Abstract
NMDA glutamate receptors play key roles in brain development, function, and dysfunction. Regulatory roles of D-serine in NMDA receptor-mediated synaptic plasticity have been reported. Nonetheless, it is unclear whether and how neonatal deficits in NMDA-receptor-mediated neurotransmission affect adult brain functions and behavior. Likewise, the role of D-serine during development remains elusive. Here we report behavioral and electrophysiological deficits associated with the frontal cortex in Pick1 knockout mice, which show D-serine deficits in a neonatal and forebrain specific manner. The pathological manifestations observed in adult Pick1 mice are rescued by transient neonatal supplementation of D-serine, but not by a similar treatment in adulthood. These results indicate a role for D-serine in neurodevelopment and provide novel insights on how we interpret data of psychiatric genetics, indicating the involvement of genes associated with D-serine synthesis and degradation, as well as how we consider animal models with neonatal application of NMDA receptor antagonists.
Keywords: PICK1, D-serine, NMDA, developmental trajectory, prefrontal cortex, prepulse inhibition
Introduction
NMDA glutamate receptors mediate excitatory neurotransmission and synaptic plasticity, and their modulation by endogenous factors has a critical role in these phenomena. Specifically, NMDA receptors are required for long-term potentiation (LTP) and long-term depression (LTD), cellular processes of neural plasticity that underlie learning and memory.1,2 NMDA receptors are activated by glutamate and require several co-agonists, including D-serine.3 A crucial role of D-serine in neural plasticity has also been demonstrated.4–6 On the other hand, excess NMDA receptor mediated signaling can lead to excitotoxicity, which may underlie many brain disorders, including stroke, Alzheimer’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS).7–10 D-serine is also known to play a role in this toxic cascade in a context-dependent manner, including in some cases of stroke and ALS.11–13
During brain development, NMDA receptors are crucial for the generation of spontaneous synchronous network activity in the neonatal brain.14–17 NMDA receptors are also essential for the conversion of silent to active synapses by recruiting AMPA receptors.18 However, it is unclear whether and how neonatal deficits in NMDA-receptor-mediated neurotransmission affect adult brain functions and behavior. Likewise, the role of D-serine during development remains elusive. Given that NMDA receptors are modulated by D-serine, animal models with transient deficits in D-serine during neonatal stages may provide a good tool to address this question.
PICK1 is a multifunctional scaffold protein that interacts with many proteins, including several types of glutamate receptors and serine racemase, the D-serine synthesizing enzyme.19,20 PICK1 has been extensively studied in the context of neural plasticity,21–32 and mice deficient in Pick1 are also available.22 Nonetheless, the physiological role of PICK1-serine racemase protein interactions remains elusive. In cell cultures, PICK1 can augment the enzymatic activity of serine racemase and D-serine synthesis.21 Pick1 knockout mice display a significant reduction in D-serine levels, which is specific to the forebrain during the neonatal stage: the level of D-serine is normal in adult Pick1 mice.21
Here we aimed to address the significance of D-serine in brain development on adult brain function and behavior, by using Pick1 knockout mice. As the first step, we examined behavioral and electrophysiological characteristics associated with the frontal cortex of the Pick1 mice. We then tested whether the representative deficits in adulthood are normalized by transiently supplying D-serine only during the neonatal stage.
Materials and Methods
Animals
We used C57BL/6J male Pick1 knockout mice,21 which have been previously characterized in the context of hippocampal and cerebellar neural plasticity.23,33 For all groups, pups were weaned on postnatal day 21, genotyped and housed in sex-matched groups of five in standard mouse cages in accordance with protocols approved by the Johns Hopkins University, the University of Maryland, and RIKEN Animal Care and Use Committees.
Behavioral Tests
Behavioral tests were conducted on male Pick1 knockout mice and control littermates in adulthood (over 2 months of age), with a one-week interval between tests. We conducted the assays based on our published protocols with minor modifications.34–40
Open field test
Horizontal and vertical locomotor activity were assessed for 2 h using activity chambers equipped with infrared beams (San Diego Instruments). Horizontal and vertical movements, stereotypies, and time spent in the center or along the walls of the chamber (thigmotaxis) were automatically recorded.
Prepulse inhibition (PPI) of the acoustic startle
The PPI test was performed as previously described.35,40 Two identical startle chambers (San Diego Instruments) were used for measuring startle reactivity. During each PPI session, a mouse was exposed to the following types of trials: pulse alone (a 120 dB, 100 ms, broadband burst); the omission of stimuli (no-stimulus trial); and four prepulse–pulse combinations (prepulse–pulse trials) consisting of a 20 ms broadband burst used as a prepulse and presented 80 ms before the pulse using one of five prepulse intensities (74, 78, 82, 86, and 90 dB). Each session consisted of six presentations of each type of trial presented in a pseudorandom order. The background noise presented throughout the entire session was 70 dB.
Forced Swim Test (FST)
The FST was employed to evaluate depression-associated responses. Latency to immobility and total immobility were analyzed during the last 4 min of the 6 min test using the SMART program (San Diego Instruments).
Elevated plus maze (EPM)
The EPM was used to assess anxiety as described previously.35 Anxiety was measured by the percent of time spent in the open arms [calculated as 100× (time spent in the open arms/(time in the open arms + time in the closed arms)].
