Abstract
Study Objective:
Sleep disturbances are among the most common nonmotor symptoms of Parkinson disease. However, no large epidemiological data regarding the association between obstructive sleep apnea (OSA) and Parkinson disease have been reported. The goal of this study was to investigate the risk for Parkinson disease during a 5-y follow-up period after a diagnosis of OSA using a population-based dataset.
Methods:
The data for this retrospective longitudinal cohort study were retrieved from the Taiwan Longitudinal Health Insurance Database 2000. We identified 1,532 patients with OSA as the study cohort and randomly selected 7,660 patients as the comparison cohort. Each subject was individually followed up for a 5-y period to identify those in whom Parkinson disease subsequently developed. Stratified Cox proportional hazard regressions were performed as a means of comparing the 5-y risk of subsequent Parkinson disease between the study cohort and comparison cohort.
Results:
Of the 9,192 total patients, Parkinson disease developed in 0.73% during the 5-y follow-up period: 1.24% and 0.63% in the OSA and control cohorts, respectively. After censoring patients who died during the follow-up period and adjusting for socio-demographic characteristics, the hazard ratio (HR) of Parkinson disease during the 5-y follow-up period for patients with OSA was 2.26 (95% confidence interval [CI] = 1.32–3.88) compared with comparison patients. In addition, among females, the adjusted HR of Parkinson disease was 3.54 (95% CI = 1.50–8.34) for patients with OSA compared to patients without OSA. However, among males, there was no significantly increased hazard of Parkinson disease for patients with OSA compared to those without OSA.
Conclusions:
Female patients with OSA were found to be at a significant risk of subsequent Parkinson disease during a 5-y follow-up period.
Citation:
Sheu JJ, Lee HC, Lin HC, Kao LT, Chung SD. A 5-year follow-up study on the relationship between obstructive sleep apnea and Parkinson disease. J Clin Sleep Med 2015;11(12):1403–1408.
Keywords: epidemiology, obstructive sleep apnea, Parkinson disease, parkinsonism, sleep apnea
Parkinson disease (PD) is a neurological syndrome characterized by movement disturbances including slow movements, muscle rigidity, tremors, and postural instability. There are a number of underlying etiologies leading to PD, and common recognized causes include drugs and substances,1 infections,2 and vascular events.3 Idiopathic PD, a parkinsonian syndrome without an obvious known cause, is the second most common degenerative neurological disorder affecting 1%–2% of adults older than 60 y.4 Recently, increasing evidence has further indicated that environmental toxins, oxidative stress, and inflammatory mechanisms might play key roles in the pathophysiology of PD.5
Obstructive sleep apnea (OSA) is a very common sleep disorder with a prevalence of 24% in men and 9% in women of middle age, and the prevalence in the elderly is probably higher.6,7 Several lines of evidence have suggested that OSA induces oxidative stress and inflammation.8 These pathological processes have been found to be etiologically involved in OSA and PD.5,8
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep disturbances are among the common symptoms of Parkinson disease. However, no large epidemiological data regarding the association between obstructive sleep apnea (OSA) and Parkinson disease have been reported.
Study Impact: This population-based study provides epidemiological evidence of a link between OSA and a subsequent Parkinson disease diagnosis. Female patients with OSA were found to be at a significant risk of subsequent Parkinson disease during a 5-y follow-up period.
However, to our knowledge, large epidemiological data regarding the association between OSA and PD are still lacking, even though OSA and PD might share similar pathological mechanisms. To date, only a few studies have investigated the occurrence of sleep disturbances in patients with PD. In previous studies, in addition to motor difficulties, sleep disturbances often cause significant disability in PD patients, and result in substantial adverse effects on their quality of life.9 Sleep disturbances such as insomnia, excessive daytime sleepiness, sleep apnea syndrome, restless legs syndrome, and rapid eye movement (REM) sleep behavior disorder are among the most common nonmotor symptoms of PD, with the prevalence in the range of 40%–90%.10 Sleep disturbances may occur in the early stage of PD and become more significant as PD progresses. In addition, some types of sleep disturbances, e.g., REM sleep behavior disorder and excessive daytime sleepiness, may antedate the onset of motor symptoms of PD by several years and therefore be thought of as predictors for the development of PD.11
Therefore, the goal of this study was to investigate the risk for subsequent PD in patients with OSA compared to patients without OSA during the same period, using a large, nationwide population-based dataset in Taiwan.
