Abstract
The combination of decreased amyloid β42 (Aβ42) and increased total tau proteins (T-Tau) and phosphorylated tau (P-Tau) in cerebrospinal fluid (CSF) has recently been considered as a biological diagnostic criterion of Alzheimer’s disease (AD). Previous studies showed significant heterogeneity in CSF Aβ42 levels to discriminate AD from non-AD patients. It was also suggested that the CSF amyloid peptide β42/β40 ratio has better diagnostic performance than Aβ42 alone. The objective of the present study was to investigate the potential added value of determining CSF amyloid β40 peptide (Aβ40) for biological diagnosis of AD when CSF Aβ42 levels failed. CSF AD biomarkers were run in 2,171 samples from 1,499 AD and 672 non-AD patients. The following pathologic thresholds were used to define an AD-positive CSF biomarker profile: T-Tau ≥ 400 ng/L, P-Tau181 ≥ 60 ng/L, and Aβ42 ≤ 700 ng/L. CSF Aβ40 was assayed in AD patients with CSF Aβ42 levels above 700 ng/L and non-AD patients with CSF Aβ42 levels below 700 ng/L. CSF Aβ40 levels were higher in AD than non-AD patients. The receiver operator characteristic curves of CSF Aβ40 and the Aβ42/Aβ40 ratio defined AD cut-off values at 12,644 ng/L and 0.06, respectively. In AD patients with non-pathological CSF Aβ42, CSF Aβ40 concentration was able to correct 76.2% of cases when expressed as CSF Aβ42/Aβ40 ratio and 94.7% of cases when used alone. Using CSF Aβ42 and then CSF Aβ40, the percentage of misinterpreted AD patients fell to 1.0%. CSF Aβ40 concentration improved interpretation of Aβ42 level for the diagnosis of AD. CSF Aβ40 alone showed better diagnostic performance than the amyloid peptide Aβ42/Aβ40 ratio. The added value of determining CSF Aβ40 in AD diagnosis now needs confirming in a cohort of definite AD patients and to be completed with novel amyloid cascade biomarkers.
Keywords: dementia, Alzheimer, Aβ42, Aβ40, cerebrospinal fluid
Introduction
According to the revised criteria for Alzheimer’s disease (AD), definite diagnosis is founded on neuropathology as gold standard, when patients meet the clinical and cognitive criteria for AD dementia (1). Diagnosis of AD onset during the patient’s lifetime is said to be “possible” or “probable.” Amyloid β42 (Aβ42), total Tau (T-Tau), and phosphorylated Tau proteins (P-Tau) assay in cerebrospinal fluid (CSF) is recommended to increase the level of diagnostic certainty for AD in atypical clinical phenotypes, for inclusion of patients in clinical trials and to improve AD diagnosis at the earliest stages of the disease (1–5). A positive AD CSF biomarker profile was defined as increased CSF Tau and/or P-Tau181 and decreased CSF Aβ42 concentrations (1, 6–8). However, researchers and clinicians continue to debate the sensitivity and specificity of various biomarkers, and especially CSF Aβ42. A recent meta-analysis highlighted significant heterogeneity in CSF Aβ42 values between different disease groups (9), reporting sensitivity and specificity ranging from 71 to 91% and 44 to 82%, respectively. Moreover, Rosen et al. showed that “normal” CSF Aβ42 levels were observed in AD patients, leading to misinterpretation of the AD CSF biomarker profile in 23.2% of AD patients (10).
One of the crucial challenges to improve screening in clinical trials is to identify an accurate CSF biomarker reflecting amyloid pathology. There is now strong evidence that CSF Aβ42 levels depend not only on impaired brain clearance in Alzheimer’s pathophysiology, but also on the total load of amyloid peptides, which shows large interindividual variability (11–14). Gamma-secretase cleaves amyloid precursor protein (APP) at several sites, resulting in different C-terminally truncated Aβ variants: amyloid β40 (Aβ40) is the most abundant amyloid peptide in CSF (15), while Aβ42 accounts for only about 10% of the total Aβ peptide population (12, 16–18). Total Aβ concentration was found not to vary significantly between various dementia disorders (11, 18, 19), and Aβ40 concentration did not differ between AD (or presymptomatic AD) patients, healthy controls, and non-AD dementia patients (19–23). CSF Aβ40 concentration could, therefore, be considered to most closely reflect total Aβ load in the brain (13). Previous studies showed that the Aβ42/Aβ40 ratio in CSF is reduced in AD patients, and its assessment improves AD diagnostic accuracy (21–25). More recently, a few studies demonstrated added value for CSF Aβ40 or CSF Aβ42/Aβ40 ratio for differential diagnosis of AD using CSF P-Tau181 levels or in ambiguous AD CSF biomarker profiles (26–28). Therefore, the objective of the present study was to investigate whether determining CSF Aβ40 level and CSF Aβ42/Aβ40 ratio could improve diagnosis in AD patients without low CSF Aβ42 levels.
