Abstract
Background and Objectives
Atrial fibrillation (AF) occurs frequently after successful radiofrequency ablation (RFA) of cavotricuspid isthmus-dependent atrial flutter (CTI-AFL). Renal impairment has been implicated in the development of AF. The purpose of this study is to clarify the impact of impaired renal function on the incidence of AF after RFA of CTI-AFL.
Subjects and Methods
Between January 2001 and December 2013, 240 non-dialysis patients with no prior history of AF {mean age 55.9±15.2 years old; male, 192 (80.0%)} who had undergone successful CTI-AFL ablation were included in the present study. The baseline estimated glomerular filtration rate was calculated, and patients were divided into those with impaired renal function (<60 mL/min/1.73 m2) and those with preserved renal function (≥ 60 mL/min/1.73 m2). The incidence of AF was retrospectively analyzed.
Results
69 (28.8%) patients experienced new onset AF during a median follow-up duration of 26 months (inter-quartile, 7-53). The incidence of AF was significantly higher in patients with impaired renal function than in those with preserved renal function {13/25 (52.0%) versus 56/215 (26.0%), log rank p=0.019}. Age, CHADS2 score, impaired renal function, and left atrial diameter were significantly associated with the incidence of AF in univariate Cox regression analysis. Multivariate analysis showed that age was the only significant predictor of AF incidence (hazard ratio, 1.024; 95% confidence interval, 1.004-1.044, p=0.020).
Conclusion
Patients with impaired renal function may require careful attention for the incidence of new onset AF following successful RFA of CTI-AFL.
Keywords: Atrial fibrillation, Atrial flutter, Renal insufficiency, Catheter ablation
Introduction
Atrial fibrillation (AF) occurs frequently after successful radiofrequency ablation (RFA) of cavotricuspid isthmus-dependent atrial flutter (CTI-AFL). Previous studies have shown that the incidence of AF is 20% to 58% among patients treated with catheter ablation of CTI-AFL.1),2),3),4),5),6),7),8) In long-term follow-up studies of 5 to 8 years, the incidence of AF has been reported to be approximately 70% after cavotricuspid isthmus (CTI) ablation.4),9)
Impaired renal function is implicated in the development and maintenance of AF. Indeed, previous studies have reported that renal impairment is significantly associated with the prevalence of AF.9),10),11),12) Moreover, impaired renal function has been reported to predict AF recurrence after successful catheter ablation or electrical cardioversion of AF.9),13),14)
Therefore, here we seek to clarify the impact of impaired renal function on the incidence of AF after successful RFA of CTI-AFL.
Subjects and Methods
Study population
Between January 2001 and December 2013, a total of 318 non-dialysis patients underwent successful CTI-AFL ablation at the Asan Medical Center, Seoul, Korea. Among these, patients who had a prior history of AF (n=74) or follow-up loss (n=4) were excluded. Consequently, 240 patients (192 males, mean age: 55.9±15.2 years old) who underwent successful RFA of isolated CTI-AFL were included in the present study. Clinical data and echocardiographic studies were retrospectively analyzed. A 12-lead electrocardiogram (ECG) or Holter monitoring were examined at three- or six-month intervals following successful RFA by the attending physician. This study was approved by the Institutional Review Board of the Asan Medical Center.
Definitions
CTI-AFL was documented on a 12-lead ECG based on the presence of predominantly negative "saw tooth" flutter waves in inferior leads, and a positive deflection in V1 with an atrial rate of 240-300 beats per minute.15) AF was characterized by 1) irregular R-R intervals (when atrioventricular conduction was present), 2) absence of distinct repeating P waves, and 3) irregular atrial activity on 12-lead ECG.15)
Electrophysiological study and catheter ablation
Informed written consent for catheter ablation was obtained from all patients before the procedure. All antiarrhythmic drugs were discontinued five half-lives before the ablation. All procedures were performed under sedation with midazolam and analgesia with fentanyl. Programmed atrial stimulation was performed to induce CTI-AFL in patients with a sinus rhythm. CTI-AFL was confirmed by observation of the activation sequence of the flutter circuit determined using a duo-decapolar catheter placed around the tricuspid annulus and entrainment maneuvers on CTI. Anatomically-guided linear RFA of the CTI was performed from the tricuspid annulus to the inferior vena cava with an irrigated ablation catheter (Thermocool, Biosense Webster, Diamond Bar, CA, USA, or Coolflex, St. Jude Medical, St. Paul, MN, USA).16) The procedural endpoint was a bidirectional conduction block through CTI, which was assessed by differential pacing.17) For the first 4 weeks following ablation, patients received oral anticoagulants with a vitamin K antagonist to raise INR to 2.0-3.0. Further long-term anticoagulants were administered at the discretion of the attending physician.