METHODS
Database
The data for this retrospective longitudinal cohort study were retrieved from the Taiwan Longitudinal Health Insurance Database (LHID2000), which includes claims data for 1 million beneficiaries. These 1 million beneficiaries were randomly sampled from the Registry for Beneficiaries for the year 2000 from the 23.72 million enrollees under the Taiwan National Health Insurance (NHI) program (coverage rate of the Taiwanese population was > 98% in 2000) by the Taiwan National Health Research Institute (NHRI). The Taiwan NHRI demonstrated that there was no significant difference in sex distribution between enrollees in the LHID2000 and the NHI program.
This study was exempt from full review by the Institutional Review Board of the National Defense Medical Center because the LHID2000 consists of deidentified secondary data released to the public for research purposes.
Study Sample
This study was designed to include a study cohort and a comparison cohort. We selected the study cohort by identifying 2,955 patients who had received a first-time diagnosis of OSA (ICD-9-CM codes 327.23, 780.51, 780.53, or 780.57) in an ambulatory care visit from January 1, 2001 to December 31, 2007. In Taiwan, when a patient is suspected of having OSA, the physician gives the patient a temporary diagnosis of OSA. This allows the physician to perform related clinical tests and polysomnography (PSG) for confirmation, without receiving possible fines for performing unnecessary or inappropriate procedures. Thereafter, a patient receives another OSA diagnosis in their next ambulatory visit if he or she is confirmed to have OSA after these tests. Therefore, this study limited the study cohort to those patients who had received ≥ 2 diagnoses of OSA (n = 1,636) in order to increase the OSA diagnostic validity. We further excluded patients younger than 18 y (n = 84). We then assigned their first ambulatory care visit for receiving an OSA diagnosis as the index date. In addition, we identified and excluded those patients who had a history of PD (ICD-9-CM code 332) prior to the index date (n = 20). As a result, 1,532 patients with OSA were included in the study cohort.
The comparison cohort was likewise retrieved from the LHID2000. We first excluded all patients who had ever received a diagnosis of OSA or were younger than 18 y. The SAS Proc SurveySelect program (SAS System for Windows, version 8.2, SAS Institute, Cary, NC, USA) was used to randomly extract 7,660 patients (five for every patient with OSA) matched in terms of sex, age group (younger than 30 y, 30–39 y, 40–49 y, 50–59 y, 60–69 y, and older than 69 y), and the index year. The index year for the study cohort was the year in which they received their first-time diagnosis of OSA. For the comparison cohort, the index year was simply a matched year in which the comparison patients visited a physician. We further defined their first ambulatory care visit that occurred in the index year as their index date. Furthermore, all selected comparison patients were confirmed not to have a history of PD prior to their index date.
Thereafter, each sampled patient (n = 9,192) was individually tracked for a 5-y period from their index date to discriminate those patients who subsequently received a diagnosis of PD during the follow-up period.
Statistical Analysis
We performed all statistical analyses using the SAS system. The Kaplan-Meier method and log-rank test were used to compare the time to the development of PD and survival time among patients with and those without OSA. We further used the stratified Cox proportional hazard regression (stratified by sex, age group, and the year of the index date) to compute the risk of PD during the 5-y follow-up period among patients with and those without OSA. In addition, we censored those patients who died during the follow-up period (103 from the study cohort [6.7% of patients with OSA] and 746 from the comparison cohort [9.7% of patients without OSA]). Hazard ratios (HRs) along with 95% confidence intervals (CIs) were used to present the risk of PD, using a significance level of 0.05.
