Abstract
Emerging evidence indicates that protein synthesis and degradation are necessary for the remodeling of synapses. These two processes govern cellular protein turnover, are tightly regulated, and are modulated by neuronal activity in time and space. The anisotropic anatomy of the neurons presents a challenge for the study of protein turnover, but the understanding of protein turnover in neurons and its modulation in response to activity can help us to unravel the fine-tuned changes that occur at synapses in response to activity. Here we review the key experimental evidence demonstrating the role of protein synthesis and degradation in synaptic plasticity, as well as the turnover rates of specific neuronal proteins.
Keywords: neuron, protein degradation, protein synthesis, protein turnover, synapse, synaptic plasticity
Introduction
The human brain is composed of a trillion neurons with complex and intricate arbors, which are interconnected by synapses (1). Synapses are highly dynamic in number and shape as a consequence of the continuous remodeling of neural circuits. A change in synaptic transmission elicited by neural activity is collectively called “synaptic plasticity,” and learning and memory rely, at least in part, on this process. Synapses are made up of proteins, including receptors for neurotransmitters, scaffolding molecules, and signaling molecules. All proteins have a finite lifetime: they are synthesized and degraded continuously to maintain cellular function and viability. This continuous process is called “protein turnover.” However, why is protein turnover important for cells? Even when the cells are in a basal state, the protein pool is dynamic and the coordination between protein synthesis and degradation maintains a steady-state level of proteins that is constantly renewed (2). Protein turnover is not only important to maintain protein concentrations in the cell, but also to allow for changes. Modulation of the proteome is necessary for most cellular responses, involving modifications in general or specific protein turnover (3, 4). Neurons also have the ability to modulate and adjust their proteome in response to specific cues, for example, synaptic remodeling in response to patterns of action potentials in neurons.
One complication of protein turnover in neurons is that synapses can be located up to hundreds of microns from the cell body. Where are proteins synthesized and degraded? Are proteins transported over the long distance from the soma to dendrites or axons, and if so, how do they reach their specific location within the intricate axonal and dendritic arbors. In fact, to overcome these challenges, neurons have very effective transport mechanisms to deliver proteins to remote regions of axons or dendrites; this has been recently reviewed in Refs. 5 and 6. Moreover, some proteins, such as receptors or scaffolding proteins, are in continuous movement in and out of the synapse with rapid rates (reviewed in Refs. 7 and 8). In addition to protein movement, neurons use local translation and degradation in dendrites and axons, allowing for a fine-tuned local protein turnover. Here we present an overview focused on the role of protein synthesis and proteasomal degradation, two main pathways controlling protein turnover, and how these two processes might work together to achieve neuronal plasticity. The role of autophagy in protein degradation is not discussed here due to space limitations.
Local Computational and Cell Biological Units
To understand the problem of regulating synaptic protein turnover in response to synaptic activity, it is necessary to know which neuronal locations process information and possess the machinery for protein turnover, and then study protein turnover within those regions. Within dendrites, individual spines are the sites of excitatory synapses. Nevertheless, recent advances allowing for the stimulation of individual synapses have shown that dendritic branches and the associated synapses can be independently regulated by synaptic activity (9, 10). Thus, the dendritic branch and its associated cluster of synapses may represent the fundamental computational unit for neurons. From this, one might predict that the turnover of some proteins changes only when needed by specific branches or an activated group of synapses within them. This is paralleled by cell biological studies that have documented the localization of both the protein synthesis machinery and the ubiquitin proteasome system (UPS)3 component in dendrites. In addition, recent high resolution in situ hybridization data indicate that mRNA molecules are distributed in local domains, along the proximal-distal dendritic axis (11). These data suggest a local sharing of protein synthesis and degradation machinery within the dendrite, giving to dendrite branches autonomy for the regulation of the protein turnover. Thus, to understand protein synthesis or degradation in response to neuronal activity, the best approach would be to study separately the dendrites/axons and soma, and ideally study in situ the activated clusters of synapses in comparison with non-activated synapses. This approach is becoming more feasible with advances in microscopy, protein labeling, and local synaptic activation techniques.
Local Protein Synthesis
The first evidence for dendritic translation in response to neuronal stimulation comes from the work of Feig and Lipton in 1993 (12). These authors applied electrical stimulation together with a muscarinic acetylcholine agonist (carbachol) to hippocampal slices and detected an increase in the incorporation of [3H]leucine after 3 min, although there was no change in synaptic transmission. In 1996, Kang and Schuman (13) discovered that local protein synthesis in hippocampal slices is necessary for synaptic plasticity induced by BDNF, and unlike other forms of plasticity, BDNF-induced plasticity requires protein synthesis. These two pioneering studies have been supported by several others confirming the role of protein synthesis in plasticity; for example, serotonin-induced long term facilitation (LTF) (similar to long-term potentiation, or LTP, in mammals) of sensory motor synapses in the marine mollusk Aplysia californica is protein synthesis-dependent (14), as well long-term depression (LTD) dependent of metabotropic glutamate receptor (mGluR) in hippocampal neurons (15). In addition, the blockade of spontaneous release of neurotransmitter by the presynaptic terminals induces translation in the postsynaptic terminal to enhance the responsiveness to the decreased input (homeostatic plasticity) (16, 17). Collectively, these studies provide strong evidence that protein synthesis can be modulated by various neuronal stimuli. The postsynaptic spine is biochemically isolated from the dendrite by the spine neck, and the molecular transport across the neck is restricted and modulated by neuronal activity (18). Within the relatively small volume of the spine, many chemical reactions take place including protein translation and degradation. Supporting the idea that proteins can be translated in spines, polyribosomes and smooth endoplasmic reticulum have been found in some spine heads (19). Furthermore, ultra-structural studies performed by Ostroff et al. (20) found that LTP induction in hippocampal slices increased the percentage of spines containing polyribosomes. Strikingly, the postsynaptic densities (PSDs) in spines containing polyribosomes were larger after LTP stimulation, suggesting that local translation serves to promote the growth of the PSD. Interestingly, another study demonstrated that NMDA receptor activation promotes the recruitment and sequestration of proteasomes into spines (21). These data suggest that protein translation and degradation within dendrites and spines could effect rapid changes in protein turnover. Thus far, it has been difficult to observe protein translation directly in spines. There is, however, ample evidence for specific protein synthesis in synaptosomes (biochemical preparations of detached synaptic spines containing presynaptic terminals) and synaptoneurosomes (subcellular preparation enriched in presynaptic structures with attached postsynaptic densities). These preparations retain a resting membrane potential and release neurotransmitters when electrically stimulated (22). Using these synaptic preparations, the mRNAs present at the synapses have been described (23, 24), and an