Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is a risk factor for stroke, which is modulated by accompanying nocturnal hypoxemia. White matter hyperintensities (WMH) share many of the same risk factors as stroke. The purpose of this study was to investigate whether OSA and nocturnal hypoxemia are associated with white matter disease in patients with minor stroke and transient ischemic attack.
Methods:
Patients with minor stroke or TIA were recruited. Level 3 diagnostic sleep testing was used to diagnose OSA and quantify nocturnal hypoxemia. Significant OSA was defined as respiratory disturbance index ≥ 15, and nocturnal hypoxemia was defined as oxyhemoglobin saturation < 90% for ≥ 12% of total monitoring time. WMH were assessed and quantified on FLAIR MRI. The volume of WMH was compared between those with and without significant OSA and between those with and without nocturnal hypoxemia.
Results:
One hundred nine patients were included. Thirty-four (31%) had OSA and 37 (34%) had nocturnal hypoxemia. Total WMH volume was significantly greater in the OSA than in the non-OSA groups (p = 0.04). WMH volume was also significantly higher in the hypoxic than the non-hypoxic groups (p = 0.001). Mutivariable analysis with adjustment for age, hypertension, and diabetes showed that nocturnal hypoxemia was independently associated with WMH volume (p = 0.03) but OSA was not (p = 0.29).
Conclusions:
We conclude that nocturnal hypoxemia, predominantly related to OSA, is independently associated with WMH in patients who present with minor ischemic stroke and TIA and may contribute to its pathogenesis.
Citation:
Patel SK, Hanly PJ, Smith EE, Chan W, Coutts SB. Nocturnal hypoxemia is associated with white matter hyperintensities in patients with a minor stroke or transient ischemic attack. J Clin Sleep Med 2015;11(12):1417–1424.
Keywords: stroke, TIA, MRI, obstructive sleep apnea, white matter disease, hypoxia
Obstructive sleep apnea (OSA) increases the risk of vascular disease including hypertension, coronary artery disease, and stroke.1–3 It is characterized by repetitive apneas due to airway closure that are associated with intermittent hypoxemia, which is thought to modulate the increased risk of vascular disease.4,5 OSA may occur in up to 20% of the general population, 72% of those following an established stroke, and 62% of those who have suffered a transient ischemic attack or minor stroke.6–10 Furthermore, OSA may be asymptomatic in many stroke patients.10
White matter hyperintensities (WMH) of presumed vascular origin in the brain represent areas of abnormal white matter with ischemic demyelination11–13 and are diagnosed by either magnetic resonance imaging (MRI) or computed tomography (CT). Although WMH can be associated with several medical conditions including hypertension, aging, and diabetes mellitus, they are also associated with an increased risk of stroke.14–18 Since the etiology of WMH is not fully explained by conventional risk factors, we postulated that OSA with associated nocturnal hypoxemia may also contribute to its pathogenesis. Previous publications that have evaluated the relationship between OSA and WMH have yielded conflicting results, with some studies reporting an increased risk of WMH19,20 while others have not.4,21 Potential reasons for these discordant results include the subjective visual assessment of WMH volumes and lack of correction for total brain volume, small sample sizes, inaccurate assessment of OSA and nocturnal hypoxemia, and poor adjustment for confounding comorbidities.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The etiology of white matter hyperintensities (WMH), which represent areas of ischemic demyelination in the brain, is not fully understood. Nocturnal hypoxemia associated with obstructive sleep apnea (OSA) increases the risk of stroke and may also contribute to the pathogenesis of WMH.
Study Impact: WMH were associated with nocturnal hypoxemia in patients who presented with a TIA or minor stroke. Treatment of OSA may reduce the risk of recurrent neurological disease in this vulnerable patient population.
This study was designed to address the limitations of previous studies. We hypothesized that patients who present with transient ischemic attack (TIA) or minor stroke and who also have OSA and/or nocturnal hypoxemia have a higher volume of WMH that is independent of other comorbidities.
METHODS
Patient Recruitment
This was a sub-study of the prospective CATCH (CT And MRI in the Triage of TIA and minor Cerebrovascular events to identify High-risk patients) study.22 We recruited minor stroke (National Institutes of Health Stroke Scale (NIHSS) < 4) and high-risk TIA (motor or speech symptoms lasting ≥ 5 min) patients assessed within 24 h of symptom onset and who had no preexisting disability (premorbid modified Rankin score [mRS] 0 or 1). Patients had a brain MRI scan within 48 h of symptom onset and completed nocturnal monitoring for sleep apnea (described below). Patients were excluded if they were receiving treatment with continuous positive airway pressure (CPAP) or oxygen therapy, both of which would have confounded diagnostic sleep testing. All patients received routine medical management of their stroke or TIA. The research study was approved by the University of Calgary Conjoint Health Research Ethics Board, and all patients provided written informed consent.