RESULTS
Table 1 shows the demographic characteristics for all sampled patients according to the presence of OSA. The mean age was 46.5 y with a standard deviation of 13.9 y, and the overwhelmingly majority were male (69.6%). After being matched for sex, age group, and the year of the index date, there were significant differences in monthly income (p < 0.001), geographic region (p < 0.001), and urbanization level (p < 0.001) between patients with and those without OSA. Patients with OSA were more likely to reside in central Taiwan and more urbanized communities than patients without OSA.
Table 1.
Demographic characteristics for the sampled patients stratified by the presence/absence of obstructive sleep apnea, 2001–2007 (n = 9,192).
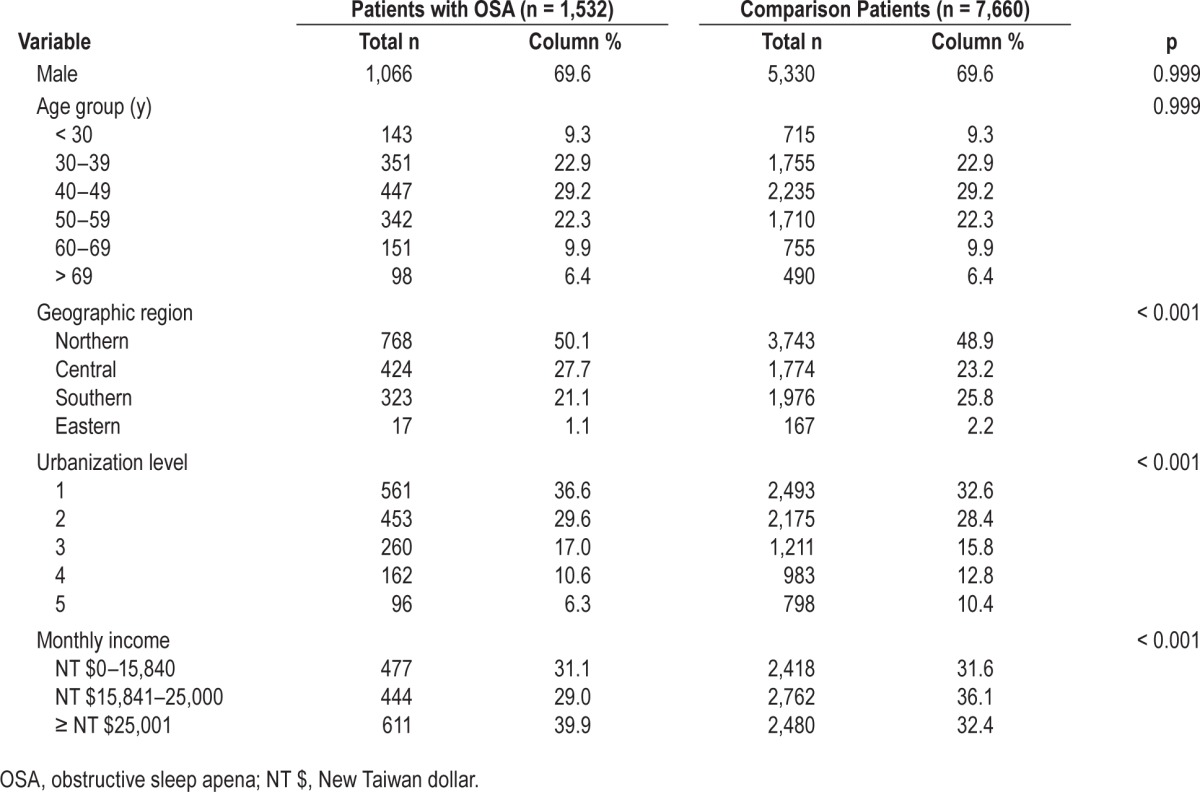
Table 2 shows the incidence of PD during the 5-y follow-up period between patients with and those without OSA. Of the 9,192 patients, 0.73% (n = 67) subsequently received a diagnosis of PD during the 5-y follow-up period: 1.24% of patients with OSA and 0.63% of those without. The Kaplan-Meier analysis demonstrated a significantly decreased PD-free survival in patients with OSA compared to those without OSA (Figure 1; log-rank p < 0.001). Furthermore, we found that of the 67 sampled patients who subsequently received a diagnosis of PD during the 5-y follow-up period, the mean time between the index date and the first diagnosis of PD was 945 days for all patients (standard deviation = 554 days), and 870 and 975 days for patients with and those without OSA, respectively (p = 0.488).