Y-maze
Spontaneous alternation as a measure of spatial working memory was assessed in the Y-maze. The spontaneous alternation behavior was calculated as the number of triads (visiting three different arms on consecutive choices, e.g., ABC, but not ABB or ACC) divided by the total number of arms entered minus two.41
Quantitative real time PCR
Quantitative real-time PCR was performed by the ABI Prism 7900HT sequence detection system (Applied Biosystems). Note, we performed this molecular study with frontal cortex homogenates from mice that had been injected with saline for another assay. The primers used for the study were as follows:
Stargazin (forward): 5’-ccagcaagaagaacgaggaa-3’
Stargazin (reverse): 5’-ttgcttgcacagacctttga-3’
Parvalbumin (forward): 5’-ttctggacaaagacaaaagtgg-3’
Parvalbumin (reverse): 5’-tgaggagaagcccttcagaat-3’
Gad67 (forward): 5’-tggagcagatcctggttgact-3’
Gad67 (reverse): 5’-gccattcaccagctaaaccaa-3’
Actin (forward): 5’-gatgacgatatcgctgcgctggtcg-3’
Actin (reverse): 5’-gcctgtggtacgaccagaggcatacag-3’
Electrophysiology
Adult mice were anesthetized with chloral hydrate (400 mg/kg i.p.) and perfused with ice cold artificial cerebrospinal fluid (ACSF, in mM: 125 NaCl, 25 NaHCO3, 10 glucose, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 3 MgCl2, 0.075 Na2S2O5, pH 7.4). Coronal brain slices (300 µm) containing the medial prefrontal cortex were made with a Vibratome and incubated for at least 1 h at 35°C. Recordings were made using 35°C O2-saturated ACSF, but with CaCl2 adjusted to 2 mM and MgCl2 to 1 mM. Recording electrodes were made from glass pipettes filled with (in mM) 115 K-gluconate, 10 HEPES, 2 MgCl2, 20 KCl, 2 Mg-ATP, 2 Na2-ATP, 0.3 GTP (pH 7.3; 280 mOsm) and Neurobiotin (0.125%). Prefrontal cortical pyramidal neurons were identified under visual guidance using infrared-differential interference contrast (IR-DIC) video microscopy (Olympus BX50-WI). Whole-cell current-clamp signals were amplified (10x) with a Multiclamp 700B (Axon Instruments), digitized with an A/D converter (Digidata, Axon Instruments) and sampled with Axoscope 9.0 (Axon Instruments) at 20 KHz.42
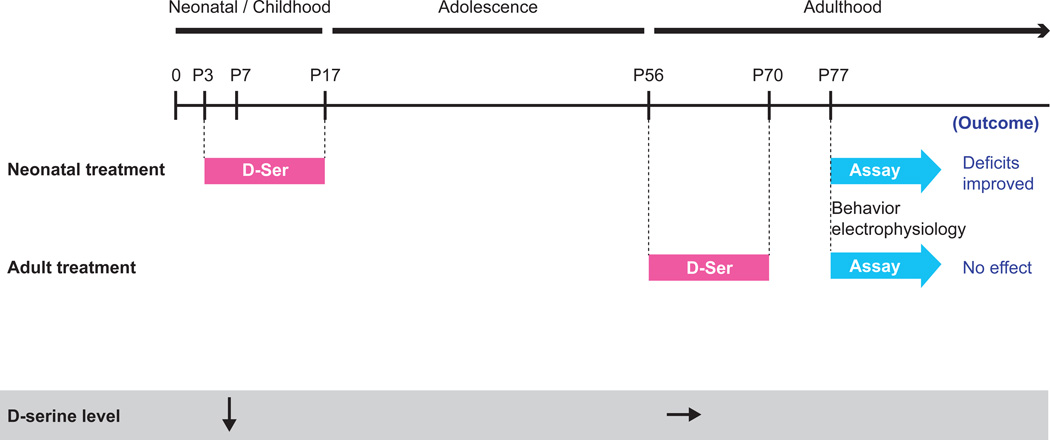
Neonatal or adult D-serine treatment (Fig. 3)
Figure 3. Time line for D-serine treatment and summary of results.
We have previously published that Pick1 knockout mice have reduced D-serine levels at P7 (neonatal stage), but not in adulthood (P56) (Hikida 2008). Based on those results, here we tested whether supplementation of D-serine during the neonatal period (P3–P17) would improve behavioral and electrophysiological deficits seen in adulthood and indeed it did. As a control experiment we also treated the mice with D-serine in adulthood (P56–P70), followed by a washout period of 1 week, and found no effect on behavior or electrophysiology.
D-serine (500 mg/kg; Sigma-Aldrich) was dissolved in sterile physiological saline and administered intraperitoneally (i.p.), using a 30-gauge needle, every day for 2 weeks beginning on postnatal day 3 (P3). The dose was chosen according to a representative publication, which showed that 300–900 mg/kg of D-serine ameliorated a PPI deficit induced by dizocilpine in adult mice.43 The effects of D-serine were evaluated in adulthood using the PPI and Y-maze tests and electrophysiological recordings assessing NMDA modulation of pyramidal cell firing in prefrontal cortical slices.
Since it was a control experiment, the conditions of the adult D-serine treatment were the same as the neonatal treatment (500 mg/kg i.p daily for 2 weeks) except for the timing: it started at the age of 8 weeks. After two weeks of treatment we waited one week to avoid acute effects of the D-serine and then did either electrophysiology or behavioral testing (Y-maze followed by PPI after another one week interval).
Statistical Analysis
Statistical analysis was performed by ANOVA. A student’s t-test was used to compare two sets of data. Values in graphs are expressed as the mean plus the standard error of the mean (SEM). Significance levels are marked as follows: *P < 0.05.
