. Molecular & Cellular Proteomics. 2005;5(4):749–757. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Shughrue Paul J, MVL, Merchenthaler Istvan. Comparative Distribution of Estrogen Receptor-a and -b mRNA in the Rat Central Nervous System. The Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Frontiers in Neuroendocrinology. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zisch AH, Pazzagli C, Freeman AL, Schneller M, Hadman M, Smith JW, Ruoslahti E, Pasquale EB. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene. 2000;19(2):177–87. doi: 10.1038/sj.onc.1203304. [DOI] [PubMed] [Google Scholar]
- 25.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11(3):353–65. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 26.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–56. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9(4):443–56. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 28.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277(5331):1508–10. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 29.Tremblay GB, Tremblay A, Labrie F, Giguere V. Ligand-independent activation of the estrogen receptors alpha and beta by mutations of a conserved tyrosine can be abolished by antiestrogens. Cancer Res. 1998;58(5):877–81. [PubMed] [Google Scholar]
- 30.Bi R, Foy MR, Thompson RF, Baudry M. Effects of estrogen, age, and calpain on MAP kinase and NMDA receptors in female rat brain. Neurobiology of Aging. 2003;24(7):977–983. doi: 10.1016/s0197-4580(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 31.Abidi Parveen, SL-S, Cortez Yuan, Han Jiahuai, Azhar Salman. Evidence that age-related changes in p38 MAP kinase contribute to the decreased steroid production by the adrenocortical cells from old rats. Aging Cell. 2008;7(2):168–178. doi: 10.1111/j.1474-9726.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- 32.Suh Y. Age-specific changes in expression, activity, and activation of the c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinases by methyl methanesulfonate in rats. Mechanisms of Ageing and Development. 2001;122(15):1797–1811. doi: 10.1016/s0047-6374(01)00301-3. [DOI] [PubMed] [Google Scholar]
- 33.Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. The Journal of Physiology. 2003;547(3):977–987. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonyi A, MK, Sun GY. Extracellular signal-regulated kinase 2 mRNA expression in the rat brain during aging. Neurochemical Research. 2003;28(9):1375–8. doi: 10.1023/a:1024948532633. [DOI] [PubMed] [Google Scholar]
- 35.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 36.Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol. 2006;20(7):1479–93. doi: 10.1210/me.2005-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tremblay AM, Wilson BJ, Yang XJ, Giguere V. Phosphorylation-dependent sumoylation regulates estrogen-related receptor-alpha and -gamma transcriptional activity through a synergy control motif. Mol Endocrinol. 2008;22(3):570–84. doi: 10.1210/me.2007-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vivar OI, Zhao X, Saunier EF, Griffin C, Mayba OS, Tagliaferri M, Cohen I, Speed TP, Leitman DC. Estrogen Receptor Binds to and Regulates Three Distinct Classes of Target Genes. Journal of Biological Chemistry. 2010;285(29):22059–22066. doi: 10.1074/jbc.M110.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao C, Gao H, Liu Y, Papoutsi Z, Jaffrey S, Gustafsson JA, Dahlman-Wright K. Genome-Wide Mapping of Estrogen Receptor- -Binding Regions Reveals Extensive Cross-Talk with Transcription Factor Activator Protein-1. Cancer Research. 2010;70(12):5174–5183. doi: 10.1158/0008-5472.CAN-09-4407. [DOI] [PubMed] [Google Scholar]
- 40.Cui Y, Zhang M, Pestell R, Curran EM, Welshons WV, Fuqua SA. Phosphorylation of estrogen receptor alpha blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64(24):9199–208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]
- 41.Picard N, Charbonneau C, Sanchez M, Licznar A, Busson M, Lazennec G, Tremblay A. Phosphorylation of activation function-1 regulates proteasome-dependent nuclear mobility and E6-associated protein ubiquitin ligase recruitment to the estrogen receptor beta. Mol Endocrinol. 2008;22(2):317–30. doi: 10.1210/me.2007-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuiper GG, Gustafsson JA. The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410(1):87–90. doi: 10.1016/s0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- 43.Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol. 1998;19(4):253–86. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- 44.Levy N, Paruthiyil S, Zhao X, Vivar OI, Saunier EF, Griffin C, Tagliaferri M, Cohen I, Speed TP, Leitman DC. Unliganded estrogen receptor-β regulation of genes is inhibited by tamoxifen. Molecular and Cellular Endocrinology. 2010;315(1–2):201–207. doi: 10.1016/j.mce.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 45.Lund TD, Rovis T, Chung WCJ, Handa RJ. Novel Actions of Estrogen Receptor-β on Anxiety-Related Behaviors. Endocrinology. 2005;146(2):797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 46.Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERalpha) and beta (ERbeta): subtype-selective ligands and clinical potential. Steroids. 2014;90:13–29. doi: 10.1016/j.steroids.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomas Barkhem BC, Nilsson Yvonne, Enmark Eva, Gustafsson Jan-Åke, Nilsson Stefan. Differential Response of Estrogen Receptor a and Estrogen Receptor b to Partial Estrogen Agonists/Antagonists. Molecular Pharmacology. 1998;54(1):105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- 48.Watters JJ1, CJ, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138(9):4030–3. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 49.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389(6652):753–8. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 50.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95(7):927–37. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Chirgadze NY, Briggs SL, Khan S, Jensen EV, Burris TP. A second binding site for hydroxytamoxifen within the coactivator-binding groove of estrogen receptor beta. Proceedings of the National Academy of Sciences. 2006;103(26):9908–9911. doi: 10.1073/pnas.0510596103. [DOI] [PMC free article] [PubMed] [Google Scholar]