Table 2.
Crude and covariate-adjusted hazard ratios for Parkinson disease among the sampled patients during the 5-year follow-up period.
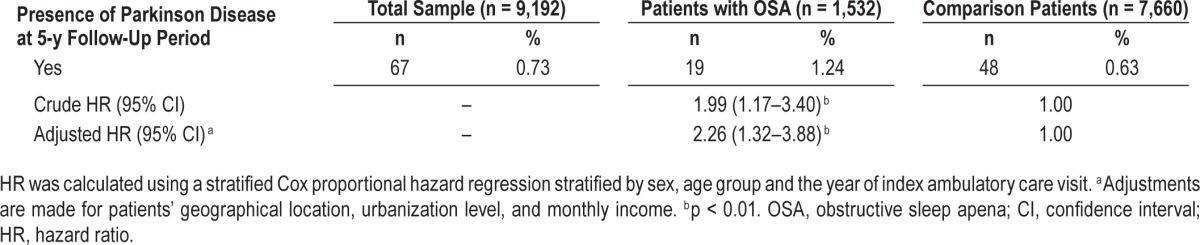
Figure 1. Five-year Parkinson disease-free survival rates.
Five-year Parkinson disease-free survival rates for patients with and those without obstructive sleep apnea are shown.
Table 2 also presents the HRs of PD during the 5-y follow-up period between patients with and those without OSA. The stratified Cox proportional analysis (stratified by age, sex, and the year of the index date) suggested that the HR of PD for patients with OSA was 2.26 (95% CI = 1.32–3.88) compared to patients without OSA after censoring individuals who died during the follow-up period and adjusting for the patients' monthly income, geographical location, and urbanization level.
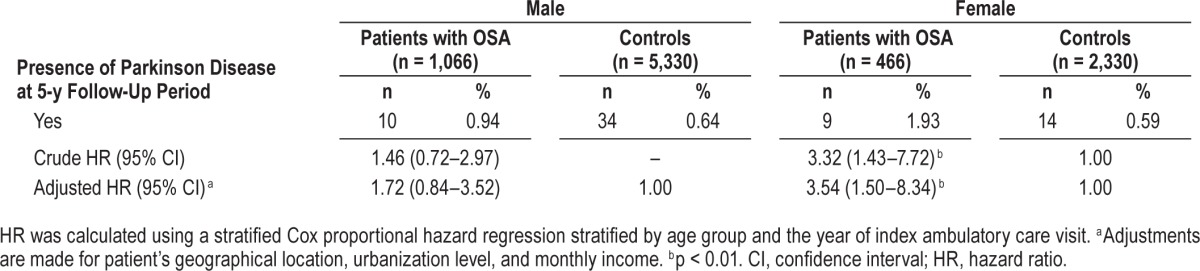
Table 3 presents the HRs of PD between patients with and those without OSA according to sex. We found that among females, the adjusted HR of PD was 3.54 (95% CI = 1.50–8.34) for patients with OSA compared to patients without OSA. However, among males, there was no significantly increased hazard of PD for patients with OSA compared to those without OSA.
Table 3.
Crude and covariate-adjusted hazard ratios for Parkinson disease among the sampled patients during the 5-year follow-up period.