Results
Behavioral and molecular alterations in adult Pick1 knockout mice
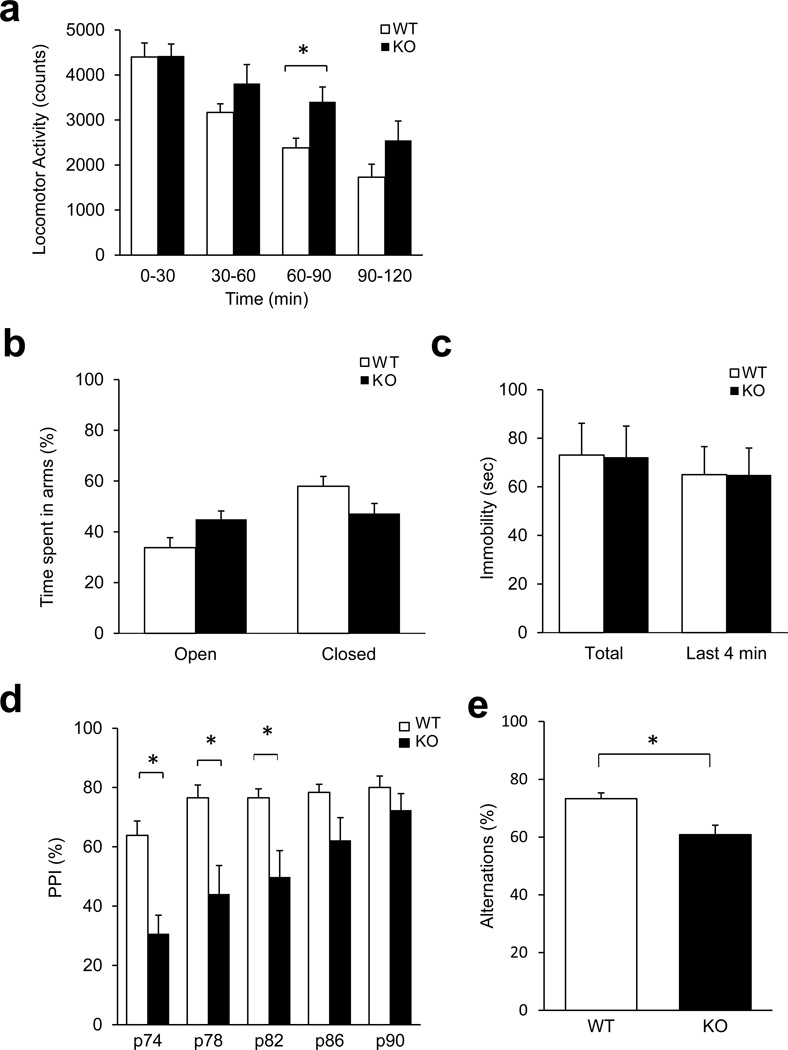
We analyzed the behavioral consequences of deleting the Pick1 gene by performing behavioral tests relevant to diverse aspects of mental conditions. Pick1 knockout mice did not differ from wild-type littermates in open field activity over 2 h (Supplementary Fig. 1), but when we divided the 2 h into four bins of 30 min, we found them to be significantly hyperactive during the 60–90 min interval (Fig. 1A, Supplementary Fig. 1). The hyperactivity of Pick1 knockout mice was unlikely related to altered emotionality, as no significant group differences were seen in the elevated plus maze and the forced swim test (Fig. 1B, C, Supplementary Fig. 2). We also assessed prepulse inhibition (PPI) of the acoustic startle response, a measure of sensorimotor gating, which is reportedly impaired in patients with several neuropsychiatric disorders, including schizophrenia (Fig. 1D).44 Two-way repeated measures ANOVA showed a significant group effect, F(1,89)=6.04, p=0.026 and significant group by prepulse interaction, F(4,89)=6.21, p<0.001. Post-hoc tests demonstrated that, compared to controls, knockouts had significantly decreased PPI at the prepulse intensities of 74, 78 and 82 dB. Notably, Pick1 knockout mice showed an increase in startle response, indicating that impaired PPI was not due to insufficient startle response (Supplementary Fig. 3). In addition, Pick1 knockout mice also exhibited significantly fewer spontaneous alternations in the Y-maze compared to wild-type mice (Fig. 1E), which is a sign of working memory deficit.44,45 Abnormalities in PPI and working memory could result from deficits of the prefrontal cortex.46 We also conducted an exploratory expression study for candidate molecules (AMPA receptor regulator Stargazin and interneuron markers of Parvalbumin and Gad67): real-time PCR indicated an increase in the expression of Stargazin and Parvalbumin, but not that of Gad67, in adult Pick1 knockout mice (Supplementary Fig. 4).
Figure 1. Behavioral changes in adult male Pick1 knockout mice in adulthood.
A) Horizontal locomotor activity. There was a significant group by interval interaction [F(23, 383)=1.83, p=0.012]. Pairwise multiple comparison procedures (Holm-Sidak method) showed significantly more ambulatory activity in Pick1 knockout mice at the 60–90 min interval (*p<0.05), p=0.054 for the 90–120 interval, n=8 per group.
B) Behavior in the elevated plus maze. No difference between wild-type and Pick1 knockout and mice was observed. n=7 per group.
C) Time immobile in the forced swim test. No differences between wild-type and Pick1 knockout mice was observed. n=8 per group.
D) Prepulse inhibition (PPI) of the acoustic startle. Pick1 knockout mice demonstrated significantly lower PPI at the prepulse intensities of 74, 78 and 82 dB. *p<0.05 vs. wild-type at the same prepulse intensity. n=11 (knockout), n=8 (wild-type).
E) Spontaneous alternations in the Y maze. Pick1 knockout mice showed significantly fewer alternations in the Y maze compared to wild-type mice, *p<0.05, n=8 per group.
Alterations in NMDA-elicited firing of prefrontal cortical pyramidal neurons in adult Pick1 knockout mice
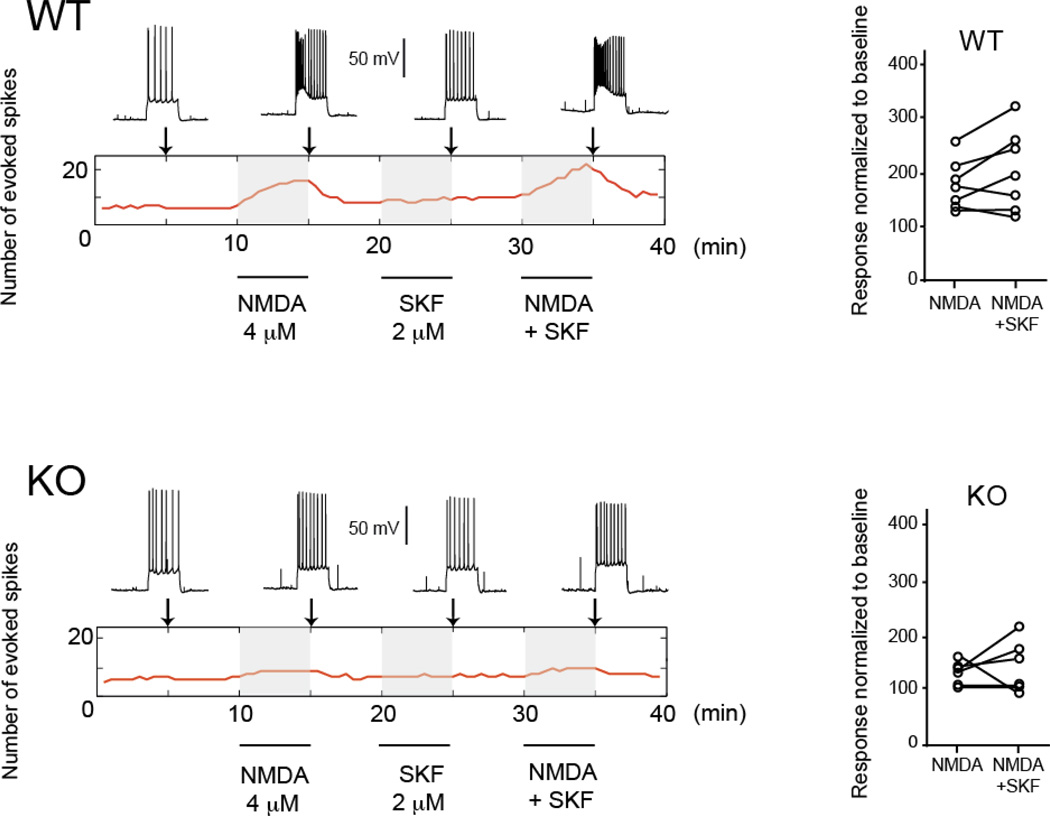
As behavioral deficits in Pick1 mice suggested altered prefrontal cortical function, we hypothesized that pyramidal neurons in the prefrontal cortex of Pick1 knockout mice may display altered physiological properties. To address this question, we tested the excitability of pyramidal neurons in brain slices containing the prefrontal cortex of adult Pick1 knockout mice and wild-type littermates. The number of action potentials evoked with intracellular current injections before and after bath application of NMDA (4 µM) was examined as a readout of cell excitability (Fig. 2, Supplementary Table 1). We also tested whether the modulation of NMDA responses by D1 dopamine receptors is affected in Pick1 knockout mice by adding the selective D1 agonist SKF38393 (2 µM).42
Figure 2. NMDA effects on prefrontal cortex pyramidal cell excitability in adult Pick1 knockout mice.
