Abstract
Aim
To review the recent evolution of spine SBRT with emphasis on single dose treatments.
Background
Radiation treatment of spine metastases represents a challenging problem in clinical oncology, because of the high risk of inflicting damage to the spinal cord. While conventional fractionated radiation therapy still constitutes the most commonly used modality for palliative treatment, notwithstanding its efficacy in terms of palliation of pain, local tumor control has been approximately 60%. This limited effectiveness is due to previous lack of technology to precisely target the tumor while avoiding the radiosensitive spinal cord, which constitutes a dose-limiting barrier to tumor cure.
Materials and methods
A thorough review of the available literature on spine SBRT has been carried out and critically assessed.
Results
Stereotactic body radiotherapy (SBRT) emerges as an alternative, non-invasive high-precision approach, which allows escalation of tumor dose, while effectively sparing adjacent uninvolved organs at risk. Engaging technological advances, such as on-line Cone Beam Computed Tomography (CBCT), coupled with Dynamic Multi-Leaf Collimation (DMLC) and rapid intensity-modulated (IMRT) beam delivery, have promoted an interactive image-guided (IGRT) approach that precisely conforms treatment onto a defined target volume with a rapid dose fall-off to collateral non-target tissues, such as the spinal cord. Recent technological developments allow the use of the high-dose per fraction mode of hypofractionated SBRT for spinal oligometastatic cancer, even if only a few millimeters away from the tumor.
Conclusion
Single-dose spine SBRT, now increasingly implemented, yields unprecedented outcomes of local tumor ablation and safety, provided that advanced technology is employed.
Keywords: SBRT, SRS, Single-fraction, Stereotactic radiotherapy, Radiosurgery, IGRT
1. Background
Skeletal metastases are a common complication of cancer which occurs in up to 40% of oncological patients.1 Disease histology most often metastatic to the spine includes the breast, lung, prostate, and renal cell carcinomas. It has been estimated that approximately 10% of cancer patients will develop spine metastases and their management represents one of the more vexing clinical problems in clinical oncology. Treatment options include pain management with narcotics, steroids, radiation therapy, surgery, or combinations of the above. Surgery is usually indicated in patients who present with a good performance status and acute neurological impairment, requiring decompression and stabilization of the spine. For unfit patients, or those with a mechanically stable spinal metastasis with or without malignant cord compression, conventional fractionated radiation therapy has been, so far, the mainstay of the palliative management. Although the issue of dose–pain response has not been fully established yet, several prospective studies indicate that a single dose of 8–10 Gy is equivalent to the classically used regimen of fractionated 30 Gy in 10 fractions.2, 3, 4, 5 However, long-term control of symptoms, even for favorable histologies is, at best, approximately 60%, with a median duration of palliation of approximately 4 months.6, 7
The limited effectiveness of fractionated radiotherapy is inherent to the technological restrictions of this approach. A fundamental issue is the uncertainties in targeting deep-seated tumors, conventionally guided by skin markings established during the simulation procedure. Small daily variations in the location of the skin markings relative to the target mandates the use of expanded safety margins around the tumor to avoid geographical tumor miss. Such safety margins frequently capture significant portions of the spinal cord, which restricts the treatment dose to levels lower than required for tumor ablation.8, 9 The technological revolution of image-guided treatment delivery systems, entailing new abilities to visualize the tumor during treatment with on-board imaging and to employ computer-driven technology to re-adjust tumor targeting on-line have promoted new standards of tumor ablation with ionizing radiation. It is now possible to achieve an extreme precision of target coverage with tumoricidal dose levels, while sparing the cord even if only a few millimeters away from the tumor. The ability to “visually” dissect the tumor from adjacent normal tissues with radiation beams has promoted and accelerated the implementation of new applications to ablate human tumors, such as with hypofractionated SBRT.
The use of this approach has expanded in the past decade to the management of patients with oligometastatic presentations (defined as initial 1–5 synchronous lesions), which appear to have a potential for long-term survival if the metastatic lesions are therapeutically controlled. Oligometastatic disease has been postulated to represent a biologically distinct phenotype with limited spread, constituting an intermediate phase in the pathway of metastatic transformation preceding the acquisition of a potential for widespread dissemination by metastatogenic tumor clonogens.10, 11 Large surgical series have shown long-term disease-free survival of ≥20% at 10–20 years following resection of oligometastatic deposits in the liver or lung, and several prospective clinical studies, currently underway, have been designed to test the curative potential of high-dose SBRT instead of surgery.12 The strict criteria employed to ablate oligometastatic tumor deposits by SBRT accommodate the special needs of local tumor control in the spine, critical for prevention of cord compression by tumor extension into the epidural space. The purpose of this review is to summarize the growing body of literature on spine SBRT with specific emphasis on the emerging new approach with single dose IGRT, an SBRT application that has attracted a great deal of attention because of its unique contribution to the quality of care in this clinical setting.
2. Technical and dosimetric considerations in spine SBRT
SBRT was developed in the mid-1990s using concepts that had been already established in the treatment of intracranial lesions with stereotactic radiosurgery (SRS). The first description of a linear-accelerator-based spinal SBRT was published in 1995 by Hamilton et al.13 Transition to linear accelerators has been critical, as it enabled the use of treatment delivery tools not available on SRS units to conform to complex target shapes and organ mobility. Indeed, the implementation of new beam shaping and image guidance technologies have rapidly enhanced the sophistication and efficacy of SBRT in reducing safety margins while precisely conforming to the three-dimensional tumor outline. On-line Cone Beam Computed Tomography (CBCT), Dynamic Multi-Leaf Collimation (DMLC), rapid intensity-modulated (IMRT) beam delivery with flattening Filter-Free (FFF) beams and on-line tracking tools have promoted new standards of local tumor cure with on-line image guided radiotherapy (IGRT).
