Abstract
Objective
The objective of this study was to investigate whether total lesion glycolysis (TLG) and metabolic tumor volume (MTV) measured by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) could predict the aggressiveness and lymph node metastasis (LNM) in patients with incidentally detected differentiated thyroid carcinoma.
Methods
A total 358 patients with focal FDG-avid thyroid incidentaloma during cancer evaluation were enrolled. Among 235 patients in whom fine-needle aspiration biopsy was performed, 51 patients underwent total thyroidectomy with LN dissection. We analyzed the relationship between volume-based parameters and clinicopathologic characteristics.
Results
The mean age and tumor size were 57.1 ± 11.3 years and 1.15 ± 0.81 cm, respectively. The prevalence of malignancy was 21.7 % (51/235). When SUVmax > 5.91, MTV2.5 > 2.05 cm3, and TLG2.5 > 9.09 were used as cutoff points, sensitivity, specificity, and area under curve (AUC) for prediction of lateral LNM were 77.9, 69.1 %, 0.716 (P = 0.047), 77.8, 88.1 %, 0.839 (P < 0.001), 77.8, 85.1 %, and 0.815 (P = 0.002), respectively. However, MTV and TLG had no value in prediction of central LNM, extrathyroidal extension, and multifocality. On comparison ROC curve analysis, the MTV and TLG showed the statistical differences for the prediction of lateral LNM compared with SUVmax (all P’s < 0.05).
Conclusions
This study has shown for the first time that volume-based PET functional parameters had a significant value for the prediction of lateral LNM in incidentally detected PTC. These results suggest that higher MTV and TLG can be potential new risk factors for preoperative risk stratification. The usefulness of TLG and MTV in preoperative risk stratification in patients with PTC needs to be confirmed in further large studies.
Keywords: Metabolic tumor volume, Total lesion glycolysis, Papillary thyroid carcinoma, Lymphatic metastasis
Introduction
Fluorodeoxyglucose labeled with 18Fluorine (18F-FDG) positron emission tomography/computed tomography (PET/CT) has an advantage over conventional modalities such as CT or magnetic resonance imaging (MRI) in that it enables quantification of the metabolic activity of a tumor. Functional imaging of FDG PET/CT can provide metabolic information on malignant tissues and make it possible to reflect the tumor burden more accurately [1]. Therefore, volume-based PET parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) have been developed to measure metabolic activity in an entire tumor mass [1, 2].
MTV is a semi-quantitative parameter of metabolic activity of tumors determined by 18F-FDG PET/CT images. MTV potentially has clinical value in the evaluation of tumor biology, evaluation of response to treatment, and prognostication in various cancers [3–6]. As another semi-quantitative parameter, TLG is called the Larson–Ginsberg index, which was calculated by multiplying the mean standardized uptake value (SUVmean) by the MTV. TLG corresponds to the cell mass of the target lesion associated with FDG uptake. TLG has been suggested to better reflect global metabolic activity in whole tumors [1]. Thus, TLG have the potential to become valuable imaging biomarkers in human solid tumors as prognostic biomarkers, adding value to clinical staging, for treatment response assessment and for treatment optimization [7].
Preoperative detection of lateral lymph node metastasis (LNM) in differentiated thyroid carcinoma (DTC) was very important for surgical plan. In addition, lateral LNM of DTC may reflect the biological aggressiveness of the carcinoma [8]. LNM may be preoperatively evaluated by imaging tests including ultrasonography (US), CT, magnetic resonance imaging (MRI), and PET/CT. Although US is the most routinely recommended of these imaging methods for preoperative evaluation of LNM in patients with papillary thyroid carcinoma (PTC) [9, 10], no other study has attempted to adapt volume-based PET parameters such as TLG and MTV for prediction of lateral LNM and characterization of DTC. The objective of this study was to investigate whether TLG and MTV measured by 18F-FDG PET/CT could predict the aggressiveness and lateral LNM in patients with incidentally detected DTC.
Materials and methods
Patients
From August 2010 to April 2013, 18,172 subjects (7589 men and 10583 women) with known or suspected cancer and healthy people in Pusan National University Hospital were enrolled in the current study. The purpose of 18F-FDG PET/CT scan was as follows: initial staging of the cancer in 9064, treatment response determination in 5186, detection of recurrent and metastatic diseases in 3306, evaluation for cancer of unknown primary origin in 383, and screening for health checkup in 233 subjects. This study was approved by the Institutional Review Board of Pusan National University Hospital, and all subjects gave their informed consent to participate in the study.
Three hundred fifty eight patients (374 nodules) have focal FDG-avid thyroid nodule in 18F-FDG PET/CT scan. Among these 358 patients, cytological diagnosis with fine-needle aspiration biopsy (FNAB) was performed in 235 patients. The results of FNAB were 36 malignant, 12 suspicious malignant, 4 follicular neoplasm, 13 atypia of undetermined significance (AUS), 153 benign, and 17 nondiagnostic. However, 101 patients did not receive FNAB due to underlying cancer, general condition, and follow-up loss. In addition, US was performed in 22 patients without FNAB due to typical benign ultrasonographic features. All 48 patients with FNAB results of malignant or suspicious malignant who underwent total thyroidectomy and central lymph node dissection were surgically confirmed as PTC. Two AUS nodules and one follicular neoplasm were confirmed as PTC and minimally invasive follicular thyroid carcinoma (FTC) by surgery, respectively. All lateral LNM had been localized by US and confirmed by FNAB prior to surgery. Especially, thirteen patients (20 lateral LNs) have FDG-avid lateral lymph node in 18F-FDG PET/CT scan. Among these patients, 9 patients underwent lateral LN dissection for lateral LN metastasis confirmed by preoperative US-guided FNA with Tg assay. The prevalence of malignancy was 21.7 % (51/235). Finally, we analyzed 51 patients in this study.
