Abstract
A central theme in nervous system function is equilibrium: synaptic strengths wax and wane, neuronal firing rates adjust up and down, and neural circuits balance excitation with inhibition. This push/pull regulatory theme carries through to the molecular level at excitatory synapses, where protein function is controlled through phosphorylation and dephosphorylation by kinases and phosphatases. However, these opposing enzymatic activities are only part of the equation as scaffolding interactions and assembly of multi-protein complexes are further required for efficient, localized synaptic signaling. This review will focus on coordination of postsynaptic serine/threonine kinase and phosphatase signaling by scaffold proteins during synaptic plasticity.
Keywords: A-kinase anchoring protein (AKAP), Ca2+/calmodulin-dependent protein kinase II (CaMKII), calcineurin, calcium channel, excitation-transcription coupling, glutamate receptor, phosphoprotein phosphatase 1 (PP1), protein kinase A (PKA), scaffold protein, synaptic plasticity
Control of Synaptic Strength through Balanced Phosphorylation/Dephosphorylation
A defining aspect of the mammalian brain is its profound capacity for experience-dependent plasticity that modifies the strength of specific synaptic connections between neurons. Two well studied opposing forms of synaptic plasticity at excitatory synapses are long-term potentiation (LTP)2 and long-term depression (LTD), which strengthen and weaken synapses, respectively. LTP and LTD have been most heavily studied in a brain region called the hippocampus where they support spatial and declarative learning and memory. LTP and LTD are induced by Ca2+ influx through postsynaptic NMDA-type ionotropic glutamate receptors (NMDARs) and are expressed by long-lasting increases or decreases, respectively, in the function of AMPA-type ionotropic glutamate receptors (AMPARs) that mediate the bulk of excitatory synaptic transmission (1, 2).
NMDARs are heterotetrameric assemblies most commonly containing two GluN1 and two GluN2A-2D subunits and are permeable to Na+, K+, and Ca2+. At hippocampal synapses, NMDARs are assembled from GluN1, GluN2A, and GluN2B subunits. AMPARs are heterotetrameric assemblies of GluA1–GluA4 subunits, with most being permeable only to Na+ and K+ due to inclusion of GluA2 subunits that prevent Ca2+ influx (3). However, hippocampal neurons can also express small numbers of Ca2+-permeable AMPARs lacking GluA2 subunits (i.e. GluA1 homomers) that primarily reside in extrasynaptic and intracellular locations but can be recruited to synapses during plasticity and following neuronal injuries (4). Intriguingly, there is much commonality in the molecular mechanisms underlying the ostensibly antagonistic processes of LTP and LTD; both require correlated pre- and postsynaptic activity leading to NMDAR Ca2+ influx and are mediated by overlapping sets of enzymes. However, it is the ability of the synapse to detect subtle differences in Ca2+ and other second messengers and efficiently transduce these signals to discrete downstream signaling pathways that permits diametrically opposed outcomes to arise from grossly similar synaptic stimuli.
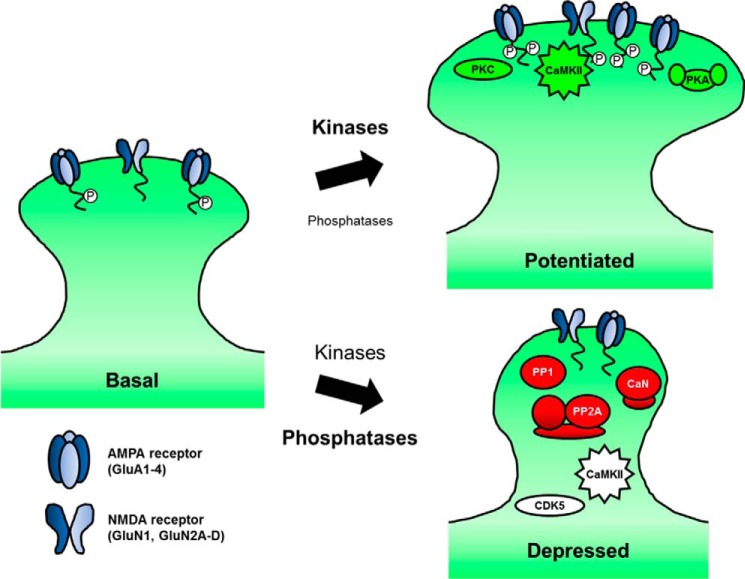
Ultimately, excitatory synaptic plasticity must add, remove, or modify AMPARs to alter synaptic strength. Although AMPAR regulation during plasticity is covered in-depth elsewhere in this series (see Roche and colleagues (101)), it bears mentioning here. Brief, strong NMDAR Ca2+ influx can activate a host of kinases that increase AMPAR activity during LTP through phosphorylating both AMPARs (2, 5–7) and other regulatory proteins (5, 8, 9). AMPAR GluA1 subunits, in particular, are phosphorylated on several C-terminal tail residues to alter channel biophysical properties and synaptic localization. For example, Ca2+-calmodulin-dependent protein kinase II (CaMKII) and PKC phosphorylate GluA1 on Ser-831 and Ser-818 (PKC only) to increase single channel conductance and synaptic incorporation during LTP. GluA1 is also phosphorylated on Ser-845 by the cAMP-dependent protein kinase (PKA), which increases channel open probability and stimulates recycling exocytosis to prime AMPARs for synaptic insertion during LTP (reviewed in Refs. 5–7). Thus, although CaMKII may be the most important, direct transducer of NMDAR Ca2+ signaling during LTP, multiple postsynaptic kinases collectively promote potentiation (Fig. 1).
FIGURE 1.
Postsynaptic phosphorylation/dephosphorylation signaling during synaptic plasticity. During synaptic potentiation, brief, strong cytosolic Ca2+ predominantly activates kinases such as CaMKII, PKC, and PKA. AMPA (GluA1–4 subunits) and NMDA-type (GluN1 and GluN2A-2D subunits) glutamate receptors are among the many synaptic targets that are phosphorylated, promoting stronger synaptic transmission. Conversely, during the modest, prolonged influx of Ca2+ that initiates synaptic depression, phosphatase activity in general outweighs kinase activity; CaN, PP1, and PP2A dephosphorylate receptors, scaffolds, and other synaptic proteins, resulting in smaller dendritic spines and diminished synaptic strength. However, kinases also play roles in synaptic depression.
Conversely, LTD can be elicited by weak but sustained NMDAR Ca2+ influx. Under these conditions, protein phosphatases 1 (PP1), 2A (PP2A), and calcineurin (CaN; also known as PP2B) become activated (10–12) (Fig. 1). Consequently, dephosphorylation of AMPARs as well as other postsynaptic targets is generally favored during LTD. In particular, GluA1 Ser-845 dephosphorylation is critical for AMPAR removal during LTD through promoting endocytosis and preventing recycling to favor receptor degradation (reviewed in Refs. 5–7). Because of its direct activation by Ca2+ and CaM, CaN is probably the most important, direct transducer of NMDAR Ca2+ signaling during LTD, but other phosphatases and kinases, including CaMKII (discussed below), also play essential roles during LTD (Fig. 1). Thus, an overriding question addressed in this review is how are ubiquitous second messenger systems (cAMP and Ca2+) and signaling enzymes with broad substrate specificity (PKA, CaMKII, PP1, and CaN) organized at synapses to coordinate very specific, localized signaling events during LTP and LTD?