increase in specific mRNAs after neuronal activation has been demonstrated (25). Furthermore, CaMKIIα (26, 27), PSD95 (27), Arc (28), and several other proteins are synthesized in these synaptic preparations, and their translation is modulated by stimulation. In line with this, a proteomic study in synaptosomes from the squid optic lobe after metabolic labeling with [35S]Met showed de novo synthesis of 80 protein species, including chaperones such as HSP70 and mitochondrial and cytoskeletal proteins (29). This study expands the protein families translated in synaptic compartments beyond exclusively synaptic proteins or neuron-specific proteins. Nevertheless, the role of these proteins in synaptic function has not been studied. The development of new techniques improving the isolation of synaptosomes will contribute to a more precise study of their protein content and the newly synthesized proteins in response to different neuronal stimulus (30). Altogether, these findings support the notion that local protein synthesis has a role in synaptic plasticity, but how is this local translation regulated in response to synaptic activity? The localization of mRNAs is thought to play a major role in the regulation of local translation (for example, see Ref. 31). One important feature of the mRNA is its ability to be spatially localized; this feature derives from untranslated information in the 3′ or 5′ ends where multiple regulatory elements are located. The transport, localization, stabilization, and translation of the mRNA are often regulated by these elements (32). Recent studies with high resolution in situ hybridization data and deep sequencing show that mRNA molecules are distributed in local domains, and more than 2500 mRNAs can be detected in the neuropil (11). In addition to all these regulatory layers associated with the mRNA, there is a regulation in response to activity at the level of translation factors; some of the best known are eEF2 and its kinase eEF2K, (33–35). Phosphorylation of eEF2 is increased in response to NMDA receptor (NMDAR) activation; this inhibits the elongation of most of mRNAs but increases the elongation of some dendritic mRNAs such as Arc or CaMKIIα (36, 37). Other translation factors with a putative role in synaptic plasticity are eIF2α (38, 39) and 4EBP (40). The regulation of protein synthesis in response to synaptic activity is extensively studied, and is a tightly regulated process able to adjust protein synthesis in response to specific neuronal stimuli.
Local Protein Degradation
Protein degradation is one crucial component governing protein turnover. The UPS is an important mechanism for cytosolic protein degradation (41). In HeLa cells, the proteins that comprise the proteasome represent 0.6% of the total cellular proteins (42). Although the study of protein degradation by the UPS in neurons has gained attention during the last several years, the mechanisms underlying proteasome-mediated degradation, local polyubiquitination, and the influence of neuronal activity on local or global protein degradation remain poorly understood. There is, however, increasing evidence for a role of the UPS in neuronal development, neurotransmitter release, synapse vesicle recycling, and learning.
The UPS pathway is composed of several enzymes including the proteasome, ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), ubiquitin-ligase enzymes (E3), and deubiquitinases. All these enzymatic activities give to the pathway a fine-tuned precision for the temporal and spatial regulation of protein degradation. However, is this pathway regulated by synaptic activity, and if so, which steps of the pathway are regulated? Indeed, in vivo studies have shown that inhibition of the proteasome has consequences for learning. In rats, bilateral infusion of the proteasome inhibitor lactacystin (administered 4–7 h after training but not after 10 h) to the CA1 region of the hippocampus caused full retrograde amnesia for a one-trial inhibitory avoidance training, indicating that the ubiquitin-proteasome cascade is crucial for long-term memory (LTM) in the behaving animal (43). Likewise, incubation of rat hippocampal slices with proteasome inhibitors decreased LTP at the Schaeffer collateral-CA1 synapses (44). There is also evidence for a role of deubiquitinating enzymes in learning. One example that, interestingly, involves transcription is Ap-uch (an ortholog of the mammalian deubiquitinase UCH-L1). During LTF in A. californica, the transcription factor CREB (cAMP-response element-binding protein) is activated, stimulating the transcription of Ap-uch, and this increases the degradation of protein kinase A regulatory subunits, leading to an increase in the catalytic activity of PKA that is responsible for LTF maintenance (45, 46). Similarly, in mice, UCH-L1 inhibitors reduced LTP in hippocampal slices (47). Another example of a deubiquitinase enzyme that regulates plasticity comes from the Usp14ax-J mice, defective in the Usp14 deubiquitinase. These mice have defects in hippocampal short-term synaptic plasticity but not in long-term plasticity (48). In addition, E3 enzymes have also been related to plasticity. Hundreds of E3 ligase enzymes have been identified. These enzymes provide specific ubiquitination for particular proteins, representing one of the most complex regulatory steps in the pathway. One example are the APC/C ligases; these multisubunit RING finger E3 ligases comprise 12 different subunits, and some of them target the APC/C to different substrates. The cytosolic APC/C-Cdc20 has a role in dendritic morphogenesis (49), and the nuclear APC/C-Cdh1 regulates axonal growth (50). Furthermore, APC/C-Cdh1 is required for associative fear memory and LTP in the amygdala in mice (51), and for mGluR-dependent LTD in the hippocampus (52). Another example is the E6-AP ligase (Ube3A). Mutations in this protein are associated with deficits in contextual learning (fear conditioning) and with decreased LTP (53). Interestingly, this ligase is associated with the neurological disorder known as Angelman syndrome (54). E6-AP ligase mediates the polyubiquitination of Arc, and its disruption increases Arc expression and thereby decreases the number of AMPA receptors at synapses (55). In addition, regulation of ubiquitination is sometimes also regulated by other post-transcriptional modifications such as phosphorylation; this is the case for the actin-binding protein SPAR. The degradation of this protein is regulated by an elegant mechanism, which involves activity-dependent induction of a kinase that phosphorylates SPAR, promoting its polyubiquitination and subsequent degradation, culminating in the loss of spines (56). Finally, in the last step of the pathway, the proteasome itself, we find another layer of regulation that is also critical for local control of the synaptic proteome. Recent findings show that the proteasome complex is regulated by at least four different mechanisms: by its subunit composition, its proteolytic activity, its location within the cell, and its interaction with other proteins, all of which are regulated by neuronal activity. These facts were demonstrated by several studies; among them Tai el at. (57) found that the treatment of neurons with the glutamate receptor agonist NMDA causes disassembly of the 26S proteasome, decrease in its proteolytic activity, and dissociation from the proteasome of the E3 ligases UBE3A and HUWE1, which in non-stimulated conditions co-sediment with proteasomes. In contrast, a different study showed that after the blockade of neuronal activity in cultured hippocampal neurons using tetrodotoxin (a blocker of the voltage-gated sodium channels), there is a decrease in the degradation rate of the chimeric proteasome substrate GFPu, whereas an increase in neuronal activity (using bicuculline, a competitive antagonist of GABAA receptors) increases the degradation of GFPu (58). Interestingly, proteasome inhibitors block spine outgrowth in response to glutamate, through a mechanism that involves the phosphorylation of the proteasome by CaMKIIα (59). Furthermore, the sequestration of the proteasome in spines is controlled by neuronal activity through CaMKIIα, which also regulates its proteolytic activity (21, 60). These data suggest a very tight regulation of proteasome degradation in response to the activation\inhibition of specific neurotransmitter receptors that has both early and late components.
