Abstract
Purpose
Due to the negative effects of meniscectomy, there is a need for an adequate material to replace damaged meniscal tissue. To date, no material tested has been able to replace the meniscus sufficiently. Therefore, a new silk fibroin scaffold was investigated in an in vivo sheep model.
Methods
Partial meniscectomy was carried out to the medial meniscus of 28 sheep, and a scaffold was implanted in 19 menisci (3-month scaffold group, n = 9; 6-month scaffold group, n = 10). In 9 sheep, the defect remained empty (partial meniscectomy group). Sham operation was performed in 9 animals.
Results
The silk scaffold was able to withstand the loads experienced during the implantation period. It caused no inflammatory reaction in the joint 6 months postoperatively, and there were no significant differences in cartilage degeneration between the scaffold and sham groups. The compressive properties of the scaffold approached those of meniscal tissue. However, the scaffolds were not always stably fixed in the defect, leading to gapping between implant and host tissue or to total loss of the implant in 3 of 9 cases in each scaffold group. Hence, the fixation technique needs to be improved to achieve a better integration into the host tissue, and the long-term performance of the scaffolds should be further investigated.
Conclusion
These first in vivo results on a new silk fibroin scaffold provide the basis for further meniscal implant development. Whilst more data are required, there is preliminary evidence of chondroprotective properties, and the compressive properties and biocompatibility are promising.
Keywords: Meniscus, Meniscal replacement, Silk fibroin, Scaffold, Osteoarthritis
Introduction
The menisci play a key role in load distribution in the knee joint [20, 36]. Meniscal lesions treated by partial or total meniscectomy compromise this function. It is well known that this can hasten the onset of osteoarthritis [3], which makes the need for new treatment strategies of great clinical relevance. Therefore, various materials have been developed and investigated to fill meniscal defects [33]. However, only few strategies showed a chondroprotective function [11, 34]. To date, scaffolds made of processed natural materials, e.g. small intestine submucosa, tendon tissue and isolated tissue components have also been used as meniscus replacement [26]. The Collagen Meniscus Implant (CMI, also known as Menaflex®) has already been used clinically for some years. Although some good results have been reported concerning tissue regeneration and pain relief scoring in several clinical trials [16, 31, 32, 42] and experimentally [14], the mechanical functionality and chondroprotective effect of the implant has not yet been confirmed experimentally or in clinical studies [25, 28]. The implantation of the CMI resulted in the formation of scar tissue, which has inferior mechanical properties compared to meniscal tissue [28]. Therefore, it is assumed that the CMI contributes little to load transmission in the knee joint [4]. In addition, non-degradable and biodegradable synthetic polymers have been developed for the replacement of damaged meniscal tissue. Amongst this scaffold class, a polycaprolactone–polyurethane scaffold (Actifit®) has already been investigated in several preclinical and clinical studies [13, 24, 40]. The mechanical properties of the polycaprolactone–polyurethane material are significantly less than those of the meniscus [38], and whether this scaffold can protect the articular cartilage from degeneration, and thus has advantages over partial meniscectomy, still needs to be confirmed [24].
Because to date no material has clearly demonstrated satisfactory replacement of damaged meniscal tissue, the present study investigated a novel scaffold formed from the structural protein, silk fibroin. Several materials manufactured from silk fibroin have already demonstrated good biocompatibility and excellent mechanical properties in other biomedical applications [1, 23]. The aim of this in vivo study was to analyse the suitability of the new silk fibroin scaffolds in partial meniscus replacement. Specifically, we hypothesized that implantation of a silk fibroin scaffold as a partial meniscal replacement device prevents the development of articular cartilage degeneration by comparison with partial meniscectomy.
Materials and methods
Study design
Thirty-seven skeletally mature (4.2 ± 0.9 years) female Merino sheep with an average weight of 88 ± 11 kg were used for the investigations. The animals were randomly divided into four groups (Table 1). All groups underwent a medial arthrotomy including the release of the medial collateral ligament. Group 1 (3-month scaffold group; sc3), group 3 (6-month partial meniscectomy group; pm) and group 4 (6-month scaffold group; sc6) underwent partial meniscectomy, and in groups 1 and 4, a silk scaffold was then implanted into the meniscal defect. Group 2 (6-month sham surgery group; sh) served as a control to demonstrate that the arthrotomy itself does not lead to degenerative changes in the stifle joint. After 3 and 6 months, respectively, the animals were killed and both hind limbs were collected and prepared for macroscopic, biomechanical, immunochemical and histological analysis.
Table 1.
Distribution of the animals in the four treatment groups
| Treatment | Observation period | n | n corr* | |
|---|---|---|---|---|
| Group 1 (sc3) | Scaffold | 3 months | 9 | 9 |
| Group 2 (sh) | Sham | 6 months | 9 | 9 |
| Group 3 (pm) | Partial meniscectomy | 6 months | 9 | 8 |
| Group 4 (sc6) | Scaffold | 6 months | 10 | 9 |
* n corr corrected number after exclusion of two animals
Materials
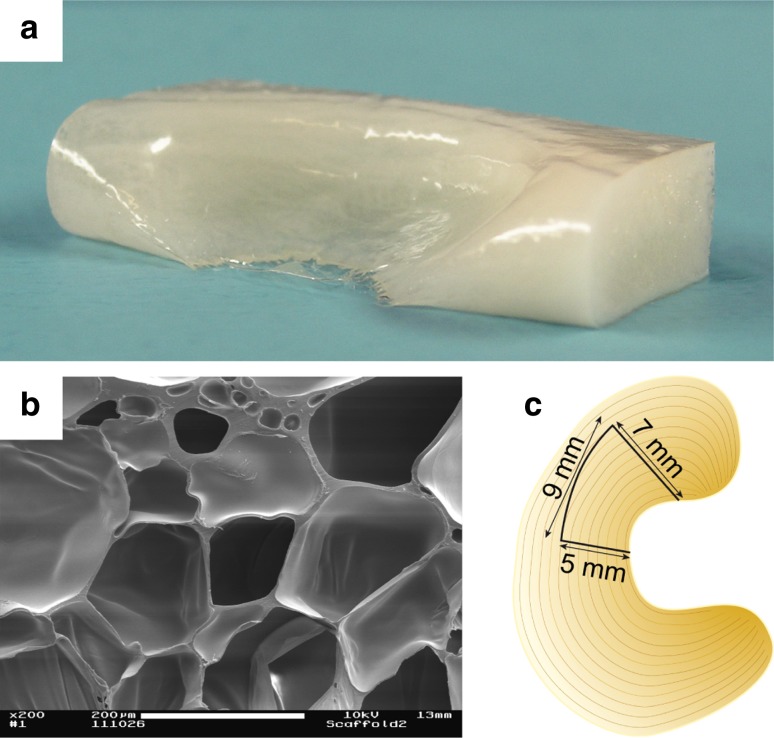
Silk fibroin scaffolds were manufactured from Bombyx mori raw silk. After degumming of raw silk fibres as described by Hakimi et al. [12], the resulting silk fibroin was processed into a porous matrix. Silk fibroin devices were then cut to size (Fig. 1a) and immersed in 0.9 % phosphate-buffered saline before being irradiated at 25 KGy to sterilize. Sterilized scaffolds were stored at 4 °C until used for surgery. An original sheep meniscus taken from the same race and age as used for the animal study was employed as a template for the scaffold mould. Since shape and size of the menisci varied only little between animals of the same age and race, this method provided the best possible fit for the animal experiment. Prior to the in vivo study the scaffolds’ structural, mechanical and biological properties were investigated. The porosity of the silk fibroin scaffolds was determined using high-resolution micro-CT (SkyScan, Belgium). The scaffolds showed a pore size ranging from 150 to 300 μm, a porosity of 85 % and very smooth surfaces (Fig. 1a, b). Cylindrical samples of the scaffold were tested in a materials testing machine (Z010, Zwick GmbH & Co. KG, Germany) and the compression test (unconfined compression) revealed a Young’s modulus of 1.1 ± 0.2 MPa at strains <10 %, which was in the range of moduli obtained for human menisci at 12 % strain [6].