Coordinated Regulation of Postsynaptic PKA, PKC, and Calcineurin Signaling by AKAP79/150
Important answers to the above question may be found in the network of scaffolding interactions found in the postsynaptic density (PSD), a structure located at the tip of dendritic spines opposite the presynaptic terminal (see also Spence and Soderling (102) in this issue for more on dendritic spine structure). Scaffold proteins in this PSD network position signaling enzymes to respond to second messengers and exert rapid effects on synaptic substrates. The PKA-PKC-CaN complex assembled by A-kinase anchoring protein (AKAP) 79/150 (human 79/rodent 150; also known as AKAP5) is a prototypical example of a postsynaptic scaffold-organized signaling complex (Fig. 2) (13).
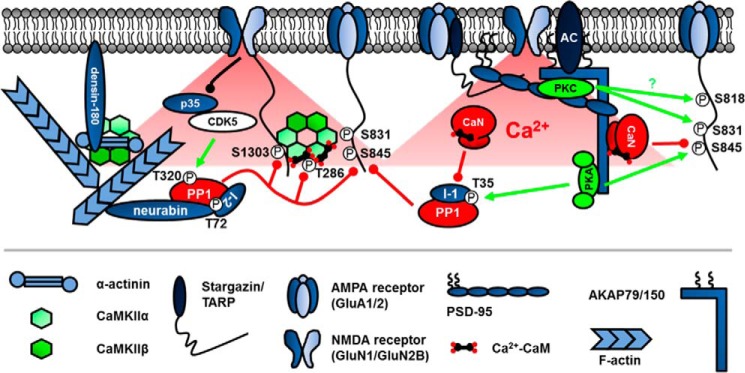
FIGURE 2.
Kinase and phosphatase scaffolding is critical for postsynaptic signaling during plasticity. Enzymes are targeted to Ca2+ and cAMP sources and important substrates through association with synaptic scaffolds such as AKAP79/150 and PSD-95 as well as actin-binding proteins such as neurabin and α-actinin. CaMKII is unique in that it serves as an enzyme, self-scaffold, and actin organizer. Circled P represents phosphorylation. Green lines represent phosphorylation, and red lines represent dephosphorylation. The black line represents degradation of p35 that inactivates CDK5 kinase activity. The specific AMPARs and NMDARs depicted are GluA1/2 and GluN1/2B. TARP is an abbreviation for transmembrane AMPA receptor regulatory protein.
First characterized by its ability to bind PKA through its C-terminal amphipathic α-helical domain (14), AKAP79/150 was subsequently shown to bind CaN (15) through a variant of the PXIXIT motif found in other CaN-binding proteins (16–18) and PKC through its N-terminal membrane-targeting domain (19). AKAP79/150 also acts as a structural scaffolding hub as it binds F-actin (20), the plasma membrane lipid phosphatidylinositol-4,5-bisphosphate (21), synaptic adhesion molecules (22), and PSD-95 family scaffold proteins that link to NMDARs and AMPARs (23). Given its collection of anchored enzymes and linkage to glutamate receptors, it is not surprising that AKAP79/150 plays an integral role in synaptic plasticity. Indeed, experiments using AKAP150 knock-out mice demonstrated roles for this scaffold in hippocampal LTD and spatial learning (24). Additional analysis of AKAP150 C-terminal truncation knock-in mice lacking the PKA anchoring site showed more specifically that AKAP-PKA signaling promotes GluA1 Ser-845 phosphorylation and supports LTP, LTD, and reversal learning (25–27). Furthermore, studies employing RNAi with mutant replacement (28, 29) and an AKAP150 knock-in mouse lacking the PXIXIT-like CaN docking motif (30) reveal that anchored CaN mediates GluA1 Ser-845 dephosphorylation and AMPAR endocytosis to promote LTD and constrain LTP. In addition, genetic disruption of AKAP-CaN or -PKA anchoring alters spatial and contextual fear learning and memory.3 Finally, although AKAP79/150-anchored PKC can phosphorylate GluA1 Ser-831 in heterologous cells and cultured neurons, the role of AKAP-PKC anchoring in synaptic plasticity and cognition has not been addressed (31). Taken together, these studies illustrate the critical role that scaffolding can play in locally balancing phosphorylation and dephosphorylation to control synaptic plasticity.
In addition to positioning kinases and phosphatases, AKAPs can bind other key components of the cAMP signaling pathway, including G protein-coupled receptors, adenylyl cyclases (ACs), and phosphodiesterases. AKAP79/150 binds to the β2-adrenergic receptor (β2AR) (32) and multiple AC isoforms (33, 34). β2ARs couple to AC-cAMP-PKA signaling to enhance LTP and learning and memory through GluA1 phosphorylation (35, 36). In a series of elegant experiments comparing AKAP150 knock-out mice with AKAP150-PKA binding-deficient mice, AKAP knockouts exhibited a greater deficiency in β2AR enhancement of GluA1 Ser-845 phosphorylation and LTP (37). The less severe phenotype of the PKA binding-deficient mice was attributed to preserved interaction with AC, resulting in normal β2AR-stimulated cAMP production that likely signaled, albeit less effectively, through other pools of PKA. Of note, AKAP250/Gravin (also known as AKAP12) can also associate with β2ARs to facilitate LTP regulation (38). Thus, there is likely interplay between these two AKAP-PKA complexes in postsynaptic LTP regulation. Interestingly, in many of the above studies, AKAP79/150-anchored PKA and CaN were found to impact LTP/LTD through preferential control of GluA1 Ca2+-permeable AMPARs, perhaps because these GluA1 homomers can be phosphorylated on four Ser-845 sites as compared with only two in GluA1/2 heteromers (26, 30, 37).
Due to their micrometer size, dendritic spines themselves are microdomains for compartmentalized signaling, but it is clear that AKAP79/150 and other PSD scaffold proteins nucleate postsynaptic signaling complexes that function on the molecular/nanometer scale. Such intra-spine nano-domain signaling may occur near receptors in the PSD, in extrasynaptic regions of the spine plasma membrane, or in spine-localized endosomes. AKAP79/150 also serves as an excellent example of postsynaptic nano-targeting, as its own localization is fine-tuned by reversible palmitoylation of its N-terminal targeting domain by the palmitoyl-acyltransferase DHHC2 (39, 40). AKAP79/150 palmitoylation is not required for general targeting to the plasma membrane but is necessary for specific localization to plasma membrane lipid rafts (which are associated with the PSD) and recycling endosomes (40, 41). Importantly, AKAP79/150 palmitoylation in endosomes is required for stimulation of recycling exocytosis and delivery of the AKAP and GluA1 to synapses during chemical LTP induction (39, 40). However, in general, our understanding of the trafficking of AKAP79/150 and other postsynaptic scaffolds lags behind our understanding of the AMPAR trafficking that they control. We do know that AKAP79/150 can be uncoupled from PSD-95 scaffolds and removed from both the postsynaptic membrane and the endosomes during chemical LTD induction through inhibition of its N-terminal targeting interactions via a combination of depalmitoylation (39), phospholipase C cleavage of phosphatidylinositol-4,5-bisphosphate, and CaN-dependent F-actin reorganization. Importantly, this inhibition of AKAP79/150 membrane targeting during LTD may prevent PKA-mediated re-phosphorylation of GluA1 that would promote recycling and reverse LTD (reviewed in Ref. 13). Interestingly, changes in AKAP79/150 synaptic localization, PKA and CaN signaling, and GluA1 Ser-845 phosphorylation have also recently been implicated in regulating GluA1 synaptic localization during slower, homeostatic forms of synaptic plasticity that scale synaptic strength up or down across all inputs in response to chronic increases or decreases in overall neuronal activity, respectively (42, 43).