Although it is known that a large amount of protein degradation occurs via the action of the proteasome pathway, how degradation itself is regulated by activity remains an interesting question. For example, Ehlers (61) found that treatment of neurons with activity blockers resulted in a decrease of ∼50% in the polyubiquitinated proteins in PSD fractions, whereas treatment of neurons with activity inducers results in an increase in polyubiquitinated proteins. Nevertheless, only a handful of proteins have been described to change their polyubiquitination status in response to neuronal activity, including scaffold molecules such as Shank, AKAP79\150, GKAP, and PSD95 (61, 62). In conclusion, more extensive studies identifying proteasome substrates in the context of neuronal activity would contribute to a better understanding of the role of proteasome pathway in synaptic plasticity. Something more complex would be the investigation of the regulatory elements of the pathway in a spatiotemporal context. For example, where within the neuron are specific proteins ubiquitylated and degraded? Also, a more detailed knowledge of the proteasomal subunit composition and its processing state is desirable. For example, does the protein composition of the proteasome and tightly associated proteins change depending on its location within neurons? Is the proteasome more active in specific locations? Are the proteasomal activity and subunit composition modulated by synaptic plasticity? In this context, a novel technique that allows for the detection and quantification of conformational states of proteasomes in situ using electron cryotomography with a Volta phase plate could be useful (63). Using this technique, the authors found that in intact hippocampal neurons, only 20% of the 26S proteasomes are engaged in substrate processing, suggesting that the capacity of the proteasome is only partially used in the conditions of this study. This technique could also be used to study structural changes in the proteasome in response to activity (63). In conclusion, and similar to protein synthesis, protein degradation is strongly regulated in neurons.
Coordination of Protein Synthesis and Degradation
As explained above, it is clear that local protein synthesis and local protein degradation play a role at synapses, implying that crosstalk between these two systems must occur to maintain protein concentrations in the appropriate range. Indeed, the co-application of proteasome blockers and translational inhibitors restores late LTP, which is otherwise blocked when these inhibitors are applied separately. This suggests that a balance between protein synthesis and degradation is needed for late LTP to occur (64), but the underlying molecular mechanisms are not known. However, it is clear that the UPS is responsible for the degradation of many proteins that are essential for protein synthesis; consequently, many steps of protein synthesis are controlled by the UPS (65, 66). Intriguingly, some of the proteins encoded by the immediate early genes (IEG) with a role in neuronal plasticity, such as Arc (67) or the transcription factor ApC/EBP (68), are degraded by the UPS under specific conditions. Furthermore, Egr1, another IEG protein, regulates the expression of some of the proteasome subunit genes (69). The UPS also has a role in RNA regulation because some RNA-binding proteins (RBPs) are degraded through this pathway; these proteins have diverse functions, such as the regulation of mRNA stability, splicing, or transport. One example is the RBP FMRP (fragile X mental retardation protein), a regulator of dendritic translation that is degraded by the proteasome (70). Similarly, Mov10 (homolog of the Drosophila DExD box protein Armitage), a component of the RISC (RNA-induced silencing complex), is degraded in response to NMDA treatment by the UPS, and its degradation relieves translational silencing of specific mRNAs such as CaMKIIα. As a consequence, after NMDA stimulation, there is a 30% reduction in the translation of the CaMKIIα in neurons treated with a proteasome inhibitor as compared with neurons treated only with NMDA (71). Finally, mRNA stability is also regulated by the UPS. There are RBPs that bind to AU-rich elements, which control mRNA stability. Interestingly, 5–8% of human genes encode transcripts that contain these elements. One example of an AU-rich element-containing mRNA is the mRNA encoding for ApC\EBP protein; this protein plays a role in synaptic plasticity, and one of the RBPs that regulates ApC\EBP mRNA stability is ApAUF1 (72). Some of the isoforms of mammalian AUF1 are well established proteasome substrates (73). As such, the UPS is potentially able to regulate the ApC/EBP mRNA and protein. Taken together, these data point to a clear coordination between protein synthesis and protein degradation, but how these two processes are co-regulated by synaptic activity remains to be elucidated.