Fig. 1.
Silk fibroin scaffold (a) before adjustment of implant size; SEM image (b) showing the porosity of the silk scaffold, scale bar 200 μm; diagram of medial meniscus with location and size of resected tissue (c)
Operation
All sheep were operated on the right stifle joint under general anaesthesia. After medial incision of skin and fasciae, the medial collateral ligament was released including its femoral bony attachment to gain access to the medial compartment of the joint. Then, the joint capsule was opened. Flexion and external rotation of the stifle joint enabled access to the medial meniscus. In the meniscectomy group, and both scaffold groups, a 9 mm × 7 mm × 5 mm piece of meniscus was resected at the anterior horn of the medial meniscus (Fig. 1c). A small millimetre scale was used to achieve these dimensions as close as possible. The meniscal explant served as a template to adjust the shape of the scaffold implant by use of a scalpel. The adjusted scaffold was implanted into the defect of the scaffold group animals with two non-penetrating horizontal mattress sutures. The meniscal defect remained untreated in the meniscectomy group. In the sham group, no intervention at the meniscus was carried out. The degree of cartilage degeneration in the visible area of the joint was recorded for each sheep. After closure of the joint capsule, the medial collateral ligament was reattached with a claw plate and a bicortical bone screw. Finally, fasciae and skin were sutured in separate layers. Full weight bearing was permitted immediately after surgery, and there was no restriction to the movement of the operated limb.
Macroscopic evaluation
Both the knee region and the stifle joint itself were examined macroscopically after killing. In the area of the surgical access, particular attention was paid to any disturbance to wound healing and signs of inflammation. Changes to the joint capsule such as colour changes, thickening, fibrosis, calcification or villus formation were recorded. The consistency of the synovial fluid was also evaluated. The scaffold’s shape and its attachment to the meniscal tissue were assessed and observations were noted. The articular cartilage of the tibial plateau and femoral condyles was examined for signs of degeneration.
Biomechanical testing
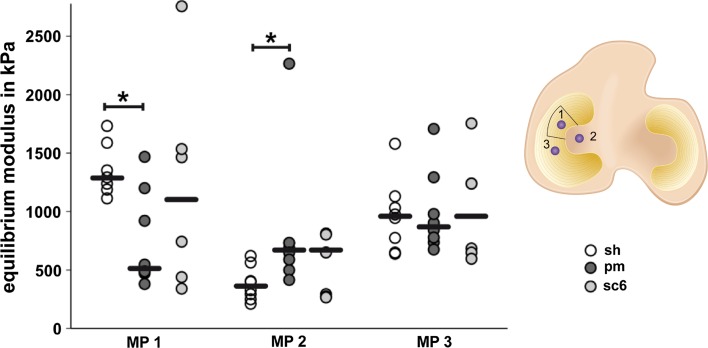
Indentation testing of the tibial condyle was performed to determine the equilibrium modulus (E eq) of the articular cartilage in groups 2 (sh), 3 (pm) and 4 (sc6). Medial tibial condyles were separated from the tibia and mounted in a transparent testing chamber filled with saline. The testing chamber allowed exact alignment of the cartilage surface parallel to the indenter at all locations. For each tibial plateau, three locations were tested (Fig. 3a). The indentation tests were performed using a materials testing machine (Z010, Zwick GmbH & Co. KG, Germany) with a flat-ended steel indenter (∅ 1.5 mm). The compressive force was measured by a 50-N load cell KAP-Z, AST GmbH, Germany, 0.05 % accuracy) and the displacement by a laser sensor (optoNCDT2200-20, Micro-Epsilon GmbH &Co.KG, Germany, 0.3 μm resolution, 0.03 % accuracy). Cartilage thickness was measured according to the needle method described by Hoch et al. [17]. After applying a 0.2-N preload, the cartilage was loaded with a constant speed (100 % of cartilage thickness per minute) until a load of 4.8 N was applied. The resulting strain was sustained for 20 min, and the decrease in force was recorded. The equilibrium modulus (E eq) of the cartilage was calculated using the equation according to Hooke’s law:
where F R = equilibrium force, d = cartilage thickness, A = surface area of the indenter, Δl = indentation depth.
Fig. 3.
Indentation test of the tibial cartilage; equilibrium modulus in the sham (sh), partial meniscectomy (pm) and scaffold 6 months (sc6) group at measuring points 1, 2 and 3, single values and medians, *p < 0.05
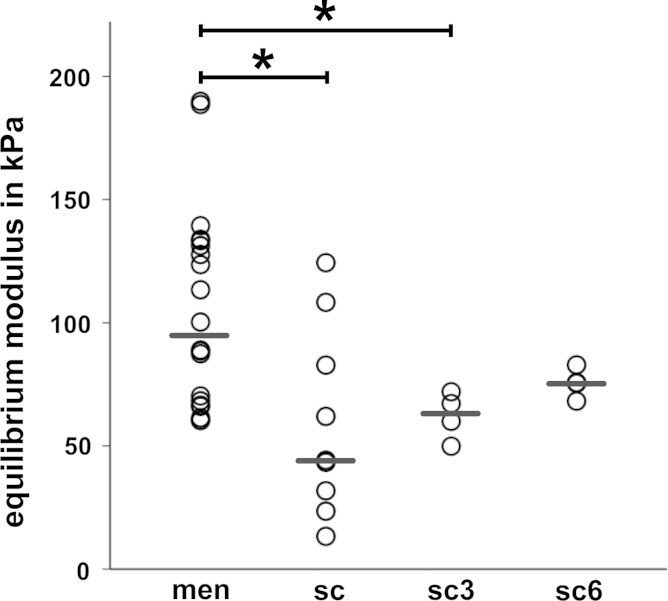
Tissue punches (∅ 3 mm) of meniscus and scaffold were mechanically tested under confined-compression in the materials testing machine to determine the equilibrium modulus (E eq). The punches were removed from the resected meniscus tissue after surgery and cut coplanar to the tibial surface. The resulting cylinders were placed into a 3-mm diameter hole in a plastic test chamber with the cut surface facing down. The test chamber was filled with saline. A porous ceramic cylinder was placed between specimen and stamp to allow fluid flow during testing. After applying a 0.5-N preload, a stress-relaxation test under confined-compression was performed at 20 % strain. The strain was kept constant for 15 min, and the decrease in force was recorded over the testing period. Similarly, samples of the scaffolds before and after implantation were tested with the smooth surface facing down during testing to allow fluid flow out of the scaffolds. The equilibrium modulus of the samples was calculated for each strain level as described above.