A critical element in implementing SBRT for spine tumors has been the progressive accumulation of data on dose-volume radiation tolerance of the spinal cord. Data from conventional fractionation regimens (1.8–2.0 Gy per fraction) indicate that spinal cord tolerance may be higher than previously appreciated, with a risk of myelopathy of <1% at 54 Gy and of 10% at 61 Gy.14 However, an exponential increase in the risk of cord damage occurs as the dose per fraction increases and the number of fractions is reduced, such as practiced in the delivery of high dose SBRT. Information regarding the dose tolerance of the spinal cord to high-dose radiation fractions is beginning to emerge. Macbeth et al., analyzing data from three randomized trials of palliative radiotherapy for non-small cell lung cancer, reported 0% (0/114) risk of collateral radiation-induced myelitis when the dose to the spine was 10 Gy in a single fraction.15 While the estimated cumulative risk of myelopathy at 2 years was 2.2% in 524 patients treated with 17 Gy in two fractions. Other studies have suggested that the spinal cord can tolerate at least a single dose of 10 Gy to 10% of the cord segment that is adjacent to a targeted tumor plus 6 mm above and below this segment, with a risk of radiation-induced myelopathy of <0.5%.16 Sahgal et al. reported Dose-Volume Histograms (DVH) of nine patients who developed radiation myelopathy post-SBRT and compared them with a cohort of 66 spine SBRT patients who did not develop the toxicity.17 A logistic regression model was developed to establish the probability of myelopathy using the thecal sac point maximum (Pmax) volume. A risk of ≤5% was observed when limiting the thecal sac Pmax volume dose to 12.4 Gy in a single fraction, 17.0 Gy in 2 fractions, 20.3 Gy in 3 fractions, 23.0 Gy in 4 fractions, and 25.3 Gy in 5 fractions.
Despite a reasonably accurate spatial precision in tumor targeting with the use of modern technology, minute deviations during spine SBRT may translate into significant dosimetric perturbations during spine SBRT because of extremely steep dose gradients in the context of a close proximity between the target and the spinal cord. The advent of automated robotic couches with six degrees of freedom (6DoF) enables rapid on-line target repositioning following CBCT, improving the accuracy target coverage and spinal cord avoidance. An analysis of set-up inaccuracies during spine irradiation has documented translational errors of up to 1 mm with a vector of 1.8 ± 1.0 mm and rotational errors of up to 1.6 ± 1.3° which may yield up to 18% errors in D(95).18 Clearly, the accurate detection and appropriate correction of such inconsistencies are of utmost importance for the safety of the treatment.
3. Rationale of single dose versus hypofractionated regimens
Numerous published series of spine SBRT have used a variety of schedules, but a clear-cut definition of the optimal number of sessions, fraction size and total dose are still lacking. A recent pooled analysis of published series using both single fraction at various dose levels and multi-fraction regimens (up to 5 sessions) entailed 1775 lesions in 1388 patients.19 Follow-up was mostly short-termed (<18 months in 12/15 studies) yielding an overall local control rate of 90% and a risk of radiation myelopathy of 0.4%, with no apparent differences in early outcomes between single dose and 3–5 fraction of SBRT. Prospective randomized trials are few and largely still underway. The RTOG 0631 prospective phase III trial compares 8 Gy with 16 Gy in single fractions to establish dose level effect on pain.20 A prospective randomized phase III trial at Memorial Sloan Kettering Cancer Center (MSKCC) in New York compares 24 Gy in a single fraction versus 3 sessions of 9 Gy (total 27 Gy) in effecting durable local control in oligometastatic tumors, including oligometastatic spine disease (MSKCC 10–154). This study targets 200 patients, and spine lesions are eligible if the cord is >3 mm away from the edge of the planned target volume (PTV). The spinal cord constraint is defined as a point Dmax of 15 Gy to the organ based on MRI segmentation.
While prospective studies comparing the most effective single dose versus hypofractionated SBRT options are not available, retrospective analyses have provided an insight into this issue. An apparent advantage for ultra-high single dose SBRT was recently reported by Folkert et al. in patients treated for spine and para-spinal metastatic sarcoma.21 Soft tissue and bone sarcomas are notoriously radio-resistant by classical radiobiological ranking, and conventional radiation treatment (20–40 Gy at 2–5 Gy per fraction) is sub-curative, resulting in low rates of local control. In this series, 88 patients with 120 discrete metastases received hypofractionation (3–6 fractions; median dose, 28.5 Gy) or single fraction (median dose, 24 Gy). With a median follow-up time of 12.3 months, the one-year local control rate for the whole group was 87.9%, with single dose patients showing superior local control of 90.8% versus 84.1% for hypofractionation (p = .007). It should be noted that the single dose levels (median 24 Gy, range 18–24 Gy) were at the high end of therapeutically feasible range, while the fractionated dose levels (median 28.5 Gy, range 24–36 Gy) were at the intermediate, arguably less effective, range.
The issue of dose level is central to the evaluation of SBRT outcome. A dose escalation study of single dose SBRT for oligometastatic lesions in non-spinal locations has been reported from MSKCC.22 At all dose levels >90% of relapses occurred within 24 months post-treatment. A steep curve of actuarial local control at ≥36 months by treatment dose was observed, with 25% local cures at the dose range of 18–20 Gy, 69% at 21–22 Gy, and 88% in tumors receiving the 23–24 Gy range (p < 0.05). Yamada et al. from MSKCC showed excellent response rates at high doses in patients treated exclusively to the spine, reporting a 95% actuarial local control at 3 years for 67 lesions treated with 24 Gy, compared to 80% in 36 lesions treated with 18–23 Gy.23 The high rate of local control with ultra-high single doses was validated in an independent series at the Champalimaud Centre of the Unknown (CCU) in Lisbon, observing a 94% 2-year actuarial control in 235 oligometastatic lesions receiving 24 Gy SBRT. Interestingly, all of the above studies showed that at high dose, all tumor types, whether sensitive or resistant to classical fractionated radiotherapy, responded with ∼90% local cure, a phenomenon that challenges classical tenets of clinical radiobiology.