18F-FDG PET-CT imaging
18F-FDG PET/CT images were obtained with a dedicated PET-CT scanner (Biograph 40, SIEMENS, Knoxville, TN, USA). Standard patient preparation included at least 8-hour fasting and a serum glucose level of less than 6.6 mmol/L before 18F-FDG administration. PET/CT imaging was performed 60 min after injection of 18F-FDG (5 MBq per kilogram of body weight). CT scan (120 kV, 30 mA) with slice width of 4 mm was obtained before the PET acquisition for attenuation correction. Emission scan time per bed position was 3 min; six bed positions were acquired. PET data were obtained using a high-resolution whole-body scanner with an axial field of view of 21.6 cm. The average axial resolution varied between 2.0 mm full width at half maximum in the center and 2.4 mm at 28 cm. The average total PET-CT examination time was 20 min.
18F-FDG PET/CT image analysis
PET/CT data sets were evaluated by 2 nuclear physicians blinded to all imaging studies and clinical and pathologic results. Decisions concerning the analysis of 18F-FDG PET/CT data sets were reached by consensus. In 18F-FDG PET/CT images, thyroid uptake was considered to be present when there was increased uptake in the thyroid gland above physiologic background. Focal thyroid uptake was defined as FDG uptake in less than one lobe of the thyroid gland. This definition was used in an attempt to separate patients with thyroiditis such as Hashimoto’s thyroiditis, which usually manifests with diffuse FDG thyroid uptake [11]. When focal FDG activity was seen in the thyroid region, the fused images and the CT images were evaluated to ensure that uptake was from within the thyroid gland. In addition, if there were multiple thyroid nodules in focal FDG uptake lesions in both thyroid glands, we evaluated the largest thyroid nodule with focal intense FDG uptake. There was no artifact from the clavicle encountered in PET/CT image.
The PET/CT data sets were analyzed semi-quantitatively by the use of the maximum standardized uptake value (SUVmax) of 18F-FDG uptake. Semi-quantitative evaluation was performed with a syngo Multimodality Workplace (MMWP) (© Siemens AG.) The SUVmax and SUVmean were semi-quantitatively used to determine 18F-FDG avidity. The SUVs were determined by the region-of-interest technique. SUVmean was defined as the mean tumor concentration of FDG (kBq/mL) divided by the injected dose (kBq), corrected for patient body weight(g). To calculate SUVmax, manually defined circular regions of interest (ROI) were drawn on the attenuation-corrected emission images throughout the axial plane in which a suspicious lesion could be delineated.
Measurement of metabolic tumor volume and total lesion glycolysis on 18F-FDG PET/CT
MTV and TLG were obtained with each threshold of SUV as 2.5, 3.0, 3.5, and 4.0.
We drew the volume of interest(VOI), which showed equal or greater than absolute SUV of 2.5, 3.0, 3.5, and 4.0 on each axial image of PET-CT. MTV was determined using a fixed background SUV cutoff, all voxels containing SUV values above this threshold constituting the MTV. The VOI was drawn so as to include hypermetabolic thyroid nodule. The boundaries were drawn large enough to include the thyroid tumor in the PET images. The contouring margins around the target lesion of thyroid were automatically produced. The voxels presenting SUV intensity of equal or greater than each threshold of the SUVmax of the primary tumor on each axial image of PET/CT were incorporated to define the MTV.
As previously described [12], after all hypermetabolic tumor foci were segmented, MTV was calculated by summation of all the voxels of each slice of PET/CT. If SUVmax of the primary tumor was lower than that of the threshold, we regarded the MTV of the lesion as 0. We did not use point spread function (PSF) reconstruction algorithm for determination of MTV in this study. TLG was calculated by multiplying the SUVmean value of the tumor by the MTV of the tumor.
Statistical analysis
Statistical analyses were performed using commercially available software (MedCalc 12.3, Mariakerke, Belgium). Continuous data are expressed as mean ± SD for normally distributed values and median (range) for nonparametric values (skewed variables). Categorical data were presented as frequency and percentage. Receiver operating characteristic (ROC) analyses were performed to determine the optimal cutoff values of MTV and TLG yielding the maximal sensitivity and specificity for prediction of pathologic lateral LN metastasis of thyroid cancer. ROC curves for each parameter were derived and evaluated by comparing the areas under the curve (AUCs). Patients were classified into two groups, namely those with low and high values of SUVmax, MTV, and TLG according to ROC cutoff values. Various volumetric parameters and clinicopathologic characteristics were evaluated using the Chi-square test or Fisher’s exact test between lateral LN positive and negative, as appropriate. To compare the semi-quantitative indices according to tumor size, we performed Kruskal–Wallis tests. We used comparison ROC curve analysis to test the statistical significance of the difference among the parameters of F-18 FDG PET-CT. Statistical significance was defined as P < 0.05.