Top: Examples of traces used to assess excitability 5 min before 4 µM NMDA application (left), after 5 min of NMDA perfusion, after 5 min of the D1 agonist SKF38393 (SKF, 2 µM), and during the combined perfusion of 4 µM NMDA and 2 µM SKF in a prefrontal cortical slice from a wild-type mouse. The rectangular area with the red line indicates the changes in the number of evoked action potentials over time with the three treatments (NMDA alone, SKF alone, NMDA+SKF, shaded areas). The vertical arrows indicate the time at which the traces on top were obtained. Right, population data indicating normalized responses to baseline after NMDA alone and NMDA + SKF. Bottom: Similar display for a representative prefrontal cortex prefrontal cortex pyramidal neuron in a Pick1 knockout mouse (left) and population data for NMDA and NMDA + SKF for all knockout mice recorded, showing a reduced NMDA effect in slices from Pick1 knockout mice.
The resting membrane potential was not different between wild-type and Pick1 knockout mice, and was not affected by the addition of NMDA and SKF38393 (wild-type: −63.3 ± 1.5 mV at baseline, −62.0 ± 2.2 mV with NMDA, −60.3 ± 3.6 mV with NMDA and SKF38393; Pick1 knockout mice: −65.8 ± 3.1 mV at baseline, −63.5 ± 3.1 mV with NMDA, −62.0 ± 5.7 mV with NMDA and SKF38393). Thus, basic membrane properties of prefrontal pyramidal neurons were not affected in Pick1 knockout mice.
We then tested whether Pick1 knockout mice exhibited altered modulation of excitability by NMDA in pyramidal neurons. The number of action potentials evoked by a constant-amplitude current pulse was markedly increased by application of NMDA in wild-type mice (from 6.6 ± 0.5 to 11.4 ± 2.8 spikes; a 73% increase from baseline) (Fig. 2, Supplementary Table 1). Although the D1 agonist SKF38393 alone did not change the number of action potentials, the combined addition of NMDA and SKF38393 synergistically augmented the number of action potentials (to 13.1 ± 4.8; a 101% increase from baseline) in wild-type mice. In Pick1 knockout mice, however, the increase in the number of action potentials by NMDA was significantly attenuated (from 5.8 ± 1.7 to 7.8 ± 2.3; a 33% increase from baseline), and the addition of SKF38393 to NMDA only slightly elevated the response (to 8.5 ± 3.4; a 45% increase from baseline). A two-way ANOVA with genotype and drug treatment as factors revealed genotype differences (ANOVA F1,22=5.82, p=0.024), indicating that NMDA responses are impaired in Pick1 knockout mice. There was no significant interaction between genotype and D1 agonist treatment (p=0.914), indicating that D1 modulation of NMDA responses was not affected in Pick1 knockout mice.
Influence of neonatal D-serine on behavioral defects in adult Pick1 knockout mice
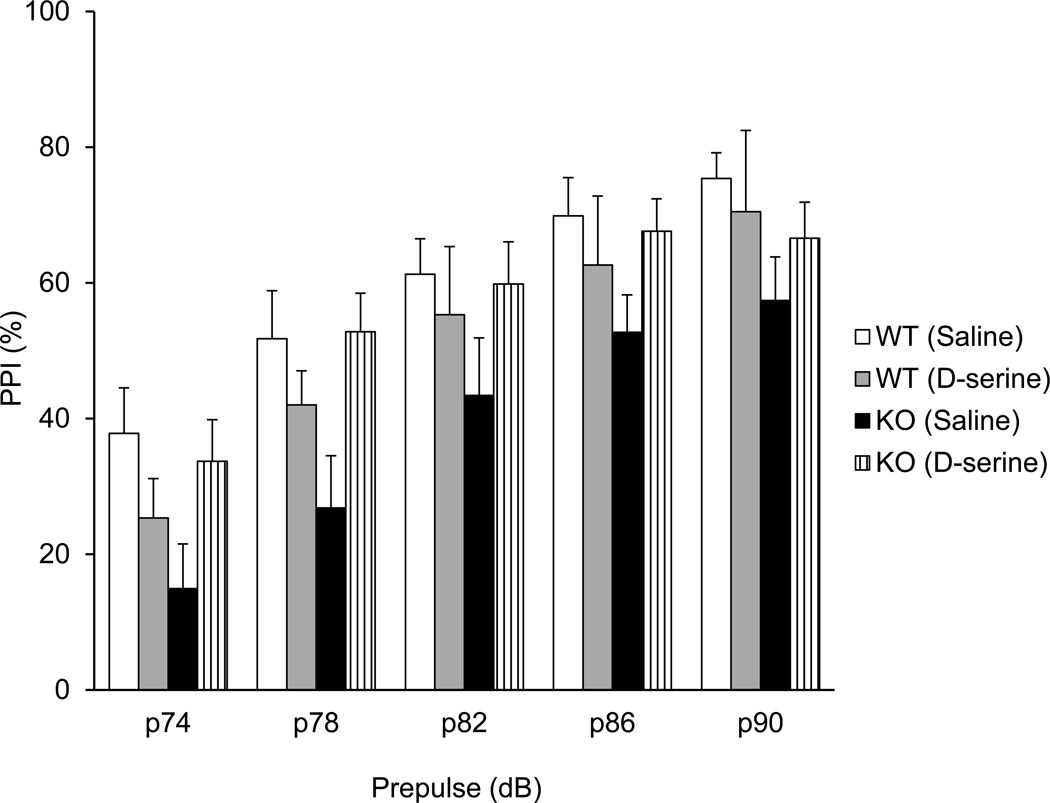
Here we show that Pick1 knockout mice have prefrontal cortex-associated behavioral deficits in adulthood. Which mechanism(s) underlie these deficits? One possibility is that down-regulation of D-serine during development, at the neonatal stage, may contribute to these adult deficits in Pick1 knockout mice. To address this question, we administered D-serine (500 mg/kg, i.p.) daily to pups beginning on postnatal day 3 (P3) for 2 weeks, and then maintained the animals until adulthood without any further supplementation of D-serine (Fig. 3). We did not observe any adverse