Regulation of Postsynaptic CaMKII Signaling
CaMKII is dodecameric holoenzyme assembled from α, β, γ, and δ isoforms. In most neurons, CaMKII contains α > β ≫ γ/δ isoforms. Due to its enrichment at synapses and mechanisms of Ca2+ regulation, CaMKII (α in particular) has attracted substantial attention in the synaptic plasticity field (44–46). In response to Ca2+ elevation, Ca2+-CaM binding to CaMKII displaces the autoinhibitory domains to permit active site access for both exogenous substrates and Thr-286 (Thr-287 on β, γ, δ) in the autoinhibitory domain of neighboring Ca2+-CaM-bound subunits. Autophosphorylation of Thr-286 modifies CaMKII function in two ways: it enhances Ca2+-CaM binding affinity (so-called CaM trapping) and prevents the autoinhibitory domain from fully occupying the active site, generating what is referred to as CaMKII “autonomy” wherein the kinase remains partially active after CaM dissociation. Importantly, CaMKII autonomy functions as a form of “molecular memory” of past Ca2+ signals and plays crucial roles in both LTP induction and learning and memory (47, 48). CaMKII Thr-286 can be dephosphorylated by either PP1 or PP2A, but PP1 appears to play a more prominent role in dephosphorylation of CaMKII in the PSD (49). Following strong synaptic stimulation, more CaMKII is also rapidly recruited to the PSD (50–53), where it phosphorylates not only AMPARs and its auxiliary subunits (8, 9) but also small GTPase regulators (54, 55) and adhesion molecules (56). With so many important targets, how is CaMKII postsynaptic signaling controlled? Because there are a number of comprehensive reviews on this topic (44–46), here we will primarily focus on specific postsynaptic scaffolding interactions that control CaMKII signaling (Fig. 2).
The CaMKII holoenzyme configuration permits CaMKII α and β subunits to serve as scaffolds for one another through both common and distinct interacting proteins. F-actin binding is one such interaction that is distinctly controlled by β versus α subunits. CaMKII can associate indirectly with F-actin through the adapter protein α-actinin (57) but can also bind F-actin directly through its β isoform (58–61). Upon activation by Ca2+-CaM, CaMKIIβ unbinds from F-actin, allowing the kinase to redistribute and establish new interactions. In particular, CaMKII activation promotes binding to the PSD scaffold densin-180; however, because densin-180 also binds α-actinin, a ternary complex results that can still be linked to F-actin but also further associate with NMDARs through CaMKII binding to GluN2B subunit (57, 62, 63). However, adding to this complexity, there are secondary interactions of CaMKII with the GluN1 subunit that are instead inhibited by α-actinin binding to GluN1 (64). Importantly, CaMKII and F-actin have reciprocal effects on one another; CaMKII is positioned by its interactions with F-actin but in turn can directly bundle and stabilize actin fibers through its β isoform in a Ca2+-CaM-regulated manner (59–61). Indeed, CaMKIIβ is important for the integrity of the actin cytoskeleton, and its loss destabilizes spines (61). Interestingly, this effect of CaMKIIβ loss on spines can be rescued with a kinase-inactive mutant, highlighting the function of CaMKII as a structural scaffold. The importance of this kinase-independent role is further supported by comparing the phenotypes of CaMKIIβ knock-out mice with those expressing kinase-inactive CaMKIIβ (65). Although CaMKIIβ knock-out impairs CaMKII synaptic localization, LTP, and learning, none of these functions are disrupted by the kinase-inactive mutation.
As alluded to above, another key CaMKII postsynaptic interaction is with the NMDAR GluN2B subunit, which contains a binding site in its C-terminal tail near the CaMKII phosphorylation site Ser-1303 (66–69). Recruitment of activated CaMKII to GluN2B occurs rapidly following Ca2+ stimulation but, like Thr-286 phosphorylation, outlasts the Ca2+ signal, thus permitting postsynaptic CaMKII to participate in yet another form of “molecular memory.” Indeed, CaMKII binding to GluN2B has been implicated in LTP maintenance and memory consolidation. In one study, a high dose of an inhibitor peptide (tatCN21), which competes with GluN2B for CaMKII binding, was found to acutely disrupt both GluN2B-CaMKII binding and LTP maintenance (70). In a second study, CaMKII binding-deficient GluN2B knock-in mice exhibited reductions in GluA1 Ser-831 phosphorylation, LTP, and memory consolidation (71). Collectively, these findings support a model in which CaMKII enzymatic and non-enzymatic functions may work together to process and store information at excitatory synapses.
Abundant evidence exists for the role of CaMKII in LTP, but there is accumulating evidence that it also participates in LTD (72). Intriguingly, a newly characterized Ser-567 phospho-site in GluA1, which results in AMPAR synaptic exclusion (73), was recently shown to be phosphorylated by autonomous CaMKII under NMDAR LTD conditions (72). In addition, GluA1 Ser-567 exhibits distinct characteristics as compared with typical CaMKII substrates implicated in LTP (i.e. Ser-831) in that Ca2+-CaM stimulation does not elevate Ser-567 phospho-levels above those obtained with autonomous CaMKII. Thus, an intriguing new hypothesis is that there are two classes of CaMKII substrates that are differentially regulated by stimulated versus autonomous kinase activity to favor either LTP or LTD, respectively. These recent findings also provide a clear exception to the over-simplified rule that kinases mediate synaptic potentiation and phosphatases mediate synaptic depression.
Regulation of Postsynaptic PP1 Signaling
Another challenge in understanding plasticity is that synaptic changes are often controlled through multiple parallel and overlapping signaling pathways, such as CaN, PP1, and PP2A phosphatases that all participate in LTD (10–12). Like control of CaN signaling by AKAP79/150, PP1 and PP2A signaling also rely heavily on binding partners to regulate activity and subcellular targeting, but because less is known about regulation of PP2A signaling during LTD, here we will only discuss PP1. Prominent among postsynaptic PP1 regulatory proteins are the related F-actin-binding proteins neurabin-1 (Fig. 2) and spinophilin (also called neurabin-2), which anchor PP1 through modular binding motifs with the loose consensus sequence (K/R)(V/I)X(F/W) that is commonly abbreviated as RVXF (74). Importantly, disruption of the interaction between PP1 and neurabin/spinophilin using competing RVXF binding motif peptides can block LTD (75). In addition, specific interference with neurabin-PP1 association by mutation inhibits LTD, whereas overexpression of wild-type neurabin-1 enhances LTD and promotes dephosphorylation of GluA1 Ser-845 and Ser-831 (76, 77). Overall, these findings support a model where neurabin-1 recruits PP1 to synapses to promote AMPAR dephosphorylation during LTD.