Results
Patient characteristics
Patient and tumor characteristics are shown in Table 1. The mean age was 57.1 ± 11.3 years (range 34–88 years). The mean tumor size was 1.15 ± 0.81 cm (range 0.4–4.5). The proportion of patients with papillary thyroid microcarcinoma (PTMC) was 68 % (34/50) with the exception of one minimally invasive FTC. According to the pathologic results, the lateral LNM was present in 9 of 51 patients (17.6 %). The proportion of advanced American Joint Committee on Cancer (AJCC) stage (stage III and IV) was 62.0 % (31/50).
Table 1.
Clinical characteristics of the patients
| Characteristics | Value |
|---|---|
| Total number of patients | 51 |
| Age, year, mean ± SD (range) | 57.1 ± 11.3 (34–88) |
| No. of women (%) | 45 (88.2) |
| Tumor size, cm, mean ± SD (range) | 1.15 ± 0.81 (0.4–4.5) |
| SUVmax, median (range) | 5.67 (1.99–52.25) |
| MTV2.5, cm3, median (range) | 0.95 (0–29.35) |
| MTV3, cm3, median (range) | 0.72 (0–26.44) |
| MTV3.5, cm3, median (range) | 0.51 (0–24.86) |
| MTV4, cm3, median (range) | 0.39 (0–23.25) |
| TLG2.5, median (range) | 3.98 (0–407.08) |
| TLG3, median (range) | 3.34 (0–398.98) |
| TLG3.5, median (range) | 2.80 (0–393.78) |
| TLG4, median (range) | 1.99 (0–387.58) |
| Multifocality (%) | 16 (31.4) |
| Extrathyroidal extension (%) | 23 (45.1) |
| LN metastasis (%) | 26 (51.0) |
| Lateral LN metastasis (%) | 9 (17.6) |
| Advanced stage (%)a | 30 (62.0) |
Data are expressed as mean ± SD and median (range) for continuous variables and frequency (%) for categorical variables
SUV standardized uptake value, MTV metabolic tumor volume, TLG total lesion glycolysis
aAdvanced stage, TNM stage III and IV
Comparison of semi-quantitative indices according to pathologic tumor size
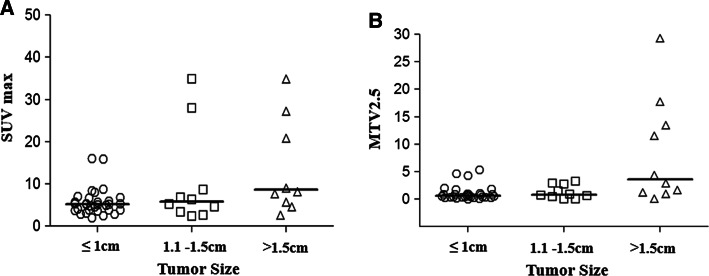
Figure 1 demonstrates the differences of SUVmax and MTV according to pathologic tumor size. The medians of SUVmax and MTV2.5 were significantly higher in patients who had a tumor greater than 1.5 cm in size as compared to those who had a tumor ≤1.5 cm in size (P < 0.001).
Fig. 1.
The differences of semi-quantitative parameters (a SUVmax, b MTV2.5) according to tumor size
Association of FDG uptake of lateral lymph node and FNA results of lateral lymph node
Table 2 shows the association between FDG uptake of lateral LN and US-guided FNA results. Among 51 patients, twenty lateral LNs with FDG uptake were detected in 13 patients (25.5 %). The mean number of FDG uptake of lateral LN per patients was 1.54 ± 0.66 (range 1–3). The sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of FDG uptake for lateral LNM were 66.7, 83.0, 80.4, 46.2, and 92.1 %, respectively. However, three patients who had US-guided FNA-confirmed lateral LNM did not show FDG uptake in lateral LNs on F18-FDG PET/CT.
Table 2.