effects of neonatal injections (data not shown). We evaluated the effects of this neonatal treatment with D-serine by monitoring possible improvement in PPI and spontaneous alternations in the Y-maze in adult mice. Compared to saline treatment, D-serine treatment in the neonatal stage significantly increased the PPI in Pick1 knockout, but not in wild-type mice (Fig. 4). We assessed the PPI effects separately for Pick1 knockout mice and wild-type mice, as our pilot data had not revealed effects of D-serine on wild-types; this design increases the power of analysis. Two-way repeated measures ANOVA of the data for knockout mice revealed a significant effect of treatment, F(1,99)=4.8, p=0.041, but not for wild-type mice, F(1,64)=0.6, p=0.47. A trend of beneficial effect of neonatal D-serine treatment was also seen in the adult behaviors in Y-maze (Supplementary Fig. 5). Note, due to unexpected baseline changes elicited by neonatal injection itself, this statement on Y-maze is not conclusive.
Figure 4. Effect of neonatal D-serine treatment on PPI in adulthood.
D-serine 500 (mg/kg) was injected daily for 2 weeks starting on postnatal day 3. The PPI deficit was significantly rescued in adult Pick1 knockout mice treated with D-serine in the neonatal stage. Two-way repeated measures ANOVA of the data for knockout mice revealed a significant effect of treatment, F(1,99)=4.8, p=0.041. n=8, wild-type with saline treatment; n=8, wild-type with D-serine treatment; n=5, Pick1 knockout mice with saline treatment; n=13, Pick1 knockout mice with D-serine treatment.
We also tested the impact of adult D-serine administration (500 mg/kg, i.p.; daily beginning at the age of 8 weeks for 2 weeks) on these behaviors. In regard to PPI, we cannot make any meaningful statements because repeated injections in adulthood significantly altered the baseline response in both wild-type and knockout mice (Supplementary Fig. 6A). Meanwhile, we observed conclusive evidence that adult D-serine treatment did not rescue the deficit in spontaneous alternations in Pick1 knockout mice (Supplementary Fig. 6B).
Influence of neonatal D-serine on electrophysiological defects in adult Pick1 knockout mice
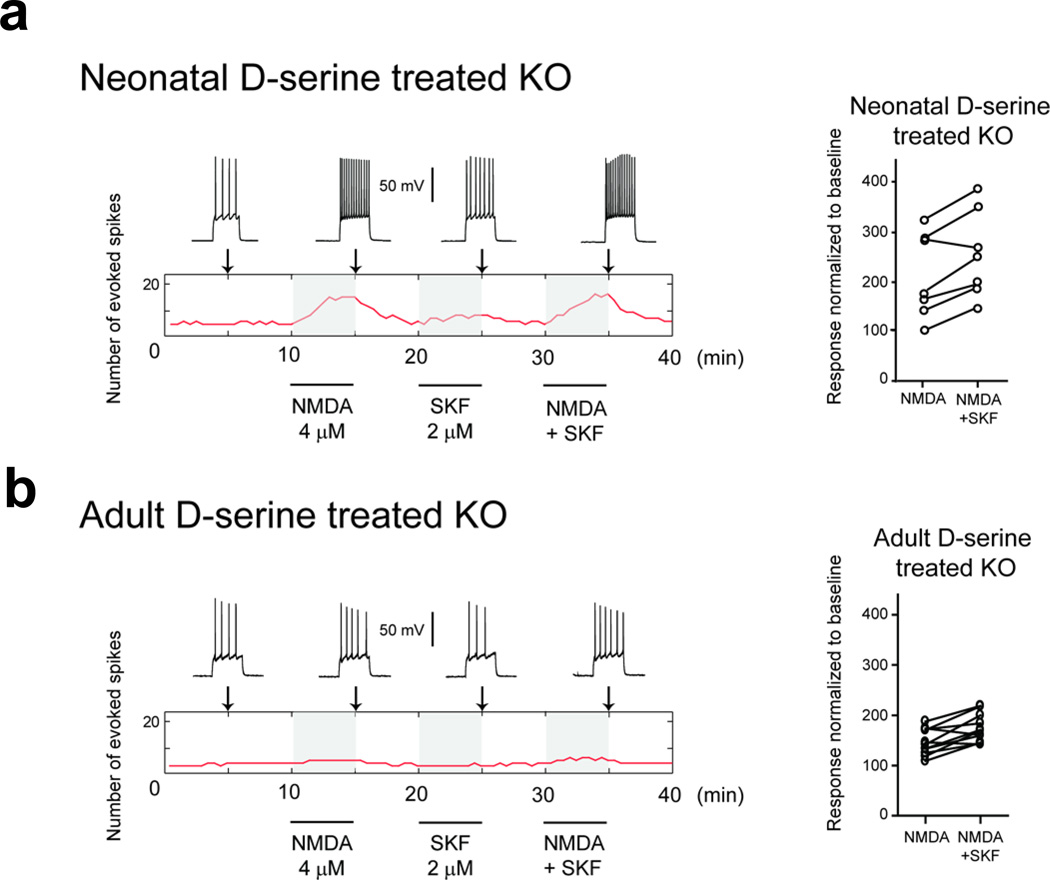
We next addressed whether neonatal D-serine treatment of Pick1 knockout mice may normalize the electrophysiological deficits observed in adult mice. We prepared prefrontal cortical slices from D-serine-treated Pick1 knockout mice, and conducted electrophysiological assessments similar to those presented in Fig. 2. The resting membrane potentials of Pick1 knockout mice treated with D-serine during the neonatal period and adulthood were −66.6 ± 4.7 and −67.9 ± 3.1 mV, respectively. Unlike untreated Pick1 knockout mice, NMDA (4 µM) elicited strong increases in evoked action potential firing in slices from D-serine-treated Pick1 knockout mice. The number of action potentials evoked by current injection rose from 3.9 ± 1.2 to 7.4 ± 3.2 with NMDA administration (89% increase from baseline) (Fig. 5A, Supplementary Table 1). The addition of SKF38393 to NMDA slightly increased the number of action potentials to 8.9 ± 3.6. A two-way ANOVA revealed differences between treated and untreated Pick1 knockout mice (F1,22=7.91, p=0.010). These results indicate that D-serine treatment during development rescued adult NMDA function in Pick1 knockout mice.