Thus, another key question is how is PP1 enzymatic activity regulated by synaptic activity when complexed with scaffolds such as neurabin? Historically, models for NMDAR activation of PP1 during hippocampal LTD have implicated CaN-mediated dephosphorylation of PP1 inhibitory PKA substrate inhibitor-1 (I-1) (10). Also, PKA phosphorylation of neurabin-1 can inhibit its PP1 binding, a mechanism that may favor LTP (76, 78). However, several additional PP1 regulatory mechanisms have recently received attention in NMDAR signaling, including PP1 binding to inhibitor-2 (I-2), possibly in a ternary complex with neurabin-1, and PP1 inhibitory phosphorylation on Thr-320. In particular, recent work identified cyclin-dependent kinase 5 (CDK5) as the kinase for PP1 Thr-320 and demonstrated that when CDK5 is inhibited by synaptic NMDAR activation via proteasomal degradation of its p35 subunit, PP1 auto-dephosphorylates to become active (79). This study also uncovered a requirement for association of PP1 with I-2, which was increased by I-2 Thr-72 dephosphorylation, to promote PP1 signaling during NMDAR LTD; this mechanism stands in contrast with reversal of PP1 association with I-1 through Thr-35 dephosphorylation. Collectively, these studies indicate that a kinase (CDK5) and multiple PP1-binding proteins conspire to regulate postsynaptic PP1 activity (Fig. 2). Interestingly, another recent study found that PP1 is also required for homeostatic synaptic downscaling, but in this case, PP1 is activated by release from I-2 inhibition through Ser-43 phosphorylation by myosin light-chain kinase (80).
Coordinated Kinase and Phosphatase Signaling in Postsynaptic Excitation-Transcription Coupling
Longer-lasting forms of plasticity that are maintained for hours, days, months, or even years, such as the late phase of LTP, require not only initial changes in AMPAR synaptic localization that occur during the early-phase of LTP but also subsequent gene transcription (81) and protein synthesis (covered by Alvarez-Castelao and Schuman (103) in this issue). Several pathways linking acute changes in neuronal activity to persistent alterations in gene transcription rely on local Ca2+ influx through voltage-gated L-type calcium channels (LTCCs) to trigger phosphorylation or dephosphorylation of transcription factors, such as Ca2+/cAMP-responsive element-binding protein (CREB), CREB-regulated transcriptional coactivator (CRTC1), and nuclear factor of activated T-cells (NFAT) (82–84). In particular, a privileged role for LTCC Ca2+ signaling to CREB in neuronal excitation-transcription (E-T) coupling associated with LTP/LTD and long-term memory has been known for almost 25 years (85–88).
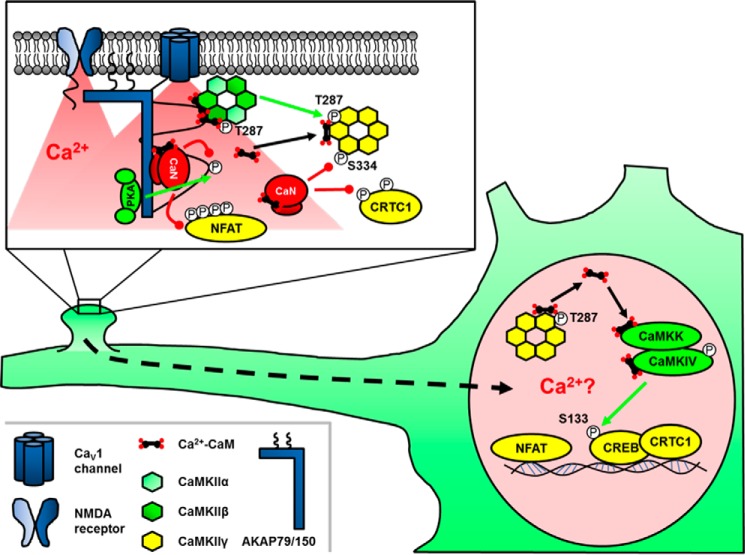
The transcriptional activating function of CREB itself can be controlled by phosphorylation on Ser-133 by a variety of kinases including PKA, CaMKs, and ERK (81). However, studies of E-T coupling have revealed vital roles for not only kinases but also phosphatases in activating CREB-dependent transcription. In a variety of neuron types, LTCCs activate both CaN and CaMKII, which can have either opposing or cooperative roles in regulating CREB-mediated transcription (83, 89, 90). However, recent studies in sympathetic and cortical neurons found that LTCC Ca2+ influx recruits CaMKIIβ to the channel to trans-phosphorylate Thr-287 in the autoinhibitory domain of CaMKIIγ and promote Ca2+-CaM trapping, whereas concomitant activation of CaN dephosphorylates Ser-334 in a nuclear localization sequence on CaMKIIγ. Subsequent CaMKIIγ translocation and shuttling of CaM to the nucleus then activates CaMK kinase (CaMKK) and CaMKIV to phosphorylate CREB (91). Interestingly, a kinase-inactive mutant of CaMKIIγ, which could not phosphorylate itself or other substrates but still served as substrate for CaMKIIβ, was able to signal to CREB, thus providing additional support for a CaM shuttling rather than an enzymatic role for CaMKIIγ. Thus, phospho-regulation of CaMKIIγ at two critical sites allows it to shuttle CaM to the nucleus and induce gene transcription. However, the molecular details of how changes in CaMKIIγ phosphorylation state and CaM binding are so precisely regulated to only load CaM at the channel and then release it in the nucleus await further investigation. In addition, according to this mechanism, only CaMKII holoenzymes exclusively composed of the γ isoform must be able to enter the nucleus (Fig. 3); otherwise it is hard to rule out additional signaling by enzymatically active CaMKIIβ that enters the nucleus in association with CaMKIIγ.
FIGURE 3.
Signaling by kinases and phosphatases in postsynaptic excitation-transcription coupling. Anchored kinases and phosphatases finely tune Ca2+ influx through LTCCs. The calcium-activated phosphatase CaN dephosphorylates nuclear localization sequences on the transcription factors NFAT and CRTC1, permitting their translocation to the nucleus. LTCC-calcium influx also activates CaMKIIα/β that can phosphorylate and “lock” CaMKIIγ in a conformation that tightly binds Ca2+-CaM. Subsequent CaN-mediated dephosphorylation of the nuclear localization sequence of CaMKIIγ drives nuclear translocation and delivery of Ca2+-CaM to CaMKIV, which phosphorylates and actives CREB. Green lines represent phosphorylation, and red lines represent dephosphorylation.