Macroscopic grading of articular cartilage after staining with India Ink
India Ink (Pelikan mbH & Co. KG., Germany) was applied to the cartilage surfaces of formaldehyde-fixed femoral condyles and tibial plateaus to facilitate the macroscopic grading of cartilage degeneration. The carbon particles of the ink bound to degenerated and fibrillated cartilage areas but not to the smooth, intact cartilage surface. One drop of ink was applied to each specimen and gently rubbed in using a wet cloth. After brief rinsing under running water, photographs of condyles and plateaus were taken. Using an image analysis software (Leica GmbH, Germany), the area of degenerated (i.e. ink stained) cartilage was determined. Total cartilage area was also determined. Then, the proportion of degenerated cartilage area in total cartilage area was calculated and served as a measure for the degree of cartilage degeneration.
Biochemical analysis of synovial fluid
Prostaglandin E2 (PGE2), Interleukin 1β (IL 1β) and Interleukin 6 (IL 6) contents were quantified as representative inflammatory markers in synovial fluid pre- and postoperatively using commercially available assay kits (PGE2: Biotrend Chemikalien GmbH, Germany; IL 1β and IL 6: Cusabio Biotech Co., China). All tests were performed following the manufacturer’s instructions.
Histology
Synovial membranes, scaffolds and tibial cartilage were assessed histologically. Some problems during sample processing, e.g. breaking of the cartilage-subchondral-bone slices or separation of the cartilage layer occurred. Therefore, the number of slices that were evaluated per group was low in some cases. Staining with haematoxylin eosin (H&E) was performed for differential analysis of tissue structures, and cartilage sections were additionally stained with Safranin-O fast green (Saf-O) for the assessment of glycosaminoglycan content. Sections of synovial membrane, scaffold and surrounding meniscal tissue were analysed qualitatively with a light microscope (Leica GmbH, Germany). The tibial cartilage sections of groups 2 (sh), 3 (pm) and 4 (sc6) were assessed semi-quantitatively using the Mankin grading system, and the Fibrillation Index (FI, quotient of cartilage surface and tidemark lengths) of the cartilage was determined histomorphometrically [27, 30].
Institutional review board
All procedures were performed according to the national and international regulations for the care and use of laboratory animals and were approved by the Regional Administrative Authority in Tübingen, Germany (registration number 1020).
Statistical analysis
A power analysis was designed based on the results of an earlier study on meniscal replacement in an ovine model [24], which resulted in a required group size of seven animals. To allow for the possibility of loss of animals during the study period, we increased the group size to at least nine animals. Statistical analysis was performed using SPSS Statistics (IBM SPSS Statistics, USA). Since the data were not normally distributed, a Mann–Whitney U test was performed to determine differences between the groups. To detect differences within each group a Wilcoxon signed-rank test was performed. In all cases, a significance level of p = 0.05 was used.
Results
Clinical observations
All animals were partially weight bearing after 4 days and fully weight bearing without visible lameness by 7 days postoperatively. No disturbance of wound healing occurred, and all animals showed a good general condition for the whole test period. One animal died during anaesthesia due to apnoea and cardiac arrest. The physical condition of two animals deteriorated 3 weeks postoperatively. These animals had to be euthanized prior to the termination of the study. In both cases, no obvious correlation between the physical deterioration and the surgical procedure was found. Thus, the number of evaluated animals was reduced to n corr = 34 (Table 1).
Macroscopic findings
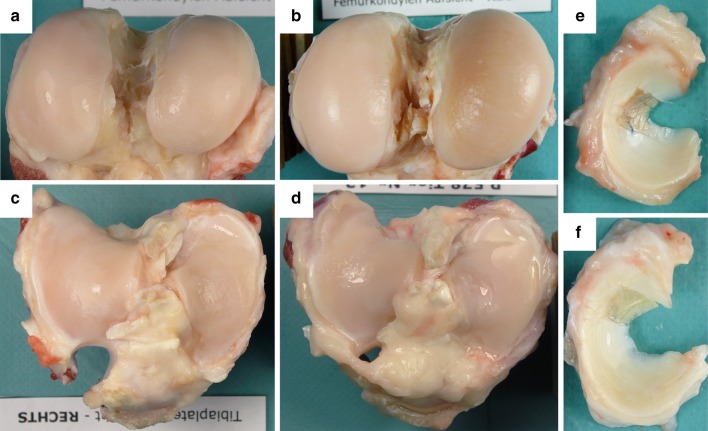
In post-mortem, no signs of inflammation were visible in the knee region in any of the four groups. The joint capsule showed a moderate, fibrotic thickening at the medial aspect of the joint in most cases, but no other changes were visible macroscopically. The synovial fluid of all animals appeared normal, being of clear, light yellowish to light reddish colour and of a characteristic viscous consistency. The scaffold was in place in six animals of the 3-month, and in six animals of the 6-month, scaffold groups (Fig. 2e, f). In some of these animals, the scaffold filled the defect completely, but in some animals, the scaffold was not firmly attached to the meniscus, particularly at the posterior edge. In both scaffold groups (groups 1 and 4), the scaffold did not fill the meniscal defect in three animals at termination of the study. Since it is not known at which point the scaffolds had dislocated from the defect, these six animals were excluded from further analysis. No regenerative tissue was found in the meniscal defects of the partial meniscectomy group. Large inter-individual differences in the macroscopic degree of cartilage degeneration were visible, consistent with the observations recorded during the surgical procedure. Between the sham and the 6-month scaffold groups, no differences in the degree of cartilage degeneration were observed macroscopically (Fig. 2a–d).
Fig. 2.
Photographs of femoral condyles (a, b) and tibial plateaus (c, d); no macroscopically visible differences of cartilage degeneration between the 6-month scaffold (a, c) and the sham (b, d) group; photographs of medial menisci of the 3-month (e) and 6-month scaffold (f) groups
Indentation test of medial tibial condyle
At the measuring point underneath the meniscal defect (MP 1), the median E eq of the partial meniscectomy group was significantly lower than the modulus of the sham group (p < 0.05, Fig. 3). After 6 months, the median modulus of the cartilage in the group treated with a scaffold was not statistically different from that of the sham group. The median E eq of the sham group was significantly lower than the E eq of the partial meniscectomy group at the central measuring point (MP 2, p < 0.05), but no differences in E eq were present between the three groups at the posterior measuring point (MP 3).