Association of FDG uptake of lateral lymph node and ultrasonography-guided FNA results in patients with FDG positivity of lateral lymph node
| Patient no | Location of lateral LN | SUVmax of lateral LN | FNA result |
|---|---|---|---|
| 1 | Rt. II | 3.4 | Reactive |
| 2 | Lt. II | 5.3 | Metastasis |
| Lt. III | 16.0 | Metastasis | |
| 3 | Rt.IB | 4.7 | Metastasis |
| 4 | Lt.II | 3.5 | Reactive |
| Lt.III | 3.1 | Metastasis | |
| 5 | Rt.IB | 3.6 | Reactive |
| 6 | Rt.III | 7.0 | Reactive |
| 7 | Rt.II | 3.8 | Reactive |
| Lt.II | 3.2 | Reactive | |
| 8 | Lt.V | 12.7 | Reactive |
| 9 | Lt.III | 3.1 | Reactive |
| 10 | Rt.II | 6.8 | Metastasis |
| Lt.II | 4.0 | Metastasis | |
| 11 | Lt.II | 4.6 | Reactive |
| 12 | Rt.II | 9.2 | Metastasis |
| Rt.IV | 11.6 | Metastasis | |
| Rt.III | 2.8 | Metastasis | |
| 13 | Lt.II | 10.3 | Metastasis |
| Lt.III | 5.8 | Metastasis |
The classification of cervical lymph nodes from consensus statement on the terminology and classification of central neck dissection for thyroid cancer. 2009 The American Thyroid Association Surgery Working Group
FDG fluorodeoxyglucose, LN lymph node, FNA fine-needle aspiration, SUV standardized uptake value
ROC analyses of SUVmax and volume-based parameters for poor pathologic characteristics
The ability of the SUVmax, MTV, and TLG of the primary tumor to predict poor pathologic characteristics including central LNM, extrathyroidal extension (ETE), and multifocality was depicted by the ROC curve. When SUVmax > 4.6 was used as the cutoff point, the area under curve (AUC) for prediction of central LNM was 0.576 (95 % CI 0.429–0.713) (P = 0.361). When SUVmax > 6.68 was used as the cutoff point, the AUC for prediction of ETE was 0.589 (95 % CI 0.442–0.724) (P = 0.292). When SUVmax > 9.07 was used as the cutoff point, the AUC for prediction of multifocality was 0.511 (95 % CI 0.367–0.653) (P = 0.909). The MTV had no predictive value in central LNM (AUC = 0.535, P = 0.679), ETE (AUC = 0.571, P = 0.401), and multifocality (AUC = 0.501, P = 0.992). In addition, the TLG had no predictive value in central LNM (AUC = 0.536, P = 0.664), ETE (AUC = 0.607, P = 0.199), and multifocality (AUC = 0.510, P = 0.914).
Comparison of clinicopathologic characteristics and semi-quantitative metabolic parameters according to lateral lymph node metastasis
Table 3 shows the comparison between SUVmax, MTV, TLG, and various clinical characteristics, including age, tumor size, ETE, central LNM, and multifocality according to lateral LNM. Lateral LN metastases were common in the group of tumor greater than 1 cm in size (P = 0.020) and central LNM group (P = 0.047). However, there was no significant difference of age (P = 0.947), sex (P = 0.140), ETE (P = 0.268), and multifocality (P = 0.701) between two groups. The lateral LNM group showed statistically significant higher values of SUVmax (P = 0.020), all MTV (all P’s < 0.001), and all TLG (all P’s < 0.001) than those of the lateral LN-negative group.
Table 3.
Comparison of clinicopathologic characteristics and semi-quantitative metabolic parameters according to lateral lymph node metastasis
| Variables | Lateral lymph node metastasis | P | |
|---|---|---|---|
| Negative number (%) | Positive number (%) | ||
| Age (years) | |||
| >45 | 37 (82.2) | 8 (17.8) | 0.947 |
| ≤45 | 5 (83.3) | 1 (16.7) | |
| Sex | |||
| Male | 12 (70.6) | 5 (29.4) | 0.140 |
| Female | 30 (88.2) | 4 (11.8) | |
| Tumor size (cm) | |||
| >1 | 13 (65.0) | 7 (35.0) | 0.020 |
| ≤1 | 29 (93.5) | 2 (6.5) | |
| ETE | |||
| Presence | 17 (73.9) | 6 (26.1) | 0.268 |