Protein Turnover
As mentioned above, protein synthesis and degradation define protein turnover rates. Interestingly, different protein turnover rates have been observed in different tissues (74). For example, one study found slower protein turnover rates in the brain with an average lifetime of 9 days, whereas the average protein lifetime is 3 days in liver and 3.5 days in blood (75). These differences are not only due to stable proteins that are uniquely expressed in the brain, but also to the fact that some ubiquitous proteins have longer half-lives in the brain, by a factor of 2–5. This is the case for a number of subunits of the proteasome, for which average half-lives of 8 and 4 days have been measured in brain and liver, respectively (75). Taken together, these data suggest a special protein turnover mechanism in the brain, as compared with other tissues. Additionally, the studies described in the previous sections suggest that some proteins may display different turnover rates depending on their location in the neuron, and/or depending on the activation state of the neuron. Furthermore, the turnover rates of some proteins may differ among synapses or dendritic branches, as a function of their activation state. In this context, post-translational modifications or protein-protein interactions can have a role in the differential modulation protein stability. This idea is supported by some apparent discrepancies in the half-life of the same protein, depending on the cellular fraction studied (Table 1). One good example is the synaptic protein PSD95 with a reported half-life in total protein extracts of ∼88 h (76), and ∼8 h in synaptic fractions (61). In this context, it is important to note that the techniques used in these studies were different, and the time of protein labeling was also different (7 days versus 12 h); this could result in the labeling of different populations of PSD95 with distinct turnover rates (for a review of strategies for turnover analysis, see Ref. 77). Interestingly, another study found a slower GluR1 turnover rate after synapse formation: in mature neuronal cultures (11 DIV), GluR1 has a half-life of ∼31 h; in contrast, in neurons cultured for 4 days, the reported GluR1 half-life is ∼12 h (78). The occurrence of different turnover rates for the same protein among different cellular compartments has been recently demonstrated in HeLa cells, comparing the nucleus, nucleolus, and cytoplasmic protein turnover rates (79). In this study, ribosomal proteins exhibited a fast turnover in the nucleolus (6 h) and slower turnover in the cytoplasm (>30 h); the authors also found differences in the turnover rates of some proteasomal subunits.
TABLE 1.
General protein half-lives in neurons, and specific protein half-lives for endogenous neuronal proteins
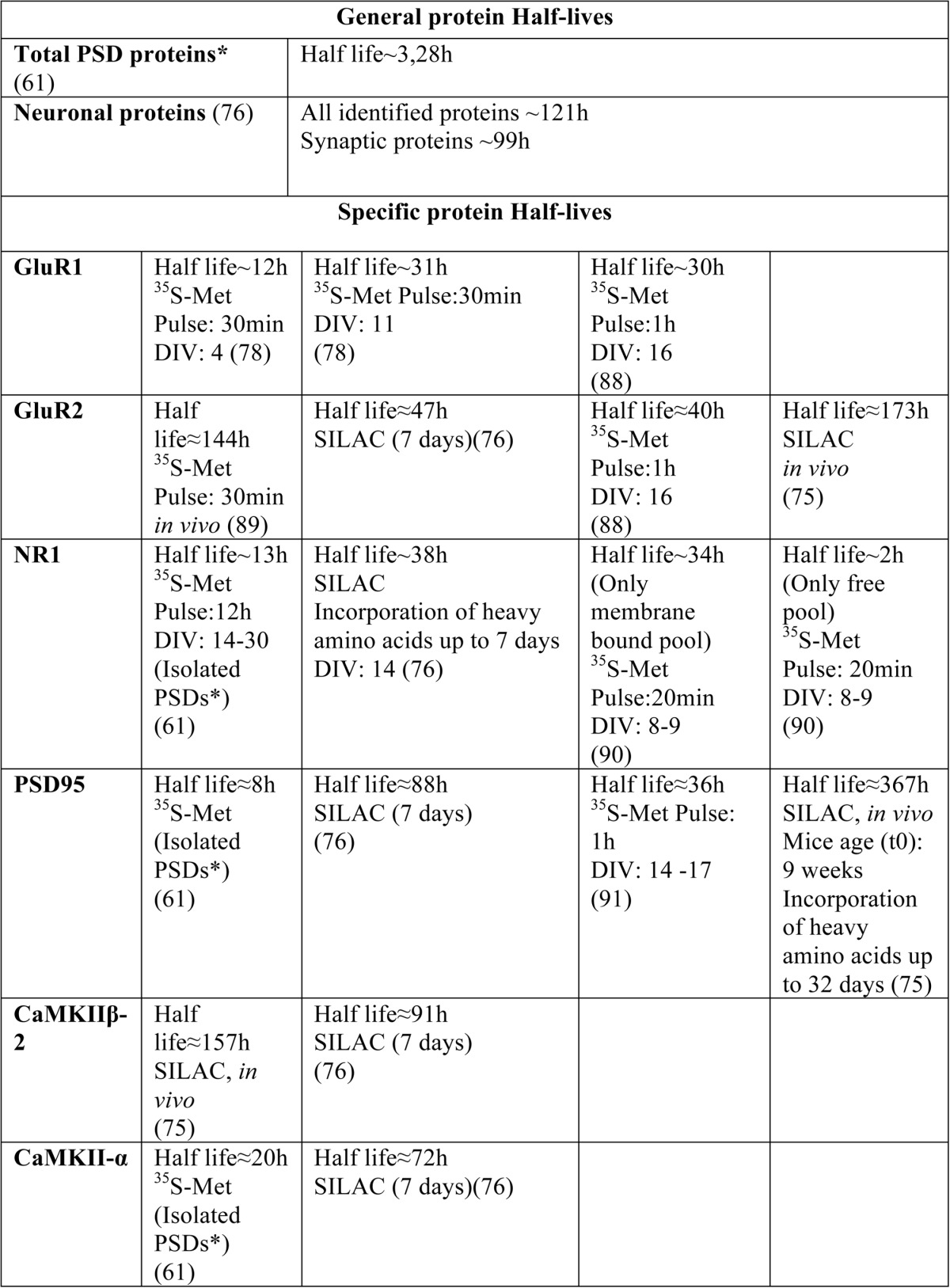
* In this study, synaptic half-lives show degradation and protein movement out of synapse.
Additionally, the fate of some molecules can also be dependent on their age. For example, in a recent study, it was demonstrated that PSD95 proteins less than 6 h old are found in immature synapses, whereas they are less abundant at mature spines where older populations of PSD95 are preferentially found. Therefore, new molecules of PSD95 not only replace the older ones, but also are located at different places, and may have different functions in different areas (80).