Figure 5. Rescue of electrophysiological deficits in adulthood by neonatal D-serine treatment, but not by adult D-serine treatment.
Responses to NMDA application in prefrontal cortical brain slices from adult Pick1 knockout mice that had been treated with D-serine. Data are presented in the same format of those in Fig. 2.
A) Neonatal D-serine treatment (from postnatal day 3 to day 16) rescued the electrophysiological changes in Pick1 knockout mice.
B) Adult D-serine treatment did not rescue the electrophysiological changes in Pick1 knockout mice.
In contrast, when we conducted D-serine treatment in adulthood, responses of neurons were noticeably blunted in Pick1 knockout mice: to NMDA (5.7 ± 1.7 at baseline to 8.2 ± 3.2 spikes for a 46% increase in action potential firing) or NMDA plus SKF38393 (9.7 ± 3.6, a 70% increase from baseline) (Fig. 5B, Supplementary Table 1). To statistically confirm the contrastive responses between neonatal and adult D-serine treatment to Pick1 mice, we employed a two-way ANOVA. First, a significant effect of D-serine treatment (F2,42=7.502, p=0.002) was revealed in Pick1 mice. Second, post hoc comparisons using Tukey’s HSD indicate that neonatal treatment rescued the electrophysiological deficits in untreated Pick1 mice (p=0.002), whereas this rescue was absent upon the treatment in adulthood (p=0.52). Thus, we conclude that neonatal, but not adult, D-serine treatment rescues NMDA function in adult Pick1 knockout mice.
Discussion
It has been an unanswered question whether D-serine during brain development plays a role in adult brain function and behavior, although its physiological and pathological role in adult animals have been well studied.6,43,47–50 To address this question, here we utilized Pick1 knockout mice in which the D-serine level is selectively reduced in the forebrain only during the neonatal stage.21 Adult Pick1 knockout mice showed several behavioral abnormalities and electrophysiological deficits in the prefrontal cortex. Importantly, neonatal, but not adult, D-serine supplement rescued such adult deficits. These observations suggest a novel role for D-serine during brain development, with significant influence on adult brain function and behavior.
How does altered D-serine during development affect adult performance? The most probable scenario is that neonatal D-serine influences NMDA receptors, which in turn affects brain network formation. In Pick1 knockout mice, a neonatal-specific deficit in D-serine may lead to transient hypo-NMDA function during a critical period for proper brain development. Alternatively, D-serine may influence brain development by an unknown mechanism independent of its action on NMDA receptors. The next question, although it is beyond the scope of the present study, is to characterize how neonatal D-serine administration to Pick1 knockout mice may change (possibly normalize) neural network formation during the developmental trajectory, in comparison with non-treated Pick1 knockout and wild-type mice. By comparing these data with those from mice with direct genetic modulation of NMDA receptors, we may be able to clarify whether the effects of neonatal D-serine supply are via NMDA receptors.
Acute or sub-chronic administration of phencyclidine (PCP), a NMDA receptor antagonist, to rodents can produce behaviors resembling phenomena observed in schizophrenia.51 Clinical observation of PCP-induced psychosis and schizophrenia-like manifestation in normal subjects with non-competing NMDA receptor antagonists indicate a possible role of adult NMDA neurotransmission in the disease.52,53,54 The current view on the mechanisms by which NMDA antagonists may produce psychosis relates to their ability to selectively suppress local inhibitory processes yielding cortical disinhibition and these effects are evident in adolescents and adults.55 Thus, the mechanism leading to behavioral deficits in Pick1 knockout mice is different from those elicited by adult and adolescent administration of NMDA receptor antagonists that functionally impair glutamatergic neurotransmission.56 Instead, as discussed above, neonatal D-serine and NMDA receptors are likely to influence neural circuit formation during neurodevelopment, secondarily affecting adult brain function and behavior in neonatal manipulations that reduce NMDA receptor activity. Thus, neonatal administration of PCP may be a useful model for adult mental illnesses,57–60 but is justified in the context of neurodevelopment, distinct from models with adult and adolescent administration of NMDA receptor antagonists.
As PICK1 is a multifunctional protein, the phenotypes of Pick1 knockout mice reflect not only D-serine associated abnormalities but also deficits elicited by many other mechanisms. Thus, in the present study, we conducted neonatal supplement of D-serine to address the primary cause of D-serine. Nonetheless, one may wonder why we did not use conditional knockout mice lacking serine racemase,61 the synthesizing enzyme for D-serine, for this study. This is in part because we currently do not have access to the conditional mice. Furthermore, as Serine racemase generates not only D-serine but also pyruvate, keto-acid, and ammonia,62 its knockout may not be a tool to purely address the role for D-serine. Thus, both conditional serine racemase knockout and Pick1 knockout mice are complementary towards investigating the neonatal role for D-serine.