Materials and Methods
Cerebrospinal fluid samples were collected between October 2010 and January 2013 from 2,171 patients who underwent lumbar puncture (LP) for routine clinical diagnosis of AD in the Neurochemistry Unit and Biochemistry Department of the University Hospital of Lyon (France). Patients were included in a multicenter memory clinic and had at least 2 years’ follow-up. They were classified into two groups: 1,499 AD and 672 non-AD patients. The non-AD group consisted of 259 patients with probable frontotemporal lobar degeneration (FTLD), 119 with probable dementia with Lewy bodies (DLB), 159 with normal pressure hydrocephalus (NPH), and 135 with psychiatric disorders.
The patients’ age, gender, and mini mental state evaluation (MMSE) score were recorded when the LP was performed. At that time, initial diagnosis was based on medical history, caregiver interviews, neurologic examination, neuropsychological battery evaluation, and brain imaging. Clinical diagnosis was made in multidisciplinary team meeting, comprising neurologists, neuropsychologists, and radiologists, and confirmed on follow-up. Dementia was defined according to DSM IV-TR criteria (29), and all AD patients were classified as having AD dementia with evidence of the AD pathophysiological process (1). Patients with mild cognitive impairment were excluded. The non-AD patients diagnosed with FTLD and DLB met the international criteria (30, 31). The non-AD patients with psychiatric disorders or NPH with cognitive complaints unrelated to AD or other degenerative disease were age matched with AD patients, and showed no progression of cognitive impairment within 2 years after CSF analysis.
This study, based on routine biological analyses, was not considered as “biomedical research” under French regulations, and therefore did not require informed consent. Samples were, however, stored in a biobank with authorization from the French Ministry of Health (Declaration number DC-2008-304). Authorization for handling personal data was granted by the French data protection commission [Commission Nationale de l’Informatique et des Libertés (CNIL)].
All patients underwent LP to collect CSF using a standard procedure. CSF collection, sampling, and storage were performed according to the international consensus (32, 33). All CSF samples were collected in Sarstedt polypropylene tubes (ref. 62.610.201) showing low adsorption of amyloid peptides (7). CSF biomarker analyses were performed, blind to clinical diagnosis, in the Neurochemistry Unit and Biochemistry Department of the University Hospital of Lyon. This department is involved in two external quality control schemes, one at French national level (working group of the French Society of Clinical Biology: Société Française de Biologie Clinique) and the other with the Alzheimer’s Association QC program (34). CSF concentrations of Aβ42, T-Tau, and P-Tau181 were measured using the standardized commercially available sandwich ELISA kit (INNOTEST®) according to the manufacturer’s procedures (Fujirebio, Ghent, Belgium).
For each CSF sample, Aβ42, T-Tau, and P-Tau181 biomarkers were simultaneously analyzed. As previously described (7), the cut-off values defining positive AD CSF biomarker profile were: T-Tau ≥ 400 ng/L, P-Tau181 ≥ 60 ng/L, and Aβ42 ≤ 700 ng/L.
Aβ40 level in CSF was quantified using ELISA tests [Human Amyloid b (1–40) (N) Assay kit, IBL, Japan] in AD patients with CSF Aβ42 levels above 700 ng/L and in non-AD patients with CSF Aβ42 levels below 700 ng/L.
Statistical Analysis
Chi-square test, Mann–Whitney U test, Kruskal–Wallis test, and receiver operator characteristic (ROC) analyses were performed using MedCalc version 11.3.1.0 (http://www.medcalc.be). Differences were considered statistically significant at p < 0.05. ROC curves were applied to define optimal biomarker cut-off values to discriminate between AD and non-AD groups. The cut-off value was defined as the value corresponding to the highest average for sensitivity and specificity. Accuracy was calculated as the sum of true positives and true negatives in the total number of patients (35).
Results
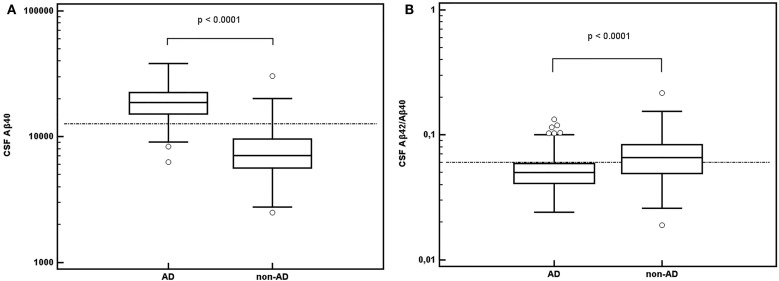
Cerebrospinal fluid data according to diagnostic group are summarized in Table 1 and Figure 1.