Stress-relaxation test of meniscus and scaffold
Due to difficulties in preparation of some scaffold samples, only four of the three and four of the 6-month implanted scaffolds were included in the evaluation of the stress-relaxation test. As expected, the meniscal tissue showed a significantly higher equilibrium modulus than the scaffold at all strain levels (p < 0.05, Fig. 4). However, the equilibrium modulus of the scaffolds implanted in the stifle joint increased over the implantation period, and after 6 months of implantation, there was no longer a significant difference between the meniscal tissue (95 kPa) and the scaffold (76 kPa).
Fig. 4.
Biomechanics of meniscus and scaffold: equilibrium modulus of meniscal tissue (men), scaffold material (sc), scaffold material after 3- (sc3) and 6-month (sc6) implantation; results shown only for the 20 % strain level; single values and median, *p < 0.05
Macroscopic grading of articular cartilage after staining with India Ink
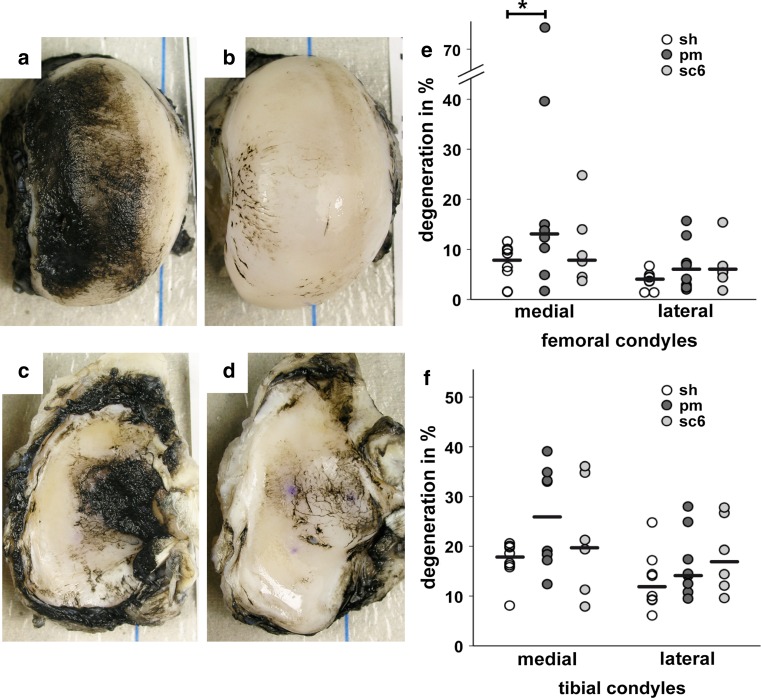
The degenerated cartilage area on the medial femoral condyles of the partial meniscectomy group was almost twice as large as the degenerated cartilage area of the sham and the 6-month scaffold group (p < 0.05, Fig. 5e). Single animals showed particularly extensive cartilage degeneration (Fig. 5a). The degenerated cartilage area of the medial femoral condyles was thus significantly larger in the partial meniscectomy group than in the sham group. The medial tibial condyles of the partial meniscectomy group also showed considerably more degenerative changes than the condyles of the sham and the 6-month scaffold group (Fig. 5f), although these differences were not statistically significant. There were no statistical differences between all groups in the lateral compartment (Fig. 5e, f).
Fig. 5.
India Ink staining: photographs of medial femoral (a, b) and tibial condyles (c, d) stained with India Ink; staining was more extensive in the partial meniscectomy group (a, c) than in the 6-month scaffold group (b, d); the relative proportion of degenerative area to total cartilage area is shown in graph e for the femoral condyles and in graph f for the tibial plateaus of sham (sh), partial meniscectomy (pm) and 6-month scaffold (sc6) groups; single values and medians, *p < 0.05
Biochemical findings
IL 1β concentrations in the synovial fluid were below the detection limit of the ELISA kit pre- and postoperatively in all groups. The IL 6 concentrations were in a relatively narrow range (8.4–11.4 pg/ml) both pre- and postoperatively with no statistical difference between the groups. The PGE2 concentrations of the individual animals varied widely pre- and postoperatively in all four groups (0–625 pg/ml). An increase in the PGE2 content occurred postoperatively in the 3-month scaffold group to 400 % of the intraoperative level. Statistical significance could not be tested in this case due to a low number of samples (n = 4), which was caused by insufficient retrieval of synovial fluid. However, the level dropped to intraoperative values after 6 months.
Histological findings
A hyperplasia of the synovial cells and a villous proliferation of the synovial membrane were visible in individual animals of all four groups but was most frequently observed in the 3-month scaffold group. In this group, occasional inflammatory cells were found in the synovial membrane of three animals. No inflammatory cells occurred in the sham, partial meniscectomy and 6-month scaffold group.