Consequently, a systematic comparison of the protein turnover rates in synaptic preparations versus total protein extracts from neurons at different maturity states could contribute to a better understanding of how this process is regulated in neurons. Furthermore, in recent years, new and exciting alternatives for the measurement of protein turnover in situ have been developed. An interesting example is the new microscopy technique denominated COIN (correlated optical and isotopic nanoscopy) (81), which detects newly synthesized proteins labeled with [15N]leucine. Using this procedure, protein turnover in several neuronal organelles was measured (15N/14N ratio). Interestingly, the authors focused on the presynaptic compartment and found some synapses with stronger protein turnover as compared with the neighboring axon, and clusters of bassoon molecules with different incorporation ratios of [15N]leucine, indicating the coexistence in the synaptic area of protein groups with low and high turnover rates (81). A more established technique developed by Schuman and colleagues (82), fluorescent non-canonical amino acid tagging (FUNCAT), consists of the tagging of proteins with non-canonical amino acids and subsequent detection by fluorescent labeling in situ. This technique has been successfully applied to primary neurons, organotypic slices, and in vivo in larval zebrafish (82, 83). Combining FUNCAT labeling with the proximity ligation assay technique (84), it is now possible to detect in situ the synthesis and degradation of specific proteins (85). In a similar way, combining puromycin labeling with proximity ligation assay technique, the synthesis of specific proteins can be detected within 0.5–1 min (85, 86). Furthermore, photocaged puromycin that can be locally activated allows for protein synthesis detection in a spatially restricted manner (87). Similarly, the TimeSTAMP technique allows for the tracking of an exogenously expressed protein fused to drug-controlled tags for detection of proteins synthesized at defined time points; these fusion proteins can be detected by live microscopy or electron microscopy (80). Finally, for studying the modulation of the synaptic protein turnover by mass spectrometry, the use of quantitative non-canonical amino acid tagging (QuaNCAT) is an elegant technique; it combines biorthogonal non-canonical amino acid tagging (BONCAT) with stable isotope labeling of amino acids in cell culture (SILAC), constituting a powerful assay to measure protein turnover (76). Collectively, these techniques open new exciting approaches for the study of local protein metabolic turnover in cell lysates and in situ.
Concluding Remarks
Protein synthesis and degradation work together to maintain and regulate synaptic protein turnover; both systems are modulated by different forms of synaptic plasticity. Of special interest is knowing how the components of both pathways are distributed in the different neuronal compartments, such as synapses, dendrites, axons, or the cell body. A deeper knowledge of this distribution would help us understand the local regulation of protein synthesis and degradation. In addition, it will be important to study whether there are compartment-specific characteristics of the synthesis and degradation machinery such as specific protein isoforms, specific post-transcriptional modifications, or specific interactors. Also, in the case of mRNA, the identification of specific elements regulating the location, its stability, and its availability to be translated is of great interest. Despite increasing attention on the role of protein synthesis and degradation in neuronal function and plasticity, many questions remain open. For example, how are protein synthesis components coordinated for controlling the synthesis of specific sets of proteins required in each location? How and where are protein synthesis and degradation components influenced by post-translational modifications or protein-protein interactions? How is all this modified by neuronal activity to regulate the synthesis and degradation of specific proteins at specific locations? In addition, how is the destruction of proteins coordinated with the creation of new ones, and how is all this coordinated to achieve synaptic plasticity? A good starting point to answer some of these questions would be to know which proteins are modified in response to the different plasticity paradigms. A detailed study of those proteins and their interactors would help us to understand the spatial and temporal regulation of protein turnover controlled by protein synthesis and degradation and its role in synaptic plasticity.
Acknowledgments
We thank Cyril Hanus for the critical reading of the manuscript. We apologize to those whose work could not be cited because of space limitations.
This is the fourth article in the Thematic Minireview series “Molecular Mechanisms of Synaptic Plasticity.” The authors declare that they have no conflicts of interest with the contents of this article.
- UPS
- ubiquitin proteasome system
- LTF
- long-term facilitation
- LTD
- long-term depression
- LTP
- long-term potentiation
- PSD
- postsynaptic density
- CaMKIIα
- Ca2+-calmodulin-dependent protein kinase IIα
- APC/C
- anaphase-promoting complex
- ApC/EBP
- Aplysia CCAAT enhancer-binding protein
- GluR
- glutamate receptor
- RBP
- RNA-binding protein
- SILAC
- stable isotope labeling of amino acids in cell culture
- DIV
- days in vitro.
References
- 1.Peters A., and Palay S. L. (1996) The morphology of synapses. J. Neurocytol. 25, 687–700 [DOI] [PubMed] [Google Scholar]
- 2.Eagle H., Piez K. A., Fleischman R., and Oyama V. I. (1959) Protein turnover in mammalian cell cultures. J. Biol. Chem. 234, 592–597 [PubMed] [Google Scholar]
- 3.Jovanovic M., Rooney M. S., Mertins P., Przybylski D., Chevrier N., Satija R., Rodriguez E. H., Fields A. P., Schwartz S., Raychowdhury R., Mumbach M. R., Eisenhaure T., Rabani M., Gennert D., Lu D., Delorey T., Weissman J. S., Carr S. A., Hacohen N., and Regev A. (2015) Dynamic profiling of the protein life cycle in response to pathogens. Science 347, 1259038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fierro-Monti I., Racle J., Hernandez C., Waridel P., Hatzimanikatis V., and Quadroni M. (2013) A novel pulse-chase SILAC strategy measures changes in protein decay and synthesis rates induced by perturbation of proteostasis with an Hsp90 inhibitor. PLoS ONE 8, e80423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzoli S. O. (2014) Synaptic vesicle recycling: steps and principles. EMBO J. 33, 788–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanus C., and Schuman E. M. (2013) Proteostasis in complex dendrites. Nat. Rev. Neurosci. 14, 638–648 [DOI] [PubMed] [Google Scholar]