Sleep Questionnaire and Nocturnal Cardiopulmonary Monitoring for Sleep Apnea
Patients completed a questionnaire which included the Ep-worth Sleepiness Scale,23 sleep history, past medical history, and current medications. Patients were instructed how to use a level 3 sleep monitor (Remmers Sleep Recorder Model 4.2, Saga Tech Electronics, Calgary, AB, Canada), which was used to diagnose OSA and to quantify the severity of nocturnal hypoxemia. This device consists of an oximeter to record oxyhemoglobin saturation (SpO2) and heart rate variability, a pressure transducer to record nasal flow, a microphone to record snoring, and a body position sensor. The Remmers Sleep Recorder has been validated by comparison to attended polysomnography.24,25 The respiratory disturbance index is calculated based on the number of episodes of oxyhemoglobin desaturation > 4% divided by the duration of the recording. We performed nocturnal monitoring on 2 consecutive nights and averaged the results. If only one night was completed, the results from the single night were used. If only one night of monitoring was performed and the study was not technically adequate, the patient was asked to repeat the study and was excluded if they refused. Our criteria for a technically adequate study were: (1) > 5 h of interpretable recordings of airflow and oximetry, and (2) self-reported sleep for > 75% of the monitoring time. The raw data were reviewed by a sleep medicine physician (PJH), who confirmed that the estimated RDI was accurate and determined whether apnea was central (Cheyne-Stokes respiration [CSR]) or OSA, based on the morphology of the airflow recordings. Specifically, nasal pressure recordings with a characteristic crescendo/decrescendo pattern and no evidence of airflow limitation were classified as CSR, whereas recordings without a crescendo/decrescendo pattern and with airflow limitation were classified as OSA. Finally, 2 measurements of SpO2 were used to quantify the severity of nocturnal hypoxemia: (1) mean SpO2 (average SpO2 during the entire recording) and (2) the duration of SpO2 < 90% during the recording.
Assessment of OSA and Hypoxemia
Patients were stratified based on their RDI and severity of nocturnal hypoxemia. These criteria were used independently of each other. Patients with RDI ≥ 15 were defined as the OSA group (as this is consistent with clinically significant OSA),26 and those with RDI < 15 were defined as the non-OSA group. Patients with oxyhemoglobin saturation < 90% for ≥ 12% of total monitoring time were defined as the hypoxemic group, as this level of hypoxemia has been correlated with cardiovascular outcomes in previous studies,27 and those with oxyhemoglobin saturation < 90% for < 12% of total monitoring time were defined as the non-hypoxemic group. Patients with CSR were excluded from analysis as decided a priori in the study design. The objective of the study was to investigate whether OSA and associated hypoxemia predispose patients with vascular disease to develop WMH as a complication of their sleep disordered breathing. Cheyne-Stokes respiration is more likely to be a consequence than a cause of cardiovascular or cerebrovascular disease, and consequently inclusion of patients with CSR may confound interpretation of the results.
Imaging and White Matter Lesion Volume Measurement
All patients received a multimodal 3T MRI brain scan within 48 h of stroke or TIA symptom onset. The 3T MRI scanner (Signa; GE Medical Systems) consisted of high performance gradients (40 mT/m; 184 μs rise time) and a standard quadrature head coil. Patients received sagittal T1 localizer, axial diffusion- tensor imaging (DTI) (B = 1000), axial 3D pre and post gadolinium time-of-flight MR angiography, axial T2 spoiled gradient-recalled echo (SPGR), and axial fluid-attenuated inversion recovery (FLAIR). The FLAIR sequence (TE/TR = 140/9000 ms, slice thickness = 5.0 mm) was used to investigate for presence of white matter lesions (Figure 1). WMH were defined according to consensus standards28 and measured quantitatively using a computer software tool called Quantomo (Version 1.0; Cybertrials, Inc, Calgary, Canada).29 Quantomo was developed using 3-D threshold-based region growing segmentation algorithm incorporated from the open-source Insight Segmentation and Registration Toolkit (ITK; National Library of Medicine, Bethesda, MD). The user interface was developed with Xcode using Objective-C and Cocoa (Apple Inc., Cupertino, CA). In this method, WMHs are segmented by placing a single seed within each visible WMH, with identification of the WMH borders by the automated computer algorithm. The WMH borders can be adjusted as necessary by the user to correct errors such as misidentification of skull as WMH. WMH volumes were quantified using the spatial dimensions of the voxels in each MRI slice. Acute DWI lesions that were also visible on FLAIR were not included in these measurements. A trained medical student blind to all clinical data made all measurements. A test of inter-rater reliability on 10 scans showed an excellent correlation (intra-class correlation coefficient 0.99) between WMH volumes generated by the medical student compared to a neurologist with 10 years' experience in research in cerebral small vessel disease.