Table 1.
Demographic, pathologic, and biological parameters of study populations.
| AD | Non-AD | ||
|---|---|---|---|
| Gender | n | 1,499 | 672 |
| M/F | 643/856 | 358/314 | |
| Age (years) | n | 1,499 | 672 |
| Mean | 71.6 | 70.0 | |
| SD | 9.5 | 10.6 | |
| MMSE score (/30) | n | 1,093 | 488 |
| Mean | 20.2 | 21.6 | |
| SD | 5.6 | 5.5 | |
| T-Tau (ng/L) | n | 1,499 | 672 |
| Median | 650 | 230 | |
| 25th–75th P | 487–913 | 168–311 | |
| P-Tau181 (ng/L) | n | 1,499 | 672 |
| Median | 83 | 38 | |
| 25th–75th P | 68–109 | 30–48 | |
| Aβ42 (ng/L) | n | 1,499 | 672 |
| Median | 539 | 807 | |
| 25th–75th P | 443–663 | 570–1,056 | |
| Aβ40 (ng/L) | n | 281 | 244 |
| Median | 19,198 | 7,112 | |
| 25th–75th P | 15,162–22,409 | 5,643–9,636 | |
| Aβ42/Aβ40 ratio | n | 281 | 244 |
| Median | 0.053 | 0.066 | |
| 25th–75th P | 0.041–0.059 | 0.049–0.084 |
AD, Alzheimer’s disease; MMSE, mini mental state evaluation; M, male; F, female; SD, standard deviation; P, percentile.
Figure 1.
CSF Aβ42/Aβ40 ratio (A) and CSF Aβ40 concentrations in nanograms per liter (B) in AD and non-AD populations. Abbreviation: AD: Alzheimer’s disease.
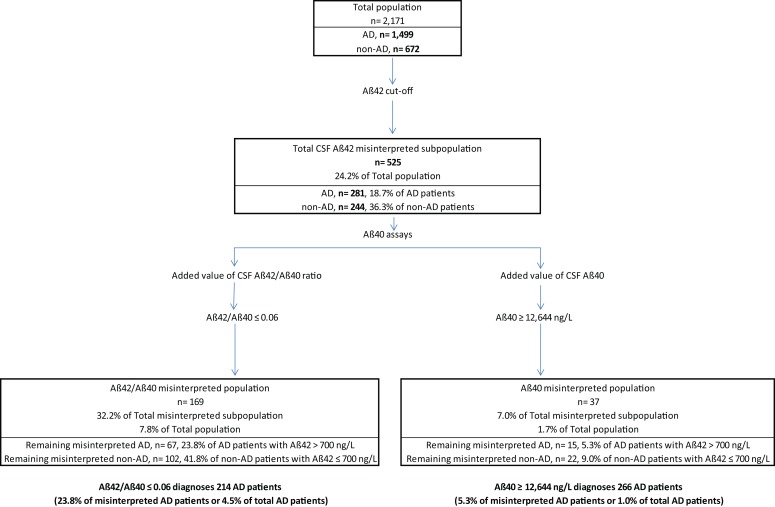
About 81.3% of AD patients (1,218/1,499) fulfilled the pathological CSF Aβ42 criteria; the remaining 18.7% (281/1,499) presented CSF Aβ42 levels above cut-off (>700 ng/L). 63.7% of non-AD patients (428/672) presented CSF Aβ42 levels above 700 ng/L; 36.3% (244/672) had CSF Aβ42 levels below 700 ng/L (Figure 2). CSF Aβ40 levels were then determined in these 525 patients: 281 AD patients (>700 ng/L) and 244 non-AD patients (≤700 ng/L).
Figure 2.
Patient classification based on the determination of cerebrospinal fluid (CSF) amyloid peptides. First, according to CSF Aβ42 levels, we obtained a percentage of misinterpreted patients with discordant results regarding clinical diagnosis. The CSF Aβ40 assay was performed in this subpopulation. Performance in accurately classifying patients was tested for CSF Aβ42/Aβ40 ratio and for CSF Aβ40 alone. Both CSF Aβ42/Aβ40 ratio and CSF Aβ40 could reclassify a high percentage of patients. CSF Aβ40 provided the best correct classification rate. Abbreviation: AD: Alzheimer’s disease.
