With recent changes in recommended criteria for the scoring of respiratory events, comparisons between the results of the scorings have been performed in some retrospective studies.1–3 These comparative studies are very useful because they link previous data to our current data in terms of disease severity and prevalence for sleep disordered breathing. One may even speculate about conversion factors. One well-designed retrospective comparison was published by Duce et al. in this issue.1
DISCUSSION
Equipment Related Issues
Differences in scoring respiratory events had been reported previously.4 We can identify several different reasons for this. First, different equipment is used in sleep studies for assessing respiration. This had been addressed in reviews when trying to identify the best and the most feasible methods for sleep studies.4,5 There is a consensus that the reference for oronasal airflow is a mask with a pneumotachograph. Because this is not practically applicable in clinical sleep studies, other sensors are used. These include thermal sensors, pressure sensors (nasal prongs), and snoring and air vibration sensors. Good validations are available for pressure sensors and for some thermal sensors. There is a consensus that the reference for respiratory effort is esophageal pressure. Again, because this is not practically applicable in clinical sleep studies, inductive plethysmography, piezo sensors, strain gauges, and impedance sensors are used. Validation studies have proven that inductive plethysmography is the best alternative to esophageal pressure.
Scoring, Software, and Definition Related Issues
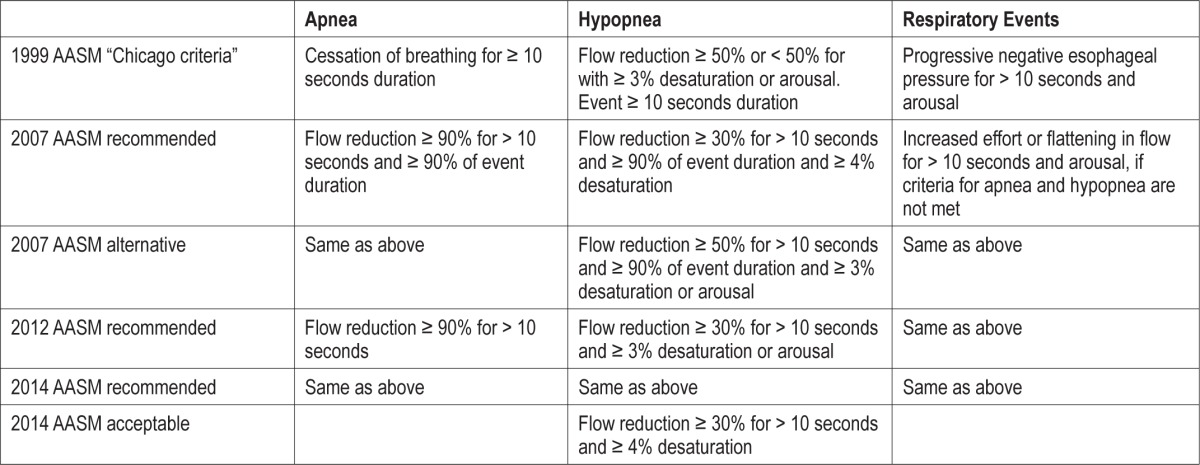
The second reason for differences in scoring results lies in the scoring process itself. Independent whether scoring was performed automatic or visual, it is based on certain definitions. An overview of the definitions as they have evolved is given in Table 1. Definitions (and software algorithms) always rely on some sort of thresholds, and thresholds are often arbitrary. No study has shown that a 30% reduction in flow is superior to scoring 33% reduction in flow. Criteria were often selected due to experience and practicability.
Table 1.
Development of criteria for the scoring of respiratory events over time.
Apnea, Hypopnea, RDI, Surrogates
A bigger problem is some signals provide only surrogates for respiratory events. A heart rate-based apnea/hypopnea evaluation, a respiratory sound-based apnea/hypopnea evaluation, or a pulse attenuation evaluation for apnea/hypopnea events are surrogates.6 Surrogates may serve very well against the reference standard and may do their job well in clinical practice.6 For summing up these surrogates the term “respiratory disturbance index (RDI)” was coined, being different from AHI. This opens our eyes to the problem that nasal prongs or thermal sensors provide surrogates for airflow as well. This issue was much debated during the development of the AASM rules for scoring published in 1999, and as a consequence, no significant effort was made to count apnea and hypopnea events differently at that time.7 Respiratory related arousals may be a different issue. With this reflection, all events are finally surrogates for a pathology called sleep disordered breathing.
Consequences in Terms of Treatment Decisions
Using apnea or hypopnea events, depending on sensors and definitions as surrogates for a pathology, we have to arrive at a clinical decision for treatment. This is done quite well, and evidence on AHI number-based treatment have helped to prove success of various treatments.8 However changing definitions will result in treating more people or fewer people, as perfectly concluded by Duce et al.1 Whether we diagnose and treat more patients with sleep apnea overall by changing AHI thresholds for sleep apnea severity becomes a health economy problem. Such decisions, if taken, should clearly state which event scoring criteria they are based upon.