Genetic studies have suggested that genes encoding key molecules of D-serine synthesis, degradation, and regulation (e.g., D-amino acid oxidase, serine racemase, and possibly G72) are frequently associated with bipolar disorder and schizophrenia.63–65 A recent genome wide association study has indicated 108 chromosomal loci that associate with schizophrenia, which include the locus of the serine racemase gene.66 In addition, a chromosomal abnormality associated with schizophrenia is reportedly linked to down-regulation of phosphoserine aminotransferase1, PSAT1, a key enzyme for the synthesis of L-serine, the precursor of D-serine.70 Some studies have found reduced D-serine in the cerebrospinal fluid of patients with schizohprenia,67,68 whereas, other studies including a meta-analysis found no differences.69 Accordingly, the Pick1 knockout mice with D-serine deficit specific to neonatal stage may model schizophrenia in which adult D-serine level is normal.
Thus far, NMDA hypofunction has been modeled primarily using experimental animals, which include the serine racemase knockout mice in which D-serine deficiencies are present throughout life. The Pick1 knockout model offers a unique opportunity to investigate the role of D-serine deficiency limited to the neonatal stage for behavior during adulthood. Our findings demonstrate that neonatal D-serine deficiency, on its own, is sufficient to induce some behavioral phenotypes, such as impaired prepulse inhibition and spontaneous alternation that may serve as pathophysiological markers for schizophrenia or other major mental disorders, and that such effects are rescued by neonatal D-serine treatment. Future studies are needed to determine the relative importance of neonatal vs. adult D-serine deficiency in the pathology of major mental illness.
Supplementary Material
Acknowledgements
We thank Ms. Yukiko Lema for organizing the figures and manuscript. This work was supported by USPHS grants MH-084018 (A.S.), MH-094268 Silvo O. Conte center (A.S.), MH-069853 (A.S.), MH-085226 (A.S.), MH-088753 (A.S.), MH-092443 (A.S.), MH-091387 (A.S.), MH-057683 (P.O.), MH-083728 (M.V.P.), Stanley (A.S.), RUSK (A.S.), S-R foundations (A.S.), NARSAD (A.S., P.O., M.V.P.), Maryland Stem Cell Research Fund (A.S.), MEXT KAKENHI (T.T.), JST CREST (T.T.), and Naito and Uehara Memorial Foundations (J.N.).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supplementary information is available at Molecular Psychiatry’s website.
References
- 1.Paré D. Presynaptic induction and expression of NMDA-dependent LTP. Trends Neurosci. 2004;27:440–441. doi: 10.1016/j.tins.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Anwyl R. Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog Neurobiol. 2006;78:17–37. doi: 10.1016/j.pneurobio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 4.Snyder SH, Kim PM. D-amino acids as putative neurotransmitters: focus on D-serine. Neurochem Res. 2000;25:553–560. doi: 10.1023/a:1007586314648. [DOI] [PubMed] [Google Scholar]
- 5.Wolosker H. D-serine regulation of NMDA receptor activity. Sci STKE. 2006;2006:pe41. doi: 10.1126/stke.3562006pe41. [DOI] [PubMed] [Google Scholar]
- 6.Martineau M, Baux G, Mothet JP. D-serine signalling in the brain: friend and foe. Trends Neurosci. 2006;29:481–491. doi: 10.1016/j.tins.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 8.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 9.Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration: insights from Huntington's disease. Trends Neurosci. 2010;33:513–523. doi: 10.1016/j.tins.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- 12.Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasabe J, Chiba T, Yamada M, Okamoto K, Nishimoto I, Matsuoka M, et al. D-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J. 2007;26:4149–4159. doi: 10.1038/sj.emboj.7601840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 15.Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- 17.Allène C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y, et al. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci. 2008;28:12851–12863. doi: 10.1523/JNEUROSCI.3733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voigt T, Opitz T, de Lima AD. Activation of early silent synapses by spontaneous synchronous network activity limits the range of neocortical connections. J Neurosci. 2005;25:4605–4615. doi: 10.1523/JNEUROSCI.3803-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii K, Maeda K, Hikida T, Mustafa AK, Balkissoon R, Xia J, et al. Serine racemase binds to PICK1: potential relevance to schizophrenia. Mol Psychiatry. 2006;11:150–157. doi: 10.1038/sj.mp.4001776. [DOI] [PubMed] [Google Scholar]
- 20.Focant MC, Goursaud S, Boucherie C, Dumont AO, Hermans E. PICK1 expression in reactive astrocytes within the spinal cord of amyotrophic lateral sclerosis (ALS) rats. Neuropathology and applied neurobiology. 2013;39:231–242. doi: 10.1111/j.1365-2990.2012.01282.x. [DOI] [PubMed] [Google Scholar]
- 21.Hikida T, Mustafa AK, Maeda K, Fujii K, Barrow RK, Saleh M, et al. Modulation of D-serine levels in brains of mice lacking PICK1. Biol Psychiatry. 2008;63:997–1000. doi: 10.1016/j.biopsych.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, et al. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Suh YH, Pelkey KA, Lavezzari G, Roche PA, Huganir RL, McBain CJ, et al. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron. 2008;58:736–748. doi: 10.1016/j.neuron.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, et al. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, et al. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Xiao N, Kam C, Shen C, Jin W, Wang J, Lee KM, et al. PICK1 deficiency causes male infertility in mice by disrupting acrosome formation. J Clin Invest. 2009;119:802–812. doi: 10.1172/JCI36230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clem RL, Anggono V, Huganir RL. PICK1 regulates incorporation of calcium-permeable AMPA receptors during cortical synaptic strengthening. J Neurosci. 30:6360–6366. doi: 10.1523/JNEUROSCI.6276-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atianjoh FE, Yaster M, Zhao X, Takamiya K, Xia J, Gauda EB, et al. Spinal cord protein interacting with C kinase 1 is required for the maintenance of complete Freund's adjuvant-induced inflammatory pain but not for incision-induced post-operative pain. Pain. 151:226–234. doi: 10.1016/j.pain.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu ZL, Huang C, Fu H, Jin Y, Wu WN, Xiong QJ, et al. Disruption of PICK1 attenuates the function of ASICs and PKC regulation of ASICs. Am J Physiol Cell Physiol. 299:C1355–C1362. doi: 10.1152/ajpcell.00569.2009. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Petralia RS, Takamiya K, Xia J, Li YQ, Huganir RL, et al. Preserved acute pain and impaired neuropathic pain in mice lacking protein interacting with C Kinase 1. Mol Pain. 7:11. doi: 10.1186/1744-8069-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anggono V, Clem RL, Huganir RL. PICK1 loss of function occludes homeostatic synaptic scaling. J Neurosci. 