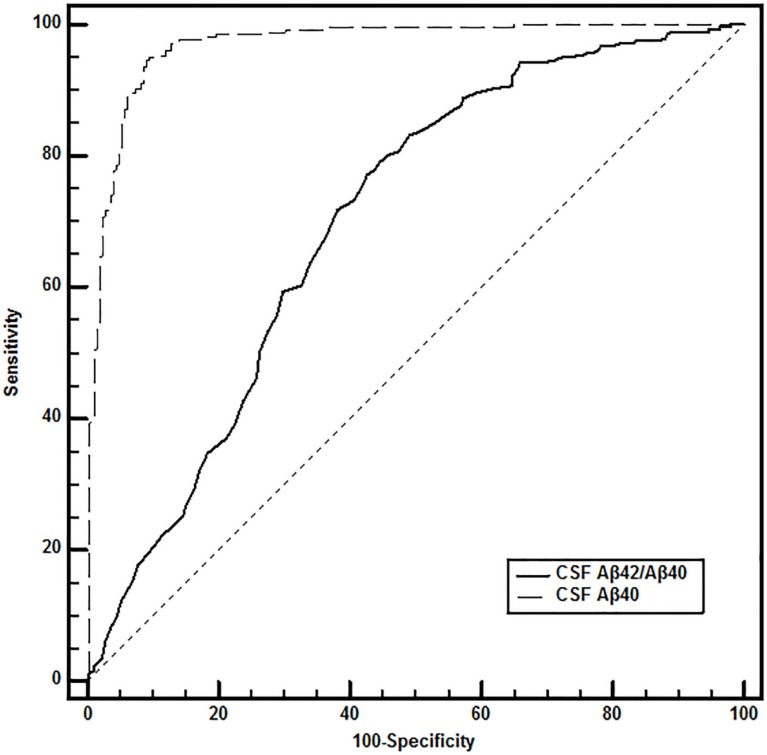
The ROC curves of CSF Aβ40 level and the Aβ42/Aβ40 ratio determined AD cut-off values of ≥12,644 ng/L and ≤0.06, respectively (Figure 3).
Figure 3.
Receiver operating characteristic curve comparison for AD diagnosis in the “discordant CSF Aβ42 values” subpopulation. DeLong et al.’s (1988) method was used to compare the values of the area under the curve (AUC). In the 525 selected patients, accuracy of diagnostic performance was significantly higher for CSF Aβ40 compared to CSF Aβ42/Aβ40 ratio, with 94.7% sensitivity and 91.0% specificity for CSF Aβ40 ≥12,644 ng/L (AUC, 0.969) compared to 76.2 and 58.2%, respectively for CSF Aβ42/Aβ40 ratio ≤0.06 (AUC, 0.700).
In the overall population, the percentage of patients in whom amyloid pathology was misinterpreted fell from 24.2% (525/2,171) using CSF Aβ42 alone to 7.8% (169/2,171) when it was followed by CSF Aβ42/Aβ40 ratio, and to 1.7% (37/2,171) when followed by CSF Aβ40 (Figure 2). In patients in whom CSF Aβ40 level was determined (n = 525), sensitivity and specificity for AD diagnosis were 76.2 and 58.2%, respectively (accuracy, 0.678) using the CSF Aβ42/Aβ40 ratio, and 94.7 and 91.0%, respectively (accuracy, 0.930) using CSF Aβ40 determination.
About 58.2% of the 244 non-AD patients with CSF Aβ42 levels below 700 ng/L (142/244) had CSF Aβ42/Aβ40 ratios higher than 0.06 and 91.0% (222/244) had CSF Aβ40 levels below 12,644 ng/L.
About 76.2% of AD patients (214/281) had CSF Aβ42/Aβ40 ratios below 0.06 and 94.7% (266/281) had CSF Aβ40 levels higher than 12,644 ng/L. In the overall AD population, percentage misinterpretation fell from 18.7% (281/1,499) with CSF Aβ42 alone to 4.5% (67/1,499) using CSF Aβ42 and then CSF Aβ42/Aβ40 ratio and 1.0% (15/1,499) using CSF Aβ42 and then CSF Aβ40 (Figure 2).
Discussion
We investigated the potential added value of CSF Aβ40 assay to improve the interpretation of Aβ42 level. The main finding was that CSF Aβ40 appeared to be an interesting complementary biomarker. CSF Aβ40 levels were higher in AD than non-AD patients. Thus, determining CSF Aβ40 concentrations corrected biological diagnosis in AD patients with non-pathological CSF Aβ42 levels in 76.2% of cases using the CSF Aβ42/Aβ40 ratio and in 94.7% using CSF Aβ40 alone; using CSF Aβ42 and then CSF Aβ40, percentage misinterpretation fell to 1.0%.
Cerebrospinal fluid Aβ42 concentrations led to misinterpretation of the AD CSF biomarker profile in 24.2% of our total population and notably in 18.7% of AD patients. This low performance of CSF Aβ42 is in perfect agreement with previous reports (7, 10, 18, 20, 36, 37). The presence of CSF Aβ42 concentrations ≤700 ng/L in non-AD patients could reflect low total CSF amyloid load, while CSF Aβ42 >700 ng/L in AD patients could result from high amyloid load. This concept justifies CSF Aβ40 assay to complete amyloid pathway interpretation.