Figure 1. T2 FLAIR sequence shown in Quantomo.
(A) FLAIR before measurement. (B) FLAIR after WMH measurement. Purple regions represent WMH regions that were automatically selected by the computer, red regions represent manual traced regions. WMH regions that were overestimated were blocked out using the blocker tool (green region).
Statistical Analysis
All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). The results were expressed as the mean ± standard deviation or as median (interquartile range) where appropriate. Pearson correlation coefficient tests were used for continuous variables, Wilcoxon rank-sum tests for dichotomous variables, and the Kruskal-Wallis test for categorical variables. A p value < 0.05 was considered statistically significant. Percent WMH was first logarithmically transformed to achieve a normal distribution. To account for differences in baseline brain size, WMH were expressed as a percentage of total brain volume. Because the percent WMH was right skewed, we logarithmically transformed it to an approximately normal distribution for univariate analyses and multivariate modeling. Multivariable linear regression was used to determine whether OSA or hypoxemia were independent predictors of percent WMH. Because OSA and nocturnal hypoxemia represent inter-correlated aspects of abnormal sleep physiology after stroke, we built separate models for each risk factor rather than including both OSA and hypoxemia in the same model. Models were also adjusted for other known predictors of WMH including age, hypertension, and diabetes that were available in the dataset.
RESULTS
Study Population
One hundred twenty-four of the 510 patients in the CATCH study were enrolled. We excluded 5 patients who were unable to use the Remmers Sleep recorder and 10 patients with CSR. The remaining 109 patients comprised the final study population.
Of the 109 patients, 78 (72%) were male, mean age was 64.6 ± 12.7 years, 18% were diabetic, 64% hypertensive, and 15% were current smokers. Baseline NIHSS total was 0 in 35 (32%), 1 in 21 (19%), 2 in 39 (31%), and 3 in 19 (17%) patients. Patients underwent their level 3 sleep test a median of 76 days (25th percentile 39 days, 75th percentile 110 days) after their stroke or TIA