The above CaMKII-CaMKIV signaling mechanism also likely contributes only to an initial, rapid phase of CREB activation (seconds to minutes) following excitation where Ca2+ increases not only near LTCCs but also globally to maintain Ca2+-bound CaM all the way to the nucleus. In contrast, more prolonged CREB activation (minutes to hours) in response to local LTP induction and restricted Ca2+ influx in dendrites is thought to be mediated by local, postsynaptic CaMKII activation of the ERK pathway followed by slower, longer-distance translocation of ERK signaling from dendrites to nucleus to phosphorylate CREB (87, 92, 93). In addition, the CREB co-activator CRTC1 is dephosphorylated by CaN in response to LTCC Ca2+ influx triggered by synaptic input to dendrites and then also slowly translocates distally to the nucleus where it is required for CREB-dependent gene expression underlying fear memory (83, 94). Thus, although many key players in CREB E-T coupling have been identified, future work must further explore the mechanisms of cellular and synaptic organization that permit signaling to CREB over these different distance and time scales.
A parallel E-T coupling system, in which more has already been learned regarding the contribution of postsynaptic organization to nuclear signaling, is NFAT translocation to the nucleus (over minutes to an hour) following brief, local LTCC Ca2+ influx in neurons. Work over the past decade has revealed the complexity and importance of an AKAP79/150-organized signaling complex in both PKA/CaN bi-directional regulation of neuronal LTCC activity and NFAT-mediated E-T coupling (Fig. 3) (18, 95, 96). AKAP79/150 directly binds the LTCC CaV1.2 through a C-terminal leucine zipper and anchors CaN through a PXIXIT-like motif that is very similar to the CaN docking sequences found in the NFAT transcription factors themselves (17). However, despite essentially competing with NFAT for CaN binding and also suppressing PKA-mediated enhancement of LTCC activity, AKAP79/150-CaN anchoring is critical for NFAT activation by LTCC Ca2+ influx (18, 95). How exactly is LTCC-CaN-NFAT signaling promoted by the AKAP? Guided by the crystal structure of the AKAP-CaN complex, mutations in the PXIXIT-like motif designed to either increase or decrease CaN anchoring affinity, were both, surprisingly, found to inhibit NFAT activation. In particular, increasing anchoring affinity immobilized CaN in spines and prevented NFAT translocation to the nucleus (97). Thus, AKAP-CaN anchoring is by necessity dynamic and promotes NFAT signaling by balancing strong recruitment of CaN near its upstream activator, the LTCC, with its efficient release to communicate with its downstream effector NFAT.
Building on this theme of dynamics and balance in local signaling complexes, additional research found that disruption of AKAP79/150-PKA anchoring, both through acute overexpression and through knock-in of PKA anchoring-deficient mutants, also prevents NFAT signaling, a deficit attributable to profound decreases in basal LTCC phosphorylation, current density, and depolarization-evoked Ca2+ influx (98, 99). Thus, neuronal LTCC-NFAT transcriptional signaling requires precise organization and balancing of the phosphatase activity of CaN in the channel nano-environment, which is required for NFAT activation, with the opposing kinase activity of PKA, which is needed to prevent CaN from suppressing channel function. Importantly, these studies of AKAP79/150 provide some of the most complete and detailed molecular mechanisms to date explaining how local Ca2+ signaling by LTCC plays such a privileged role in neuronal E-T coupling.
Conclusions and Future Directions
It is clear that postsynaptic kinase/phosphatase signaling balance is key to synaptic plasticity regulation on multiple time scales through controlling both local signal transduction confined to synapses as well as distal communication with the nucleus. In all cases, the high degree of signaling specificity and efficiency required is conferred by regulatory binding partners/scaffolds that are as important as the activities of the kinases and phosphatases themselves in determining impacts on synaptic function. Indeed, distinct and even opposing types of plasticity can involve the same kinase and phosphatase players, but the actions of these players appear to be directed by scaffolding interactions toward one pathway or another to achieve different outcomes. Future research should further elucidate how scaffold proteins play key roles in such local, postsynaptic decision making through interrogating signaling complexes on the nanometer scale using new super-resolution microscopy methods (100). In addition, it will be important to further explore how scaffold-directed phosphorylation intersects with signaling through other reversible post-translational modifications, such as palmitoylation and ubiquitination (6)(see also Alvarez-Castelao and Schuman (103) in this issue). Finally, given that many neuropsychiatric and neurological disorders are characterized by alterations in synaptic plasticity, a better understanding of how kinase/phosphatase signaling pathways are organized at synapses will hopefully identify novel drug targets and therapies for brain diseases. Precisely targeting synaptic scaffolding interactions could have many advantages, in terms of improved efficacy and specificity, over globally inhibiting kinase and phosphatase catalytic activity using existing pharmacology.
Acknowledgments
We thank Drs. Matthew Kennedy and Ulli Bayer for critically reading this manuscript. Although we attempted to be as inclusive and comprehensive as possible and highlight both past and recent publications in the field, we apologize for omission of any other important contributions and references from this minireview. Due to space constraints, we were required to limit the number of citations and focus our review on specific topics and examples from the literature.
This work was supported by National Institutes of Health Grants NS040701 and MH102338 (to M. L. D.). This is the second article in the Thematic Minireview series “Molecular Mechanisms of Synaptic Plasticity.” The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
M. L. Dell'Acqua, unpublished observations.
- LTP
- long-term potentiation
- LTD
- long-term depression
- AC
- adenylyl cyclase
- AKAP
- A-kinase anchoring protein
- β2AR
- β2-adrenergic receptors
- CaM
- calmodulin
- CaMK
- Ca2+-calmodulin-dependent protein kinase
- CaN
- calcineurin
- CDK5
- cyclin-dependent kinase 5
- CREB
- Ca2+/cAMP-responsive element-binding protein
- CRTC1
- CREB-regulated transcriptional co-activator
- E-T
- excitation-transcription
- I-1
- inhibitor-1
- I-2
- inhibitor-2
- LTCC
- L-type calcium channel
- NFAT
- nuclear factor of activated T-cells
- PP1
- protein phosphatase 1
- PP2A
- protein phosphatase 2A
- PP2B
- protein phosphatase 2B
- PSD
- postsynaptic density
- NMDAR
- NMDA-type ionotropic glutamate receptor
- AMPAR
- AMPA-type ionotropic glutamate receptor.