Similar patterns of local cure were, however, observed with hypofractionated SBRT schemes when high dose per fraction was used. McCammon et al. reported a 3-year actuarial local control rate of 89.3% in 105 primary or metastatic lesions to the lung and liver treated with 3 fractions of 18–20 Gy, compared with 59.0% in 59 lesions treated with 3 fractions of 14–16 Gy and 8.1% in 82 lesions exposed to ≤12 Gy per fraction in 3 sessions.24 However, while hypofractionation does provide equivalent local control to single-dose SBRT, a high dose per fraction, exceeding 18 Gy, is required. In this context, it should be noted that the AAPM guidelines for SBRT dose constraints for critical normal organs has identified 14–15 Gy as an approximate common threshold for a maximal safe point dose to the so-called serial normal tissues, which include the CNS tissues.25 Hence, the delivery of 3 fractions of 16–20 Gy requires the same elaborate and diligent IGRT-based treatment delivery at each fraction as for single-dose 24 Gy treatment, to avoid normal tissue toxicity. Ultra-high single dose should, therefore, be regarded as more cost-effective and potentially safer than hypofractionated SBRT in achieving an iso-therapeutic effect. It is for this reason that perspectives of single dose SBRT have attracted much attention and experimental activity in the search for optimizing the treatment approach in metastatic disease of the spine.
4. Outcomes of single dose SBRT for spine tumors
The two main therapeutic targets of single dose SBRT for spine metastases, namely pain control and spine stabilization to avoid cord compression, have each been explored in series of phase I and II studies (Table 1), but outcome data of phase III studies are not available as of yet. While a dose escalation study for durable tumor control has established a dose response relationship,23 a similar relationship for pain control has not been fully established. A study from the Henry Ford Hospital in Detroit reported a trend for improved pain relief with higher radiation doses.26 In this study, 61 spinal lesions in 49 patients were treated with escalating doses (2 Gy increments) within the range of 10–16 Gy (6–20 lesions in each dose bin), delivered to the involved spinal segment alone. Pain relief was evaluated using a verbal/visual analog scale, with pain intensity description ranging from zero (no pain) to 10 (the worst imaginable pain). A univariate analysis failed to show a significant decrease in pain score with dose, although a strong trend in this direction was observed at doses ≥14 Gy. Overall, pain relief, when achieved, was rapid, and at 4 weeks a complete pain relief was documented in 37.7%, partial relief in 47.6%, and worsening of pain in 1.6%. The median duration of pain relief was 13.6 months, and pain control was durable at one year in 84%. The 1-year survival in this group of patients was 74.3%, and there was no evidence for neurological toxicity.
Table 1.
Phase I and II studies assessing single dose stereotactic irradiation to the spine.
| Author, year | No. patients/no. lesions | Prior RT | Dose/coverage | Constraints/dose to spinal cord | Histology | Median F/U (months) | Local control |
|---|---|---|---|---|---|---|---|
| Gerszten et al., 2005 | 50/68 | 48/68 | 12.5–22.5 Gy (mean, 19 Gy)/80% IDL | 13 Gy (max. dose actually received) | Breast | 16 | 100% |
| Gerszten et al., 2005 | 28/36 | 23/36 | 17.5–25 Gy (mean, 21.9 Gy)/80% IDL | 13.1 Gy (max. dose actually received) | Melanoma | 13 | 93% |
| Gerszten et al., 2006 | 77/87 | 70/87 | 15–25 Gy (mean, 20 Gy)/80% IDL | 12 Gy (max. dose actually received) | Lung | 16 | 100% |
| Gerszten et al., 2007 | 393/500 | 344/500 | 12.5–25 Gy (mean, 20 Gy)/80% IDL | NR (mean volume of spinal canal dose >8 Gy 0.6 cm3 | Mixed | 21 | 88% |
| Yamada et al., 2008 | 93/103 | 0/103 | 18–24 Gy/100% IDL | 14 Gy (max. dose) | Mixed | 15 | 90% |
| Amdur et al., 2009 | 21/25 | 12/25 | 15 Gy/95% PTV | ≤12 Gy to 0.1 mL (no previous RT) ≤5 Gy to 0.5 mL (if previous RT) |
Mixed | 8 | 95% |
| Yamada et al., 2011 | 412/362 | 0/363 | 18–24 Gy/100% IDL | 14–15 Gy (max point dose) | Mixed | 36 | 90% (98% in breast and prostate) |
| Garg et al., 2012 | 61/63 | 0/63 | 16–24 Gy/80–90% | ≤10 Gy to 0.01 cm3 ≤12 Gy (spinal cord + 2 mm) |
Mixed (renal versus non-renal) | 19.7 | 88% |
| Ryu et al., 2014 | 39/NR | NR | 16 Gy/90% PTV (accepted >80%) | ≤10% partial spinal cord, max 10 Gy ≤0.35 cm3 absolute spinal cord, max 10 Gy |
NR (no exclusion criteria for histology) | NR | NR |
IDL, isodose line.
