Abstract
Background
The outstanding innovations made by early diagnosis, novel surgical techniques, effective chemotherapy regimens and conformal radiotherapy, have significantly improved patients overall survival and quality of life. Multidisciplinary approach to cancer has also led to an increased prevalence of patients with few, organ-confined metastases, who can experience long-term survival even if their disease is no longer localized. Liver is one of the most common site for metastatic disease from several cancers, and when metastatic disease is confined to liver, given the ability of this organ to regenerate almost to its optimal volume, surgical resection represents the standard of care because is associated with a better prognosis. Approximately 70–90% of liver metastases, however, are unresectable and a safe, effective alternative therapeutic option is necessary for these patients.
Materials and methods
A review of the current literature was performed to analyze the role of SBRT in treating liver metastases from different cancers. A literature search using the terms “SBRT” and “liver metastases” was carried out in PUBMED.
Results
Stereotactic body radiation therapy has shown to provide promising results in the treatment of liver metastases, thanks to the ability of this procedure to deliver a conformal high dose of radiation to the target lesion and a minimal dose to surrounding critical tissues.
Conclusion
Stereotactic body radiation therapy is a non-invasive, well-tolerated and effective treatment for patients with liver metastases not suitable for surgical resection.
Keywords: SBRT, Liver, Oligometastatic patients
1. Background
Over the last decades, innovations made by modern oncology, in terms of early diagnosis, novel surgical techniques, effective chemotherapy regimens and conformal radiotherapy, have significantly improved patients overall survival and quality of life.1 The multidisciplinary approach to cancer has also led to an increased prevalence of patients with few, organ-confined metastases, who can experience long-term survival even if their disease is no longer localized. The concept of oligometastatic state was first proposed in 1995 by Hellman and Weichselbaum, who suggested the existence of an intermediate tumour state for most cancers, standing between purely localized lesions and a widely metastatic disease.2
An attractive consequence of the presence of this clinically significant oligometastatic state is that this subpopulation of patients should be amenable to curative therapeutic strategies. The successful surgical resection or radiation ablation of one or a small number of pulmonary, hepatic, or even brain metastases is the evidence of a limited form of the oligometastatic state.3
Liver is the most common site of metastastic disease from most cancers and when metastatic disease is confined to this organ, surgical resection has proved to obtain an improved survival, as compared to other therapeutic strategies.4, 5 Colorectal cancer (CRC) is one of the tumours that most often presents with solitary or oligometastasic disease, commonly in the liver. It is estimated, indeed, that 30–70% of patients with CRC will develop liver metastases and in this subset of patients, the overall survival is about 31% and 1% at 1 year and at 4 years, respectively, with a median survival ranging from 6 to 12 months.6 Historically, surgical resection of CRC liver metastases improves overall survival, with 1 and 5-year rates of 90–95% and 30–60%, respectively, with a median overall survival of 40–53 months.7, 8, 9, 10, 11, 12, 13
For partially resectable or unresectable patients, the introduction of modern chemotherapy regimens in the induction treatment of colorectal liver metastases allowed downsizing the disease, favouring surgical resection and, potentially, survival.14 On the other hand, chemotherapy can also worsen hepatic function and increase the rate of perioperative complications. Recent studies have demonstrated that chemotherapy may produce multiple toxic effects on the underlying liver parenchyma, ranging from steatosis to steatohepatitis and sinusoidal obstruction syndrome.15
Only 10–50% of patients are suitable for surgical resection because of technical difficulties, unfavourable tumour factors or patient comorbidities.12, 13 In these patients, a safe and effective alternative therapeutic option is necessary.
In the last decade, minimally invasive loco-regional approaches were introduced as an alternative to surgery, including radiofrequency ablation (RFA), which causes tissue focal coagulative necrosis.
Nevertheless, this technique has some limits, mostly related to lesion size and location (lesions higher than 3 cm of diameter or in proximity of major blood vessels, the main biliary tract or the gallbladder, or just beneath the diaphragm).20, 21, 22, 23
This review aims to better define the role of stereotactic body radiotherapy in the treatment of colorectal cancer liver metastases through a revision of the current literature, in order to provide practice guidelines on the use of this novel strategy.