event. Thirty-three (30%) patients had no OSA (RDI < 5), 42 (39%) had mild OSA (5 ≤ RDI < 15), 18 (17%) had moderate OSA (15 ≤ RDI ≤ 30), and 16 (15%) had severe OSA (RDI > 30). Thirty-seven (34%) patients were found to have nocturnal hypoxemia as previously defined. Higher RDI scores were moderately correlated with lower mean SpO2 (r = −0.41, p < 0.001) and higher duration of time with SpO2 < 90% (r = 0.54, p < 0.001). Median WMH volume was 4.23 mL (interquartile range, IQR, 1.77–9.37 mL) and median WMH volume as a percentage of total brain volume was 0.33% (IQR 0.14% to 0.71%). As expected, WMH volume as a percentage of brain volume was correlated with age (r = 0.69, p < 0.001) and was higher in patients with a history of hyper-tension (p < 0.001) and diabetes (p = 0.01)
Comparison between OSA and non-OSA Groups
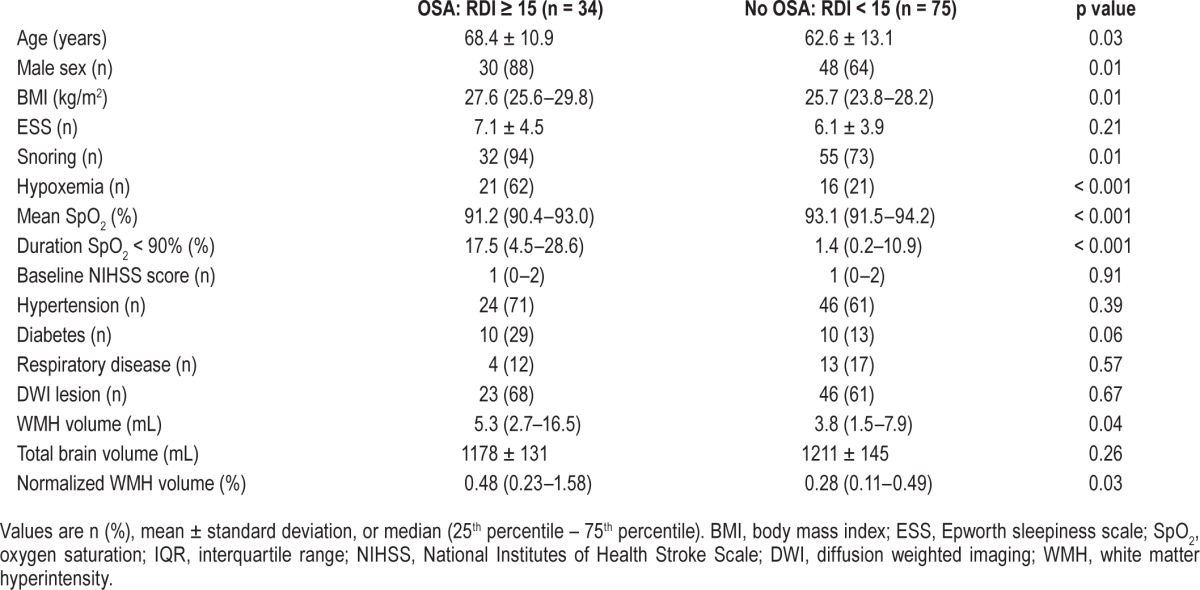
Patients with OSA were older, heavier, more frequently male, and had more severe nocturnal hypoxemia (Table 1). The prevalence of hypertension was high but not significantly different between the groups. The prevalence of diabetes was higher in those with OSA but did not reach statistical significance. Total WMH volume and WMH volume corrected for brain volume were significantly greater in the OSA group.
Table 1.
Characteristics of patients with and without OSA.
Comparison between Hypoxemic and Non-Hypoxemic Groups
Thirty-seven (34%) patients had nocturnal hypoxemia (Table 2) and were older, heavier, and more frequently male. Despite a higher prevalence of sleep apnea in the hypoxemic group, symptoms of sleep apnea including daytime sleepiness (reflected by the ESS), and snoring were not significantly different. WMH volumes (total volume or total as a percentage of brain volume) were significantly higher in the hypoxemic group but acute stroke lesions on diffusion weighted imaging (DWI) and total brain volume were similar in both groups.
Table 2.
Characteristics of patients with and without hypoxemia.

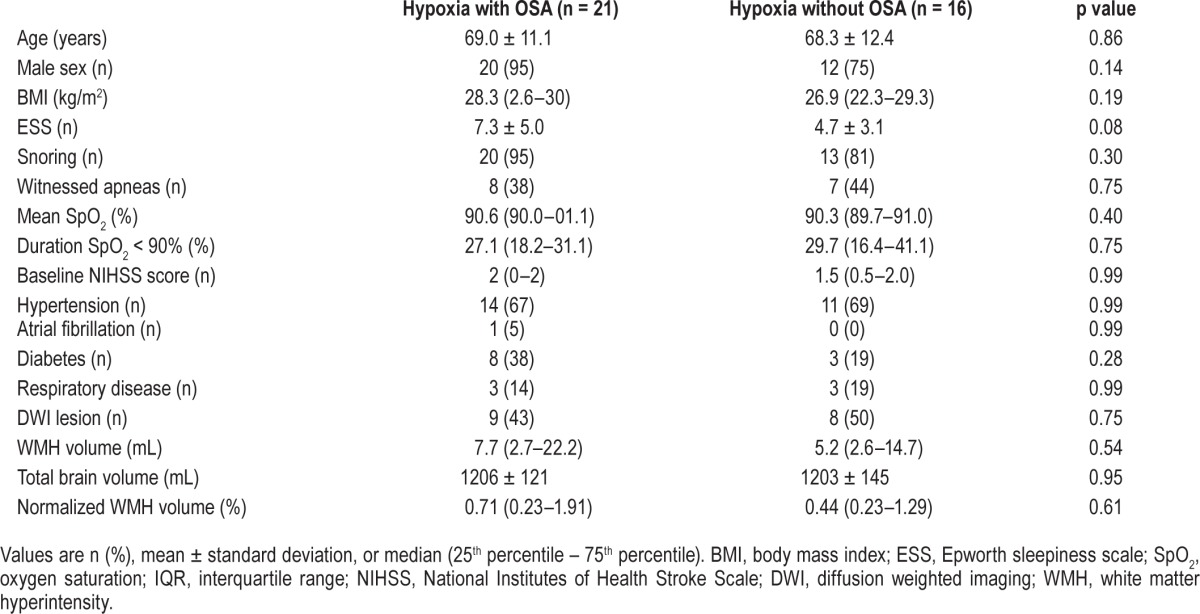
Comparison between Hypoxemic Patients with and without OSA
There were no differences in patient demographics, features of OSA, comorbidities, or brain volumes between hypoxemic patients with OSA and hypoxemic patients without OSA (Table 3).