References
- 1.Malenka R. C., and Bear M. F. (2004) LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 [DOI] [PubMed] [Google Scholar]
- 2.Huganir R. L., and Nicoll R. A. (2013) AMPARs and synaptic plasticity: the last 25 years. Neuron 80, 704–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dingledine R., Borges K., Bowie D., and Traynelis S. F. (1999) The glutamate receptor ion channels. Pharmacol. Rev. 51, 7–61 [PubMed] [Google Scholar]
- 4.Liu S. J., and Zukin R. S. (2007) Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 30, 126–134 [DOI] [PubMed] [Google Scholar]
- 5.Lee H. K. (2006) Synaptic plasticity and phosphorylation. Pharmacol. Ther. 112, 810–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu W., and Roche K. W. (2012) Posttranslational regulation of AMPA receptor trafficking and function. Curr. Opin. Neurobiol. 22, 470–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anggono V., and Huganir R. L. (2012) Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 22, 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomita S., Stein V., Stocker T. J., Nicoll R. A., and Bredt D. S. (2005) Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron 45, 269–277 [DOI] [PubMed] [Google Scholar]
- 9.Opazo P., Labrecque S., Tigaret C. M., Frouin A., Wiseman P. W., De Koninck P., and Choquet D. (2010) CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67, 239–252 [DOI] [PubMed] [Google Scholar]
- 10.Mulkey R. M., Endo S., Shenolikar S., and Malenka R. C. (1994) Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369, 486–488 [DOI] [PubMed] [Google Scholar]
- 11.Nicholls R. E., Alarcon J. M., Malleret G., Carroll R. C., Grody M., Vronskaya S., and Kandel E. R. (2008) Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron 58, 104–117 [DOI] [PubMed] [Google Scholar]
- 12.Beattie E. C., Carroll R. C., Yu X., Morishita W., Yasuda H., von Zastrow M., and Malenka R. C. (2000) Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat. Neurosci. 3, 1291–1300 [DOI] [PubMed] [Google Scholar]
- 13.Sanderson J. L., and Dell'Acqua M. L. (2011) AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist 17, 321–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr D. W., Stofko-Hahn R. E., Fraser I. D., Cone R. D., and Scott J. D. (1992) Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins. Characterization of AKAP 79. J. Biol. Chem. 267, 16816–16823 [PubMed] [Google Scholar]
- 15.Coghlan V. M., Perrino B. A., Howard M., Langeberg L. K., Hicks J. B., Gallatin W. M., and Scott J. D. (1995) Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science 267, 108–111 [DOI] [PubMed] [Google Scholar]
- 16.Dell'Acqua M. L., Dodge K. L., Tavalin S. J., and Scott J. D. (2002) Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315–360. J. Biol. Chem. 277, 48796–48802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H., Rao A., and Hogan P. G. (2011) Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 21, 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveria S. F., Dell'Acqua M. L., and Sather W. A. (2007) AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 55, 261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klauck T. M., Faux M. C., Labudda K., Langeberg L. K., Jaken S., and Scott J. D. (1996) Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science 271, 1589–1592 [DOI] [PubMed] [Google Scholar]
- 20.Gomez L. L., Alam S., Smith K. E., Horne E., and Dell'Acqua M. L. (2002) Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J. Neurosci. 22, 7027–7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dell'Acqua M. L., Faux M. C., Thorburn J., Thorburn A., and Scott J. D. (1998) Membrane-targeting sequences on AKAP79 bind phosphatidylinositol-4,5-bisphosphate. EMBO J. 17, 2246–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorski J. A., Gomez L. L., Scott J. D., and Dell'Acqua M. L. (2005) Association of an A-kinase-anchoring protein signaling scaffold with cadherin adhesion molecules in neurons and epithelial cells. Mol. Biol. Cell 16, 3574–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colledge M., Dean R. A., Scott G. K., Langeberg L. K., Huganir R. L., and Scott J. D. (2000) Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 27, 107–119 [DOI] [PubMed] [Google Scholar]
- 24.Tunquist B. J., Hoshi N., Guire E. S., Zhang F., Mullendorff K., Langeberg L. K., Raber J., and Scott J. D. (2008) Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc. Natl. Acad. Sci. U.S.A. 105, 12557–12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisenhaus M., Allen M. L., Yang L., Lu Y., Nichols C. B., Su T., Hell J. W., and McKnight G. S. (2010) Mutations in AKAP5 disrupt dendritic signaling complexes and lead to electrophysiological and behavioral phenotypes in mice. PLoS ONE 5, e10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y., Allen M., Halt A. R., Weisenhaus M., Dallapiazza R. F., Hall D. D., Usachev Y. M., McKnight G. S., and Hell J. W. (2007) Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J. 26, 4879–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y., Zhang M., Lim I. A., Hall D. D., Allen M., Medvedeva Y., McKnight G. S., Usachev Y. M., and Hell J. W. (2008) AKAP150-anchored PKA activity is important for LTD during its induction phase. J. Physiol. 586, 4155–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jurado S., Biou V., and Malenka R. C. (2010) A calcineurin/AKAP complex is required for NMDA receptor-dependent long-term depression. Nat. Neurosci. 13, 1053–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya S., Biou V., Xu W., Schlüter O., and Malenka R. C. (2009) A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat. Neurosci. 12, 172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanderson J. L., Gorski J. A., Gibson E. S., Lam P., Freund R. K., Chick W. S., and Dell'Acqua M. L. (2012) AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. J. Neurosci. 32, 15036–15052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tavalin S. J. (2008) AKAP79 selectively enhances protein kinase C regulation of GluR1 at a Ca2+-calmodulin-dependent protein kinase II/protein kinase C site. J. Biol. Chem. 283, 11445–11452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser I. D., Cong M., Kim J., Rollins E. N., Daaka Y., Lefkowitz R. J., and Scott J. D. (2000) Assembly of an AKAP/β2-adrenergic receptor signaling complex facilitates receptor phosphorylation and signaling. Curr. Biol. 10, 409–412 [DOI] [PubMed] [Google Scholar]
- 33.Efendiev R., Samelson B. K., Nguyen B. T., Phatarpekar P. V., Baameur F., Scott J. D., and Dessauer C. W. (2010) AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and -6 to α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J. Biol. Chem. 285, 14450–14458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauman A. L., Soughayer J., Nguyen B. T., Willoughby D., Carnegie G. K., Wong W., Hoshi N., Langeberg L. K., Cooper D. M., Dessauer C. W., and Scott J. D. (2006) Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol. Cell 23, 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian H., Matt L., Zhang M., Nguyen M., Patriarchi T., Koval O. M., Anderson M. E., He K., Lee H. K., and Hell J. W. (2012) β2-Adrenergic receptor supports prolonged theta tetanus-induced LTP. J. Neurophysiol. 107, 2703–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu H., Real E., Takamiya K., Kang M. G., Ledoux J., Huganir R. L., and Malinow R. (2007) Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 131, 160–173 [DOI] [PubMed] [Google Scholar]