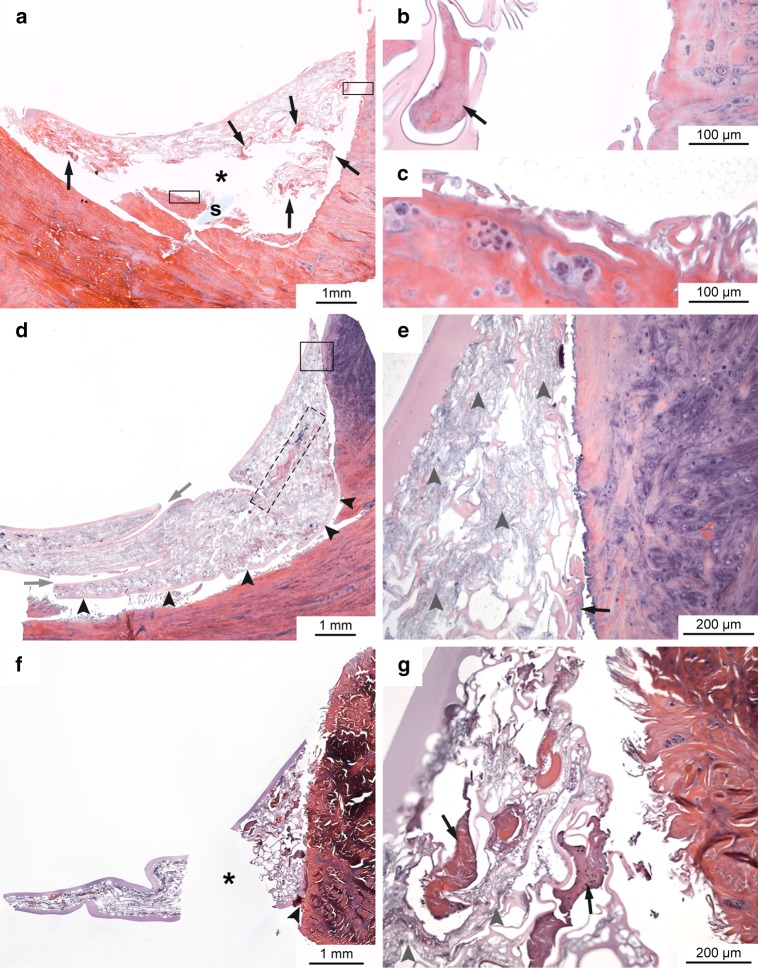
In both scaffold groups, no signs of inflammation were present in the meniscal tissue that had been in contact with the scaffold in vivo. The nature of the single scaffolds differed greatly in both scaffold groups (Table 2). The surfaces of some scaffolds were more or less smooth (Fig. 6a), but other scaffolds had up to 1-mm deep folds at their tibial and femoral surfaces (Fig. 6d, f). Furthermore, differences in pore size and condition of pore walls occurred within the individual scaffolds as well as between different scaffolds. Macropores were formed by pore walls >10 μm. These macropores were compressed to a differing degree, some were empty, but some were filled with amorphous material possibly representing protein-rich fluid, fibroblast- or fibrochondrocyte-like cells and fibrous tissue (Fig. 6a, b, d, e–g). Only single inflammatory cells occurred in the 3- and 6-month implanted scaffolds. Some areas of the scaffolds consisted mainly of micropores (∅ < 10 μm) with very thin pore walls (Fig. 6d, e, g). Most of these micropores were highly compressed and contained neither cells nor tissue (Fig. 6e). Between the scaffolds and the meniscal tissue, a 80–320-μm wide gap was visible in the histological sections and no signs of a fibrous adhesion between scaffold and meniscus could be found histologically (Fig. 6a, b, d, e, g). In general, the condition of the 3-month implanted scaffolds did not differ from the condition of the 6-month implanted scaffolds (Table 2).
Table 2.
Findings of the histological examination of the scaffolds after 3 and 6 months of implantation
| Folds | Macropores | Micropores | Amorphous material | Cells/tissue | |
|---|---|---|---|---|---|
| Sc3 | |||||
| 1 | x | x | x | x | |
| 2 | x | x | x | ||
| 3 | x | x | x | x | |
| 4 | x | x | x | x | |
| 5 | x | x | x | x | |
| 6 | x | x | x | x | |
| Total | 4 | 6 | 3 | 6 | 4 |
| Sc6 | |||||
| 1 | x | x | x | x | x |
| 2 | x | x | x | x | x |
| 3 | x | x | |||
| 4 | x | x | x | x | x |
| 5 | x | x | x | x | |
| 6 | x | x | x | ||
| Total | 5 | 5 | 5 | 5 | 4 |
Fig. 6.
Histological sections of scaffolds after three (a–c) and six (d–g) months of implantation stained with HE; a horizontal section of a scaffold of the 3-month scaffold group, b, c are magnifications of a, s suture remnants; d horizontal section of a scaffold of the 6-month scaffold group, e magnification of d, grey arrows folds in the surface of the scaffold, dashed box suturing channel; f vertical section of a scaffold of the 6-month scaffold group, g magnification of f; black arrows pores filled with amorphous and/or fibrous tissue; arrowheads smooth surface of scaffold in contact with meniscus; * artefact
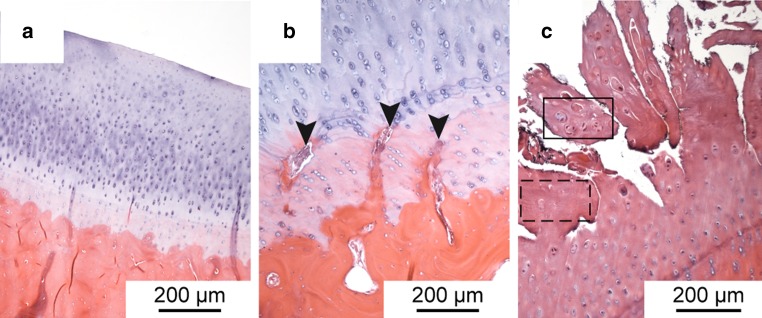
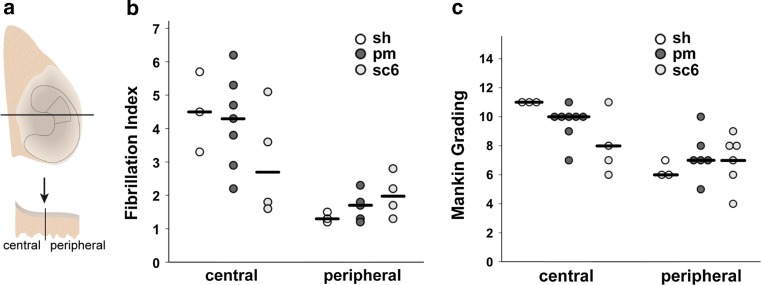
It was noticeable that the degeneration in the central area was more severe than at the periphery (Figs. 7, 8). Degenerated areas with cartilage fibrillation occurred exclusively in the central part of the condyles and were accompanied by a marked reduction in Saf-O staining (Fig. 7a, b). Cells tended to form clusters in these areas or hypocellularity was observed (Fig. 8c). Peripherally, the interruption of the tidemark by blood vessels was the most frequent change, and often no other changes were visible there (Fig. 8b). Centrally, the median Fibrillation Index and Mankin score of the 6-month scaffold group was much lower than in the sham and partial meniscectomy groups, although the difference was not statistically significant (Fig. 9b, c). Peripherally, the differences in the Fibrillation Index and the Mankin grading between the three groups were much lower (Fig. 9b, c).
Fig. 7.