The most extensive study of pain relief by single dose SBRT was reported by Gerszten et al.27 Metastatic tumors (500 lesions in 393 patients) with multiple histologies were treated with single doses of 12–22.5 Gy, prescribed to 80% of isodose surface (mean 20 Gy). Exclusion criteria were evidence of overt spinal instability and/or neurological deficit resulting from bony compression of neural structures. Maximum doses to the spinal cord and cauda equina (spinal canal) were 1.3–13.1 Gy (mean 8.6 Gy) and 1.1–13.3 Gy (mean 9 Gy), respectively. Sixty-nine percent of the lesions were re-irradiated to relieve pain relapsing after previous conventional low dose radiotherapy. Improvement of pain symptoms was defined as a benefit of at least 3 points on the 10-point visual analog scale. At a median follow-up of 21 months, (range, 3–53 months), sustained pain relief was observed in 86%. There were no neurological toxicities attributable to treatment. Long-term local control, using CT criteria, was achieved in 88% of all treated lesions. The likelihood of tumor control was affected by histology, with the breast and lung being the most radio-responsive (100% local control), while renal cell cancer and melanoma exhibited 87% and 75% local control rates, respectively. Subgroups of this cohort were also reported separately. Ninety-six percent of 68 spinal lesions in 50 breast cancer patients experienced pain relief (mean 5 points on the visual scale) at a median follow-up of 16 months (range 6–48), with no apparent acute or sub-acute neurological toxicity.28 Similarly, long-term pain improvement and no acute or sub-acute neurological toxicities were reported in 77 lung cancer patients.29 The majority of tumors in this series were non-small cell cancers (79/87, 91%), and 80% (70/87) received re-irradiation of lesions relapsing after previous conventional low dose radiotherapy. Patients with metastatic melanoma (36 lesions in 28 patients) also entertained excellent pain relief (96%), with a mean improvement of 7 points in a 10-point visual scale, and no recorded neurological toxicities.30
While the above studies combined demonstrate the efficacy of pain relief by the low and intermediate range of clinically feasible single dose SBRT, without risk of cord damage, the issue of pain relief dependence on dose has, nonetheless, not been resolved. The RTOG 0631 trial has been designed to address this issue, exploring in a prospective randomized phase III trial pain relief with single dose 8 Gy versus 16 Gy.20 An initial phase II study was designed to establish a baseline technical uniformity in accurate and reproducible delivery of 16 Gy single dose SBRT within the institutions participating in this cooperative group study, with rigorous quality control requirements. The phase II base-line study was completed with 44 patients with 1–3 spine metastases with a Numerical Rating Pain Scale (NRPS) score ≥5 accrued and treated with 16 Gy. The designed targeting accuracy ≤2 mm, target volume coverage of >90% of prescription dose, and spinal cord dose constraints (10 Gy to ≤10% of the cord volume from 5–6 mm above to 5–6 mm below the tumor, or absolute spinal cord volume <0.35 cm3) and other normal tissue dose constraints were achieved with high compliance. Eleven patients had grade 1–2 adverse events; however the majority of these were unrelated to treatment. One grade 3 cervical pain was identified as an acute adverse event associated with SBRT, but there were no other acute treatment-related toxicities. The phase III arm of the study has now been activated with an anticipated accrual of 240 patients.
While pain relief appears to be responsive to single dose SBRT, contributing significantly to alleviation of one of the more deleterious complications of cancer metastases to the spine, there is increasing awareness of the need to achieve durable local control, as improvements in systemic treatment are leading to an ever growing proportion of metastatic patients that live longer. There is a growing body of compelling evidence that spine SBRT provides durable tumor control that is far superior to that achievable with conventional radiation therapy. Studies of this notion were pioneered by Yamada et al.23 An early report on the treatment of 103 lesions in 93 patients included only radiotherapy-naive lesions that were not subject to prior surgical treatment. Exclusion criteria included high-grade epidural cord compression and mechanical instability. Whenever an involvement of the vertebral body was present, the CTV included the entire body. Planning target volume consisted of the CTV plus a 2 mm margin with avoidance of the spinal cord. Dose was prescribed to the 100% isodose line and ranged between 18 and 24 Gy (median 24 Gy). A maximum point dose of 14 Gy was used as spinal cord constraint. Patients were assessed at 3–4 months intervals. At 15 months median follow-up an actuarial local control rate of 90% was observed. Interestingly, patients who experienced local failure had a median time to failure of only 9 months from treatment time (range 5–13 months). Median overall survival was 15 months (range 6–36). On multivariate analysis, no statistically significant differences in overall survival or local control were found when stratifying the series by histology of the primary disease. However, there was a significant dose effect when comparing low dose (18–23 Gy) with high dose (24 Gy), with actuarial local control rates of 80 and 95%, respectively (p = 0.03). A recent and expanded update of this series, which now includes 412 lesions in 362 patients, confirms >90% 3-year local control in all histologies, reaching levels of 98% in breast, GI, lung and prostate tumors.31 The 3-year cumulative incidence of local recurrence in the 24 Gy group (333 lesions) is 2.4%, compared with 10.4% in the 16–23 Gy cohort (80 lesions) (p < 0.001). The median time to recurrence has not changed, with only 2/6 patients in the 24 Gy group recurring at ≥2 years. At a median follow-up of 16 months (range 2–78), 91% of lesions treated with 24 Gy show a persistent local control, 212/362 (59%) patients are alive without local relapse and the other 132 (36%) died without local relapse.
A more limited phase I and II study was reported by Garg et al.32 A total of 63 metastases in 61 patients were treated to a peripheral dose of 16–24 Gy in a single fraction. Mean follow-up was 19.7 months (range, 1.2–52.1 months; median 17.8 months). CTV was defined as the GTV plus contiguous bone marrow space with no margin for PTV. Dose prescription varied according to location (spinal versus para-spinal) and histology (renal versus non-renal). In non-renal spinal metastases, 18 Gy was prescribed to the GTV with a mean of 16 Gy to the CTV; in renal vertebral lesions the doses were 24 and 18 Gy to the GTV and CTV, respectively. The spinal cord was not to receive more than 10 Gy to more than 0.01 cm3, and maximum allowed dose to the spinal cord plus 2 mm was 12 Gy. The 18-months actuarial local control in this cohort was 88%. Interestingly, pain level at 6 months turned out to be predictive of local control: patients with pain level ≥4 fared worse compared to those with less pain (p = 0.01). Neurological function preservation correlated with overall survival (p < 0.01). None of the patients had neurological deterioration attributable to progression at the treated site. There were, however, 2 patients who experienced high-grade neurological toxicity (1 myelopathy, 1 radiculopathy).