As reported in various studies (20, 26, 27, 36), the CSF Aβ42/Aβ40 ratio showed better diagnostic performance than CSF Aβ42 alone. The CSF Aβ42/Aβ40 ratio cut-off value at 0.06 was identical to that reported by Lewczuk et al. (36). The discrepancy with Hansson et al.’s (20) 0.095 cut-off might be due to the Genetics Company ELISA kit halving the range of CSF Aβ40 levels. We found an increase in the rate of correct interpretation from 75.8% with CSF Aβ42 alone to 92.2% when CSF Aβ42 assay was followed by determining the CSF Aβ42/Aβ40 ratio, similarly to other reports (20, 28, 36).
The type of sampling and storage tubes is an important source of variability because of amyloid adsorption (33, 37, 38). CSF sample selection from biological banks should, therefore, be performed rigorously. There is parallel adsorption of CSF Aβ42 and Aβ40 onto the sampling tube surface, regardless of the type of plastic (personal data). Systematic use of the CSF Aβ42/Aβ40 ratio would provide complete interpretation of CSF amyloid biomarker results, integrating the impact of plastic tube type. In the present study, however, samples were analyzed sequentially, leading to higher between-run imprecision for the CSF Aβ42/Aβ40 ratio than for CSF Aβ42 alone [coefficient of variation (CV), 13.3 and 10.2%, respectively]. One solution to decrease the CV of the CSF Aβ42/Aβ40 ratio would be to use multiplex assays to analyze both amyloid peptides simultaneously. Unfortunately, at the moment, there is no analytical validation available for CSF Aβ42 and CSF Aβ40 in multiplex assays for in vitro diagnostic use.
In the present study, CSF Aβ40 was determined only in AD patients with CSF Aβ42 levels above 700 ng/L and in non-AD patients with levels below 700 ng/L. CSF Aβ40 concentrations were significantly higher in AD than non-AD patients. The optimal CSF Aβ40 cut-off value was 12,644 ng/L. To our knowledge, there is currently no effective CSF Aβ40 cut-off value to discriminate AD from non-AD patients reported in the literature; only a slight increase in CSF Aβ40 was found in two other studies (20, 24), and a recent study focusing on AD-MCI patients found a significant increase in CSF Aβ40 values compared to a control group (36). However, the present data contrasted with those reported in another study (26) including AD and non-AD dementia. Selection of the non-AD patient population to compare with the AD population was probably one of the major differences. Another difference may be the biological factor used for the patients’ initial classification, CSF P-Tau181 concentrations in intermediate levels (26). Similarly, Sauvee et al. suggested using the CSF Aβ42/Aβ40 ratio when data for CSF Aβ42 combined to CSF P-Tau181 are inconclusive (27). In these particular cases, adding the CSF Aβ42/Aβ40 ratio improved their proportion of interpretable biological profiles from 68 to 89% (27). Moreover, in confirmation of our sequential approach, Sauvee et al. showed that adding CSF Aβ40 peptide concentration and CSF Aβ42/Aβ40 ratio did not change their conclusions when CSF Aβ42 and CSF P-Tau181 were concordant.
In the present study, it was also interesting that 36.3% of non-AD patients presented pathological CSF Aβ42 levels. One hypothesis could concern the heterogeneity of the non-AD population, which included patients with psychiatric disorders and NPH and demented patients with neurodegenerative diseases (FTLD and DLB). CSF Aβ42 was previously reported to be less effective for differential diagnosis of the main neurodegenerative dementia than CSF Tau proteins (39–41). To discriminate AD and FTLD, CSF Aβ42 assay could then be combined with Tau proteins and expressed as T-Tau/Aβ42 and P-Tau181/Aβ42 ratios (42, 43). Typical CSF AD profiles including CSF Aβ42 and Tau proteins were reported in 47% of patients meeting clinical diagnostic criteria for DLB and in 30% of FTLD patients (41), suggesting coexisting pathologies, as strongly highlighted by postmortem studies (44, 45). NPH patients also have lower CSF amyloid peptide and Tau protein concentrations than controls (46, 47). To validate our hypothesis and strategy regarding differential diagnosis, postmortem confirmation on autopsy-proven patients should be carried out.
The diagnostic performance of CSF Aβ42 is increasingly questioned. It should be noted that biological diagnosis as performed in specialized memory clinics is also founded on the second pathway of AD pathophysiology, reflected by CSF Tau protein levels. Nevertheless, a more accurate evaluation of CSF amyloid biomarkers is important to include patients in therapeutic trials involving the amyloid cascade, using added Aβ peptides or other amyloid cascade biomarkers. For example, the soluble peptide APPβ (sAPPβ) and CSF Aβ40 come from the same enzymatic digestion of APP, and it would be interesting to assess sAPPβ to complete this study. Increased CSF sAPPβ levels were already reported in AD patients as compared to non-AD demented patients (48) and FTD patients (49).