| Absence | 25 (89.3) | 3 (10.7) | |
| CLN | |||
| Presence | 17 (70.8) | 7 (29.2) | 0.047 |
| Absence | 25 (92.6) | 2 (7.4) | |
| Multifocality | |||
| Presence | 14 (87.5) | 2 (12.5) | 0.701 |
| Absence | 28 (80.0) | 7 (20.0) | |
| SUVmax | |||
| >5.91 | 13 (65.0) | 7 (35.0) | 0.020 |
| ≤5.91 | 29 (93.5) | 2 (6.5) | |
| MTV2.5 (cm3) | |||
| >2.05 | 5 (41.7) | 7 (58.3) | 0.000 |
| ≤2.05 | 37 (94.7) | 2 (5.3) | |
| MTV3 (cm3) | |||
| >1.54 | 5 (41.7) | 7 (58.3) | 0.000 |
| ≤1.54 | 37 (94.7) | 2 (5.3) | |
| MTV3.5 (cm3) | |||
| >1.22 | 5 (41.7) | 7 (58.3) | 0.000 |
| ≤1.22 | 37 (94.7) | 2 (5.3) | |
| MTV4 (cm3) | |||
| >0.61 | 7 (50.0) | 7 (50.0) | 0.001 |
| ≤0.61 | 35 (94.6) | 2 (5.4) | |
| TLG2.5 | |||
| >9.09 | 6 (46.2) | 7 (53.8) | 0.000 |
| ≤9.09 | 36 (94.7) | 2 (5.3) | |
| TLG3 | |||
| >5.35 | 7 (50.0) | 7 (50.0) | 0.001 |
| ≤5.35 | 35 (94.6) | 2 (5.4) | |
| TLG3.5 | |||
| >4.42 | 7 (50.0) | 7 (50.0) | 0.001 |
| ≤4.42 | 35 (94.6) | 2 (5.4) | |
| TLG4 | |||
| >3.47 | 7 (50.0) | 7 (50.0) | 0.001 |
| ≤3.47 | 35 (94.6) | 2 (5.4) | |
Data are expressed as frequency (%) for categorical variables
CLN central lymph node, ETE extrathyroidal extension, SUV standardized uptake value, MTV metabolic tumor volume, TLG total lesion glycolysis
Prediction of lateral lymph node metastasis from semi-quantitative metabolic parameters of primary thyroid carcinoma
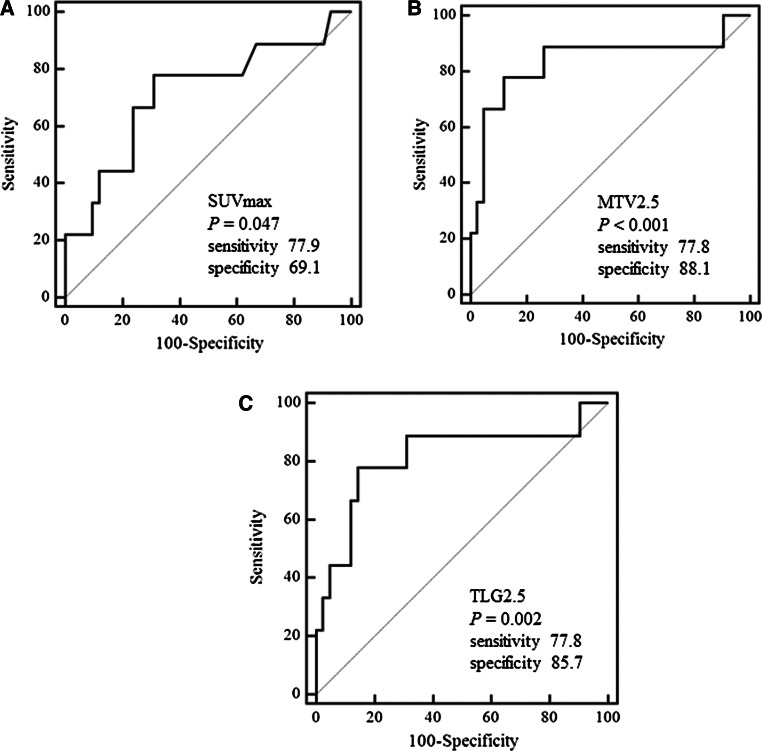
ROC analyses were performed to determine the optimal cutoff values of semi-quantitative indices for prediction of pathologic lateral LN involvement of thyroid carcinoma. Table 4 and Fig. 2 summarize the results of ROC analyses. The AUC for predicting lateral LNM with SUVmax was 0.716 (P = 0.047). The AUCs for predicting lateral LNM with MTV calculated at 2.5, 3.0, 3.5, and 4.0 of fixed SUVmax threshold were 0.839 (P < 0.001), 0.825 (P = 0.002), 0.815 (P = 0.002), and 0.798 (P = 0.003), respectively. The AUCs for predicting lateral LNM with TLG calculated at 2.5, 3.0, 3.5, and 4.0 of fixed SUVmean threshold were 0.815 (P = 0.002), 0.802 (P = 0.003), 0.802 (P = 0.003), and 0.790 (P = 0.004), respectively. Therefore, we used the MTV2.5 and TLG2.5 showing the lowest P value and the highest AUC for prediction of lateral LNM (Fig. 2).
Table 4.
Optimal cutoff values of SUVmax and volumetric parameters for prediction of the lateral lymph node metastasis
| Criteria | Cutoff value | Sensitivity (95 % CI) | Specificity (95 % CI) | AUC (95 % CI) | SE | P value |
|---|---|---|---|---|---|---|
| SUVmax | 5.91 | 77.9 % (40.0–97.2 %) | 69.1 % (52.9–82.4 %) | 0.716 (0.572–0.833) | 0.109 | 0.047 |
| MTV (cm3) | ||||||
| MTV2.5 | 2.05 | 77.8 % (40.0–97.2 %) | 88.1 % (74.4–96.0 %) | 0.839 (0.709–0.927) | 0.099 | <0.001 |
| MTV3 | 1.54 | 77.8 % (40.0–97.2 %) | 88.1 % (74.4–96.0 %) | 0.825 (0.693–0.917) | 0.104 | 0.002 |
| MTV3.5 | 1.22 | 77.8 % (40.0–97.2 %) | 88.1 % (74.4–96.0 %) | 0.815 (0.681–0.909) | 0.101 | 0.002 |
| MTV4 | 0.61 | 77.8 % (40.0–97.2 %) | 83.3 % (68.6–93.0 %) | 0.798 (0.662–0.897) | 0.101 | 0.003 |
| TLG | ||||||
| TLG2.5 | 9.09 | 77.8 % (40.0–97.2 %) | 85.7 % (71.5–94.6 %) | 0.815 (0.681–0.910) | 0.099 | 0.002 |
| TLG3 | 5.35 | 77.8 % (40.0–97.2 %) | 83.3 % (68.6–93.0 %) | 0.802 (0.666–0.900) | 0.103 | 0.003 |
| TLG3.5 | 4.42 | 77.8 % (40.0–97.2 %) | 83.3 % (68.6–93.0 %) | 0.802 (0.666–0.900) | 0.101 | 0.003 |
| TLG4 | 3.47 | 77.8 % (40.0–97.2 %) | 83.3 % (68.6–93.0 %) | 0.790 (0.653–0.891) | 0.099 | 0.004 |
AUC area under curve, CI confidence interval, MTV metabolic tumor volume (cm3), SE standard error, TLG total lesion glycolysis
Fig. 2.