Taken together, this series of publications demonstrated the safety and high efficacy of single dose SBRT in providing a durable local tumor control and prevention of direct or indirect cord compression by tumor growth. The high end of the dose range tested appears to be required to assure >90% success, and 24 Gy has consistently yielded this rate of local control even in classical radioresistant phenotypes, such as melanoma and renal cell carcinoma. This is in stark contrast with fractionated radiotherapy data, suggesting a different mechanism of tumor stem cell kill. In a retrospective analysis of 105 cases of metastatic renal cell carcinomas treated (59 spinal lesions) with either hypofractionated or high dose IGRT (dose range 18–24 Gy), Zelefsky et al. confirmed a strong predictive value of high prescription dose.33 The overall 3-year actuarial local progression-free survival for all lesions was 44%. The 3-year local control for high single-dose (24 Gy), low single-dose (<24 Gy), and hypofractionation were 88%, 21%, and 17%, respectively (high single dose versus low single dose, p = 0.001).
The assessment of treatment response is typically based on imaging and long-term follow-up. Early predictive indicators on success of treatment are highly desired, since information would provide an indication for adjuvant treatment in patients with a high risk of local failure. A recent approach to address this issue has been to measure the magnitude of change in metabolic uptake of FDG (ΔSUV) as detected by positron emission measurements post-single fraction as predictive of long-term freedom from relapse.34 The use of this biomarker is extensively studied at CCU as an adjunct to efforts in the implementation of state-of-the-art high-precision IGRT techniques. To date, 51 metastatic spine lesions have been treated with SD-IGRT with doses ranging between 16 and 24 Gy (mean, 20.5 Gy, median, 24 Gy). The most prevalent histologies were NSCLC (34%), prostate (19%), and breast (19%). Median follow-up is 18 months (range, 3–31 months). All patients have been treated with fast FFF beams and Volumetric Modulated Arc Therapy (VMAT) plans. The MRI-based maximum cord dose constraint is 15 Gy. All cases have been planned and assessed post-treatment by PET/CT. Previous data suggests that the magnitude of change in metabolic uptake (ΔSUV) post-single fraction is predictive of long-term freedom from relapse.34
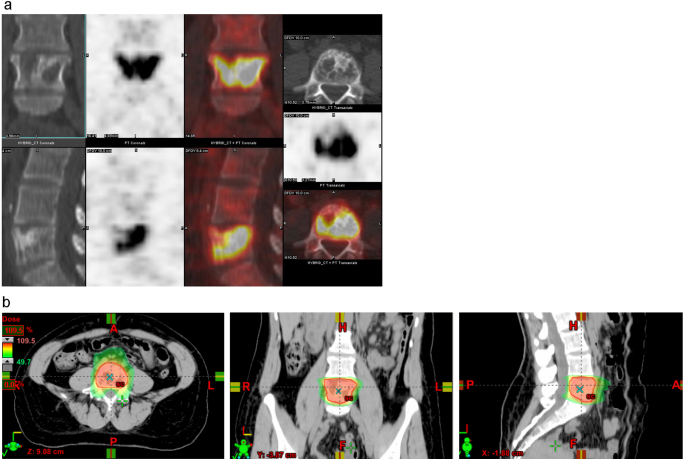
Fig. 1 shows a planning PET/CT and 3- and 24-month follow-up scans of a breast cancer solitary metastasis in the vertebral body at L5 (prescription dose 24 Gy) which achieved a complete metabolic response at 3 months post-treatment. All patients, with no exception, treated with high dose SBRT for metastatic disease at CCU were prospectively assessed with PET/CT and ΔSUV response scores, according to criteria of response correlated with traditional PERCIST. A preliminary analysis of the metastatic cohort shows that a decline of >70% (ΔSUV>70%) at 3 months post-treatment appears to be significantly associated with local tumor control, as observed at CCU in other types of treated tumors. Among patients who demonstrated an initial ΔSUV>70%, 97% achieved sustained metabolic local control compared with 60% who did not demonstrate an initial ΔSUV>70% (p = 0.01). Longer follow-up is needed to confirm the ΔSUV approach as an early biomarker of response.
Fig. 1.
(a) Baseline PET/CT. Solitary L5 metastasis from breast cancer with extensive involvement of the vertebral body (SUVmax 17.5). (b) Dose distribution of VMAT plan. Prescription dose 24 Gy to the PTV. 10 MV beam. (c) 3-Months post-single dose IGRT 24 Gy Follow-up PET/CT (SUVmax <1). (d) 2-Year follow-up PET/CT assessment (SUVmax <1).
5. Risk of vertebral fracture following high-dose spinal RT
Little data exists on the risk of vertebral fracture to the spine following high-dose SRS to the spine. Rose et al. evaluated 62 consecutive patients at 71 sites.35 Patients had no prior surgical or radiation treatment to the region of interest. Single dose prescription varied from 18 to 24 Gy as part of a deliberate dose escalation scheme. Fracture progression was noted in 27 vertebrae (39%). The risk of fracture progression was not associated with dose within this range. Multivariate logistic regression analysis showed that CT appearance, lesion location, and percent vertebral body involvement independently predicted fracture progression. Lesions located between T10 and the sacrum were 4.6 times more likely to fracture than were lesions above T10 (95% CI, 1.1–19.7). Lytic lesions were 6.8 times more likely to fracture than were sclerotic and mixed lesions. As percent vertebral body involvement increased, so did the odds of fracture. Lytic disease involving more than 40% of the vertebral body and location at or below T10 confer a high risk of fracture, the presence of which yields significantly poorer clinical outcomes. This data may be used as a guide for the indication of preventive vertebroplasty prior to spine SBRT in selected cases.