- 37.Zhang M., Patriarchi T., Stein I. S., Qian H., Matt L., Nguyen M., Xiang Y. K., and Hell J. W. (2013) Adenylyl cyclase anchoring by a kinase anchor protein AKAP5 (AKAP79/150) is important for postsynaptic β-adrenergic signaling. J. Biol. Chem. 288, 17918–17931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Havekes R., Canton D. A., Park A. J., Huang T., Nie T., Day J. P., Guercio L. A., Grimes Q., Luczak V., Gelman I. H., Baillie G. S., Scott J. D., and Abel T. (2012) Gravin orchestrates protein kinase A and β2-adrenergic receptor signaling critical for synaptic plasticity and memory. J. Neurosci. 32, 18137–18149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keith D. J., Sanderson J. L., Gibson E. S., Woolfrey K. M., Robertson H. R., Olszewski K., Kang R., El-Husseini A., and Dell'Acqua M. L. (2012) Palmitoylation of A-kinase anchoring protein 79/150 regulates dendritic endosomal targeting and synaptic plasticity mechanisms. J. Neurosci. 32, 7119–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woolfrey K. M., Sanderson J. L., and Dell'Acqua M. L. (2015) The palmitoyl acyltransferase DHHC2 regulates recycling endosome exocytosis and synaptic potentiation through palmitoylation of AKAP79/150. J. Neurosci. 35, 442–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delint-Ramirez I., Willoughby D., Hammond G. R. V., Ayling L. J., and Cooper D. M. (2011) Palmitoylation targets AKAP79 protein to lipid rafts and promotes its regulation of calcium-sensitive adenylyl cyclase type 8. J. Biol. Chem. 286, 32962–32975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diering G. H., Gustina A. S., and Huganir R. L. (2014) PKA-GluA1 coupling via AKAP5 controls AMPA receptor phosphorylation and cell-surface targeting during bidirectional homeostatic plasticity. Neuron 84, 790–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S., and Ziff E. B. (2014) Calcineurin mediates synaptic scaling via synaptic trafficking of Ca2+-permeable AMPA receptors. PLoS Biol. 12, e1001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lisman J., Yasuda R., and Raghavachari S. (2012) Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coultrap S. J., and Bayer K. U. (2012) CaMKII regulation in information processing and storage. Trends Neurosci. 35, 607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hell J. W. (2014) CaMKII: claiming center stage in postsynaptic function and organization. Neuron 81, 249–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buard I., Coultrap S. J., Freund R. K., Lee Y. S., Dell'Acqua M. L., Silva A. J., and Bayer K. U. (2010) CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J. Neurosci. 30, 8214–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giese K. P., Fedorov N. B., Filipkowski R. K., and Silva A. J. (1998) Autophosphorylation at Thr286 of the α calcium-calmodulin kinase II in LTP and learning. Science 279, 870–873 [DOI] [PubMed] [Google Scholar]
- 49.Strack S., Barban M. A., Wadzinski B. E., and Colbran R. J. (1997) Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J. Neurochem. 68, 2119–2128 [DOI] [PubMed] [Google Scholar]
- 50.Strack S., Choi S., Lovinger D. M., and Colbran R. J. (1997) Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J. Biol. Chem. 272, 13467–13470 [DOI] [PubMed] [Google Scholar]
- 51.Shen K., and Meyer T. (1999) Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284, 162–166 [DOI] [PubMed] [Google Scholar]
- 52.Shen K., Teruel M. N., Connor J. H., Shenolikar S., and Meyer T. (2000) Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat. Neurosci. 3, 881–886 [DOI] [PubMed] [Google Scholar]
- 53.Otmakhov N., Tao-Cheng J. H., Carpenter S., Asrican B., Dosemeci A., Reese T. S., and Lisman J. (2004) Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J. Neurosci. 24, 9324–9331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Z., Srivastava D. P., Photowala H., Kai L., Cahill M. E., Woolfrey K. M., Shum C. Y., Surmeier D. J., and Penzes P. (2007) Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56, 640–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Araki Y., Zeng M., Zhang M., and Huganir R. L. (2015) Rapid dispersion of SynGAP from synaptic spines triggers AMPA receptor insertion and spine enlargement during LTP. Neuron 85, 173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bemben M. A., Shipman S. L., Hirai T., Herring B. E., Li Y., Badger J. D. 2nd, Nicoll R. A., Diamond J. S., and Roche K. W. (2014) CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses. Nat. Neurosci. 17, 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walikonis R. S., Oguni A., Khorosheva E. M., Jeng C. J., Asuncion F. J., and Kennedy M. B. (2001) Densin-180 forms a ternary complex with the α-subunit of Ca2+/calmodulin-dependent protein kinase II and α-actinin. J. Neurosci. 21, 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen K., Teruel M. N., Subramanian K., and Meyer T. (1998) CaMKIIβ functions as an F-actin targeting module that localizes CaMKIIα/β heterooligomers to dendritic spines. Neuron 21, 593–606 [DOI] [PubMed] [Google Scholar]
- 59.O'Leary H., Lasda E., and Bayer K. U. (2006) CaMKIIβ association with the actin cytoskeleton is regulated by alternative splicing. Mol. Biol. Cell 17, 4656–4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin Y. C., and Redmond L. (2008) CaMKIIβ binding to stable F-actin in vivo regulates F-actin filament stability. Proc. Natl. Acad. Sci. U.S.A. 105, 15791–15796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okamoto K., Narayanan R., Lee S. H., Murata K., and Hayashi Y. (2007) The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc. Natl. Acad. Sci. U.S.A. 104, 6418–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robison A. J., Bass M. A., Jiao Y., MacMillan L. B., Carmody L. C., Bartlett R. K., and Colbran R. J. (2005) Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180, and α-actinin-2. J. Biol. Chem. 280, 35329–35336 [DOI] [PubMed] [Google Scholar]
- 63.Strack S., Robison A. J., Bass M. A., and Colbran R. J. (2000) Association of calcium/calmodulin-dependent kinase II with developmentally regulated splice variants of the postsynaptic density protein densin-180. J. Biol. Chem. 275, 25061–25064 [DOI] [PubMed] [Google Scholar]
- 64.Leonard A. S., Bayer K. U., Merrill M. A., Lim I. A., Shea M. A., Schulman H., and Hell J. W. (2002) Regulation of calcium/calmodulin-dependent protein kinase II docking to N-methyl-d-aspartate receptors by calcium/calmodulin and α-actinin. J. Biol. Chem. 277, 48441–48448 [DOI] [PubMed] [Google Scholar]
- 65.Borgesius N. Z., van Woerden G. M., Buitendijk G. H., Keijzer N., Jaarsma D., Hoogenraad C. C., and Elgersma Y. (2011) βCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting αCaMKII to synapses. J. Neurosci. 31, 10141–10148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strack S., McNeill R. B., and Colbran R. J. (2000) Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-d-aspartate receptor. J. Biol. Chem. 275, 23798–23806 [DOI] [PubMed] [Google Scholar]
- 67.Leonard A. S., Lim I. A., Hemsworth D. E., Horne M. C., and Hell J. W. (1999) Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-d-aspartate receptor. Proc. Natl. Acad. Sci. U.S.A. 96, 3239–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bayer K. U., De Koninck P., Leonard A. S., Hell J. W., and Schulman H. (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411, 801–805 [DOI] [PubMed] [Google Scholar]