Table 3.
Characteristics of hypoxemic patients with and without OSA.
Multivariable Analysis
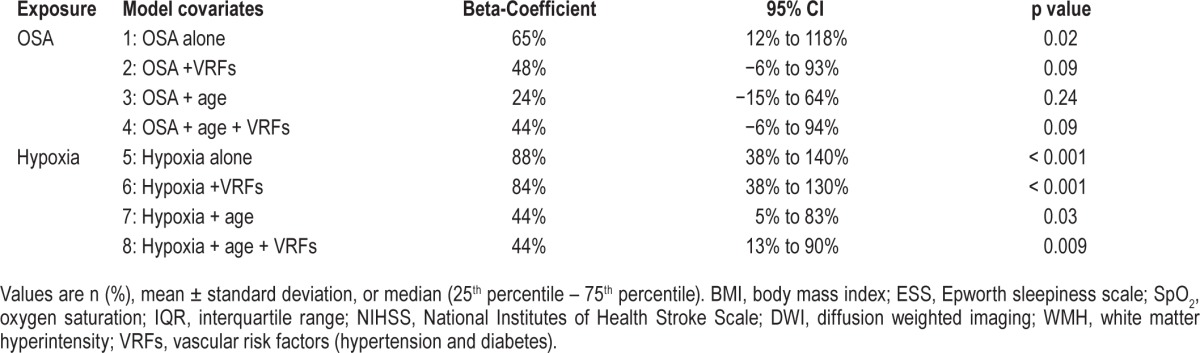
Multivariable models used to evaluate the association of OSA and nocturnal hypoxemia with WMH volume are shown in Table 4. Although OSA in isolation was significantly associated with WMH volume, this was no longer significant when adjusted for other vascular risk factors (hypertension and diabetes) and age. In contrast, patients with nocturnal hypoxemia had 44% higher WMH volume (95% CI 13% to 90%, p = 0.009) after adjustment for vascular risk factors and age.
Table 4.
Multivariable models of the association between OSA and hypoxemia and MRI white matter hyperintensity (WMH) volume.
DISCUSSION
We investigated the relationship of OSA and nocturnal hypoxemia with WMH by measuring both in an objective and blinded fashion in patients who had suffered a minor stroke or TIA. The median WMH burden in this population (4.23 cm3) was significantly higher than what has been reported in a recent population-based study of the same age (2.2 cm3),30 reflecting the increased prevalence of cerebrovascular disease in our patient cohort. Although we found that OSA was associated with an increased risk of WMH, this association lost statistical significance following adjustment for other vascular risk factors and age. In contrast, the association between nocturnal hypoxemia and WMH was significant and independent of hypertension, diabetes, and age.
Previous investigations of the relationship between OSA and WMH has shown mixed results with some finding a strong association,20,31 while others have not.4,32–34 These discrepancies are likely due to poor control for vascular risk factors and age,35 case mix differences (e.g. general population versus cohorts with large strokes), no assessment of hypoxemia, and diversity of methods and rating scales for quantifying WMH. One of the largest studies to find an independent association between OSA and WMH evaluated middle-aged to older aged adults in the general population.36 Our population was older and had overt cerebrovascular disease, which may explain the lack of an association between OSA and WMH when both age and vascular risk factors are controlled. Further, previous studies that showed a significant correlation between OSA and WMH recruited subjects with severe OSA or patients with major stroke.37 In contrast, our study population consisted entirely of patients who had suffered only a minor stroke or TIA and their prevalence of severe OSA was relatively low, which may have hindered our ability to find an association between OSA and WMH.