DISCUSSION
Data currently available regarding the association between OSA and PD were mostly obtained from cross-sectional descriptive studies or small sample case-control studies.12–16 Currently, the association between OSA and PD remains an issue of controversy, and previous studies yielded conflicting results. Maria et al.12 found there was a higher prevalence of OSA, defined as an apnea-hypopnea index (AHI) > 5, in 15 PD patients compared with 15 healthy controls.12 Shpirer et al.14 also demonstrated a greater mean AHI for PD patients compared with that of controls.14 On the contrary, compared with 50 in-hospital controls, Cochen et al.15 showed that sleep apnea was less frequent in 100 PD patients. Nevertheless, Yong et al.16 found that there was no difference in the AHI among PD patients and controls in an Asian population. A real association or causal relationship cannot be established by descriptive or case-control studies because of the small sample size or cross-sectional design. To our understanding, this study is the first population-based study to investigate the relationship between OSA and PD. In this study, we first identified patients with OSA in ambulatory care visits, and then tracked each patient for 5 y to identify those subjects who subsequently received a diagnosis of PD during the follow-up period. We found that the adjusted risk of subsequent PD for patients with OSA was 2.26 times greater than for patients without OSA. The results of our study provide the first epidemiological evidence that patients with OSA may be at greater risk for subsequent PD, although the absolute number of patients with OSA in whom PD later developed was small, and OSA might only contribute a minor risk for PD.
The actual mechanisms contributing to the association between OSA and PD are not fully understood. There is accumulative evidence that OSA is associated with cerebrovascular disease and vascular risk factors, including hypertension, diabetes, and hyperlipidemia.17–19 Vascular parkinsonism, a parkinsonian syndrome caused by cerebrovascular disease, usually occurs in the aged with vascular risk factors such as hypertension and diabetes.3,20 Thus, the reason implicating OSA in the development of PD may be attributable to the effects of cerebrovascular disease and vascular risk factors.
Previous studies proposed possible underlying pathomechanisms for the initiation and progression of PD, including oxidative stress, mitochondrial dysfunction, excitotoxicity, microglial activation, and neuroinflammation.21,22 Oxidative stress plays a significant role in several cellular processes, including perturbation of the ubiquitin-proteasome system, mis-folding and aggregation of α-synuclein, and mitochondrial dysfunction, which result in dopaminergic neurodegeneration.22 Activation of microglial cells, resident immune cells in the substantia nigra, may trigger exaggerated inflammatory responses, successively activate the surrounding microglia and astrocytes, cause an adverse effect on the remaining neurons, and therefore aggravate dopaminergic neurodegeneration in PD.21 There is increasing evidence that OSA, characterized by repetitive intermittent hypoxia during apnea and reoxygenation following apnea, activates intense local and systemic inflammatory and immune responses.23 Proinflammatory cytokines produced by peripheral inflammation can cross the disrupted blood-brain barrier and ignite deleterious effects on the nigrostriatal dopaminergic system.24 In addition, peripheral inflammatory signals can also be transmitted to the brain via the vagus nerve, consistent with the enteric autonomic nervous system route of the dual-hit hypothesis that initiates the development of PD.25 Consequently, we postulated that OSA may trigger aberrant inflammatory responses, convey inflammatory signals to the brain through humoral or neural routes, and bring about microglial activation and degeneration of dopaminergic neurons in the substantia nigra.
Interestingly, we found that females rather than males had higher risks of subsequent PD following an OSA diagnosis. Although the reason is not clear, a possible explanation is related to the underrecognition and underdiagnosis of OSA in women. A low awareness of OSA in women by patients and physicians, under-reporting of snoring and breathing pauses during sleep, and atypical presenting symptoms such as insomnia, anxiety, and depression of OSA in women all result in the clinical underdiagnosis of OSA.26 Therefore, women with OSA might not receive a diagnosis until severe symptoms occur. It is reasonable to hypothesize that severe OSA in women increases the risk of PD because of the greater degree of hypoxia, inflammation, and oxidative stress. Additionally, the age difference might be one of the potential rationales which contributed the dissimilar risk of PD between males and females. In this study, the mean age was significantly higher in females than males (47.3 versus 46.1; p < 0.001). Prior studies have reported that age was a strong factor leading to the disease severity of PD.27 As a result, it is plausible that selected females in our study have elevated risk of PD because of increasing age.