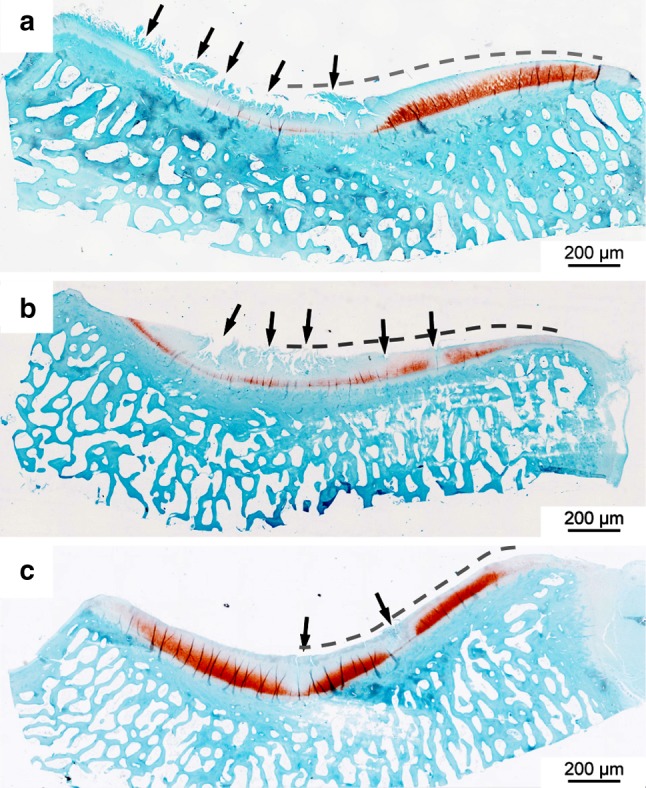
Histological sections of the medial tibial condyles stained with Saf-O; a Tibial condyle of the sham group; Saf-O intensity is reduced especially in the central part of the plateau and cartilage fibrillation (arrows) occurs; b tibial condyle of the partial meniscectomy group; Saf-O intensity is highly reduced and pronounced fibrillation occurs in the central part (arrows); c tibial condyle of the 6-month scaffold group; single clefts occur (arrows), the Saf-O intensity is reduced near the clefts. The dashed lines indicate submeniscal parts
Fig. 8.
Histological sections of the medial tibial condyles stained with HE; a tibial cartilage of the 6-month scaffold group, cartilage surface and tidemark are intact, cell number is normal; b tibial cartilage of the 6-month scaffold group, blood vessels cross the tidemark (arrowheads), cell number is normal; c highly fibrillated tibial cartilage of the sham group with cell-cluster formation (black box) and hypocellularity (dashed box)
Fig. 9.
Histological evaluation of the tibial cartilage. a Diagram for histological sampling of the medial tibial condyle with indication of meniscus and scaffold; b Fibrillation Index of the tibial cartilage in the sham (sh), partial meniscectomy (pm) and 6-month scaffold (sc6) group; c Mankin grade (min: 0, max: 14) of the medial tibial cartilage in these groups; single values and median
Discussion
The present study demonstrated that after 6-month implantation, the silk fibroin scaffolds showed compressive properties comparable to those of native meniscus. They did not cause an inflammatory reaction in the knee joint, and there was some evidence that they delay the onset of degeneration of the articular cartilage in the operated joint in the medium term partially confirming the hypothesis. The study further showed that the scaffold fixation was not yet satisfactory leading to partial gapping between scaffold and meniscal tissue, or total loss of the scaffold in a minority of cases.
In the present in vivo study, no macroscopic signs of inflammation were present after 3 and 6 months of silk fibroin scaffold implantation. The histological investigations showed only mild alterations in the synovial membrane in both scaffold groups and control groups, and only in the 3-month scaffold group were occasional inflammatory cells found. The findings of the present study are in accordance with numerous in vitro and in vivo studies that have already demonstrated the biocompatibility of silk fibroin materials [2, 29, 35], and it is notable that other meniscal scaffolds have caused mild to moderate inflammatory reactions in vivo [24, 38].
When meniscal tissue is damaged, shear forces present a particular challenge to the fixation technique since these forces cause gapping of meniscal tears [9]. Accordingly, a rigid fixation of the scaffold material to the meniscus is very important. In the present study, a non-penetrating technique was chosen to suture the scaffold to the remaining meniscus. With this technique, the suture material did not interact with the articular cartilage and the influence of scaffold implantation on cartilage health could be better determined. On the other hand, the suture material was anchored only in the porous part of the scaffold and not in the more robust scaffold surface. This and immediate postoperative load bearing may have contributed to the dislocation of three scaffolds in each group and to the fact that in some animals, the scaffold was not firmly attached to the meniscal tissue after 6 months of implantation. Additionally, due to the small size of the scaffold, only two sutures were used for fixation. With an increasing number of fixation points, the loads acting on a single suture should decrease and fixation should be more stable. Similar experimental studies with other scaffold materials used three to four sutures to fix the scaffold to the meniscal tissue [24, 28]. Nevertheless, insufficient fixation of scaffolds was observed in some cases in these studies too. Alterations to the fixation technique in combination with changes in scaffold structure such as the integration of a fibre mesh could improve fixation stability of the silk fibroin scaffolds in the future.
The resection of meniscal tissue may not have been performed into the vascularized zone of the meniscus since no regenerative tissue formation was observed in the defect of the partial meniscectomy group. In contrast, varying amounts of regenerative tissue were described in the partial meniscectomy control groups of comparable in vivo studies [7, 28], and Maher et al. [24] observed even a considerable amount of regenerative tissue in the partial meniscectomy group, indicating that meniscal resection has been carried out into the vascularized zone. The absence of regenerative tissue in the present study indicates that scaffolds were implanted into the non-vascular part of the meniscus. This, together with the advanced age of the sheep used in this study and hence lower regenerative potential, is likely to have complicated scaffold integration.
Everyday activities like stair climbing cause loads as high as 3.5-fold body weight in the knee joint [21]. Therefore, an adequate initial stability for meniscus replacement materials is essential. Due to the material properties of the scaffolds used in the present study, a very slow degradation rate can be expected [1]. Silk fibroin is known to have very great strength and elasticity [1, 23]. Hence, it can be assumed that the silk fibroin scaffold can withstand the high mechanical loads in the longer term. In order to verify whether the scaffolds are suitable to mechanically replace meniscal tissue, the equilibrium moduli of meniscus and scaffold were compared in the present study. It could be shown that the equilibrium modulus of the scaffolds increased over implantation time and approached the modulus of the meniscus. Whether the increase in stiffness is based on an alteration of material properties or on a compaction of the material due to constant loading needs to be further investigated. The mechanical properties of other scaffold materials pre- and postimplantation have only rarely been studied [25]. Tienen et al. [38] described an improvement in the mechanical properties of polycaprolactone–polyurethane and Estane scaffolds over implantation time in a canine model. However, unlike the present study, there were still major differences between the scaffold material and meniscus at the end of the study. No mechanical investigations of a widely used collagen scaffold (CMI) have been published so far [25]. This scaffold has been described as mechanically unstable [28], but nevertheless, it has already been used clinically [4]. Similarly, a polycaprolactone–polyurethane scaffold (ActiFit®) has been used in clinical studies, but no data exist, which present the mechanical properties of the material [39, 40]. In some experimental studies with various scaffold materials, even structural changes such as tearing or shrinkage were reported [18, 19, 24]. An in vivo study with a polycaprolactone-based polyurethane implant for total meniscus replacement showed that all implants were severely damaged after 6 months of implantation [13]. Efe et al. [10] reported shape irregularities and extrusions of the Actifit® polyurethane scaffold in a clinical study. Such findings have not been observed in the present study.