In conclusion, the present study offers an improvement in biological diagnosis of AD focusing on the amyloid pathway. In the misinterpretation using CSF Aβ42 levels, classification based on the CSF Aβ42/Aβ40 ratio gives good results. More interestingly, CSF Aβ40 assay alone also provides better results: the misinterpretation rate using CSF Aβ42 and then CSF Aβ40 alone falls to 1.7%. Sequential assessment of CSF Aβ40 would also provide a better cost-effectiveness ratio than systematic determination of the CSF Aβ42/Aβ40 ratio. Finally, these results need to be confirmed in a prospective study including autopsy-proven AD patients, and completed with novel amyloid cascade biomarkers.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was conducted under the EU Joint Program – Neurodegenerative Disease Research (JPND) – BIOMARKAPD JPND 0005/2011 project. The authors thank the patients and their families for their participation.
References
- 1.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement (2011) 7(3):263–9. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol (2014) 13(6):614–29. 10.1016/S1474-4422(14)70090-0 [DOI] [PubMed] [Google Scholar]
- 3.Hort J, O’Brien JT, Gainotti G, Pirttila T, Popescu BO, Rektorova I, et al. EFNS guidelines for the diagnosis and management of Alzheimer’s disease. Eur J Neurol (2010) 17(10):1236–48. 10.1111/j.1468-1331.2010.03040.x [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol (2010) 9(11):1118–27. 10.1016/S1474-4422(10)70223-4 [DOI] [PubMed] [Google Scholar]
- 5.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement (2011) 7(3):270–9. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seguin J, Formaglio M, Perret-Liaudet A, Quadrio I, Tholance Y, Rouaud O, et al. CSF biomarkers in posterior cortical atrophy. Neurology (2011) 76(21):1782–8. 10.1212/WNL.0b013e31821ccc98 [DOI] [PubMed] [Google Scholar]
- 7.Lehmann S, Schraen S, Quadrio I, Paquet C, Bombois S, Delaby C, et al. Impact of harmonization of collection tubes on Alzheimer’s disease diagnosis. Alzheimers Dement (2013) 10(5 Suppl):S390–4. 10.1016/j.jalz.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 8.Molinuevo JL, Blennow K, Dubois B, Engelborghs S, Lewczuk P, Perret-Liaudet A, et al. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement (2014) 10(6):808–17. 10.1016/j.jalz.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 9.Mo JA, Lim JH, Sul AR, Lee M, Youn YC, Kim HJ. Cerebrospinal fluid beta-amyloid1-42 levels in the differential diagnosis of Alzheimer’s disease – systematic review and meta-analysis. PLoS One (2015) 10(2):e0116802. 10.1371/journal.pone.0116802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen C, Farahmand B, Skillback T, Nagga K, Mattsson N, Kilander L, et al. Benchmarking biomarker-based criteria for Alzheimer’s disease: data from the Swedish Dementia Registry, SveDem. Alzheimers Dement (2015). 10.1016/j.jalz.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 11.Wiltfang J, Esselmann H, Bibl M, Hull M, Hampel H, Kessler H, et al. Amyloid beta peptide ratio 42/40 but not A beta 42 correlates with phospho-Tau in patients with low- and high-CSF A beta 40 load. J Neurochem (2007) 101(4):1053–9. 10.1111/j.1471-4159.2006.04404.x [DOI] [PubMed] [Google Scholar]
- 12.Wiltfang J, Esselmann H, Bibl M, Smirnov A, Otto M, Paul S, et al. Highly conserved and disease-specific patterns of carboxyterminally truncated Abeta peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer’s disease and in patients with chronic neuroinflammation. J Neurochem (2002) 81(3):481–96. 10.1046/j.1471-4159.2002.00818.x [DOI] [PubMed] [Google Scholar]
- 13.Spies PE, Slats D, Sjogren JM, Kremer BP, Verhey FR, Rikkert MG, et al. The cerebrospinal fluid amyloid beta42/40 ratio in the differentiation of Alzheimer’s disease from non-Alzheimer’s dementia. Curr Alzheimer Res (2010) 7(5):470–6. 10.2174/156720510791383796 [DOI] [PubMed] [Google Scholar]
- 14.Lewczuk P, Kamrowski-Kruck H, Peters O, Heuser I, Jessen F, Popp J, et al. Soluble amyloid precursor proteins in the cerebrospinal fluid as novel potential biomarkers of Alzheimer’s disease: a multicenter study. Mol Psychiatry (2010) 15(2):138–45. 10.1038/mp.2008.84 [DOI] [PubMed] [Google Scholar]