A particular strength of this study is the use of a population-based dataset that provided a sufficient sample size and statistical power to explore the association between OSA and PD. Nevertheless, some insufficiencies in our study should be addressed. First, OSA and PD diagnoses, which rely on administrative claims data reported by physicians or hospitals, may be less accurate than those made under standardized diagnostic criteria. However, there is no universal definition for a diagnosis of OSA. To our knowledge, most sleep centers in Taiwan follow clinical guidelines of the American Academy of Sleep Medicine for diagnosing OSA.28 In addition, to ensure diagnostic validity, we included only patients who had received two or more OSA diagnoses following a PSG examination. The NHI in Taiwan has a regular cross-checking system with full review of chart records, laboratory findings, and imaging results by specialists to prevent miscoding or inaccurate medical claims, followed by punitive measures for fraudulent coding. Moreover, from previous studies, the NHI Research Database was found to be of acceptable quality to provide reasonable estimations for epidemiological data of OSA and PD.29,30 Second, information on the severity of OSA such as AHI scores and the treatment status for OSA such as continuous positive airway pressure were not available in the database. Therefore, we were unable to evaluate the relationship between OSA severity or treatment and the subsequent risk of PD. Third, our database lacks information on individual factors such as cigarette smoking, alcohol consumption, body mass index, and dietary patterns that may potentially confound both OSA and PD.31,32 This may have compromised our findings. However, we adjusted for patient monthly income, geographical location, and urbanization level in the study to minimize the effect of sociodemographic characteristics. Finally, the study population mainly consisted of Taiwanese ethnic Chinese, and therefore, the results may lack generalizability to other ethnic populations.
Despite these limitations, our nationwide population-based study provides epidemiological evidence of a link between OSA and a subsequent PD diagnosis. Further prospective studies describing OSA severity and sex-specific differences are warranted to confirm our findings and clarify the underlying pathomechanisms.
DISCLOSURE STATEMENT
This was not an industry supported study. This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, Taiwan and managed by the National Health Research Institutes. The interpretations and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- HR
hazard ratio
- LHID 2000
Longitudinal Health Insurance Database 2000
- NHI
National Health Insurance
- OSA
obstructive sleep apnea
- PD
Parkinson disease
- REM
rapid eye movement
REFERENCES
- 1.Mena MA, de Yebenes JG. Drug-induced parkinsonism. Expert Opin Drug Saf. 2006;5:759–71. doi: 10.1517/14740338.5.6.759. [DOI] [PubMed] [Google Scholar]
- 2.Casals J, Elizan TS, Yahr MD. Postencephalitic parkinsonism-a review. J Neural Transm. 1998;105:645–76. doi: 10.1007/s007020050086. [DOI] [PubMed] [Google Scholar]
- 3.Thanvi B, Lo N, Robinson T. Vascular parkinsonism—an important cause of parkinsonism in older people. Age Ageing. 2005;34:114–9. doi: 10.1093/ageing/afi025. [DOI] [PubMed] [Google Scholar]
- 4.De Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 5.Miller RL, James-Kracke M, Sun GY, Sun AY. Oxidative and inflammatory pathways in Parkinson's disease. Neurochem Res. 2009;34:55–65. doi: 10.1007/s11064-008-9656-2. [DOI] [PubMed] [Google Scholar]
- 6.Young T, PM, Dempsey J, Skatrud J, Skatrud J, Weber S, Badr S. The occurrence of sleep disordered breathing among middle aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 7.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavie L. Intermittent hypoxia: the culprit of oxidative stress, vascular inflammation and dyslipidemia in obstructive sleep apnea. Expert Rev Respir Med. 2008;2:75–84. doi: 10.1586/17476348.2.1.75. [DOI] [PubMed] [Google Scholar]