6. High-dose spinal RT in the adjuvant setting
Gross spinal instability may require open surgery for decompressing and stabilizing the spine. Persistence of gross disease post-operatively may be addressed with adjuvant radiation therapy with the aim of improving local tumor control. In an early report, Rock et al. evaluated the combination of open surgical procedure followed with adjuvant radiosurgery in a series of 18 patients. Doses ranged between 6 and 16 Gy (mean, 11.4 Gy) prescribed to the 90% isodose line.36 This treatment paradigm was associated with a significant chance of stabilizing or improving neurological function. In this series, 92% of patients initially presenting with neurological symptoms either remained neurologically stable or improved. Local control was 94%. Overall, the technique was deemed well tolerated and associated with little to no morbidity. Moulding et al. reviewed 21 patients who had undergone “separation surgery” for radioresistant histologies.37 The radiation treatment volume was delineated based on the pre-operative imaging studies rather than the postoperative residual disease. The spinal cord and thecal sac contours were established using myelogram/CT, which provides excellent anatomic detail even in the presence of spinal implants. The GTV received 24 Gy in 16 patients and 18–21 Gy in 5 patients. Overall local control was 81% with an estimated one-year failure of 9.5%. Local control was significantly better in the cohort receiving 24 Gy compared to patients receiving less than 24 Gy (94% versus 60%, respectively). Recently, Laufer et al. retrospectively reviewed the outcomes of 186 patients suffering from metastatic epidural spinal cord compression treated with surgical decompression, instrumentation, and postoperative IGRT delivered as either single-dose of 24 Gy (40/186; 21.5%), high-dose hypofractionation in 3 fractions and a total dose of 24–30 Gy (37/186; 19.9%), or low-dose hypofractionation in 5 or 6 fractions and a total dose of 18–36 Gy (109/186; 58.6%).38 The overall local progression rate after radiation was 16% at 1 year. The only factor significantly associated with local tumor progression was the post-operative radiation dose, with the high-dose hypofractionated regimen resulting in a 4% local progression rate after 1 year, compared with the significantly higher 1-year local progression rate of 22% for the low-dose hypofractionated approach. The 1-year local progression rate for single-fraction was 9%. No other variable significantly correlated with progression-free survival, including radiation sensitivity of tumor histology, grade of epidural cord compression, extent of surgical decompression.
Some of the drawbacks of invasive surgery lie in the risks of surgical morbidity and the potential delays in receiving adjuvant irradiation. The use of less invasive, percutaneous, surgical approaches combined with SBRT is an active area of current investigation which relies on the excellent long-term tumor control provided by high dose postoperative high-dose IGRT, potentially obviating the need for extensive tumor resection in favor of a limited spinal cord decompression. Recently, Massicotte et al. reported on the role of minimal access spine surgery (MASS) in a small series as an adjunct to definitive SBRT with a median time between surgery and irradiation of only 7 days.39 The median total radiation dose was 24 Gy (range, 18–35 Gy), in a mean of 3 fractions (range, 1–5). With a median follow-up for the cohort of 13 months (range, 3–18), local control based on imaging was achieved in 7 of the 10 patients treated.
Patients with compression fractures can be treated with vertebro- or kyphoplasty followed by high-dose IGRT treatment. Percutaneous cement augmentation is effective treatment for pain, while it plays no role in local tumor control and it is, thus, typically combined with postoperative radiation. The pain relief afforded by these minimally invasive procedures may greatly improve the patient's tolerance to the immobilization required for IGRT delivery. Gerszten et al. have demonstrated the utility of high-dose irradiation following percutaneous cement augmentation in a series of 26 patients with a 92% local tumor control following single-fraction radiosurgery (at a mean of 12 days after kyphoplasty) with a mean dose of 18 Gy (range, 16–20 Gy prescribed to the 80% isodose line).40
7. Selection of candidates for high-dose spinal SBRT
Attempts to identify the best candidates for spinal SBRT have been made. A prognostic index using recursive partitioning analysis has been proposed using data from 174 patients treated typically with a single fraction with a median dose of 14 Gy (range, 8–24 Gy).41 Kaplan–Meier analysis was performed to detect any correlation between survival and histology. Histologies were divided into favorable (breast and prostate), radioresistant (renal cell, melanoma and sarcoma), and other (all other histologies). Association of the following variables with overall survival: histology, gender, age, Karnofsky performance status (KPS), control of primary, extraosseous metastases, time from primary diagnosis (TPD), dose (≤14 Gy versus >14 Gy), extent of spine disease (epidural only, bone and epidural, bone only), upfront or salvage treatment, presence of paraspinal extension, and previous surgery. Median follow-up was 8.9 months. Median overall survival time from SBRT was 10.7 months. Median overall survival intervals for favorable histologies were 14 months, 11.2 months for radioresistant histologies, and 7.3 months for other histologies (p = 0.02). Recursive partitioning analysis resulted in three classes (p < 0.0001). Class 1 was defined as TPD of >30 months and KPS of >70; Class 2 was TPD of >30 months and KPS of ≤70 or a TPD of ≤30 months and age <70 years old; Class 3 was TPD of ≤30 months and age ≥70 years old. Median OS was 21.1 months for Class 1, 8.7 months for Class 2, and 2.4 months for Class 3. This index represents a simple and yet effective approach to predict which patients may benefit most from SBRT to spinal disease.