- 7.Specht C. G., and Triller A. (2008) The dynamics of synaptic scaffolds. Bioessays 30, 1062–1074 [DOI] [PubMed] [Google Scholar]
- 8.Opazo P., Sainlos M., and Choquet D. (2012) Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr. Opin. Neurobiol. 22, 453–460 [DOI] [PubMed] [Google Scholar]
- 9.Branco T., and Häusser M. (2010) The single dendritic branch as a fundamental functional unit in the nervous system. Curr. Opin. Neurobiol. 20, 494–502 [DOI] [PubMed] [Google Scholar]
- 10.Jia H., Rochefort N. L., Chen X., and Konnerth A. (2010) Dendritic organization of sensory input to cortical neurons in vivo. Nature 464, 1307–1312 [DOI] [PubMed] [Google Scholar]
- 11.Cajigas I. J., Tushev G., Will T. J., tom Dieck S., Fuerst N., and Schuman E. M. (2012) The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron 74, 453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feig S., and Lipton P. (1993) Pairing the cholinergic agonist carbachol with patterned Schaffer collateral stimulation initiates protein synthesis in hippocampal CA1 pyramidal cell dendrites via a muscarinic, NMDA-dependent mechanism. J. Neurosci. 13, 1010–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang H., and Schuman E. M. (1996) A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 273, 1402–1406 [DOI] [PubMed] [Google Scholar]
- 14.Martin K. C., Casadio A., Zhu H., Yaping E., Rose J. C., Chen M., Bailey C. H., and Kandel E. R. (1997) Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91, 927–938 [DOI] [PubMed] [Google Scholar]
- 15.Huber K. M., Kayser M. S., and Bear M. F. (2000) Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1257 [DOI] [PubMed] [Google Scholar]
- 16.Sutton M. A., Wall N. R., Aakalu G. N., and Schuman E. M. (2004) Regulation of dendritic protein synthesis by miniature synaptic events. Science 304, 1979–1983 [DOI] [PubMed] [Google Scholar]
- 17.Sutton M. A., and Schuman E. M. (2006) Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127, 49–58 [DOI] [PubMed] [Google Scholar]
- 18.Bloodgood B. L., and Sabatini B. L. (2005) Neuronal activity regulates diffusion across the neck of dendritic spines. Science 310, 866–869 [DOI] [PubMed] [Google Scholar]
- 19.Spacek J., and Harris K. M. (1997) Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci. 17, 190–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostroff L. E., Fiala J. C., Allwardt B., and Harris K. M. (2002) Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron 35, 535–545 [DOI] [PubMed] [Google Scholar]
- 21.Bingol B., and Schuman E. M. (2006) Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature 441, 1144–1148 [DOI] [PubMed] [Google Scholar]
- 22.Blaustein M. P., and Goldring J. M. (1975) Membrane potentials in pinched-off presynaptic nerve terminals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J. Physiol. 247, 589–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chicurel M. E., Terrian D. M., and Potter H. (1993) mRNA at the synapse: analysis of a synaptosomal preparation enriched in hippocampal dendritic spines. J. Neurosci. 13, 4054–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto M., Setou M., and Inokuchi K. (2007) Transcriptome analysis reveals the population of dendritic RNAs and their redistribution by neural activity. Neurosci. Res. 57, 411–423 [DOI] [PubMed] [Google Scholar]
- 25.Håvik B., Røkke H., Bårdsen K., Davanger S., and Bramham C. R. (2003) Bursts of high-frequency stimulation trigger rapid delivery of pre-existing α-CaMKII mRNA to synapses: a mechanism in dendritic protein synthesis during long-term potentiation in adult awake rats. Eur. J. Neurosci. 17, 2679–2689 [DOI] [PubMed] [Google Scholar]
- 26.Bagni C., Mannucci L., Dotti C. G., and Amaldi F. (2000) Chemical stimulation of synaptosomes modulates α-Ca2+/calmodulin-dependent protein kinase II mRNA association to polysomes. J. Neurosci. 20, RC76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muddashetty R. S., Kelić S., Gross C., Xu M., and Bassell G. J. (2007) Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J. Neurosci. 27, 5338–5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin Y., Edelman G. M., and Vanderklish P. W. (2002) The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc. Natl. Acad. Sci. U.S.A. 99, 2368–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiménez C. R., Eyman M., Lavina Z. S., Gioio A., Li K. W., van der Schors R. C., Geraerts W. P., Giuditta A., Kaplan B. B., and van Minnen J. (2002) Protein synthesis in synaptosomes: a proteomics analysis. J. Neurochem. 81, 735–744 [DOI] [PubMed] [Google Scholar]
- 30.Biesemann C., Grønborg M., Luquet E., Wichert S. P., Bernard V., Bungers S. R., Cooper B., Varoqueaux F., Li L., Byrne J. A., Urlaub H., Jahn O., Brose N., and Herzog E. (2014) Proteomic screening of glutamatergic mouse brain synaptosomes isolated by fluorescence activated sorting. EMBO J. 33, 157–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Will T. J., Tushev G., Kochen L., Nassim-Assir B., Cajigas I. J., Tom Dieck S., and Schuman E. M. (2013) Deep sequencing and high-resolution imaging reveal compartment-specific localization of Bdnf mRNA in hippocampal neurons. Sci. Signal. 6, rs16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrett L. W., Fletcher S., and Wilton S. D. (2012) Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell. Mol. Life Sci. 69, 3613–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutton M. A., Taylor A. M., Ito H. T., Pham A., and Schuman E. M. (2007) Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron 55, 648–661 [DOI] [PubMed] [Google Scholar]
- 34.Barrera I., Hernández-Kelly L. C., Castelán F., and Ortega A. (2008) Glutamate-dependent elongation factor-2 phosphorylation in Bergmann glial cells. Neurochem. Int. 52, 1167–1175 [DOI] [PubMed] [Google Scholar]
- 35.Autry A. E., Adachi M., Nosyreva E., Na E. S., Los M. F., Cheng P. F., Kavalali E. T., and Monteggia L. M. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheetz A. J., Nairn A. C., and Constantine-Paton M. (2000) NMDA receptor-mediated control of protein synthesis at developing synapses. Nat. Neurosci. 3, 211–216 [DOI] [PubMed] [Google Scholar]