2. The role of stereotactic body radiation therapy (SBRT) for the treatment of liver metastases
Historically, radiation therapy has had a limited role in the treatment of liver metastases. The low tolerance of liver tissue to irradiation raises the risk of the radiation-induced liver disease (RILD). RILD syndrome is characterized by anicteric ascites with elevation of alkaline phosphatase and liver transaminases, which occurs two weeks to 4 months after radiotherapy and can results in liver failure and death.24 From the classic work of Ingold16 and the historical report by Emami et al.,17 several radiobiological studies demonstrated that the liver has a parallel architecture, and that a life-threatening liver toxicity, as documented in previous experiences involving the irradiation of the whole liver, is quite infrequent when only a part of liver is irradiated. Modern 3D-radiotherapy, indeed, allows delivering of a very conformal radiation dose to the tumour and a minimal radiation dose to surrounding critical tissues, thus validating the importance of the mean radiation dose delivered to normal tissue.24 Moreover, recent studies have demonstrated that an increased toxicity risk is associated to the mean dose delivered to liver.24, 37
Unlike standard radiotherapy, which delivers conventional fractions (ranging from 1.5 to 3 Gy) to a larger volume, SBRT entails precise delivery of high-dose in a single or a few fractions (1–6 fractions).25, 26
Analysis of the literature demonstrates that SBRT was used in several primary and secondary tumours.27, 28 No randomized Phase III data have been published. Liver metastases were the target of SBRT in five retrospective29, 30, 31, 32, 33 and 8 prospective34, 35, 36, 37, 38, 39, 40, 41 studies. The latter are described in detail in Table 1.
Table 1.
Outcomes of published Phase I–II trial.
| Authors | No. of patients | No. of lesions | Histology | Total dose/frs | Toxicity | Local control | Overall survival |
|---|---|---|---|---|---|---|---|
| Scorsetti, 2013 Phase II |
61 | 76 | CRC (29) Breast (11) Gyn (7) Other (14) |
75 Gy/3 frs | No RILD, 1 case G3 late toxicity (chest wall pain) | 1-yr LC, 94% | 1–yr OS 83.5% |
| Goodman, 2010 Phase I |
29 (19 Mets) | 40 | CRC (6) Pancreatic (3) Gastric (2) Ovarian (2) Other (6) |
Dose escalation, 18–30 Gy/1 fr | 4 cases of G2 late toxicity (2 GI, 2 soft tissue/rib) | 1-yr local failure, 23% | 2-yr OS 49% (Mets only) |
| Ambrosino, 2009 Prospective cohort |
27 | Not reported | CRC (11) Other (16) |
25–60 Gy/3 frs | No serious toxicity | Crude LC rate 74% | Not reported |
| Rusthoven, 2009 Phase I–II |
47 | 63 | CRC (15) Lung (10) Breast (4) Ovarian (3) Esophageal (3) HCC (2) Other (10) |
Dose escalation, 36–60 Gy/3 frs | No RILD Late G3/4 <2% |
1-yr LC, 95% 2-yr LC, 92% |
Median survival 20.5 mo |
| Lee, 2009 Phase I–II |
68 | 143 | CRC (40) Breast (12) Gallbladder (4) Lung (2) Anal canal (2) Melanoma (2) Other (6) |
Individualized dose, 27.7–60 Gy/6 frs | No RILD 10% G3/4 acute toxicity No G3/4 late toxicity |
1-yr LC, 71% | Median survival, 17.6 mo |
| Mendez Romero, 2006 Phase I–II |
25 (17 Mets) | 34 | CRC (14) Lung (1) Breast (1) Carcinoid (1) |
30–37.5 Gy/3 frs | 2 cases G3 liver toxicities | 2-yr LC, 86% | 2-yr OS, 62% |
| Hoyer, 2006 Phase II |
64 (44 Mets) | 141 (lung + liver + other sites) | CRC (44) | 45 Gy/3 frs | 1 liver failure 2 severe late GI toxicities |
2-yr LC, 79% (by tumour) and 64% (by patient) | 2-yr OS 38% |
| Herfarth, 2004 Phase I–II | 35 | Not reported | Dose escalation, 14–26 Gy/1 fr | No significant toxicity reported | 1–yr LC, 71% 18–mo LC, 67% |
1–yr OS, 72% | |
As expected, colorectal, breast and lung cancer were the primary tumours most frequently represented and treated. Patients had a good performance status (Eastern Cooperative Oncology Group 0–1 or Karnofsky >70), absent or stable extrahepatic disease, adequate hepatic volume and function and less than 5 lesions with tumour size <6 cm.