Chang et al. reported on the safety, effectiveness, and patterns of failure obtained in a Phase I/II study of SBRT for spinal metastatic tumors.42 A total of 74 lesions were treated in 63 patients with near-simultaneous computed tomography-guided SBRT. Spinal magnetic resonance imaging was conducted at the baseline and at each follow-up visit. No neuropathy or myelopathy was observed at a median follow-up of 21.3 months (range 0.9–49.6 months). The actuarial 1-year tumor progression-free incidence was 84% for all tumors. Pattern-of-failure analysis showed that 47% of the recurrences occurred in the epidural space adjacent to the spinal cord, likely due in part to tumor underdosing in the region due to spinal cord constraints which had been set to a dose of 9–10 Gy. Although this value may be regarded as overly conservative, especially in the radiation-naïve cases, this finding is particularly useful in identifying cases that may be at greater risk of local recurrence, e.g. lesions abutting ≤ 1 mm from the thecal sac.
8. Outlook for the future
This review demonstrates that single dose SBRT is rapidly gaining consensus as a method to treat metastatic tumor to the spine as a single modality or in combination with neurosurgical procedures.43 Remarkable consistency has been demonstrated in reported outcomes with durable local control rates in excess of 90% in large retrospective studies. While the perspective of using single dose SBRT in spine metastases is highly promising, the issue of tumor to cord proximity and the associated risk of collateral cord damage continues to represent a hurdle that hinders a wider application of this technique. In particular, it is the need to apply ultra-high SBRT doses that mandates meticulous and diligent use of labor-intensive techniques to deliver treatment safely.
One resolution of this dilemma may result from a new understanding of the biology of single dose radiotherapy. When treated with standard fractionation, human tumors exhibit a rank ordering of the dose required for 90% tumor cure by their inherent tumor-type specific radiosensitivity. This phenotypic feature of tumor radiobiology is not relevant to tumor response to ultra-high radiation dose, as all tumor types exhibit a near uniform response at the high range of clinically feasible single dose SBRT. This phenomenon suggests that high single dose SBRT might operate a mechanism of tumor cell lethality that differs from that operating at low dose exposures. In fact, such a mechanism has recently been described as a co-dependent dual target radiation response, with a threshold at a high radiation dose level, as such, it is not functional at the traditional low dose/fraction mode.44, 45 It has been shown that radiation exposure at the ultra-high dose level engages a microvascular response that interferes metabolically with tumor cell ability to properly repair radiation damage, resulting in tumor cell death and tumor cure. The understanding of this mechanism of action has yielded new targets for selective tumor versus normal tissue radiosensitization.46 Biological modifiers now used in experimental tumor models enable up to a 6 Gy de-escalation of the dose required for single dose tumor cure without affecting normal tissue sensitivity.47 If such tumor-selective dose reduction could convert 24 Gy tumor cure to a 15–18 Gy iso-effect in clinical settings, it would significantly simplify the treatment of spine tumors, as it will reduce or eliminate the risk of collateral cord toxicity. It should, however, be emphasized that these approaches are still at an experimental phase and must await further development before being introduced into clinical practice.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Greco C., Forte L., Erba P., Mariani G. Bone metastases, general and clinical issues. Q J Nucl Med Mol Imaging. 2011;55:337–352. [PubMed] [Google Scholar]
- 2.Greenberg H.S., Kim J.H., Posner J.B. Epidural spinal cord compression from metastatic tumor: results with a new treatment protocol. Ann Neurol. 1980;8:361–366. doi: 10.1002/ana.410080404. [DOI] [PubMed] [Google Scholar]
- 3.Young R.F., Post E.M., King G.A. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg. 1980;53:741–748. doi: 10.3171/jns.1980.53.6.0741. [DOI] [PubMed] [Google Scholar]
- 4.Maranzano E., Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 5.Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94:269–275. doi: 10.1111/j.1600-0404.1996.tb07064.x. [DOI] [PubMed] [Google Scholar]
- 6.Katagiri H., Takahashi M., Inagaki J. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42:1127–1132. doi: 10.1016/s0360-3016(98)00288-0. [DOI] [PubMed] [Google Scholar]
- 7.Maranzano E., Bellavita R., Rossi R. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol. 2005;23:3358–3365. doi: 10.1200/JCO.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 8.Faul C.M., Flickinger J.C. The use of radiation in the management of spinal metastases. J Neurooncol. 1995;23:149–161. doi: 10.1007/BF01053419. [DOI] [PubMed] [Google Scholar]
- 9.Sahgal A., Larson D.A., Chang E.L. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys. 2008;71:652–665. doi: 10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 10.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 11.Tree A.C., Khoo V.S., Eeles R.A., Ahmed M., Dearnaley D.P. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:28–37. doi: 10.1016/S1470-2045(12)70510-7. [DOI] [PubMed] [Google Scholar]
- 12.Corbin K.S., Hellman S., Weichselbaum R.R. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. J Clin Oncol. 2013;31:1384–1390. doi: 10.1200/JCO.2012.45.9651. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton A.J., Lulu B.A., Fosmire H., Stea B., Cassady J.R. Preliminary clinical experience with linear accelerator-based spinal stereotactic radiosurgery. Neurosurgery. 1995;36:311–319. doi: 10.1227/00006123-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick J.P., van der Kogel A.J., Schultheiss T.E. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76:S42–S49. doi: 10.1016/j.ijrobp.2009.04.095. [DOI] [PubMed] [Google Scholar]
- 15.Macbeth F.R., Wheldon T.E., Girling D.J. Radiation myelopathy: estimates of risk in 1048 patients in three randomized trials of palliative radiotherapy for non-small cell lung cancer. The Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:167–175. doi: 10.1016/s0936-6555(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 16.Ryu S., Jin J.Y., Jin R. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109:628–636. doi: 10.1002/cncr.22442. [DOI] [PubMed] [Google Scholar]