Estimation of renal function
Renal function was assessed by calculating the estimated glomerular filtration rate (eGFR) using the abbreviated Modification of Diet in Renal Disease Study equation: eGFR (mL/min/1.73 m2)=186.3×(serum creatinine in mg/dL-1.154)×(age-0.203)×(0.742 if female).18) Impaired renal function was defined as <60 mL/min/1.73 m2, and preserved renal function was defined as ≥60 mL/min/1.73 m2.
Clinical and echocardiographic variables
Clinical data including medical history, medication, and ECG records were analyzed retrospectively from electronic medical records. Echocardiographic variables including left ventricle ejection fraction (LV EF), left atrial (LA) diameter, and E/E' ratio were obtained from the echocardiogram prior to the procedure.
Primary endpoint
The primary endpoint was the incidence of new onset AF during follow-up after RFA of CTI-AFL. New onset AF was diagnosed on the basis of 12-lead ECGs or Holter monitoring records. Thus, all 12-lead ECGs and Holter monitoring records during the follow-up period were reviewed carefully.
Statistical analysis
Statistical analysis was performed using SPSS version 21 (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean±standard deviation for continuous variables and as frequencies for categorical variables. In comparisons of baseline variables between groups according to renal function or the development of new onset AF, continuous variables were compared using a Student's t test, and categorical variables were compared using a chi-square or Fisher's exact test. The Kaplan-Meier method was used to estimate the survival free rate of atrial fibrillation between the patients with and without impaired renal function. Furthermore, the survival rates were compared with a log rank test. The associations of clinical and echocardiographic variables with the development of new onset AF were assessed using univariate and multivariate Cox's proportional hazard models. All variables with p<0.10 in univariate analysis were subjected to multivariate analysis. A multivariate model with a backward elimination stepwise approach was used to test the independent correlation of these variables with new onset AF after RF ablation of CTI-AFL. A p<0.05 was considered statistically significant.
Results
Baseline characteristics
There were 192 men (80.0%) and 48 women (20.0%), and the mean age of the study patients was 55.9±15.2 old years. Baseline characteristics of patients with or without an impaired renal function are shown in Table 1. The patients with impaired renal function were significantly older than those with preserved renal function. Patients with impaired renal function were more likely to have hypertension, diabetes mellitus, and higher CHADS2 and CHADS2VASc scores.
Table 1. Baseline characteristics of the study patients according to renal function at the time of radiofrequency ablation of cavotricuspid isthmus-dependent atrial flutter.
| Total (n=240) |
Impaired renal function (eGFR<60 mL/min/1.73 m2) (n=25) |
Preserved renal function (eGFR≥60 mL/min/1.73 m2) (n=215) |
p | |
|---|---|---|---|---|
| Age (years) | 55.9±15.2 | 70.3±10.6 | 54.3±14.7 | <0.0001 |
| Male | 192 (80.0) | 18 (72.0) | 174 (80.9) | 0.291 |
| Body mass index (kg/m2) | 24.1±4.3 | 25.4±3.5 | 23.9±4.4 | 0.109 |
| AFL duration (months) | 11.5±22.4 | 6.8±10.1 | 12.0±23.4 | 0.271 |
| Congestive heart failure | 55 (22.9) | 9 (36.0) | 46 (21.4) | 0.100 |
| Hypertension | 79 (32.9) | 15 (60.0) | 64 (29.8) | 0.002 |
| Diabetes mellitus | 42 (17.5) | 11 (44.0) | 31 (14.4) | 0.001 |
| Stroke | 19 (7.9) | 4 (16.0) | 15 (7.0) | 0.120 |