Two trials and one pooled analysis investigated the role of SBRT on hepatic lesion from a single primary tumour type, including 44, 20 and 86 patients respectively, treated for colorectal cancer liver metastases.39, 40, 44
Two studies employed a single fraction of SBRT with a prescription dose ranging from 14 to 30 Gy. In these trials, 1-year local control ranged from 71% to 77%, 1-year OS from 72% to 80% and toxicity from G0 to G2 (2 cases of G2 gastrointestinal late toxicity and 2 cases of G2 late toxicity of soft tissue/rib).34, 36
In 5 trials and 1 pooled analysis, doses prescription ranged from 25 to 60 Gy in 3–6 fractions, with a local control rate ranging from 70% to 100% and 60% to 90% at 1 and 2 years, respectively.
One and 2 years OS rates were 70–80% and 30–73% respectively, with a median overall survival rate ranging from 10 to 34 months.35, 37, 38, 39, 40, 44
Correlation between prognostic factors and both local control and overall survival were analyzed by Rusthoven et al. A significant connection between dose prescription, tumour size and local failure rate was demonstrated. This phase I/II study enrolled 47 patients with a prescription dose of 60 Gy in 3 fractions and showed that 1-year LC decreased significantly in the subgroup of lesions with diameter >3 cm.37 In 2011, Chang et al. confirmed the correlation between dose prescription and local control and suggested a dose ≥48 Gy for a 3 fractions SBRT regimen. In this pooled analysis, the absence of active extrahepatic disease and LC was associated with improved OS.44
In 2013, the preliminary results of a phase II trial with a higher prescription dose was published.41 With a 1-year LC rate of 94%, Scorsetti et al. showed no statistical differences in local control rates for lesions with diameter ≤3 cm compared with those >3 cm, when a prescription dose of 75 Gy in 3 fractions was delivered.41
Most studies with a 3–6 fractions regimen showed a low toxicity profile with a ≥G3 toxicity rate of 1–10% and an incidence of RILD less than 1%. Lee et al.35 had no RILD in 68 patients treated with 6 fractions and median liver dose of 16.9 Gy (range, 3–22 Gy). In two Phase I/II studies,37, 41 reporting on 47 and 61 patients, respectively, no RILD was observed using a dose constraint allowing no more than 700 mL of uninvolved liver to receive 15 Gy or greater in 3 fractions. The most common G2 toxicities included a transient hepatic transaminase increase within 3 months of SBRT39, 41 and gastrointestinal, soft-tissue and bone complications, related to lesions close to the duodenum, bowel, skin and ribs. Duodenal ulceration and intestinal perforation were observed in 3 patients with maximum doses greater than 30 Gy in 3 fractions to the duodenum and bowel.40 One case of Grade 3 soft-tissue toxicity was observed for a 48 Gy dose in 3 fractions to subcutaneous tissue.37 In 2 patients, nontraumatic rib fractures were experienced for maximum doses of 51.8 Gy and 66.2 Gy in 6 fractions to 0.5 cm3.35 One patient suffered from chronic chest wall pain G3, which resolved within 1 year after SBRT with a prescription dose of 75 Gy in 3 fractions.41
3. Stereotactic body radiation therapy (SBRT) for liver metastases: patients selection
Multidisciplinary assessment is recommended to identify patients with liver metastases candidate to SBRT. According to the current literature and on the basis of lesion's number and diameter, distance from OARs, liver function and free liver volume, SBRT may be considered as follows:
-
•
Indicated: patients with a good performance status (Eastern Cooperative Oncology Group 0–1 or Karnofsky >70), number of hepatic lesions ≤3, size lesions ≤3 cm, lesion distance from OARs >8 mm, good liver function and free liver volume >1000 cm3.