We found evidence of a significant association between hypoxemia and WMH even after adjustment for vascular risk factors. Most of the hypoxemia was due to OSA since the prevalence of lung disease was low and the nocturnal oximetry profiles did not raise any suspicion of nocturnal hypoventilation. Obstructive sleep apnea is known to increase sympathetic nervous system activity, oxidative stress, and systemic inflammation, ultimately leading to vascular complications including hypertension, coronary artery disease,38 and stroke.3 However, oxidative stress may have a direct effect on the brain independent of these vascular complications, since it has been shown to contribute to the pathogenesis of demyelination and tissue injury in multiple sclerosis.39 Further, an animal model of OSA has demonstrated that chronic intermittent hypoxia leads to cell damage and death in the cortex.40 Recently, white matter damage has been correlated with indices of systemic inflammation in patients with OSA, and a pathological study of aging brains has implicated hypoxia in the pathogenesis of WMH.41,42 Consequently, there are several mechanisms through which OSA may increase the risk of WMH. These possibilities are further supported by a recent study of two elderly patient cohorts, one in the community and the other recruited from patients who had suffered a “non-disabling stroke,” which highlighted that the etiology of WMH was predominantly related to non-vascular factors that remain to be identified.43
What are the potential clinical implications of these results? Although WMH may be an incidental finding in asymptomatic individuals, they increase with age and have been associated with cognitive impairment and dementia.42 Cognitive impairment is an important and common complication of OSA that may persist in some patients after effective treatment with CPAP.44 Further, OSA may be a risk factor for progression of mild cognitive impairment to dementia and poor sleep been implicated in the pathogenesis of Alzheimer disease.45 These findings raise the possibility that WMH may modulate some of the neurological complications of OSA.
It is important to distinguish between WMH and white matter integrity, which has also been evaluated in patients with OSA.46 The degree of white matter integrity can be inferred by MRI diffusion tensor imaging (DTI), which measures the degree and directionality of water proton diffusivity.47 In the presence of highly organized white matter tracts, water protons are freer to diffuse in parallel to the white matter fibres than perpendicular to the fibres. This directionality of diffusion can be measured mathematically using constructs such as fractional anisotropy. When white matter microarchitecture is disrupted, fractional anisotropy tends to decrease and the total mean diffusivity tends to increase. Fractional anisotropy is decreased in regions of WMH, and furthermore is decreased in regions of normal-appearing white matter that become WMH in the future.48 Therefore, decreased white matter integrity precedes WMH and may be a precursor to WMH. In one trial, CPAP therapy restored more normal white matter integrity in OSA patients, suggesting that CPAP might also prevent WMH although this has not yet been evaluated in clinical trials.
We chose to use level 3 diagnostic sleep monitoring rather than polysomnography (PSG) for several reasons. Firstly, we have used level 3 monitoring extensively over the past several years both for clinical purposes and research studies. As such, we have considerable experience with this methodology, including its strengths and limitations. The main limitations of level 3 monitoring include underestimation of sleep disordered breathing and nocturnal hypoxemia due to prolonged wakefulness, loss of respiratory signals due to monitoring equipment being disconnected, and poor signal quality due to the fact that the test is not attended by technical staff. We believe that the criteria we used for technically acceptable monitoring curtailed the potential impact of these limitations on our results. Secondly, we felt that performing an ambulatory sleep study in the home would be more acceptable to patients and would improve our recruitment. Finally, provision of ambulatory monitoring enabled us to avoid the delay we would have encountered with limited access to PSG.
The strengths of our study include the semi-automated volume measurement of WMH, which provided a quantitative and objective measurement of WMH. Previous studies used visual rating scales, which are subjective and generally less reliable than quantitative volumetric measurements. Our study also has limitations, which should be addressed. Firstly, although our sample size is larger than most of the observational studies to date, our relatively small study population limited our analysis and may have precluded additional findings. Secondly, our cohort was not representative of the general population since all patients had a recent TIA or minor stroke. Although this limits the generalizability of our findings, our results are highly relevant for this population, which has a high risk of recurrent cerebrovascular disease.49 Finally, we have shown an association between nocturnal hypoxemia and white matter disease but cannot infer causality from our data. We do not believe that OSA was secondary to neurological disease, since the latter was mild and had resolved in the majority of patients by the time the sleep study was performed.