- 15.Portelius E, Westman-Brinkmalm A, Zetterberg H, Blennow K. Determination of beta-amyloid peptide signatures in cerebrospinal fluid using immunoprecipitation-mass spectrometry. J Proteome Res (2006) 5(4):1010–6. 10.1021/pr050475v [DOI] [PubMed] [Google Scholar]
- 16.Otto M, Lewczuk P, Wiltfang J. Neurochemical approaches of cerebrospinal fluid diagnostics in neurodegenerative diseases. Methods (2008) 44(4):289–98. 10.1016/j.ymeth.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 17.Gravina SA, Ho L, Eckman CB, Long KE, Otvos L, Jr, Younkin LH, et al. Amyloid beta protein (A beta) in Alzheimer’s disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43). J Biol Chem (1995) 270(13):7013–6. [DOI] [PubMed] [Google Scholar]
- 18.Lewczuk P, Wiltfang J. Neurochemical dementia diagnostics: state of the art and research perspectives. Proteomics (2008) 8(6):1292–301. 10.1002/pmic.200700703 [DOI] [PubMed] [Google Scholar]
- 19.Verbeek MM, De Jong D, Kremer HP. Brain-specific proteins in cerebrospinal fluid for the diagnosis of neurodegenerative diseases. Ann Clin Biochem (2003) 40(Pt 1):25–40. 10.1258/000456303321016141 [DOI] [PubMed] [Google Scholar]
- 20.Hansson O, Zetterberg H, Buchhave P, Andreasson U, Londos E, Minthon L, et al. Prediction of Alzheimer’s disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord (2007) 23(5):316–20. 10.1159/000100926 [DOI] [PubMed] [Google Scholar]
- 21.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol (2000) 57(1):100–5. 10.1001/archneur.57.1.100 [DOI] [PubMed] [Google Scholar]
- 22.Shoji M, Matsubara E, Kanai M, Watanabe M, Nakamura T, Tomidokoro Y, et al. Combination assay of CSF tau, A beta 1-40 and A beta 1-42(43) as a biochemical marker of Alzheimer’s disease. J Neurol Sci (1998) 158(2):134–40. 10.1016/S0022-510X(98)00122-1 [DOI] [PubMed] [Google Scholar]
- 23.Kanai M, Matsubara E, Isoe K, Urakami K, Nakashima K, Arai H, et al. Longitudinal study of cerebrospinal fluid levels of tau, A beta1-40, and A beta1-42(43) in Alzheimer’s disease: a study in Japan. Ann Neurol (1998) 44(1):17–26. 10.1002/ana.410440108 [DOI] [PubMed] [Google Scholar]
- 24.Fukuyama R, Mizuno T, Mori S, Nakajima K, Fushiki S, Yanagisawa K. Age-dependent change in the levels of Abeta40 and Abeta42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Abeta42 to Abeta40 level in cerebrospinal fluid from Alzheimer’s disease patients. Eur Neurol (2000) 43(3):155–60. 10.1159/000008157 [DOI] [PubMed] [Google Scholar]
- 25.Schoonenboom NS, Mulder C, Van Kamp GJ, Mehta SP, Scheltens P, Blankenstein MA, et al. Amyloid beta 38, 40, and 42 species in cerebrospinal fluid: more of the same? Ann Neurol (2005) 58(1):139–42. 10.1002/ana.20508 [DOI] [PubMed] [Google Scholar]
- 26.Slaets S, Le Bastard N, Martin JJ, Sleegers K, Van Broeckhoven C, De Deyn PP, et al. Cerebrospinal fluid Abeta1-40 improves differential dementia diagnosis in patients with intermediate P-tau181P levels. J Alzheimers Dis (2013) 36(4):759–67. 10.3233/JAD-130107 [DOI] [PubMed] [Google Scholar]
- 27.Sauvee M, DidierLaurent G, Latarche C, Escanye MC, Olivier JL, Malaplate-Armand C. Additional use of Abeta(4)(2)/Abeta(4)(0) ratio with cerebrospinal fluid biomarkers P-tau and Abeta(4)(2) increases the level of evidence of Alzheimer’s disease pathophysiological process in routine practice. J Alzheimers Dis (2014) 41(2):377–86. 10.3233/JAD-131838 [DOI] [PubMed] [Google Scholar]
- 28.Dumurgier J, Schraen S, Gabelle A, Vercruysse O, Bombois S, Laplanche JL, et al. Cerebrospinal fluid amyloid-beta 42/40 ratio in clinical setting of memory centers: a multicentric study. Alzheimers Res Ther (2015) 7(1):30. 10.1186/s13195-015-0114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American-Psychiatric-Association. Diagnostic and Statistical Manual of Mental Disorders (IV-TR). 4th ed Washington, DC: American-Psychiatric-Association; (2000). [Google Scholar]
- 30.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology (2005) 65(12):1863–72. 10.1212/01.wnl.0000187889.17253.b1 [DOI] [PubMed] [Google Scholar]