- 37.Chotiner J. K., Khorasani H., Nairn A. C., O'Dell T. J., and Watson J. B. (2003) Adenylyl cyclase-dependent form of chemical long-term potentiation triggers translational regulation at the elongation step. Neuroscience 116, 743–752 [DOI] [PubMed] [Google Scholar]
- 38.Costa-Mattioli M., Gobert D., Stern E., Gamache K., Colina R., Cuello C., Sossin W., Kaufman R., Pelletier J., Rosenblum K., Krnjević K., Lacaille J. C., Nader K., and Sonenberg N. (2007) eIF2α phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129, 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa-Mattioli M., Gobert D., Harding H., Herdy B., Azzi M., Bruno M., Bidinosti M., Ben Mamou C., Marcinkiewicz E., Yoshida M., Imataka H., Cuello A. C., Seidah N., Sossin W., Lacaille J. C., Ron D., Nader K., and Sonenberg N. (2005) Translational control of hippocampal synaptic plasticity and memory by the eIF2α kinase GCN2. Nature 436, 1166–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banko J. L., Merhav M., Stern E., Sonenberg N., Rosenblum K., and Klann E. (2007) Behavioral alterations in mice lacking the translation repressor 4E-BP2. Neurobiol. Learn. Mem. 87, 248–256 [DOI] [PubMed] [Google Scholar]
- 41.Lee D. H., and Goldberg A. L. (1998) Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 8, 397–403 [DOI] [PubMed] [Google Scholar]
- 42.Hendil K. B. (1988) The 19S multicatalytic “prosome” proteinase is a constitutive enzyme in HeLa cells. Biochem. Int. 17, 471–477 [PubMed] [Google Scholar]
- 43.Lopez-Salon M., Alonso M., Vianna M. R., Viola H., Mello e Souza T., Izquierdo I., Pasquini J. M., and Medina J. H. (2001) The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur. J. Neurosci. 14, 1820–1826 [DOI] [PubMed] [Google Scholar]
- 44.Karpova A., Mikhaylova M., Thomas U., Knöpfel T., and Behnisch T. (2006) Involvement of protein synthesis and degradation in long-term potentiation of Schaffer collateral CA1 synapses. J. Neurosci. 26, 4949–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hegde A. N., Goldberg A. L., and Schwartz J. H. (1993) Regulatory subunits of cAMP-dependent protein kinases are degraded after conjugation to ubiquitin: a molecular mechanism underlying long-term synaptic plasticity. Proc. Natl. Acad. Sci. U.S.A. 90, 7436–7440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hegde A. N., Inokuchi K., Pei W., Casadio A., Ghirardi M., Chain D. G., Martin K. C., Kandel E. R., and Schwartz J. H. (1997) Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell 89, 115–126 [DOI] [PubMed] [Google Scholar]
- 47.Gong B., Cao Z., Zheng P., Vitolo O. V., Liu S., Staniszewski A., Moolman D., Zhang H., Shelanski M., and Arancio O. (2006) Ubiquitin hydrolase Uch-L1 rescues β-amyloid-induced decreases in synaptic function and contextual memory. Cell 126, 775–788 [DOI] [PubMed] [Google Scholar]
- 48.Wilson S. M., Bhattacharyya B., Rachel R. A., Coppola V., Tessarollo L., Householder D. B., Fletcher C. F., Miller R. J., Copeland N. G., and Jenkins N. A. (2002) Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nat. Genet. 32, 420–425 [DOI] [PubMed] [Google Scholar]
- 49.Kim A. H., Puram S. V., Bilimoria P. M., Ikeuchi Y., Keough S., Wong M., Rowitch D., and Bonni A. (2009) A centrosomal Cdc20-APC pathway controls dendrite morphogenesis in postmitotic neurons. Cell 136, 322–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stegmüller J., Konishi Y., Huynh M. A., Yuan Z., Dibacco S., and Bonni A. (2006) Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron 50, 389–400 [DOI] [PubMed] [Google Scholar]
- 51.Pick J. E., Malumbres M., and Klann E. (2013) The E3 ligase APC/C-Cdh1 is required for associative fear memory and long-term potentiation in the amygdala of adult mice. Learn. Mem. 20, 11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang J., Ikeuchi Y., Malumbres M., and Bonni A. (2015) A Cdh1-APC/FMRP ubiquitin signaling link drives mGluR-dependent synaptic plasticity in the mammalian brain. Neuron 86, 726–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Y. H., Armstrong D., Albrecht U., Atkins C. M., Noebels J. L., Eichele G., Sweatt J. D., and Beaudet A. L. (1998) Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 21, 799–811 [DOI] [PubMed] [Google Scholar]
- 54.El Hokayem J., and Nawaz Z. (2014) E6AP in the brain: one protein, dual function, multiple diseases. Mol. Neurobiol. 49, 827–839 [DOI] [PubMed] [Google Scholar]
- 55.Greer P. L., Hanayama R., Bloodgood B. L., Mardinly A. R., Lipton D. M., Flavell S. W., Kim T. K., Griffith E. C., Waldon Z., Maehr R., Ploegh H. L., Chowdhury S., Worley P. F., Steen J., and Greenberg M. E. (2010) The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating Arc. Cell 140, 704–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pak D. T., and Sheng M. (2003) Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science 302, 1368–1373 [DOI] [PubMed] [Google Scholar]
- 57.Tai H. C., Besche H., Goldberg A. L., and Schuman E. M. (2010) Characterization of the brain 26S proteasome and its interacting proteins. Front. Mol. Neurosci. 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Djakovic S. N., Schwarz L. A., Barylko B., DeMartino G. N., and Patrick G. N. (2009) Regulation of the proteasome by neuronal activity and calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 284, 26655–26665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamilton A. M., Oh W. C., Vega-Ramirez H., Stein I. S., Hell J. W., Patrick G. N., and Zito K. (2012) Activity-dependent growth of new dendritic spines is regulated by the proteasome. Neuron 74, 1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bingol B., Wang C. F., Arnott D., Cheng D., Peng J., and Sheng M. (2010) Autophosphorylated CaMKIIα acts as a scaffold to recruit proteasomes to dendritic spines. Cell 140, 567–578 [DOI] [PubMed] [Google Scholar]
- 61.Ehlers M. D. (2003) Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 6, 231–242 [DOI] [PubMed] [Google Scholar]
- 62.Colledge M., Snyder E. M., Crozier R. A., Soderling J. A., Jin Y., Langeberg L. K., Lu H., Bear M. F., and Scott J. D. (2003) Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron 40, 595–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asano S., Fukuda Y., Beck F., Aufderheide A., Förster F., Danev R., and Baumeister W. (2015) Proteasomes. A molecular census of 26S proteasomes in intact neurons. Science 347, 439–442 [DOI] [PubMed] [Google Scholar]
- 64.Fonseca R., Vabulas R. M., Hartl F. U., Bonhoeffer T., and Nägerl U. V. (2006) A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron 52, 239–245 [DOI] [PubMed] [Google Scholar]