Receiver operating characteristic curves (ROC) using a SUVmax, b metabolic tumor volume (MTV), and c total lesion glycolysis (TLG) to predict lateral lymph node metastasis
Comparison of ROC curve analysis
Table 5 shows the results of pairwise comparison of ROC analyses of semi-quantitative parameters of 18F-FDG PET/CT for prediction of pathologic lateral LNM of thyroid carcinoma. Compared with SUVmax, the MTV2.5 and TLG2.5 showed the statistical differences for the prediction of pathologic lateral LNM of thyroid carcinoma (MTV vs. SUVmaxP < 0.0125, TLG vs. SUVmaxP = 0.0133). However, there was no significant difference between MTV2.5 and TLG2.5 (P = 0.1298).
Table 5.
Pairwise comparison of ROC curves of semi-quantitative indices of 18F-FDG PET-CT for the lateral lymph node metastasis
| MTV2.5 | TLG2.5 | |
|---|---|---|
| SUVmax | ||
| DBA | 0.123 | 0.0992 |
| SE | 0.0493 | 0.0401 |
| 95 % CI | 0.0264–0.220 | 0.0207–0.178 |
| P | 0.0125 | 0.0133 |
| MTV2.5 | ||
| DBA | 0.0238 | |
| SE | 0.0157 | |
| 95 % CI | −0.0069 to 0.0546 | |
| P | 0.1298 | |
DBA difference between areas, CI confidence interval, MTV metabolic tumor volume, SE standard error, SUV standardized uptake value, TLG total lesion glycolysis
Discussion
The value of preoperative F18-FDG PET/CT in PTC remains unclear. As one of the candidate predictors for the evaluation of lateral LNM and local invasiveness of DTC, we evaluated whether the volume-based parameters of 18F-FDG PET/CT imaging could predict pathologic lateral LNM and aggressiveness in patients with incidentally detected DTC in this study. The current study demonstrated that the SUVmax, MTV, and TLG measured by 18F-FDG PET/CT had significant association with lateral LNM in patients with incidentally detected DTC. The MTV and TLG had significant value for the prediction of pathologic lateral LNM in comparison with SUVmax.
It is well known that PTC generally shows an indolent disease course. However, PTC frequently metastasizes or recurs to regional LN. Metastasis to lateral LN is associated with a poor prognosis [13–15]. Thus, imaging the neck before surgery for thyroid carcinoma is necessary to determine the appropriate extent of surgical resection. In addition, it is important to identify patients who are at risk of developing distant metastasis or recurrence that necessitate more aggressive surgical treatment or more intensive radioactive iodine (RAI) therapy. In this regard, 18F-FDG PET/CT scans provide anatomical as well as prognostic information that can identify the patients at higher risk of recurrence and metastatic disease [16, 17]. However, 18F-FDG PET/CT is not routinely used in daily clinical practice in characterizing thyroid nodules and preoperative investigation of thyroid carcinomas. It is well known that US is useful in the preoperative assessment of cervical LN metastasis in patients with DTC. Very recently, Lee et al. [10] demonstrated that preoperative US might be helpful in detecting nodal metastases in PTC patients.
The value of FDG uptake in preoperative risk stratification remains controversial. In this study, 25.5 % (13/51) of patients showed FDG uptake in lateral LN. Increased FDG uptake of lateral LN metastasis showed low sensitivity and PPV but high specificity and NPV for pathologically proven lateral LNM. Although three patients who had US-guided FNA-confirmed lateral LN metastasis did not show FDG uptake in lateral LNs on F18-FDG PET/CT, preoperative FDG negativity of lateral LNs suggested low possibility in lateral LNM in patients with DTC. Therefore, both preoperative F18-FDG PET-CT and US may improve sensitivity and specificity of prediction of lateral LNM in incidentally detected thyroid carcinoma.
A previous study showed that the SUV of thyroid carcinoma alone was not predictive of the presence of ETE, LNM, or the multiplicity of PTMC [18]. In contrast, Yun et al. [19] reported that visual FDG positivity is a potential new risk factor that may be useful for preoperative risk stratification of PTMC. Gender, age, and tumor size showed a correlation with either ETE or central LNM, while visually discernible FDG uptake was associated with a higher prevalence of ETE and central LNM. However, in the current study, all parameters measured by 18F-FDG PET/CT had no value in prediction of central LNM, ETE, and multifocality except lateral LNM. In particular, the MTV and TLG were better predictors of lateral LNM than SUVmax. When all patients were classified into two groups according to the presence of lateral LNM, tumor size greater than 1 cm and central LNM were significantly associated with lateral LNM. However, other clinicopathologic characteristics including age, gender, ETE, and multifocality showed no significant difference between the two groups.