-
•
Borderline: patients with 4 liver metastases, diameter >3 and ≤6 cm, OARs distance >5 and ≤8 mm, moderate liver function and free liver ≥700 and <1000 cm3.
-
•
Contra-indicated: patients with ≥ 5hepatic lesions, diameter >6 cm, OARs distance ≤5 mm, inadequate liver function and free liver volume <700 cm3.
Age is not considered in selection criteria, as even elderly and fragile patients can safely undergo SBRT. This non-invasive and well-tolerated therapy is a good option for patients unsuitable for surgery.
The intrinsic radio-sensitivity of tumours is not an issue, as SBRT can be used regardless of histopathology thanks to the use of ablative doses, with similar local control rates in radioresistant and radiosensitive primary tumour.
4. Stereotactic body radiation therapy (SBRT) for liver metastases: target definition, planning and delivery
SBRT requires precise target definition, planning and delivery.48 Practice guidelines for SBRT were published in 2010 by ASTRO and ACR.25, 26 To ensure the delivery accuracy, the target position is checked before or during SBRT treatment, by an integrated image acquisition system (image-guided radiation therapy or IGRT).25, 26
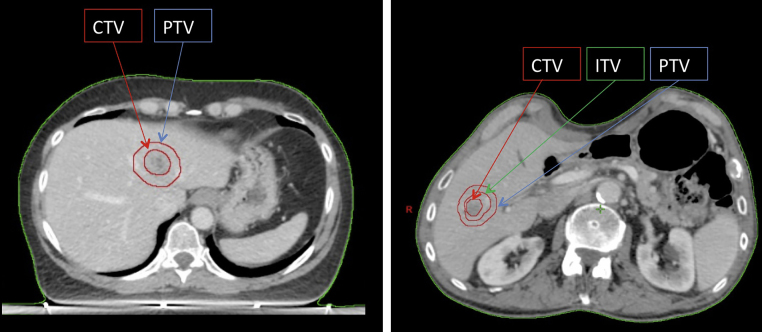
According to ICRU 82,42 the clinical target volume (CTV) is defined as equal to the gross tumour volume (GTV). During simulation phase, all the immobilization devices offered by modern technologies in order to reduce organ motion and maximize reproducibility and accuracy during each fraction, are required. An abdominal compression and a 4D-CT scan can be useful, since a significant liver motion according to respiratory cycle has been widely demonstrated.41 A three-phase contrast-enhanced CT scan should be employed, because not all hepatic lesions can be easily identified with a contrast-free CT-scan. For this reason, positron emission tomography (PET) and/or magnetic resonance (MRI) could be necessary to improve target definition. If 4D-CT scan is performed, an internal target volume (ITV) is the volume encompassing all GTVs in the different phase of respiratory cycle. ITV is defined as internal margin (IM).
A margin to compensate set-up errors is added to ITV (planning target volume, PTV). If 4D-CT scan is not acquired and ITV is not defined, the PTV is generated from CTV. A geometrical margin is added to CTV to rectify the uncertainties of set-up and internal margin.42 Fig. 1 shows these volumes.
Fig. 1.
Examples of target volumes delineation for liver SBRT.
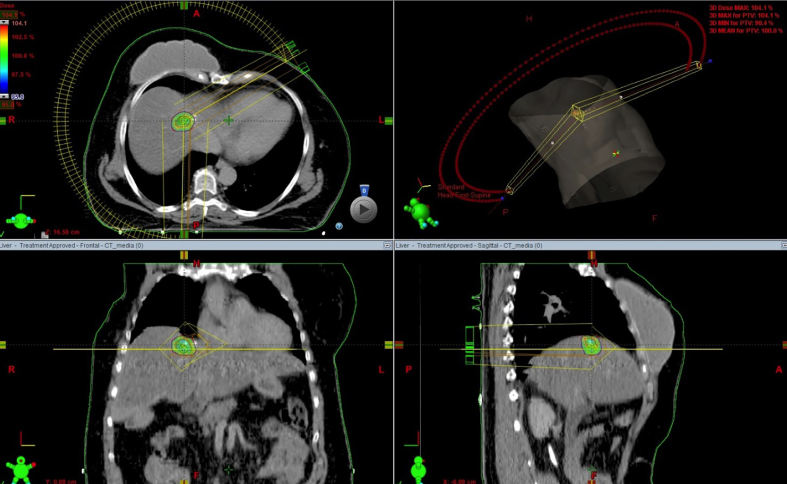
In order to obtain a high conformal dose distribution with the maximum sparing of organs at risk, intensity modulated RT (IMRT) or volumetric arc radiation therapy (VMAT) should be employed,25, 26 as showed in Fig. 2.