- 17.Sahgal A., Weinberg V., Ma L. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85:341–347. doi: 10.1016/j.ijrobp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Kim S., Jin H., Yang H., Amdur R.J. A study on target positioning error and its impact on dose variation in image-guided stereotactic body radiotherapy for the spine. Int J Radiat Oncol Biol Phys. 2009;73:1574–1579. doi: 10.1016/j.ijrobp.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Hall W.A., Stapleford L.J., Hadjipanayis C.G., Curran W.J., Crocker I., Shu H. Stereotactic body radiosurgery for spinal metastatic disease: an evidence-based review. Int J Surg Oncol. 2011;2011:979214. doi: 10.1155/2011/979214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryu S., Pugh S.L., Gerszten P.C. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1–3) spine metastases: phase 2 results. Pract Radiat Oncol. 2014;4:76–81. doi: 10.1016/j.prro.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folkert M.R., Bilsky M.H., Tom A.K. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys. 2014;88:1085–1091. doi: 10.1016/j.ijrobp.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 22.Greco C., Zelefsky M.J., Lovelock M. Predictors of local control after single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases. Int J Radiat Oncol Biol Phys. 2011;79:1151–1157. doi: 10.1016/j.ijrobp.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 23.Yamada Y., Bilsky M.H., Lovelock D.M. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 24.McCammon R., Schefter T.E., Gaspar L.E., Zaemisch R., Gravdahl D., Kavanagh B. Observation of a dose–control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2009;73:112–118. doi: 10.1016/j.ijrobp.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 25.Benedict S.H., Yenice K.M., Followill D. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 26.Ryu S., Jin R., Jin J.Y. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage. 2008;35:292–298. doi: 10.1016/j.jpainsymman.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Gerszten P.C., Burton S.A., Ozhasoglu C., Welch W.C. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 28.Gerszten P.C., Burton S.A., Welch W.C. Single-fraction radiosurgery for the treatment of spinal breast metastases. Cancer. 2005;104:2244–2254. doi: 10.1002/cncr.21467. [DOI] [PubMed] [Google Scholar]
- 29.Gerszten P.C., Burton S.A., Quinn A.E., Agarwala S.S., Kirkwood J.M. Radiosurgery for the treatment of spinal melanoma metastases. Stereotact Funct Neurosurg. 2005;83:213–221. doi: 10.1159/000091952. [DOI] [PubMed] [Google Scholar]
- 30.Gerszten P.C., Burton S.A., Belani C.P. Radiosurgery for the treatment of spinal lung metastases. Cancer. 2006;107:2653–2661. doi: 10.1002/cncr.22299. [DOI] [PubMed] [Google Scholar]
- 31.Yamada Y., Cox B.W., Zelefsky M.J. An Analysis of prognostic factors for local control of malignant spine tumors treated with spine radiosurgery. Int J Radiat Oncol Biol Phys. 2011;81:S132–S133. [Google Scholar]
- 32.Garg A.K., Shiu A.S., Yang J. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer. 2012;118:5069–5077. doi: 10.1002/cncr.27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelefsky M.J., Greco C., Motzer R. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:1744–1748. doi: 10.1016/j.ijrobp.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greco C., Fuks Z., Kim B., Larson S., Yamada Y., Zelefsky M. Post-treatment F-18 FDG-PET standardized uptake value (SUV) predicts local control following high-dose single-fraction IGRT. Int J Radiat Oncol Biol Phys. 2009;75:S535–S536. [Google Scholar]
- 35.Rose P.S., Laufer I., Boland P.J. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rock J.P., Ryu S., Shukairy M.S. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58:891–898. doi: 10.1227/01.NEU.0000209913.72761.4F. [DOI] [PubMed] [Google Scholar]
- 37.Moulding H.D., Elder J.B., Lis E. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine. 2010;13:87–93. doi: 10.3171/2010.3.SPINE09639. [DOI] [PubMed] [Google Scholar]
- 38.Laufer I., Iorgulescu J.B., Chapman T. Local disease control for spinal metastases following separation surgery and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. 2013;18:207–214. doi: 10.3171/2012.11.SPINE12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massicotte E., Foote M., Reddy R., Sahgal A. Minimal access spine surgery (MASS) for decompression and stabilization performed as an out-patient procedure for metastatic spinal tumours followed by spine stereotactic body radiotherapy (SBRT): first report of technique and preliminary outcomes. Technol Cancer Res Treat. 2012;11:15–25. doi: 10.7785/tcrt.2012.500230. [DOI] [PubMed] [Google Scholar]
- 40.Gerszten P.C., Germanwala A., Burton S.A., Welch W.C., Ozhasoglu C., Vogel W.J. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. J Neurosurg Spine. 2005;3:296–301. doi: 10.3171/spi.2005.3.4.0296. [DOI] [PubMed] [Google Scholar]
- 41.Chao S.T., Koyfman S.A., Woody N. Recursive partitioning analysis index is predictive for overall survival in patients undergoing spine stereotactic body radiation therapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2012;82:1738–1743. doi: 10.1016/j.ijrobp.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Chang E.L., Shiu A.S., Mendel E. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 43.Guckenberger M., Sweeney R.A., Flickinger J.C. Clinical practice of image-guided spine radiosurgery – results from an international research consortium. Radiat Oncol. 2011;6:172. doi: 10.1186/1748-717X-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Barros M., Paris F., Cordon-Cardo C. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 45.Fuks Z., Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Truman J.P., García-Barros M., Kaag M. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS ONE. 2010;5:e12310. doi: 10.1371/journal.pone.0012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stancevic B., Varda-Bloom N., Cheng J. Adenoviral transduction of human acid sphingomyelinase into neo-angiogenic endothelium radiosensitizes tumor cure. PLoS ONE. 2013;8:e69025. doi: 10.1371/journal.pone.0069025. [DOI] [PMC free article] [PubMed] [Google Scholar]