- 65.Brooks S. A. (2010) Functional interactions between mRNA turnover and surveillance and the ubiquitin proteasome system. Wiley Interdiscip. Rev. RNA 1, 240–252 [DOI] [PubMed] [Google Scholar]
- 66.Durairaj G., and Kaiser P. (2014) The 26S proteasome and initiation of gene transcription. Biomolecules 4, 827–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soulé J., Alme M., Myrum C., Schubert M., Kanhema T., and Bramham C. R. (2012) Balancing Arc synthesis, mRNA decay, and proteasomal degradation: maximal protein expression triggered by rapid eye movement sleep-like bursts of muscarinic cholinergic receptor stimulation. J. Biol. Chem. 287, 22354–22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamamoto N., Hegde A. N., Chain D. G., and Schwartz J. H. (1999) Activation and degradation of the transcription factor C/EBP during long-term facilitation in Aplysia. J. Neurochem. 73, 2415–2423 [DOI] [PubMed] [Google Scholar]
- 69.James A. B., Conway A. M., and Morris B. J. (2006) Regulation of the neuronal proteasome by Zif268 (Egr1). J. Neurosci. 26, 1624–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hou L., Antion M. D., Hu D., Spencer C. M., Paylor R., and Klann E. (2006) Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 51, 441–454 [DOI] [PubMed] [Google Scholar]
- 71.Banerjee S., Neveu P., and Kosik K. S. (2009) A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron 64, 871–884 [DOI] [PubMed] [Google Scholar]
- 72.Lee Y. S., Choi S. L., Jun H., Yim S. J., Lee J. A., Kim H. F., Lee S. H., Shim J., Lee K., Jang D. J., and Kaang B. K. (2012) AU-rich element-binding protein negatively regulates CCAAT enhancer-binding protein mRNA stability during long-term synaptic plasticity in Aplysia. Proc. Natl. Acad. Sci. U.S.A. 109, 15520–15525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laroia G., and Schneider R. J. (2002) Alternate exon insertion controls selective ubiquitination and degradation of different AUF1 protein isoforms. Nucleic Acids Res. 30, 3052–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lajtha A., Latzkovits L., and Toth J. (1976) Comparison of turnover rates of proteins of the brain, liver and kidney in mouse in vivo following long term labeling. Biochim. Biophys. Acta 425, 511–520 [DOI] [PubMed] [Google Scholar]
- 75.Price J. C., Guan S., Burlingame A., Prusiner S. B., and Ghaemmaghami S. (2010) Analysis of proteome dynamics in the mouse brain. Proc. Natl. Acad. Sci. U.S.A. 107, 14508–14513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen L. D., Zuchman R., Sorokina O., Müller A., Dieterich D. C., Armstrong J. D., Ziv T., and Ziv N. E. (2013) Metabolic turnover of synaptic proteins: kinetics, interdependencies and implications for synaptic maintenance. PLoS ONE 8, e63191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Claydon A. J., and Beynon R. (2012) Proteome dynamics: revisiting turnover with a global perspective. Mol. Cell. Proteomics 11, 1551–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mammen A. L., Huganir R. L., and O'Brien R. J. (1997) Redistribution and stabilization of cell surface glutamate receptors during synapse formation. J. Neurosci. 17, 7351–7358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boisvert F. M., Ahmad Y., Gierliński M., Charrière F., Lamont D., Scott M., Barton G., and Lamond A. I. (2012) A quantitative spatial proteomics analysis of proteome turnover in human cells. Mol. Cell. Proteomics 11, M111.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Butko M. T., Yang J., Geng Y., Kim H. J., Jeon N. L., Shu X., Mackey M. R., Ellisman M. H., Tsien R. Y., and Lin M. Z. (2012) Fluorescent and photo-oxidizing TimeSTAMP tags track protein fates in light and electron microscopy. Nat. Neurosci. 15, 1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saka S. K., Vogts A., Kröhnert K., Hillion F., Rizzoli S. O., and Wessels J. T. (2014) Correlated optical and isotopic nanoscopy. Nat. Commun. 5, 3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dieterich D. C., Hodas J. J., Gouzer G., Shadrin I. Y., Ngo J. T., Triller A., Tirrell D. A., and Schuman E. M. (2010) In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat. Neurosci. 13, 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hinz F. I., Dieterich D. C., Tirrell D. A., and Schuman E. M. (2012) Non-canonical amino acid labeling in vivo to visualize and affinity purify newly synthesized proteins in larval zebrafish. ACS Chem. Neurosci. 3, 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K. J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L. G., and Landegren U. (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 [DOI] [PubMed] [Google Scholar]
- 85.Tom Dieck S., Kochen L., Hanus C., Heumüller M., Bartnik I., Nassim-Assir B., Merk K., Mosler T., Garg S., Bunse S., Tirrell D. A., and Schuman E. M. (2015) Direct visualization of newly synthesized target proteins in situ. Nat. Methods 12, 411–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt E. K., Clavarino G., Ceppi M., and Pierre P. (2009) SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 6, 275–277 [DOI] [PubMed] [Google Scholar]
- 87.Buhr F., Kohl-Landgraf J., tom Dieck S., Hanus C., Chatterjee D., Hegelein A., Schuman E. M., Wachtveitl J., and Schwalbe H. (2015) Design of photocaged puromycin for nascent polypeptide release and spatiotemporal monitoring of translation. Angew. Chem. Int. Ed. Engl. 54, 3717–3721 [DOI] [PubMed] [Google Scholar]
- 88.Pavlopoulos E., Trifilieff P., Chevaleyre V., Fioriti L., Zairis S., Pagano A., Malleret G., and Kandel E. R. (2011) Neuralized1 activates CPEB3: a function for nonproteolytic ubiquitin in synaptic plasticity and memory storage. Cell 147, 1369–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kjøller C., and Diemer N. H. (2000) GluR2 protein synthesis and metabolism in rat hippocampus following transient ischemia and ischemic tolerance induction. Neurochem. Int. 37, 7–15 [DOI] [PubMed] [Google Scholar]
- 90.Huh K. H., and Wenthold R. J. (1999) Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-d-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J. Biol. Chem. 274, 151–157 [DOI] [PubMed] [Google Scholar]
- 91.El-Husseini A. E.-D., Schnell E., Dakoji S., Sweeney N., Zhou Q., Prange O., Gauthier-Campbell C., Aguilera-Moreno A., Nicoll R. A., and Bredt D. S. (2002) Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108, 849–863 [DOI] [PubMed] [Google Scholar]