31:2188–2196. doi: 10.1523/JNEUROSCI.5633-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volk L, Kim CH, Takamiya K, Yu Y, Huganir RL. Developmental regulation of protein interacting with C kinase 1 (PICK1) function in hippocampal synaptic plasticity and learning. Proc Natl Acad Sci U S A. 107:21784–21789. doi: 10.1073/pnas.1016103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, et al. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. 115. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- 36.Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 16:293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, et al. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 68:1172–1181. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ibi D, Nagai T, Koike H, Kitahara Y, Mizoguchi H, Niwa M, et al. Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav Brain Res. 2010;206:32–37. doi: 10.1016/j.bbr.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai T, Kitahara Y, Ibi D, Nabeshima T, Sawa A, Yamada K. Effects of antipsychotics on the behavioral deficits in human dominant-negative DISC1 transgenic mice with neonatal polyI:C treatment. Behav Brain Res. 2011;225:305–310. doi: 10.1016/j.bbr.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 40.Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreasson KI, Savonenko A, Vidensky S, Goellner JJ, Zhang Y, Shaffer A, et al. Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J Neurosci. 2001;21:8198–8209. doi: 10.1523/JNEUROSCI.21-20-08198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto K, Fujita Y, Horio M, Kunitachi S, Iyo M, Ferraris D, et al. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol Psychiatry. 2009;65:1103–1106. doi: 10.1016/j.biopsych.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 45.Wietrzych M, Meziane H, Sutter A, Ghyselinck N, Chapman PF, Chambon P, et al. Working memory deficits in retinoid X receptor gamma-deficient mice. Learn Mem. 2005;12:318–326. doi: 10.1101/lm.89805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 47.Ma TM, Abazyan S, Abazyan B, Nomura J, Yang C, Seshadri S, et al. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duffy S, Labrie V, Roder JC. D-serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- 49.Malkesman O, Austin DR, Tragon T, Wang G, Rompala G, Hamidi AB, et al. Acute d-serine treatment produces antidepressant-like effects in rodents. Int J Neuropsychopharmacol. 2012;15:1135–1148. doi: 10.1017/S1461145711001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bado P, Madeira C, Vargas-Lopes C, Moulin TC, Wasilewska-Sampaio AP, Maretti L, et al. Effects of low-dose D-serine on recognition and working memory in mice. Psychopharmacology (Berl) 2011;218:461–470. doi: 10.1007/s00213-011-2330-4. [DOI] [PubMed] [Google Scholar]
- 51.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 52.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 53.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. A.M.A. archives of neurology and psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 54.Rosenbaum G, Cohen BD, Luby ED, Gottlieb JS, Yelen D. Comparison of sernyl with other drugs: simulation of schizophrenic performance with sernyl, LSD-25, and amobarbital (amytal) sodium; I. Attention, motor function, and proprioception. A.M.A. archives of general psychiatry. 1959;1:651–656. doi: 10.1001/archpsyc.1959.03590060113013. [DOI] [PubMed] [Google Scholar]
- 55.O'Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–492. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawa A. Cortical development and glutamatergic dysregulation in schizophrenia. Biol Psychiatry. 2009;66:530–532. doi: 10.1016/j.biopsych.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deutsch SI, Burket JA, Katz E. Does subtle disturbance of neuronal migration contribute to schizophrenia and other neurodevelopmental disorders? Potential genetic mechanisms with possible treatment implications. Eur Neuropsychopharmacol. 2010;20:281–287. doi: 10.1016/j.euroneuro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Semba J, Tanaka N, Wakuta M, Suhara T. Neonatal phencyclidine treatment selectively attenuates mesolimbic dopamine function in adult rats as revealed by methamphetamine-induced behavior and c-fos mRNA expression in the brain. Synapse. 2001;40:11–18. doi: 10.1002/1098-2396(200104)40:1<11::AID-SYN1021>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 59.Harich S, Gross G, Bespalov A. Stimulation of the metabotropic glutamate 2/3 receptor attenuates social novelty discrimination deficits induced by neonatal phencyclidine treatment. Psychopharmacology (Berl) 2007;192:511–519. doi: 10.1007/s00213-007-0742-y. [DOI] [PubMed] [Google Scholar]
- 60.Nakatani-Pawlak A, Yamaguchi K, Tatsumi Y, Mizoguchi H, Yoneda Y. Neonatal phencyclidine treatment in mice induces behavioral, histological and neurochemical abnormalities in adulthood. Biol Pharm Bull. 2009;32:1576–1583. doi: 10.1248/bpb.32.1576. [DOI] [PubMed] [Google Scholar]
- 61.Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foltyn VN, Bendikov I, De Miranda J, Panizzutti R, Dumin E, Shleper M, et al. Serine racemase modulates intracellular D-serine levels through an alpha,beta-elimination activity. J Biol Chem. 2005;280:1754–1763. doi: 10.1074/jbc.M405726200. [DOI] [PubMed] [Google Scholar]
- 63.Detera-Wadleigh SD, McMahon FJ. G72/G30 in schizophrenia and bipolar disorder: review and meta-analysis. Biol Psychiatry. 2006;60:106–114. doi: 10.1016/j.biopsych.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 64.Hayden EP, Nurnberger JI. Molecular genetics of bipolar disorder. Genes Brain Behav. 2006;5:85–95. doi: 10.1111/j.1601-183X.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- 65.Craddock N, O'Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H, et al. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophrenia research. 2007;90:41–51. doi: 10.1016/j.schres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindstrom LH, Iyo M. Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Progress in neuro-psychopharmacology & biological psychiatry. 2005;29:767–769. doi: 10.1016/j.pnpbp.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 69.Brouwer A, Luykx JJ, van Boxmeer L, Bakker SC, Kahn RS. NMDA-receptor coagonists in serum, plasma, and cerebrospinal fluid of schizophrenia patients: a meta-analysis of case-control studies. Neuroscience and biobehavioral reviews. 2013;37:1587–1596. doi: 10.1016/j.neubiorev.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Ozeki Y, Pickard BS, Kano S, Malloy MP, Zeledon M, Sun DQ, et al. A novel balanced chromosomal translocation found in subjects with schizophrenia and schizotypal personality disorder: altered l-serine level associated with disruption of PSAT1 gene expression. Neurosci Res. 2011;69:154–160. doi: 10.1016/j.neures.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.