Fig. 2.
Example of liver metastases SBRT with VMAT technique.
Image guided radiation therapy (IGRT) should be performed before each daily session to check patient and tumour position; in selected patients, fiducials markers implantation could be useful for target localization.43 In patients who had previous surgery, surgical clips could be used as markers.41
5. Stereotactic body radiation therapy (SBRT) for liver metastases: dose prescription and response evaluation
There are several schedules of treatment for SBRT in liver metastases in current literature. In Chang et al. experience, local control is correlated with total dose.44 Prescription dose ≥48 Gy in 3 fractions should be considered, if allowed by normal tissue constraints. If lesion is smaller than 3 cm, a total dose of 60 Gy could be suggested.37 For lesions greater than 3 cm, a dose escalation should be cautiously evaluated up 75 Gy in 3 fractions.41
Table 2 shows recommended dose prescription according to lesion size. Table 3 summarizes recommended dose constraints for organs at risk (OARs).37, 41
Table 2.
Dose prescription for SBRT in 3 fractions recommended according to lesion size.
| Lesion size | Prescription dose |
|---|---|
| ≤3 cm | 48–60 Gy |
| >3–6 cm | 60–75 Gy |
Table 3.
Recommended OARs dose constraints for SBRT of liver metastasis in 3 fractions.
| OAR | Dose-volume limits |
|---|---|
| Healthy liver (total liver volume minus cumulative GTV) | >700 cm3 at <15 Gya |
| Stomach, duodenum, small intestine | D 3 cm3 at <21 Gyb |
| Both kidneys | V 15 Gy at <35% |
| Spinal cord | D 1 cm3 at <18 Gy |
| Heart | D 1 cm3 at <30 Gy |
| Rib | D 30 cm3 at <30 Gy |
Volume of healthy liver >1000 cm3.
Distance by GTV >8 mm.
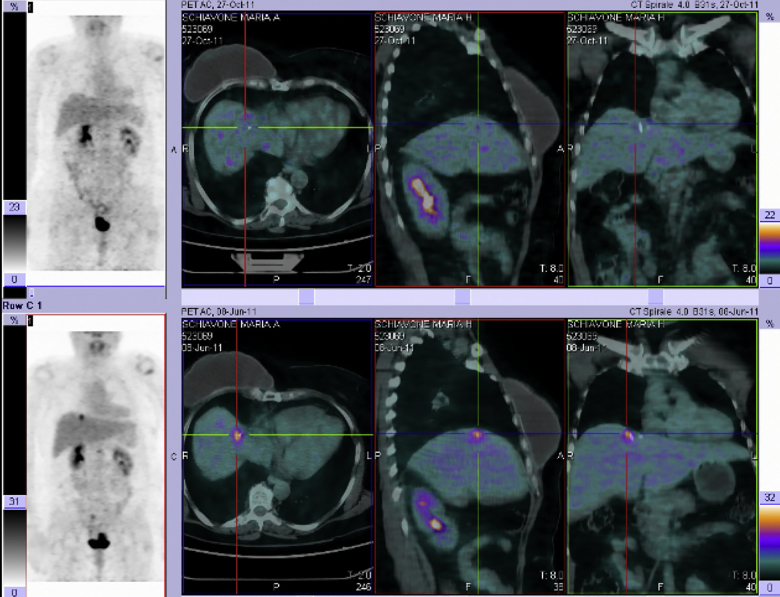
The criteria established by the European Organization for Research and Treatment of Cancer Response Evaluation Criteria in Solid Tumours (EORTC-RECIST) must be used to evaluate tumour response.18 PET Response Criteria in Solid Tumours (PERCIST) are recommended, if PET-CT is performed before SBRT.19 Fig. 3 shows the metabolic complete response of hepatic lesion 6 months after SBRT.