| Coronary artery disease | 30 (12.5) | 6 (24.0) | 24 (11.2) | 0.101 |
| Peripheral artery disease | 4 (1.7) | 1 (4.0) | 3 (1.4) | 0.358 |
| Valvular heart disease | 37 (15.4) | 4 (16.0) | 33 (15.3) | 1.000 |
| CHADS2 score | 1.0±1.2 | 2.1±1.2 | 0.9±1.1 | <0.0001 |
| CHA2DS2VASc score | 1.5±1.6 | 3.3±1.4 | 1.3±1.4 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 85.9±23.8 | 49.5±11.4 | 90.2±21.0 | <0.0001 |
| ACE inhibitor/ARB | 61 (25.4) | 7 (28.0) | 54 (25.1) | 0.754 |
| Statin | 34 (14.2) | 4 (16.0) | 30 (14.0) | 0.763 |
| Antiarrhythmic drugs | 23 (9.6) | 2 (8.0) | 21 (9.8) | 1.000 |
| LV EF (%) | 54.2±11.6 | 53.2±10.6 | 54.4±11.7 | 0.626 |
| LA diameter (mm) | 42.0±7.2 | 44.3±5.3 | 41.7±7.3 | 0.090 |
| E/E' ratio | 12.9±9.2 | 16.7±12.2 | 12.4±8.7 | 0.057 |
Data are expressed as the mean±standard deviation or as a number (%). eGFR: estimated glomerular filtration rate, AFL: atrial flutter, ACE: angiotensin converting enzyme, ARB: angiotensin receptor blocker, LV EF: left ventricle ejection fraction, LA: left atrium
Development of new onset atrial fibrillation after radiofrequency ablation of cavotricuspid isthmus-dependent atrial flutter
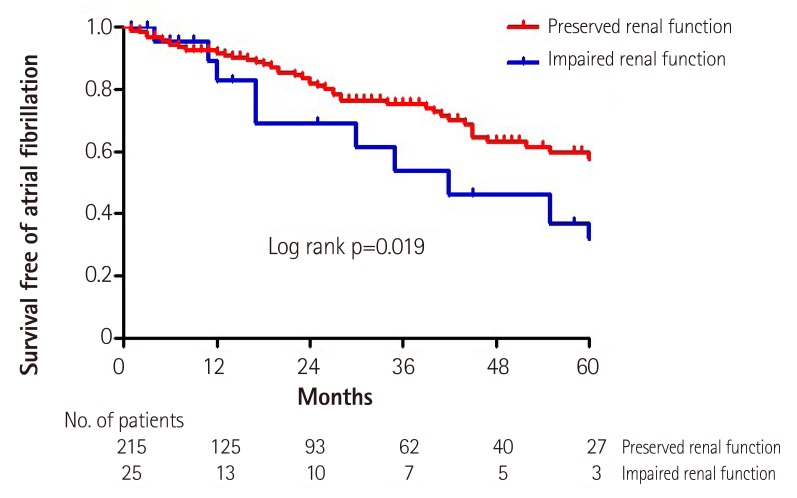
A total of 69 patients (28.8%) experienced new onset AF during a median follow-up of 26 months (inter-quartile, 7 to 53 months). The incidence of new onset AF was significantly higher in patients with impaired renal function than in those with preserved renal function {13/25 (52.0%) versus 56/215 (26.0%), log rank p=0.019}. Kaplan-Meier analysis revealed a significant difference in the incidence of new onset AF between patients with impaired renal function and those with preserved renal function (Fig. 1). Cumulative incidence rates and hazard ratios for new onset AF were significantly higher in patients with impaired renal function than in those with preserved renal function (Table 2). The estimated median duration of AF incidence in patients with impaired renal function was 47 months {95% confidence interval (CI), 28.4-66.3 months} after CTI-AFL ablation. However, the estimated median duration of AF incidence in patients with preserved renal function was 77.7 months (95% CI, 66.5-88.8 months).
Fig. 1. Kaplan-Meier curves showing the probability of survival free atrial fibrillation during post-ablation follow-up between the patients with or without impaired renal function. (< 60 mL/min/1.73 m2).
Table 2. Cumulative incidence rates and hazard ratios for new onset atrial fibrillation during follow-up.
| Patient-months | No. of events | No. /100 patient-months | HR (95% CI) | p | |
|---|---|---|---|---|---|
| Impaired renal function | 678 | 13 | 1.92 | 2.027 (1.105-3.716) | 0.022 |
| Preserved renal function | 6015 | 56 | 0.93 | 1.0 |
The HR and p of the impaired renal function group were compared with those of the preserved renal function group for the overall follow-up duration by a univariate Cox regression model. No.: number, HR: hazard ratio, CI: confidence interval
Predictors of new onset atrial fibrillation after radiofrequency ablation of cavotricuspid isthmus-dependent atrial flutter
In the univariate Cox proportional hazards model, age, CHADS2 score, impaired renal function, and LA diameter were significantly associated with new onset AF after RFA of CTI-AFL (Table 3). In multivariate analysis, impaired renal function did not predict the incidence of AF during follow-up (Table 3). On the other hand, age was the only significant predictor of the incidence of AF. LA diameter tended to be associated with AF incidence, but this was not found to be statistically significant.