- 9.Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord. 2009;24:1641–9. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 10.Iranzo de Riquer A, Bergareche A, Campos V. Sleep disorders in Parkinson disease. Neurologist. 2011;17:S38–S42. doi: 10.1097/NRL.0b013e31823966f8. [DOI] [PubMed] [Google Scholar]
- 11.Boeve BF. Idiopathic REM sleep behavior disorder in the development of Parkinson's disease. Lancet Neurol. 2013;12:469–82. doi: 10.1016/S1474-4422(13)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maria B, Sophia S, Michalis M, et al. Sleep breathing disorders in patients with idiopathic Parkinson's disease. Respir Med. 2003;97:1151–7. doi: 10.1016/s0954-6111(03)00188-4. [DOI] [PubMed] [Google Scholar]
- 13.Diederich NJ, Vaillant M, Leischen M, et al. Sleep apnea syndrome in Parkinson's disease. A case-control study in 49 patients. Mov Disord. 2005;20:1413–8. doi: 10.1002/mds.20624. [DOI] [PubMed] [Google Scholar]
- 14.Shpirer I, Miniovitz A, Klein C, et al. Excessive daytime sleepiness in patients with Parkinson's disease: a polysomnography study. Mov Disord. 2006;21:1432–8. doi: 10.1002/mds.21002. [DOI] [PubMed] [Google Scholar]
- 15.Cochen De Cock V, Abouda M, Leu S, et al. Is obstructive sleep apnea a problem in Parkinson's disease? Sleep Med. 2010;11:247–52. doi: 10.1016/j.sleep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Yong MH, Fook-Chong S, Pavanni R, Lim LL, Tan EK. Case control polysomnographic studies of sleep disorders in Parkinson's disease. PLoS One. 2011;6:e22511. doi: 10.1371/journal.pone.0022511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 18.Drager LF, Jun J, Polotsky VY. Obstructive sleep apnea and dyslipidemia: implications for atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2010;17:161–5. doi: 10.1097/MED.0b013e3283373624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neuro. 2012;3:126. doi: 10.3389/fneur.2012.00126. eCollection 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benamer HT, Grosset DG. Vascular parkinsonism: a clinical review. Eur Neurol. 2009;61:11–5. doi: 10.1159/000165343. [DOI] [PubMed] [Google Scholar]
- 21.Barcia C, Ros F, Carrillo MA, et al. Inflammatory response in Parkinsonism. J Neural Transm Suppl. 2009;73:245–52. doi: 10.1007/978-3-211-92660-4_19. [DOI] [PubMed] [Google Scholar]
- 22.Gao HM, Hong JS. Gene-environment interactions: key to unraveling the mystery of Parkinson's disease. Prog Neurobiol. 2011;94:1–19. doi: 10.1016/j.pneurobio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNicholas WT. Obstructive sleep apnea and inflammation. Prog Cardiovasc Dis. 2009;51:392–9. doi: 10.1016/j.pcad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-Romero MC, Delgado-Cortés MJ, Sarmiento M, et al. Peripheral inflammation increases the deleterious effect of CNS inflammation on the nigrostriatal dopaminergic system. Neurotoxicology. 2012;33:347–60. doi: 10.1016/j.neuro.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med. 2009;10:1075–84. doi: 10.1016/j.sleep.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Szewczyk-Krolikowski K, Tomlinson P, Nithi K, et al. The influence of age and gender on motor and non-motor features of early Parkinson's disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat Disord. 2014;20:99–105. doi: 10.1016/j.parkreldis.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 29.Sheu JJ, Wu CS, Lin HC. Association between obstructive sleep apnea and sudden sensorineural hearing loss: a population-based case-control study. Arch Otolaryngol Head Neck Surg. 2012;138:55–9. doi: 10.1001/archoto.2011.227. [DOI] [PubMed] [Google Scholar]
- 30.Chung SD, Ho JD, Hu CC, Lin HC, Sheu JJ. Increased risk of Parkinson disease following a diagnosis of neovascular age-related macular degeneration: a retrospective cohort study. Am J Ophthalmol. 2014;157:464–9. doi: 10.1016/j.ajo.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Veldman BA, Wijn AM, Knoers N, Praamstra P, Horstink MW. Genetic and environmental risk factors in Parkinson's disease. Clin Neurol Neurosurg. 1998;100:15–26. doi: 10.1016/s0303-8467(98)00009-2. [DOI] [PubMed] [Google Scholar]
- 32.Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51:285–93. doi: 10.1016/j.pcad.2008.08.001. [DOI] [PubMed] [Google Scholar]


