Background: NgR is expressed on microglia and regulates cell adhesion and migration behavior.
Results: Nogo/NgR signal induced the expression of proinflammatory factors in microglia through NF-κB and STAT3 signal pathways.
Conclusion: Nogo peptide could directly take part in CNS inflammatory process by influencing the expression of proinflammatory factors in microglia.
Significance: Nogo/NgR signal might be involved in multiple processes in various inflammation-associated CNS diseases.
Keywords: microglia, neurodegenerative disease, neuroinflammation, NF-kappaB, STAT3, NgR, Nogo peptide
Abstract
Microglia have been proposed to play a pivotal role in the inflammation response of the CNS by expressing a range of proinflammatory enzymes and cytokines under pathological stimulus. Our previous study has confirmed that Nogo receptor (NgR), an axon outgrowth inhibition receptor, is also expressed on microglia and regulates cell adhesion and migration behavior in vitro. In the present study, we further investigated the proinflammatory effects and possible mechanisms of Nogo on microglia in vitro. In this study, Nogo peptide, Nogo-P4, a 25-amino acid core inhibitory peptide sequence of Nogo-66, was used. We found that Nogo-P4 was able to induce the expression of inducible nitric-oxide synthase and cyclooxygenase-2 and the release of proinflammatory cytokines, including IL-1β, TNF-α, NO, and prostaglandin E2 in microglia, which could be reversed by NEP1–40 (Nogo-66(1–40) antagonist peptide), phosphatidylinositol-specificphospholipase C, or NgR siRNA treatment. After Nogo-P4 stimulated microglia, the phosphorylation levels of NF-κB and STAT3 were increased obviously, which further mediated microglia expressing proinflammatory factors induced by Nogo-P4. Taken together, we concluded that Nogo peptide could directly take part in CNS inflammatory process by influencing the expression of proinflammatory factors in microglia, which were related to the NF-κB and STAT3 signal pathways. Besides neurite outgrowth restriction, the Nogo/NgR signal might be involved in multiple processes in various inflammation-associated CNS diseases.
Introduction
Nogo-A, a myelin-associated inhibitory molecule, plays an important role in various cellular and molecular events contributing to the failure of CNS axon regrowth and reconnection after transection (1). Nogo receptor (NgR)4 is a neuronal surface glycosylphosphatidylinositol-linked receptor, which binds with high affinity to Nogo-66 (2). Accumulating reports have demonstrated that interrupting the function of the Nogo-A/NgR signal on neurons enables the extension of axons over the insulted zone, which is beneficial for the regeneration of the neurite and the functional improvement of the injured CNS (3). Recently, increasing numbers of in vitro studies have identified the expression of NgR on immune cells, including macrophage (4), microglia (5), dendritic cells (6), and peripheral blood mononuclear cells (7), implying that NgR might play a more widespread role in neuroinflammation under a range of neurological conditions.
CNS inflammation plays a significant role in the progression of numerous CNS diseases, including brain trauma, spinal cord injury, ischemia, and degenerative disorders, although the mechanisms are not yet fully understood (8–13). Microglia, the resident CNS macrophage-like cell, has been proposed to participate in the innate immune response in the CNS (14). Overactivated microglia causes uncontrolled inflammatory response, including the release of proinflammatory and/or cytotoxic factors, leading to neuronal damage and tissue injury (15–18).
Evidence from animal models has elucidated that the efflux of macrophage out of the sites of inflammation in peripheral nerves and the termination of inflammation are mediated by NgR (4). Our previous work also demonstrated that NgR is expressed on microglia, and binding of NgR with Nogo-66 inhibits the adhesion and migration of microglia (5), suggesting that NgR will possibly take part in neuroinflammation via mediating microglia adhesion and migration. So far, although several reports have implied the relationship between the Nogo-A signal and CNS inflammation, there is few direct evidence and mechanisms associated with the role of Nogo in neuroinflammation have been provided. In the present study, we explored the effects of Nogo-P4 on the expression of inflammatory mediators in microglia, including iNOS, COX-2, IL-1β, TNF-α, NO, and PGE2. Nogo-P4 is a 25-amino acid inhibitory peptide sequence (residues 31–55 of Nogo-66), an active segment of Nogo-66, and has the core inhibitory activity of Nogo-66, a potent inhibitory component of Nogo-A (19). We also demonstrated that both the NF-κB pathway and STAT3 pathway were responsible for the expression of inflammatory mediators triggered by Nogo-P4.