It is well established that ETE is an important adverse prognostic factor for patients with PTC [20, 21]. However, recent studies reported that microscopic ETE might not be a prognostic factor to predict tumor recurrence in patients with PTC [22, 23]. Thus, it is unclear that microscopic ETE is associated with poor prognostic factor. No significant predictive value of volume-based parameters for ETE observed in the current study is thought to be that most ETE in our patients (22/23) is minimal microscopic extrathyroidal invasion into the strap muscle.
It is still unclear whether or not FDG positivity of DTC in the preoperative 18F-FDG PET-CT indicates tumor aggressiveness. The reason of the discrepancy between previous studies [4–7] and our findings in DTC could be that tumor size of incidentally detected PTC was relatively small compared to other cancers. The partial volume effect (PVE) can have a major effect on the measurement of tumor uptake with PET. The PVE may affect MTV or TLG [24]. The PVE strongly depends on the size of the tumor. The smaller the tumor, the greater the underestimation of the uptake value [25]. In addition, the accuracy of PET-based automatic or semiautomatic delineation methods can be affected by many factors, including image resolution and reconstruction settings [26, 27].
This study has some limitations. First, all patients with focal FDG-avid thyroid incidentaloma did not underwent FNAB and surgery to confirm malignancy due to underlying cancer and general condition. Second, the limited sample size of surgically confirmed DTC and the retrospective design are also limitations of this study. Third, a large proportion of the patients studied in this series have had former cancer treatment which might have influenced measured metabolic parameters. Despite these limitations, we have, for the first time, found the clinical implication of volume-based parameters of 18F-FDG PET/CT in preoperative risk stratification of incidentally detected thyroid cancers.
In conclusion, this study has shown that volume-based PET functional parameters such as TLG and MTV can provide metabolic information of incidentally detected PTC. The TLG and MTV had significant value for the prediction of lateral LNM in comparison with SUVmax. These results suggest that higher MTV and TLG can be potential new risk factors for preoperative risk stratification. Therefore, thyroid cancer with high MTV and TLG should be closely examined for LNM by preoperative neck US. Future large prospective studies will provide greater clarity regarding the value of these parameters of preoperative 18F-FDG PET-CT in patients with incidentally detected DTC.
Complaince with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Contributor Information
Bo Hyun Kim, Email: pons71@hanmail.net.
Seong-Jang Kim, Phone: +82-51-240-7389, Email: growthkim@daum.net, Email: growthkim@pusan.ac.kr.
Keunyoung Kim, Email: 4mura2@hanmail.net.
Heeyoung Kim, Email: dinggury84@hanmail.net.
So Jung Kim, Email: laocone@naver.com.
Won Jin Kim, Email: bearshow00@hanmail.net.
Yun Kyung Jeon, Email: puritystar@hanmail.net.
Sang Soo Kim, Email: drsskim7@gmail.com.
Yong Ki Kim, Email: yongki@pusan.ac.kr.
In Joo Kim, Email: injkim@pusan.ac.kr.
References
- 1.Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2(3):159–171. doi: 10.1016/S1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 2.Erdi YE, Macapinlac H, Rosenzweig KE, Humm JL, Larson SM, Erdi AK, et al. Use of PET to monitor the response of lung cancer to radiation treatment. Eur J Nucl Med. 2000;27(7):861–866. doi: 10.1007/s002590000258. [DOI] [PubMed] [Google Scholar]
- 3.Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, et al. Prognostic significance of metabolic parameters measured by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer. 2011;73(3):332–337. doi: 10.1016/j.lungcan.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Dibble EH, Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med. 2012;53(5):709–715. doi: 10.2967/jnumed.111.099531. [DOI] [PubMed] [Google Scholar]
- 5.Hs I. Kim SJ, Kim IJ, Kim K. Predictive value of metabolic tumor volume measured by 18F-FDG PET for regional lymph node status in patients with esophageal cancer. Clin Nucl Med. 2012;37(5):442–446. doi: 10.1097/RLU.0b013e318238f703. [DOI] [PubMed] [Google Scholar]
- 6.Romesser PB, Qureshi MM, Shah BA, Chatburn LT, Jalisi S, Devaiah AK, et al. Superior prognostic utility of gross and metabolic tumor volume compared to standardized uptake value using PET/CT in head and neck squamous cell carcinoma patients treated with intensity-modulated radiotherapy. Ann Nucl Med. 2012;26(7):527–534. doi: 10.1007/s12149-012-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van de Wiele C, Kruse V, Smeets P, Sathekge M, Maes A. Predictive and prognostic value of metabolic tumour volume and total lesion glycolysis in solid tumours. Eur J Nucl Med Mol Imaging. 2013;40(2):290–301. doi: 10.1007/s00259-012-2280-z. [DOI] [PubMed] [Google Scholar]