- 69.O'Leary H., Liu W. H., Rorabaugh J. M., Coultrap S. J., and Bayer K. U. (2011) Nucleotides and phosphorylation bi-directionally modulate Ca2+/calmodulin-dependent protein kinase II (CaMKII) binding to the N-methyl-d-aspartate (NMDA) receptor subunit GluN2B. J. Biol. Chem. 286, 31272–31281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanhueza M., Fernandez-Villalobos G., Stein I. S., Kasumova G., Zhang P., Bayer K. U., Otmakhov N., Hell J. W., and Lisman J. (2011) Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J. Neurosci. 31, 9170–9178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halt A. R., Dallapiazza R. F., Zhou Y., Stein I. S., Qian H., Juntti S., Wojcik S., Brose N., Silva A. J., and Hell J. W. (2012) CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 31, 1203–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coultrap S. J., Freund R. K., O'Leary H., Sanderson J. L., Roche K. W., Dell'Acqua M. L., and Bayer K. U. (2014) Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell Rep. 6, 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu W., Isozaki K., Roche K. W., and Nicoll R. A. (2010) Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc. Natl. Acad. Sci. U.S.A. 107, 22266–22271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peti W., Nairn A. C., and Page R. (2013) Structural basis for protein phosphatase 1 regulation and specificity. FEBS J. 280, 596–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morishita W., Connor J. H., Xia H., Quinlan E. M., Shenolikar S., and Malenka R. C. (2001) Regulation of synaptic strength by protein phosphatase 1. Neuron 32, 1133–1148 [DOI] [PubMed] [Google Scholar]
- 76.Hu X. D., Huang Q., Roadcap D. W., Shenolikar S. S., and Xia H. (2006) Actin-associated neurabin-protein phosphatase-1 complex regulates hippocampal plasticity. J. Neurochem. 98, 1841–1851 [DOI] [PubMed] [Google Scholar]
- 77.Hu X. D., Huang Q., Yang X., and Xia H. (2007) Differential regulation of AMPA receptor trafficking by neurabin-targeted synaptic protein phosphatase-1 in synaptic transmission and long-term depression in hippocampus. J. Neurosci. 27, 4674–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McAvoy T., Allen P. B., Obaishi H., Nakanishi H., Takai Y., Greengard P., Nairn A. C., and Hemmings H. C. Jr. (1999) Regulation of neurabin I interaction with protein phosphatase 1 by phosphorylation. Biochemistry 38, 12943–12949 [DOI] [PubMed] [Google Scholar]
- 79.Hou H., Sun L., Siddoway B. A., Petralia R. S., Yang H., Gu H., Nairn A. C., and Xia H. (2013) Synaptic NMDA receptor stimulation activates PP1 by inhibiting its phosphorylation by Cdk5. J. Cell Biol. 203, 521–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siddoway B. A., Altimimi H. F., Hou H., Petralia R. S., Xu B., Stellwagen D., and Xia H. (2013) An essential role for inhibitor-2 regulation of protein phosphatase-1 in synaptic scaling. J. Neurosci. 33, 11206–11211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greer P. L., and Greenberg M. E. (2008) From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron 59, 846–860 [DOI] [PubMed] [Google Scholar]
- 82.Bading H., Ginty D. D., and Greenberg M. E. (1993) Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260, 181–186 [DOI] [PubMed] [Google Scholar]
- 83.Ch'ng T. H., Uzgil B., Lin P., Avliyakulov N. K., O'Dell T. J., and Martin K. C. (2012) Activity-Dependent Transport of the Transcriptional Coactivator CRTC1 from Synapse to Nucleus. Cell 150, 207–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Graef I. A., Mermelstein P. G., Stankunas K., Neilson J. R., Deisseroth K., Tsien R. W., and Crabtree G. R. (1999) L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature 401, 703–708 [DOI] [PubMed] [Google Scholar]
- 85.Murphy T. H., Worley P. F., and Baraban J. M. (1991) L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron 7, 625–635 [DOI] [PubMed] [Google Scholar]
- 86.Deisseroth K., Bito H., and Tsien R. W. (1996) Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron 16, 89–101 [DOI] [PubMed] [Google Scholar]
- 87.Dolmetsch R. E., Pajvani U., Fife K., Spotts J. M., and Greenberg M. E. (2001) Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 294, 333–339 [DOI] [PubMed] [Google Scholar]
- 88.Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Müller J., Stiess M., Marais E., Schulla V., Lacinova L., Goebbels S., Nave K. A., Storm D. R., Hofmann F., and Kleppisch T. (2005) Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. 25, 9883–9892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bito H., Deisseroth K., and Tsien R. W. (1996) CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87, 1203–1214 [DOI] [PubMed] [Google Scholar]
- 90.Wheeler D. G., Barrett C. F., Groth R. D., Safa P., and Tsien R. W. (2008) CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J. Cell Biol. 183, 849–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma H., Groth R. D., Cohen S. M., Emery J. F., Li B., Hoedt E., Zhang G., Neubert T. A., and Tsien R. W. (2014) γCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159, 281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wiegert J. S., Bengtson C. P., and Bading H. (2007) Diffusion and not active transport underlies and limits ERK1/2 synapse-to-nucleus signaling in hippocampal neurons. J. Biol. Chem. 282, 29621–29633 [DOI] [PubMed] [Google Scholar]
- 93.Zhai S., Ark E. D., Parra-Bueno P., and Yasuda R. (2013) Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines. Science 342, 1107–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nonaka M., Kim R., Fukushima H., Sasaki K., Suzuki K., Okamura M., Ishii Y., Kawashima T., Kamijo S., Takemoto-Kimura S., Okuno H., Kida S., and Bito H. (2014) Region-specific activation of CRTC1-CREB signaling mediates long-term fear memory. Neuron 84, 92–106 [DOI] [PubMed] [Google Scholar]
- 95.Zhang J., and Shapiro M. S. (2012) Activity-dependent transcriptional regulation of M-type (Kv7) K+ channels by AKAP79/150-mediated NFAT actions. Neuron 76, 1133–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hall D. D., Davare M. A., Shi M., Allen M. L., Weisenhaus M., McKnight G. S., and Hell J. W. (2007) Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry 46, 1635–1646 [DOI] [PubMed] [Google Scholar]
- 97.Li H., Pink M. D., Murphy J. G., Stein A., Dell'Acqua M. L., and Hogan P. G. (2012) Balanced interactions of calcineurin with AKAP79 regulate Ca2+-calcineurin-NFAT signaling. Nat. Struct. Mol. Biol. 19, 337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murphy J. G., Sanderson J. L., Gorski J. A., Scott J. D., Catterall W. A., Sather W. A., and Dell'Acqua M. L. (2014) AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. Cell Rep .7, 1577–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dittmer P. J., Dell'Acqua M. L., and Sather W. A. (2014) Ca2+/calcineurin-dependent inactivation of neuronal L-type Ca2+ channels requires priming by AKAP-anchored protein kinase A. Cell Rep. 7, 1410–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toomre D., and Bewersdorf J. (2010) A new wave of cellular imaging. Annu. Rev. Cell Dev. Biol. 26, 285–314 [DOI] [PubMed] [Google Scholar]
- 101.Lussier M. P., Sanz-Clemente A., and Roche K. W. (2015) Dynamic regulation of NMDA and AMPA receptors by posttranslational modifications. J. Biol. Chem. 48, 28596–28603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spence E. F., and Soderling S. H. (2015) Actin Out: regulation of the synaptic cytoskeleton. J. Biol. Chem. 48, 28613–28622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alvarez-Castelao B., and Schuman E. R. (2015) The regulation of synaptic protein turnover. J. Biol. Chem. 48, 28623–28630 [DOI] [PMC free article] [PubMed] [Google Scholar]