Table 3. Univariate and multivariate Cox proportional hazards model for the incidence of atrial fibrillation during follow-up.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (years) | 1.025 (1.006-1.045) | 0.011 | 1.024 (1.004-1.044) | 0.020 |
| Male | 1.538 (0.806-2.936) | 0.192 | ||
| Body mass index (kg/m2) | 1.017 (0.965-1.073) | 0.522 | ||
| AFL duration (months) | 1.004 (0.994-1.014) | 0.419 | ||
| Congestive heart failure | 1.516 (0.888-2.590) | 0.127 | ||
| Hypertension | 1.027 (0.617-1.711) | 0.919 | ||
| Diabetes mellitus | 1.503 (0.844-2.678) | 0.166 | ||
| Stroke | 1.798 (0.766-4.221) | 0.178 | ||
| Coronary artery disease | 1.148 (0.601-2.196) | 0.676 | ||
| Peripheral artery disease | 0.048 (0.000-541.844) | 0.524 | ||
| Valvular heart disease | 1.075 (0.596-1.940) | 0.809 | ||
| CHADS2 score | 1.247 (1.007-1.543) | 0.043 | ||
| CHA2DS2VASc score | 1.158 (0.988-1.358) | 0.071 | ||
| eGFR<60 mL/min/1.73 m2 | 2.027 (1.105-3.716) | 0.022 | ||
| ACE inhibitor/ARB | 1.400 (0.821-2.388) | 0.216 | ||
| Statin | 0.678 (0.324-1.420) | 0.303 | ||
| Antiarrhythmic drugs | 0.952 (0.454-1.955) | 0.896 | ||
| LV EF (%) | 0.993 (0.973-1.013) | 0.478 | ||
| LA diameter (mm) | 1.041 (1.003-1.080) | 0.034 | 1.037 (0.998-1.077) | 0.066 |
| E/E' ratio | 1.002 (0.975-1.030) | 0.884 | ||
HR: hazard ratio, CI: confidence interval, AFL: atrial flutter, ACE: angiotensin converting enzyme, ARB: angiotensin receptor blocker, LV EF: left ventricle ejection fraction, LA: left atrium
Recurrence of cavotricuspid isthmus-dependent atrial flutter
CTI-AFL recurred in 11 patients (4.6%) after flutter ablation, at a median follow-up time of 10 months (inter-quartile, 1 to 21 months). The recurrence rate of CTI-AFL was not significantly different between patients with impaired renal function and those with preserved renal function {2/25 (8.0%) versus 9/215 (4.2%), log rank p=0.352}.
Discussion
Major findings
The major findings of the present study are (1) new onset AF developed frequently (28.8%) after successful RFA of CTI-AFL during follow-up (median 26 months, inter-quartile, 7 to 53 months), (2) patients with impaired renal function had twice the AF incidence than those with preserved renal function (52% versus 26%, log rank p=0.019), and (3) age was a significant predictor of new onset AF after successful RFA of CTI-AFL.
Relationship between atrial fibrillation and cavotricuspid isthmus-dependent atrial flutter
AF occurred frequently after CTI-AFL ablation. The incidence rate of new onset AF in our cohort is similar to that reported in previous studies.3),6) AF and CTI-AFL are known to be closely interconnected. Wu et al.19) suggested a mechanism for the conversion of AFL to AF involving progressive shortening of the action potential duration, which was characterized by detachment of stationary re-entry from the pectinate muscle and the generation of multiple wave breaks, as well as the formation of multiple, isolated, stationary wave fronts with different activation cycle lengths. On the other hand, Waldo and Feld20) suggested that, in the vast majority of instances, there can be no atrial flutter (AFL) without a preceding AF, because a preceding AF develops a line of block between the superior and inferior vena cava that completes the AFL re-entrant circuit. Furthermore, they indicated that this may be the reason why AF occurs after an attempt to cure AFL by ablation of the CTI, because after the latter AF can no longer "evolve" to AFL and thus clinically manifests.