In summary, we found that nocturnal hypoxemia, which was predominantly due to OSA, was associated with WMH independent of other risk factors such as hypertension, diabetes, and age. This implies that nocturnal hypoxemia, which is often clinically silent, contributes to the pathogenesis of WMH, and should be considered as part of the spectrum of cerebrovascular complications of OSA. Future research is required to determine the physiological and cognitive impacts of WMH in patients who present with TIA and minor stroke, and whether treatment of OSA and nocturnal hypoxemia improves clinical outcomes such as cognitive function and stroke recurrence.
DISCLOSURE STATEMENT
This study was funded by Canadian Institute of Health Research (CIHR) and a Pfizer Cardiovascular research award. Dr. Coutts, received salary support from Alberta-Innovates-Health Solutions and the Heart and Stroke Foundation of Canada's Distinguished Clinician Scientist award, supported in partnership with the Canadian Institute of Health Research (CIHR), Institute of Circulatory and Respiratory Health and AstraZeneca Canada Inc. Dr. Patel received funding from a Canadian Stroke Network summer studentship. Dr. Smith received salary support from Alberta-Innovates-Health solutions and the Canadian Institutes of Health Research. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- BMI
body mass index
- CATCH
CT and MRI in the triage of TIA and minor cerebrovascular events to identify high-risk patients
- CPAP
continuous positive airway pressure
- CSR
Cheyne-Stokes respiration
- CT
computed tomography
- DTI
diffusion tensor imaging
- DWI
diffusion weighted imaging
- ESS
Epworth Sleepiness Scale
- FLAIR
fluid-attenuated inversion recovery
- MRI
magnetic resonance imaging
- mRS
modified Rankin score
- NIHSS
National Institutes of Health Stroke Scale
- OSA
obstructive sleep apnea
- PSG
polysomnography
- RDI
respiratory disturbance index
- SPGR
spoiled gradient-recalled echo
- SpO2
oxyhemoglobin saturation
- TIA
transient ischemic attack
- WMH
white matter hyperintensities
REFERENCES
- 1.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 3.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 4.Davies CW, Crosby JH, Mullins RL, et al. Case control study of cerebrovascular damage defined by magnetic resonance imaging in patients with OSA and normal matched control subjects. Sleep. 2001;24:715–20. doi: 10.1093/sleep/24.6.715. [DOI] [PubMed] [Google Scholar]
- 5.Pialoux V, Hanly PJ, Foster GE, et al. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med. 2009;180:1002–9. doi: 10.1164/rccm.200905-0671OC. [DOI] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Christiansen P, Larsson HB, Thomsen C, Wieslander SB, Henriksen O. Age dependent white matter lesions and brain volume changes in healthy volunteers. Acta Radiol. 1994;35:117–22. [PubMed] [Google Scholar]
- 9.Chan W, Coutts SB, Hanly P. Sleep apnea in patients with transient ischemic attack and minor stroke: opportunity for risk reduction of recurrent stroke? Stroke. 2010;41:2973–5. doi: 10.1161/STROKEAHA.110.596759. [DOI] [PubMed] [Google Scholar]
- 10.Arzt M, Young T, Peppard PE, et al. Dissociation of obstructive sleep apnea from hypersomnolence and obesity in patients with stroke. Stroke. 2010;41:e129–34. doi: 10.1161/STROKEAHA.109.566463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke. 1986;17:1084–9. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- 12.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–3. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 13.Junque C, Pujol J, Vendrell P, et al. Leuko-araiosis on magnetic resonance imaging and speed of mental processing. Arch Neurol. 1990;47:151–6. doi: 10.1001/archneur.1990.00530020047013. [DOI] [PubMed] [Google Scholar]
- 14.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 15.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–51. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.van Harten B, Oosterman JM, Potter van Loon BJ, Scheltens P, Weinstein HC. Brain lesions on MRI in elderly patients with type 2 diabetes mellitus. Eur Neurol. 2007;57:70–4. doi: 10.1159/000098054. [DOI] [PubMed] [Google Scholar]
- 17.van Swieten JC, Kappelle LJ, Algra A, van Latum JC, Koudstaal PJ, van Gijn J. Hypodensity of the cerebral white matter in patients with transient ischemic attack or minor stroke: influence on the rate of subsequent stroke. Dutch TIA Trial Study Group. Ann Neurol. 1992;32:177–83. doi: 10.1002/ana.410320209. [DOI] [PubMed] [Google Scholar]