Consequences in Terms of Prevalence
Changing criteria for respiratory events results in totally different prevalence values for sleep disordered breathing. An example for this is given by Heinzer et al.3 In their Swiss general population cohort study, they found a prevalence of 23.4% in women and 49.7% in men for AHI ≥ 15/hour using the AASM 2012 criteria.9 In the supplement to their paper, the authors compared the AASM 2012, AASM 2007 recommended,10 and AASM 1999 criteria.7 While AASM 2012 and AASM 1999 did not differ significantly, the AASM 2007 criteria resulted in roughly three-fold lower AHI values. Using surrogate respiratory event recording and evaluation in a selected urban population, we can see a 55% prevalence for moderate and severe sleep apnea.6 Where is the truth? This clearly demonstrates that setting scoring criteria is also a health policy issue.
Consequences in Terms of Cardiovascular Risk
In the study by Marin, evidence was given that severe sleep apnea (AHI > 30/h) has increased cardiovascular mortality.12 Without apnea, with treated sleep apnea, and with some mild degrees of sleep apnea, we have lower cardiovascular risk. What we also know is subjects with mild and moderate severity of sleep apnea have a high night-to-night variability of their AHI.11 What needs further investigation is who is in danger for an increased cardiovascular risk. Counting respiratory events is a surrogate, currently the best surrogate we have. But what we do not know is how the risk changes based on these numbers. Data suggest that there is a steady increase: the more respiratory events, the higher the risk. But there may be a threshold for AHI. There may be a big variation in respiratory events that do not increase cardiovascular risk in mild and moderate sleep apnea. Changing criteria for scoring respiratory events does not help to solve this problem.
CONCLUSION
All the definitions for respiratory events and for thresholds defining mild, moderate, and severe sleep apnea are arbitrary. Definitely we need thresholds to convince patients and payers for health care payment decisions. However, in the end, we are still seeking better correlates for the clinical picture of obstructive sleep apnea linked with obesity, obstructive sleep apnea linked with narrow upper airways, heritable obstructive sleep apnea, central sleep apnea linked to heart failure, sleep apnea linked to hyper- or hyposensitivity in chemoreflexes, and sleep apnea linked to prolonged circulation time. Sleep stages, body position, other medications might influence the resulting respiratory events. A pure apnea hypopnea index is not the solution but currently the best available option until we succeeded in phenotyping and characterizing severity of these phenotypes.
DISCLOSURE STATEMENT
Dr. Penzel has received research support from Heinen + Löwenstein (Germany), Itamar Medical (Israel), Cidelec (France), Resmed, Philips/Respironics, ImThera, Somnodent (Australia), and Weinmann (Germany); and has financial interests in Advanced Sleep Research (Germany) and The Siesta Group (Austria). The other authors have indicated no conflicts of interest.
CITATION
Penzel T, Christoph S, Fietze I. Revise respiratory event criteria or revise severity thresholds for sleep apnea definition? J Clin Sleep Med 2015;11(12):1357–1359.
REFERENCES
- 1.Duce B, Milosavljevic J, Hukins C. The 2012 AASM respiratory event criteria increase the incidence of hypopneas in an adult sleep center population. J Clin Sleep Medr. 2015;11:1425–31. doi: 10.5664/jcsm.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redline S, Kapir VK, Sanders MH, et al. Effects of varying approaches for identifying respiratory disturbances on sleep apnea assessment. Am J Respir Crit Care Med. 2000;161:369–74. doi: 10.1164/ajrccm.161.2.9904031. [DOI] [PubMed] [Google Scholar]
- 3.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–8. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169–200. [PubMed] [Google Scholar]
- 5.Penzel T, Blau A, Garcia C, Schöbel C, Sebert M, Fietze I. Portable monitoring in sleep apnea. Curr Respir Care Rep. 2012;1:139–45. [Google Scholar]
- 6.Garg N, Rolle AJ, Lee TA, Prasad B. Home-based diagnosis of obstructive sleep apnea in an urban population. J Clin Sleep Med. 2014;10:879–85. doi: 10.5664/jcsm.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 8.Randerath WJ, Hein H, Arzt M, et al. Konsensuspapier zur Diagnostik und Therapie schlafbezogener Atmungsstörungen bei Erwachsenen. Somnologie. 2014;18:34–52. doi: 10.1055/s-0033-1359221. [DOI] [PubMed] [Google Scholar]
- 9.Berry R, Brooks R, Gamaldo C, et al. Darien, IL: American Academy of Sleep Medicine; 2012. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification, Version 2.0.3. www.aasmnet.org. [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson AL, Quan S. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specification. [Google Scholar]
- 11.Bittencourt LRA, Suchecki D, Tufik S, et al. The variability of the apnoeahypopnoea index. J Sleep Res. 2001;10:245–51. doi: 10.1046/j.1365-2869.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 12.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 13.Berry R, Brooks R, Gamaldo C, et al. Darien, IL: American Academy of Sleep Medicine; 2014. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification, Version 2.1. www.aasmnet.org. [Google Scholar]