Predictors of post-ablation atrial fibrillation
Previous studies have found that male, pre-ablation AF, a history of electrical cardioversion, number of antiarrhythmic agents, experimentally induced AF, and LA diameter and volume are related to the occurrence of AF after CTI-AFL ablation.1),3),4),5),6),7),21) In the present study, only age was a significant predictor of post-ablation AF. This discrepancy may reflect differences in population number, follow-up duration, and/or study characteristics between our study and previous studies.
Impact of impaired renal function on atrial fibrillation incidence
Previous studies have demonstrated that the prevalence of AF was higher in the non-dialysis population with chronic kidney disease, and that it gradually increased with decreasing eGFR.10),12) In addition, impaired renal function is a significant predictor of AF recurrence after successful electrical cardioversion.13),14) Several mechanisms have been suggested for these associations. Firstly, chronic renal disease may activate the renin-angiotensin-aldosterone (RAS) system, leading to LV hypertrophy and fibrosis.22) Abnormal relaxation increases LV end diastolic pressure, increasing atrial pressure and stretch, thereby favoring AF occurrence.23) In addition, RAS system activation is directly associated with atrial fibrosis and electrophysiological remodeling, which may also contribute to AF.23) Secondly, inflammation may be an important contributory factor for AF and chronic renal disease.24),25) Therefore, the higher incidence of AF in individuals with impaired renal function may be a consequence of the inflammation associated with renal dysfunction. However, the present study is unable to verify this theory, because we did not evaluate inflammatory markers at baseline. Finally, both renal disease and AF have several common risk factors including old age, hypertension, coronary artery disease, and congestive heart failure.10) Moreover, impaired renal function may reflect the cumulative effects of cardiovascular risk factors and may precede the onset of AF. Actually, in our cohort, impaired renal function was significantly associated with old age, hypertension, and diabetes mellitus, which are traditional cardiovascular risk factors. In particular, calculation of the estimated renal function was dependent on the patient's age, and both variables showed definite negative correlation in Spearman correlation analysis (correlation coefficient=-0.511, p<0.0001). Therefore, impaired renal function would not be a significant predictor of new onset AF in our multivariate Cox regression model including age factor. In multivariate Cox analysis excluding age factor, impaired renal function was a borderline significant predictor for new onset AF (hazard ratio, 1.833; 95% CI, 0.994-3.381; p=0.052).
Thus, our results may be in support of a relationship between impaired renal function and the incidence of AF.
Study limitations
The present retrospective study had several inherent limitations. Firstly, follow-up evaluation of 12-lead ECGs or Holter monitoring records was not consistent or uniform. Secondly, the diagnosis of AF was only based on 12-lead ECGs or Holter monitoring records, not on patient's symptoms or cardiac implantable electronic device interrogation. Therefore, new onset AF may have been underestimated in the present study because paroxysmal AF, especially asymptomatic episodes, could have gone undiagnosed, and in addition, the time of AF occurrence may have been inaccurate. Thirdly, the majority of subjects in our cohort were male, thus the results cannot be generalized to women. Fourthly, because renal function was evaluated at the time of RFA, true renal function may have been misrepresented. Moreover, we did not have eGFR data at the time of diagnosis of new onset AF, thus, we could not evaluate the association between a change in renal function and new onset AF. Lastly, due to the fact that the number of subjects with impaired renal function was relatively small (10%), the statistical power of the association between impaired renal function and the incidence of AF was probably low. Therefore, further prospective studies using larger populations may be necessary to overcome these limitations.
Conclusion
Impaired renal function may be suggested as a borderline significant predictor of new onset AF after RFA of CTI-AFL. Patients with impaired renal function may require careful attention for the incidence of new onset AF after successful RFA of CTI-AFL.