Fig. 3.
Example of complete metabolic response in a single hepatic.
6. Conclusions
Whether or not SBRT plays a significant role in the management of liver metastases can only be evaluated by means of randomized clinical trials that compare the standard of care alone or with the addition of SBRT, such as the ongoing randomized trial NCT01233544 (RFA versus SBRT).46 Combination with chemotherapy and targeted therapy could be investigated to reduce the incidence of extra-field recurrences.47
Further studies with a homogeneous selection of patients on the basis of histopathology, previous treatments and prognosis are advisable to better understand the real impact of SBRT on survival. Literature review shows that stereotactic body radiation therapy is a non-invasive, well-tolerated and effective treatment for liver metastases. It represents a valid, promising alternative for patients not suitable for surgical resection.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.De Santis C.E., Lin C.C., Mariotto A.B., Siegel R.L. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;(June) doi: 10.3322/caac.21235. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Weichselbaum R.R., Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 3.Alongi F., Arcangeli S., Filippi A.R., Ricardi U., Scorsetti M. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist. 2012;17(8):1100–1107. doi: 10.1634/theoncologist.2012-0092. 2012-0092 [Epub 20.06.12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon S.S., Tanabe K.K. Surgical treatment and other regional treatments for colorectal cancer liver metastases. Oncologist. 1999;4:197–208. [PubMed] [Google Scholar]
- 5.Scheele J., Stangl R., Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 6.Bengmark S., Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer. 1969;23:198–202. doi: 10.1002/1097-0142(196901)23:1<198::aid-cncr2820230126>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Saltz L.B. Metastatic colorectal cancer: is there one standard approach? Oncology (Williston Park) 2005;19:1147–1154. discussion 1154, 57–58, 60. [PubMed] [Google Scholar]
- 8.Cunningham D., Pyrhonen S., James R.D. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352:1413–1418. doi: 10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 9.Chang A.E., Schneider P.D., Sugarbaker P.H. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg. 1987;206:685–693. doi: 10.1097/00000658-198712000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings L., Payes J., Cooper G: Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 11.Wei A.C., Greig P.D., Grant D. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13:668–676. doi: 10.1245/ASO.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Fong Y., Fortner J., Sun R.L. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlinson J.S., Jarnagin W.R., Dematteo R.P. Actual 10-year survival after resection of 514 colorectal liver metastases defines cure. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(29):4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 14.Leonard G., Brenner B., Kemeny N. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2005;23:2038–2048. doi: 10.1200/JCO.2005.00.349. [DOI] [PubMed] [Google Scholar]
- 15.Alberts S.R. Update on the optimal management of patients with colorectal liver metastases. Crit Rev Oncol Hematol. 2012;84(October (1)):59–70. doi: 10.1016/j.critrevonc.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Ingold J.A., Reed G.B., Kaplan H.S., Bagshaw M.A. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93(January):200–208. [PubMed] [Google Scholar]
- 17.Emami B., Lyman J., Brown A. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(May (1)):109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer E., Therasse P., Bogaerts J. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Wahl RL1, Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl. 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Meijer V.E., Verhoef C., Kuiper J.W., Alwayn I.P., Kazemier G., Ijzermans J.N. Radiofrequency ablation in patients with primary and secondary hepatic malignancies. J Gastrointest Surg. 2006;10:960–973. doi: 10.1016/j.gassur.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Gillams A.R., Lees W.R. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19:1206–1213. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 22.Siperstein A.E., Berber E., Ballem N., Parikh R.T. Survival after radiofrequency ablation of colorectal liver metastases, 10-year experience. Ann Surg. 2007;246(4):559–567. doi: 10.1097/SLA.0b013e318155a7b6. [DOI] [PubMed] [Google Scholar]