- 31.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain (2011) 134(Pt 9):2456–77. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement (2012) 8(1):65–73. 10.1016/j.jalz.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 33.del Campo M, Mollenhauer B, Bertolotto A, Engelborghs S, Hampel H, Simonsen AH, et al. Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: an update. Biomark Med (2012) 6(4):419–30. 10.2217/bmm.12.46 [DOI] [PubMed] [Google Scholar]
- 34.Mattsson N, Andreasson U, Persson S, Carrillo MC, Collins S, Chalbot S, et al. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement (2013) 9(3):251–61. 10.1016/j.jalz.2013.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metz CE. Basic principles of ROC analysis. Semin Nucl Med (1978) 8(4):283–98. 10.1016/S0001-2998(78)80014-2 [DOI] [PubMed] [Google Scholar]
- 36.Lewczuk P, Lelental N, Spitzer P, Maler JM, Kornhuber J. Amyloid-beta 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer’s disease: validation of two novel assays. J Alzheimers Dis (2015) 43(1):183–91. 10.3233/JAD-140771 [DOI] [PubMed] [Google Scholar]
- 37.Lewczuk P, Beck G, Esselmann H, Bruckmoser R, Zimmermann R, Fiszer M, et al. Effect of sample collection tubes on cerebrospinal fluid concentrations of tau proteins and amyloid beta peptides. Clin Chem (2006) 52(2):332–4. 10.1373/clinchem.2005.058776 [DOI] [PubMed] [Google Scholar]
- 38.Fourier A, Portelius E, Zetterberg H, Blennow K, Quadrio I, Perret-Liaudet A. Pre-analytical and analytical factors influencing Alzheimer’s disease cerebrospinal fluid biomarkers variability. Clin Chim Acta (2015) 449:9–15. 10.1016/j.cca.2015.05.024 [DOI] [PubMed] [Google Scholar]
- 39.Grossman M, Farmer J, Leight S, Work M, Moore P, Van Deerlin V, et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer’s disease. Ann Neurol (2005) 57(5):721–9. 10.1002/ana.20477 [DOI] [PubMed] [Google Scholar]
- 40.Koopman K, Le Bastard N, Martin JJ, Nagels G, De Deyn PP, Engelborghs S. Improved discrimination of autopsy-confirmed Alzheimer’s disease (AD) from non-AD dementias using CSF P-tau(181P). Neurochem Int (2009) 55(4):214–8. 10.1016/j.neuint.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 41.Schoonenboom NS, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM, et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology (2012) 78(1):47–54. 10.1212/WNL.0b013e31823ed0f0 [DOI] [PubMed] [Google Scholar]
- 42.de Souza LC, Lamari F, Belliard S, Jardel C, Houillier C, De Paz R, et al. Cerebrospinal fluid biomarkers in the differential diagnosis of Alzheimer’s disease from other cortical dementias. J Neurol Neurosurg Psychiatry (2011) 82(3):240–6. 10.1136/jnnp.2010.207183 [DOI] [PubMed] [Google Scholar]
- 43.Toledo JB, Brettschneider J, Grossman M, Arnold SE, Hu WT, Xie SX, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol (2012) 124(1):23–35. 10.1007/s00401-012-0983-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol (2013) 126(3):365–84. 10.1007/s00401-013-1157-y [DOI] [PubMed] [Google Scholar]
- 45.Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther (2014) 6(9):82. 10.1186/s13195-014-0082-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graff-Radford NR. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology (2014) 83(17):1573–5. 10.1212/WNL.0000000000000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeppsson A, Zetterberg H, Blennow K, Wikkelso C. Idiopathic normal-pressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology (2013) 80(15):1385–92. 10.1212/WNL.0b013e31828c2fda [DOI] [PubMed] [Google Scholar]
- 48.Gabelle A, Roche S, Geny C, Bennys K, Labauge P, Tholance Y, et al. Correlations between soluble alpha/beta forms of amyloid precursor protein and Abeta38, 40 and 42 in human cerebrospinal fluid. Brain Res (2010) 1357:175–83. 10.1016/j.brainres.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 49.Gabelle A, Roche S, Geny C, Bennys K, Labauge P, Tholance Y, et al. Decreased sAbetaPPbeta, Abeta38, and Abeta40 cerebrospinal fluid levels in frontotemporal dementia. J Alzheimers Dis (2011) 26(3):553–63. 10.3233/JAD-2011-110515 [DOI] [PubMed] [Google Scholar]