Acknowledgments
We thank Seungbong Han, PhD, Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, for statistical analysis.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Anné W, Willems R, Van der Merwe N, Van de Werf F, Ector H, Heidbüchel H. Atrial fibrillation after radiofrequency ablation of atrial flutter: preventive effect of angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and diuretics. Heart. 2004;90:1025–1030. doi: 10.1136/hrt.2003.023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinitz JS, Gerstenfeld EP, Marchlinski FE, Callans DJ. Atrial fibrillation is common after ablation of isolated atrial flutter during long-term follow-up. Heart Rhythm. 2007;4:1029–1033. doi: 10.1016/j.hrthm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Laurent V, Fauchier L, Pierre B, Grimard C, Babuty D. Incidence and predictive factors of atrial fibrillation after ablation of typical atrial flutter. J Interv Card Electrophysiol. 2009;24:119–125. doi: 10.1007/s10840-008-9323-1. [DOI] [PubMed] [Google Scholar]
- 4.Moubarak G, Pavin D, Laviolle B, et al. Incidence of atrial fibrillation during very long-term follow-up after radiofrequency ablation of typical atrial flutter. Arch Cardiovasc Dis. 2009;102:525–532. doi: 10.1016/j.acvd.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799–802. doi: 10.1111/j.1540-8167.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee YS, Hyun DW, Jung BC, et al. Left atrial volume index as a predictor for occurrence of atrial fibrillation after ablation of typical atrial flutter. J Cardiol. 2010;56:348–353. doi: 10.1016/j.jjcc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Gilligan DM, Zakaib JS, Fuller I, et al. Long-term outcome of patients after successful radiofrequency ablation for typical atrial flutter. Pacing Clin Electrophysiol. 2003;26(1 Pt 1):53–58. doi: 10.1046/j.1460-9592.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 8.Calkins H, Canby R, Weiss R, et al. Results of catheter ablation of typical atrial flutter. Am J Cardiol. 2004;94:437–442. doi: 10.1016/j.amjcard.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 9.Berkowitsch A, Kuniss M, Greiss H, et al. Impact of impaired renal function and metabolic syndrome on the recurrence of atrial fibrillation after catheter ablation: a long term follow-up. Pacing Clin Electrophysiol. 2012;35:532–543. doi: 10.1111/j.1540-8159.2012.03350.x. [DOI] [PubMed] [Google Scholar]
- 10.Deo R, Katz R, Kestenbaum B, et al. Impaired kidney function and atrial fibrillation in elderly subjects. J Card Fail. 2010;16:55–60. doi: 10.1016/j.cardfail.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananthapanyasut W, Napan S, Rudolph EH, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:173–181. doi: 10.2215/CJN.03170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iguchi Y, Kimura K, Kobayashi K, et al. Relation of atrial fibrillation to glomerular filtration rate. Am J Cardiol. 2008;102:1056–1059. doi: 10.1016/j.amjcard.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt M, Daccarett M, Rittger H, et al. Renal dysfunction and atrial fibrillation recurrence following cardioversion. J Cardiovasc Electrophysiol. 2011;22:1092–1098. doi: 10.1111/j.1540-8167.2011.02069.x. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Rieber J, Daccarett M, et al. Relation of recurrence of atrial fibrillation after successful cardioversion to renal function. Am J Cardiol. 2010;105:368–372. doi: 10.1016/j.amjcard.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 15.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Kim JJ, Kim YH, Cheong SS, et al. Radiofrequency catheter ablation in patients with atrial flutter. Korean Circ J. 1996;26:605–613. [Google Scholar]
- 17.Shah D, Haïssaguerre M, Takahashi A, Jaïs P, Hocini M, Clémenty J. Differential pacing for distinguishing block from persistent conduction through an ablation line. Circulation. 2000;102:1517–1522. doi: 10.1161/01.cir.102.13.1517. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Wu TJ, Kim YH, Yashima M, et al. Progressive action potential duration shortening and the conversion from atrial flutter to atrial fibrillation in the isolated canine right atrium. J Am Coll Cardiol. 2001;38:1757–1765. doi: 10.1016/s0735-1097(01)01606-0. [DOI] [PubMed] [Google Scholar]
- 20.Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779–786. doi: 10.1016/j.jacc.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Park SJ, Byeon K, et al. Risk factors between patients with lone and non-lone atrial fibrillation. J Korean Med Sci. 2013;28:1174–1180. doi: 10.3346/jkms.2013.28.8.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: 'Guyton revisited'. Eur Heart J. 2005;26:11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 23.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27:512–518. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 24.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 25.Goicoechea M, de Vinuesa SG, Lahera V, et al. Effects of atorvastatin on inflammatory and fibrinolytic parameters in patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(12 Suppl):S231–S235. doi: 10.1681/ASN.2006080938. [DOI] [PubMed] [Google Scholar]