The main objective of meniscus replacement is to prevent degeneration of the articular cartilage, which is frequently a consequence of partial meniscectomy in the long term [8]. Therefore, it is necessary to ensure that the replacement material does not cause cartilage defects itself but helps to delay cartilage degeneration. The ovine model is particularly suited to investigate the chondroprotective properties of a material since sheep quickly develop osteoarthritic changes after total or partial meniscectomy [5, 24]. In the present study, a highly variable degree of cartilage degeneration was noted in animals at the beginning of the study, which complicated the interpretation of the results. Sheep naturally develop osteoarthritis with increasing age [43], and the mean age of the animals in the study (4.2 years) was quite advanced. This is a major limitation of this study and should be considered in future studies using younger animals, which would provide healthy articular cartilage at the beginning of the study. Nevertheless, on average the comparison of the sham and the 6-month scaffold group showed that the silk fibroin scaffold does not cause articular cartilage degeneration. Additionally, biomechanical investigations, macroscopic grading after India Ink staining and histological analysis of the articular cartilage all indicate that the degenerative changes observed after partial meniscectomy could be delayed by silk fibroin scaffold implantation. The chondroprotective properties should be confirmed over a longer period of time in younger animals. Although other scaffolds have been intensively investigated in experimental studies and some have already been used clinically [15, 33, 41], the chondroprotective potential of these scaffold materials has not been unambiguously confirmed so far [10, 31, 37]. Zaffagnini et al. [42] reported a worsened degeneration score 2 years after lateral CMI implantation in some cases whilst Linke et al. [22] reported no significant difference in Lysholm and IKDC scores between patients receiving the CMI and the meniscectomy control group at 2-years follow up.
Conclusions
The hypothesis that implantation of a silk fibroin scaffold as a partial meniscal replacement device prevents the development of articular cartilage degeneration was partly confirmed. Although there were some indications of chondroprotective instability in scaffold fixation partly compromised the results obtained. Provided the shortcomings of the new material and its implantation technique can be overcome, the mechanical strength, biocompatibility and indications of chondroprotection suggest silk fibroin scaffolds deserve further consideration as an implant for partial meniscal replacement.
Acknowledgments
We want to thank Ursula Maile and Marion Tomo for sectioning and staining the numerous histological specimens and Patrizia Horny for the illustrations. This work was financially supported by the Wellcome Trust [08906/z/08/z].
Conflict of interest
None.
Footnotes
A correction to this article is available online at https://doi.org/10.1007/s00167-018-4878-6.
References
- 1.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. doi: 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 2.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23(20):4131–4141. doi: 10.1016/S0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 3.Andersson-Molina H, Karlsson H, Rockborn P. Arthroscopic partial and total meniscectomy: a long-term follow-up study with matched controls. Arthroscopy. 2002;18(2):183–189. doi: 10.1053/jars.2002.30435. [DOI] [PubMed] [Google Scholar]
- 4.Buma P, van Tienen T, Veth R. The collagen meniscus implant. Expert Rev Med Devices. 2007;4(4):507–516. doi: 10.1586/17434440.4.4.507. [DOI] [PubMed] [Google Scholar]
- 5.Burger C, Mueller M, Wlodarczyk P, Goost H, Tolba RH, Rangger C, Kabir K, Weber O. The sheep as a knee osteoarthritis model: early cartilage changes after meniscus injury and repair. Lab Anim. 2007;41(4):420–431. doi: 10.1258/002367707782314265. [DOI] [PubMed] [Google Scholar]
- 6.Chia HN, Hull ML. Compressive moduli of the human medial meniscus in the axial and radial directions at equilibrium and at a physiological strain rate. J Orthop Res. 2008;26(7):951–956. doi: 10.1002/jor.20573. [DOI] [PubMed] [Google Scholar]
- 7.Chiari C, Koller U, Dorotka R, Eder C, Plasenzotti R, Lang S, Ambrosio L, Tognana E, Kon E, Salter D, Nehrer S. A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthr Cartil. 2006;14(10):1056–1065. doi: 10.1016/j.joca.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Cox JS, Nye CE, Schaefer WW, Woodstein IJ. The degenerative effects of partial and total resection of the medial meniscus in dogs’ knees. Clin Orthop Relat Res. 1975;109:178–183. doi: 10.1097/00003086-197506000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Dürselen L, Hebisch A, Claes LE, Bauer G. Gapping phenomenon of longitudinal meniscal tears. Clin Biomech. 2003;18(6):505–510. doi: 10.1016/S0268-0033(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 10.Efe T, Getgood A, Schofer MD, Fuchs-Winkelmann S, Mann D, Paletta JR, Heyse TJ. The safety and short-term efficacy of a novel polyurethane meniscal scaffold for the treatment of segmental medial meniscus deficiency. Knee Surg Sports Traumatol Arthrosc. 2011;20(9):1822–1830. doi: 10.1007/s00167-011-1779-3. [DOI] [PubMed] [Google Scholar]
- 11.Haddad B, Haddad B, Konan S, Adesida A, Khan WS. A systematic review of tissue engineered meniscus and replacement strategies: preclinical models. Curr Stem Cell Res Ther. 2013;8(3):232–242. doi: 10.2174/1574888X11308030008. [DOI] [PubMed] [Google Scholar]
- 12.Hakimi O, Gheysens T, Vollrath F, Grahn MF, Knight DP, Vadgama P. Modulation of cell growth on exposure to silkworm and spider silk fibers. J Biomed Mater Res A. 2010;92(4):1366–1372. doi: 10.1002/jbm.a.32462. [DOI] [PubMed] [Google Scholar]
- 13.Hannink G, van Tienen TG, Schouten AJ, Buma P. Changes in articular cartilage after meniscectomy and meniscus replacement using a biodegradable porous polymer implant. Knee Surg Sports Traumatol Arthrosc. 2010;19(3):441–451. doi: 10.1007/s00167-010-1244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen R, Bryk E, Vigorita V. Collagen scaffold meniscus implant integration in a canine model: a histological analysis. J Orthop Res. 2013;31(12):1914–1919. doi: 10.1002/jor.22456. [DOI] [PubMed] [Google Scholar]
- 15.Hasan J, Fisher J, Ingham E. Current strategies in meniscal regeneration. J Biomed Mater Res B Appl Biomater. 2014;102(3):619–634. doi: 10.1002/jbm.b.33030. [DOI] [PubMed] [Google Scholar]