- 8.Stack BC, Jr, Ferris RL, Goldenberg D, Haymart M, Shaha A, Sheth S, et al. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid. 2012;22(5):501–508. doi: 10.1089/thy.2011.0312. [DOI] [PubMed] [Google Scholar]
- 9.Lee DW, Ji YB, Sung ES, Park JS, Lee YJ, Park DW, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol. 2013;39(2):191–196. doi: 10.1016/j.ejso.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 10.Lee YJ, Kim DW, Kyoung Park H, Kim DH, Jung SJ, Oh M, et al. Pre-operative ultrasound diagnosis of nodal metastasis in papillary thyroid carcinoma patients according to nodal compartment. Ultrasound Med Biol. 2015;41(5):1294–1300. doi: 10.1016/j.ultrasmedbio.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda S, Shohtsu A, Ide M, Takagi S, Takahashi W, Suzuki Y, et al. Chronic thyroiditis: diffuse uptake of FDG at PET. Radiology. 1998;207(3):775–778. doi: 10.1148/radiology.207.3.9609903. [DOI] [PubMed] [Google Scholar]
- 12.Kim BH, Kim SJ, Kim H, Jeon YK, Kim SS, Kim IJ, et al. Diagnostic value of metabolic tumor volume assessed by 18F-FDG PET/CT added to SUVmax for characterization of thyroid 18F-FDG incidentaloma. Nucl Med Commun. 2013;34(9):868–876. doi: 10.1097/MNM.0b013e328362d2d7. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferri EL. Long-term outcome of patients with differentiated thyroid carcinoma: effect of therapy. Endocr Pract. 2000;6(6):469–476. doi: 10.4158/EP.6.6.469. [DOI] [PubMed] [Google Scholar]
- 14.Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5(1):3–43. [PubMed] [Google Scholar]
- 15.Kim TY, Kim WG, Kim WB, Shong YK. Current status and future perspectives in differentiated thyroid cancer. Endocrinol Metab (Seoul) 2014;29(3):217–225. doi: 10.3803/EnM.2014.29.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shammas A, Degirmenci B, Mountz JM, McCook BM, Branstetter B, Bencherif B, et al. 18F-FDG PET/CT in patients with suspected recurrent or metastatic well-differentiated thyroid cancer. J Nucl Med. 2007;48(2):221–226. [PubMed] [Google Scholar]
- 17.Wang W, Larson SM, Fazzari M. Prognostic value of [18F]-fluorodeoxy glucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000;85(3):1107–1113. doi: 10.1210/jcem.85.3.6458. [DOI] [PubMed] [Google Scholar]
- 18.Jeong HS, Chung M, Baek CH, Ko YH, Choi JY, Son YI. Can [18F]-fluorodeoxyglucose standardized uptake values of PET imaging predict pathologic extrathyroid invasion of thyroid papillary microcarcinomas? Laryngoscope. 2006;116(12):2133–2137. doi: 10.1097/01.mlg.0000243043.65560.3f. [DOI] [PubMed] [Google Scholar]
- 19.Yun M, Noh TW, Cho A, Choi YJ, Hong SW, Park CS, et al. Visually discernible [18F]fluorodeoxyglucose uptake in papillary thyroid microcarcinoma: a potential new risk factor. J Clin Endocrinol Metab. 2010;95(7):3182–3188. doi: 10.1210/jc.2009-2091. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz S, Rodríguez JM, Soria T, Pérez-Flores D, Piñero A, Moreno J, et al. Extrathyroid spread in papillary carcinoma of the thyroid: clinicopathological and prognostic study. Otolaryngol Head Neck Surg. 2001;124(3):261–265. doi: 10.1067/mhn.2001.113141. [DOI] [PubMed] [Google Scholar]
- 21.Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of degree of extra-thyroidal extension of disease on papillary thyroid cancer outcome. Thyroid. 2014;24(2):241–244. doi: 10.1089/thy.2012.0567. [DOI] [PubMed] [Google Scholar]
- 22.Arora N, Turbendian HK, Scognamiglio T, Wagner PL, Goldsmith SJ, Zarnegar R, et al. Extrathyroidal extension is not all equal: implications of macroscopic versus microscopic extent in papillary thyroid carcinoma. Surgery. 2008;144(6):942–947. doi: 10.1016/j.surg.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Kim JM, Lee YY, Choi CW, Lim SM, Lee S, Cho SY, et al. The clinical importance of minimal extrathyroidal extension on tumor recurrence in patients with papillary thyroid carcinoma. Endocrinol Metab. 2010;25(4):340–346. doi: 10.3803/EnM.2010.25.4.340. [DOI] [Google Scholar]
- 24.Hatt M, Le Pogam A, Visvikis D, Pradier O. Cheze Le Rest C. Impact of partial-volume effect correction on the predictive and prognostic value of baseline 18F-FDG PET images in esophageal cancer. J Nucl Med. 2012;53(1):12–20. doi: 10.2967/jnumed.111.092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48(6):932–945. doi: 10.2967/jnumed.106.035774. [DOI] [PubMed] [Google Scholar]
- 26.Geets X, Lee JA, Bol A, Lonneux M, Grégoire V. A gradient-based method for segmenting FDG-PET images: methodology and validation. Eur J Nucl Med Mol Imaging. 2007;34(9):1427–1438. doi: 10.1007/s00259-006-0363-4. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer A, Kremp S, Hellwig D, Rübe C, Kirsch CM, Nestle U. A contrast-oriented algorithm for FDG-PET-based delineation of tumour volumes for the radiotherapy of lung cancer: derivation from phantom measurements and validation in patient data. Eur J Nucl Med Mol Imaging. 2008;35(11):1989–1999. doi: 10.1007/s00259-008-0875-1. [DOI] [PubMed] [Google Scholar]