- 23.Fiorentini G., Aliberti C., Mulazzani L. Chemoembolization in colorectal liver metastases: the rebirth. Anticancer Res. 2014;34(2):575–584. [PubMed] [Google Scholar]
- 24.Dawson L.A., Normolle D., Balter J.M. Analysis of radiation induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 25.Potters L., Kavanagh B., Galvin J.M. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76(February (2)):326–332. doi: 10.1016/j.ijrobp.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 26.Seung S.K., Larson D.A., Galvin J.M. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for the Performance of Stereotactic Radiosurgery (SRS) Am J Clin Oncol. 2013;36(June (3)):310–315. doi: 10.1097/COC.0b013e31826e053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Høyer M., Swaminath A., Bydder S. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys. 2012;82(3):1047–1057. doi: 10.1016/j.ijrobp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Nair V.J., Pantarotto J.R. Treatment of metastatic liver tumors using stereotactic ablative radiotherapy. World J Radiol. 2014;6(February (2)):18–25. doi: 10.4329/wjr.v6.i2.18. ISSN 1949-8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blomgren H., Lax I., Naslund I., Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 30.Wada H., Takai Y., Nemoto K., Yamada S. Univariate analysis of factors correlated with tumor control probability of three-dimensional conformal hypofractionated high-dose radiotherapy for small pulmonary or hepatic tumors. Int J Radiat Oncol Biol Phys. 2004;58:1114–1120. doi: 10.1016/j.ijrobp.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Wulf J., Guckenberger M., Haedinger U. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 2006;45:838–847. doi: 10.1080/02841860600904821. [DOI] [PubMed] [Google Scholar]
- 32.Katz A.W., Carey-Sampson M., Muhs A.G., Milano M.T., Schell M.C., Okunieff P. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67:793–798. doi: 10.1016/j.ijrobp.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Van der Pool A.E., Mendez Romero A., Wunderink W. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg. 2010;97:377–382. doi: 10.1002/bjs.6895. [DOI] [PubMed] [Google Scholar]
- 34.Herfarth K.K., Debus J., Wannenmacher M. Stereotactic single-dose radiation therapy of liver tumors: results of a Phase I/II trial. J Clin Oncol. 2001;19:164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 35.Lee M.T., Kim J.J., Dinniwell R. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 36.Goodman K.A., Wiegner E.A., Maturen K.E. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Rusthoven K.E., Kavanagh B.D., Cardenes H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 38.Ambrosino G., Polistina F., Costantin G. Image-guided robotic stereotactic radiosurgery for unresectable liver metastases: preliminary results. Anticancer Res. 2009;29:3381–3384. [PubMed] [Google Scholar]
- 39.Mendez Romero A., Wunderink W., van Os R.M. Quality of life after stereotactic body radiation therapy for primary and metastatic liver tumors. Int J Radiat Oncol Biol Phys. 2008;70:1447–1452. doi: 10.1016/j.ijrobp.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 40.Hoyer M., Roed H., Traberg H.A. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830. doi: 10.1080/02841860600904854. [DOI] [PubMed] [Google Scholar]
- 41.Scorsetti M., Arcangeli S., Tozzi A. Is stereotactic body radiation therapy an attractive option for unresectable liver metastasis? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys. 2013;86(2):336–342. doi: 10.1016/j.ijrobp.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 42.ICRU (International Commission on Radiation Units and Measurements) Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). ICRU Report 83. J ICRU. 2010;10:1–106. [Google Scholar]
- 43.Hennessey H., Valenti D., Cabrera T., Panet-Raymond V., Roberge D. Cardiac embolization of an implanted fiducial marker for hepatic stereotactic body radiotherapy: a case report. J Med Case Rep. 2009;3:140. doi: 10.1186/1752-1947-3-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang D.T., Swaminath A., Kozak M. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011;117(17):4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 46.http://clinicaltrials.gov/ct2/results?term=NCT+01233544&Search=Search
- 47.http://clinicaltrials.gov/ct2/results?term=NCT+00892424+&Search=Search
- 48.Rubio C., Morera R., Hernando O., Leroy T., Lartigau S.E. Extracranial stereotactic body radiotherapy. Review of main SBRT features and indications in primary tumors. Rep Pract Oncol Radiother. 2013;18(November (6)):387–396. doi: 10.1016/j.rpor.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]