- 16.Hirschmann MT, Keller L, Hirschmann A, Schenk L, Berbig R, Luthi U, Amsler F, Friederich NF, Arnold MP. One-year clinical and MR imaging outcome after partial meniscal replacement in stabilized knees using a collagen meniscus implant. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):740–747. doi: 10.1007/s00167-012-2259-0. [DOI] [PubMed] [Google Scholar]
- 17.Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4–12. doi: 10.1002/jor.1100010102. [DOI] [PubMed] [Google Scholar]
- 18.Kelly BT, Robertson W, Potter HG, Deng XH, Turner AS, Lyman S, Warren RF, Rodeo SA. Hydrogel meniscal replacement in the sheep knee: preliminary evaluation of chondroprotective effects. Am J Sports Med. 2007;35(1):43–52. doi: 10.1177/0363546506292848. [DOI] [PubMed] [Google Scholar]
- 19.Kon E, Filardo G, Tschon M, Fini M, Giavaresi G, Reggiani LM, Chiari C, Nehrer S, Martin I, Salter DM, Ambrosio L, Marcacci M. Tissue engineering for total meniscal substitution: animal study in sheep model-results at 12 months. Tissue Eng Part A. 2012;18(15–16):1573–1582. doi: 10.1089/ten.tea.2011.0572. [DOI] [PubMed] [Google Scholar]
- 20.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980;149:283–290. [PubMed] [Google Scholar]
- 21.Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, Bergmann G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech. 2010;43(11):2164–2173. doi: 10.1016/j.jbiomech.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 22.Linke RD, Ulmer M, Imhoff AB. Replacement of the meniscus with a collagen implant (CMI) Oper Orthop Traumatol. 2006;18(5–6):453–462. doi: 10.1007/s00064-006-1188-9. [DOI] [PubMed] [Google Scholar]
- 23.MacIntosh AC, Kearns VR, Crawford A, Hatton PV. Skeletal tissue engineering using silk biomaterials. J Tissue Eng Regen Med. 2008;2(2–3):71–80. doi: 10.1002/term.68. [DOI] [PubMed] [Google Scholar]
- 24.Maher SA, Rodeo SA, Doty SB, Brophy R, Potter H, Foo LF, Rosenblatt L, Deng XH, Turner AS, Wright TM, Warren RF. Evaluation of a porous polyurethane scaffold in a partial meniscal defect ovine model. Arthroscopy. 2010;26(11):1510–1519. doi: 10.1016/j.arthro.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 25.Maher SA, Rodeo SA, Potter HG, Bonassar LJ, Wright TM, Warren RF. A pre-clinical test platform for the functional evaluation of scaffolds for musculoskeletal defects: the meniscus. Hss J. 2011;7(2):157–163. doi: 10.1007/s11420-010-9188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411–7431. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523–537. doi: 10.2106/00004623-197153030-00009. [DOI] [PubMed] [Google Scholar]
- 28.Martinek V, Ueblacker P, Braun K, Nitschke S, Mannhardt R, Specht K, Gansbacher B, Imhoff AB. Second generation of meniscus transplantation: in vivo study with tissue engineered meniscus replacement. Arch Orthop Trauma Surg. 2006;126(4):228–234. doi: 10.1007/s00402-005-0025-1. [DOI] [PubMed] [Google Scholar]
- 29.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26(2):147–155. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 30.Pastoureau P, Chomel A. Methods for cartilage and subchondral bone histomorphometry. Methods Mol Med. 2004;101:79–91. doi: 10.1385/1-59259-821-8:079. [DOI] [PubMed] [Google Scholar]
- 31.Rodkey WG, DeHaven KE, Montgomery WH, 3rd, Baker CL Jr., Beck CL Jr., Hormel SE, Steadman JR, Cole BJ, Briggs KK (2008) Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J Bone Joint Surg Am 90(7):1413–1426 [DOI] [PubMed]
- 32.Rodkey WG, Steadman JR, Li ST (1999) A clinical study of collagen meniscus implants to restore the injured meniscus. Clin Orthop Relat Res (367 Suppl):S281–S292 [DOI] [PubMed]
- 33.Rongen JJ, van Tienen TG, van Bochove B, Grijpma DW, Buma P. Biomaterials in search of a meniscus substitute. Biomaterials. 2014;35(11):3527–3540. doi: 10.1016/j.biomaterials.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Scotti C, Hirschmann MT, Antinolfi P, Martin I, Peretti GM. Meniscus repair and regeneration: review on current methods and research potential. Euro Cells Mater. 2013;26:150–170. doi: 10.22203/eCM.v026a11. [DOI] [PubMed] [Google Scholar]
- 35.Seo YK, Yoon HH, Song KY, Kwon SY, Lee HS, Park YS, Park JK. Increase in cell migration and angiogenesis in a composite silk scaffold for tissue-engineered ligaments. J Orthop Res. 2009;27(4):495–503. doi: 10.1002/jor.20752. [DOI] [PubMed] [Google Scholar]
- 36.Shrive NG, O’Connor JJ, Goodfellow JW. Load-bearing in the knee joint. Clin Orthop Relat Res. 1978;131:279–287. [PubMed] [Google Scholar]
- 37.Spencer SJ, Saithna A, Carmont MR, Dhillon MS, Thompson P, Spalding T. Meniscal scaffolds: early experience and review of the literature. Knee. 2012;19(6):760–765. doi: 10.1016/j.knee.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Tienen TG, Heijkants RG, de Groot JH, Schouten AJ, Pennings AJ, Veth RP, Buma P. Meniscal replacement in dogs. Tissue regeneration in two different materials with similar properties. J Biomed Mater Res B Appl Biomater. 2006;76(2):389–396. doi: 10.1002/jbm.b.30406. [DOI] [PubMed] [Google Scholar]
- 39.Verdonk P, Beaufils P, Bellemans J, Djian P, Heinrichs EL, Huysse W, Laprell H, Siebold R, Verdonk R. Successful treatment of painful irreparable partial meniscal defects with a polyurethane scaffold: two-year safety and clinical outcomes. Am J Sports Med. 2012;40(4):844–853. doi: 10.1177/0363546511433032. [DOI] [PubMed] [Google Scholar]
- 40.Verdonk R, Verdonk P, Huysse W, Forsyth R, Heinrichs EL. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am J Sports Med. 2011;39(4):774–782. doi: 10.1177/0363546511398040. [DOI] [PubMed] [Google Scholar]
- 41.Vrancken AC, Buma P, van Tienen TG. Synthetic meniscus replacement: a review. Int Orthop. 2013;37(2):291–299. doi: 10.1007/s00264-012-1682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaffagnini S, Marcheggiani Muccioli GM, Bulgheroni P, Bulgheroni E, Grassi A, Bonanzinga T, Kon E, Filardo G, Busacca M, Marcacci M. Arthroscopic collagen meniscus implantation for partial lateral meniscal defects: a 2-year minimum follow-up study. Am J Sports Med. 2012;40(10):2281–2288. doi: 10.1177/0363546512456835. [DOI] [PubMed] [Google Scholar]
- 43.Zur G, Linder-Ganz E, Elsner JJ, Shani J, Brenner O, Agar G, Hershman EB, Arnoczky SP, Guilak F, Shterling A. Chondroprotective effects of a polycarbonate-urethane meniscal implant: histopathological results in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2010;19(2):255–263. doi: 10.1007/s00167-010-1210-5. [DOI] [PubMed] [Google Scholar]