- 18.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–9. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi K, Kario K, Hoshide S, Ishikawa J, Morinari M, Shimada K. Nocturnal hypoxia is associated with silent cerebrovascular disease in a high-risk Japanese community-dwelling population. Am J Hypertens. 2005;18:1489–95. doi: 10.1016/j.amjhyper.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Nishibayashi M, Miyamoto M, Miyamoto T, Suzuki K, Hirata K. Correlation between severity of obstructive sleep apnea and prevalence of silent cerebrovascular lesions. J Clin Sleep Med. 2008;4:242–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Ding J, Nieto FJ, Beauchamp NJ, Jr., et al. Sleep-disordered breathing and white matter disease in the brainstem in older adults. Sleep. 2004;27:474–9. doi: 10.1093/sleep/27.3.474. [DOI] [PubMed] [Google Scholar]
- 22.Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke. 2012;43:1013–7. doi: 10.1161/STROKEAHA.111.637421. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Issa FG, Morrison D, Hadjuk E, Iyer A, Feroah T, Remmers JE. Digital monitoring of sleep-disordered breathing using snoring sound and arterial oxygen saturation. Am Rev Respir Dis. 1993;148:1023–9. doi: 10.1164/ajrccm/148.4_Pt_1.1023. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez JC, Tsai WH, Flemons WW, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302–7. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flemons J BD, Redline S, et al. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;1:667–89. [PubMed] [Google Scholar]
- 27.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 28.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosior JC, Idris S, Dowlatshahi D, et al. Quantomo: validation of a computer-assisted methodology for the volumetric analysis of intracerebral haemorrhage. Int J Stroke. 2011;6:302–5. doi: 10.1111/j.1747-4949.2010.00579.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith EE, O'Donnell M, Dagenais G, et al. Early cerebral small vessel disease and brain volume, cognition, and gait. Ann Neurol. 2015;77:251–61. doi: 10.1002/ana.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kepplinger J, Barlinn K, Boehme AK, et al. Association of sleep apnea with clinically silent microvascular brain tissue changes in acute cerebral ischemia. J Neurol. 2014;261:343–9. doi: 10.1007/s00415-013-7200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hentschel F, Schredl M, Dressing H. [Sleep apnea syndrome and cerebral lesions--a prospective MRI study] Fortschritte der Neurologie-Psychiatrie. 1997;65:421–4. doi: 10.1055/s-2007-996347. [DOI] [PubMed] [Google Scholar]
- 33.Kiernan TE, Capampangan DJ, Hickey MG, Pearce LA, Aguilar MI. Sleep apnea and white matter disease in hypertensive patients: a case series. Neurologist. 2011;17:289–91. doi: 10.1097/NRL.0b013e31821a25d6. [DOI] [PubMed] [Google Scholar]
- 34.Schulz UG, Gruter BE, Briley D, Rothwell PM. Leukoaraiosis and increased cerebral susceptibility to ischemia: lack of confounding by carotid disease. J Am Heart Assoc. 2013;2:e000261. doi: 10.1161/JAHA.113.000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho ER, Kim H, Seo HS, Suh S, Lee SK, Shin C. Obstructive sleep apnea as a risk factor for silent cerebral infarction. J Sleep Res. 2013;22:452–8. doi: 10.1111/jsr.12034. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Yun CH, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep. 2013;36:709–15B. doi: 10.5665/sleep.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harbison J, Gibson GJ, Birchall D, Zammit-Maempel I, Ford GA. White matter disease and sleep-disordered breathing after acute stroke. Neurology. 2003;61:959–63. doi: 10.1212/01.wnl.0000086818.57992.b8. [DOI] [PubMed] [Google Scholar]
- 38.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 39.Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;251:261–8. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 40.Xu W, Chi L, Row BW, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–23. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 41.Chen HL, Lu CH, Lin HC, et al. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep. 2015;38:361–70. doi: 10.5665/sleep.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wharton SB, Simpson JE, Brayne C, Ince PG. Age-associated white matter lesions: the MRC Cognitive Function and Ageing Study. Brain Pathol. 2015;25:35–43. doi: 10.1111/bpa.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wardlaw JM, Allerhand M, Doubal FN, et al. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology. 2014;82:1331–8. doi: 10.1212/WNL.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36:1297–305. doi: 10.5665/sleep.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014;27:478–83. doi: 10.1097/YCO.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 47.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–48. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 48.de Groot M, Verhaaren BF, de Boer R, et al. Changes in normal-appearing white matter precede development of white matter lesions. Stroke. 2013;44:1037–42. doi: 10.1161/STROKEAHA.112.680223. [DOI] [PubMed] [Google Scholar]
- 49.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–6. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]