Experimental Procedures
Cell Culture and Treatments
Sprague-Dawley rats were purchased from Zhejiang Laboratory Animal Center (Hangzhou, China). All animal tests were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the institutional animal care and use committee of the Nanjing Medical University Experimental Animal Department.
Primary microglia culture was prepared following the method described previously with some modifications (5). Briefly, cerebral cortex from postnatal Sprague-Dawley rats (P0–P2) was stripped of meninges and chopped into small chunks. The tissue was then dissociated in trypsin-EDTA (0.25%; Sigma-Aldrich) before being seeded into poly-l-lysine (0.01 mg/ml; Sigma-Aldrich)-precoated T75 tissue culture flasks in Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F-12; Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco). Subsequent medium change was carried out after 3 days. After 2 weeks in culture, floating and weakly attached cells on the mixed primary culture cell layer were isolated by gentle shaking of the flask. The resulting cell suspension was then collected by centrifugation. About 95% of these cells were positive for CD11b (cluster of differentiation molecule 11B), a marker for microglial cell types.
BV-2 murine microglial cells (ATCC) were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% FBS (Gibco), penicillin (0.1%; Sigma-Aldrich), streptomycin (0.1%; Sigma-Aldrich) at 5% CO2 at 37 °C.
In this study, Nogo peptide, Nogo-P4 (Alpha Diagnostic International Inc.), a 25-amino acid core inhibitory peptide sequence of Nogo-66 (19), was used for stimulating microglia, and Rtn-P4 (Alpha Diagnostic International), a Reticulon 1 (Rtn1) non-inhibitory control/blocking peptide, was used as a control. The method of treating microglia was as described previously with modifications (20, 21). Briefly, 24-well plates were coated with methanol-solubilized nitrocellulose and air-dried under a sterile hood. Considering the possible anti-adhesion effects of Nogo-P4 on microglia, poly-l-lysine was used to aid in adhesion of microglia. Then Nogo-P4 or Rtn-P4 was applied at 100 μg/ml, and the protein was incubated overnight at 4 °C. Microglia freshly dissociated from primary culture or BV-2 microglial cells were added into the wells precoated with Nogo-P4 or Rtn-P4 and cultured for the indicated time. After the stimulation, cell lysis and supernatant were collected for further detection. To elucidate the signal pathway related to inflammation cytokine production induced by Nogo-P4, microglia were preincubated with the blocking agent NEP1–40 (Nogo-66(1–40) antagonist peptide) (10 μm; Sigma-Aldrich), PI-PLC (0.3 units/ml, Sigma-Aldrich), JSH-23, NF-κB activation inhibitor II (10 μm; Selleck Chemicals) or S3I-201, and STAT3 inhibitor VII (20 μm; Santa Cruz Biotechnology, Inc.) for 30 min before they were transferred into prepared wells.
siRNA Transfection
According to the gene sequence of rat NgR, a small interfering RNA (siRNA) targeting NgR (NgR siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′ (antisense)) was designed. Meanwhile, nonspecific sequences (control siRNA, 5′-GCCGAAAUCUCACUAUCCUTT-3′ (sense) and 5′-AGGAUAGUGAGAUUUCGGCTT-3′ (antisense)) were used as a control. The siRNA was synthesized by Shanghai GenePharma Co. Ltd. Transfection of siRNAs was performed using Lipofectamine® 2000 transfection reagent (Invitrogen).
BV-2 microglial cells were seeded onto 24-well plates at a density of 1 × 105 cells/well and cultured for 12 h. Before transfection, the medium was changed to a fresh one containing no serum. Treatment with control siRNA or NgR siRNA was realized at a final dose of 60 nm siRNA/well and removed 5 h post-transfection. These cells were subsequently cultured for 36 h. After transfection, BV-2 microglial cells were added into the wells precoated with Nogo-P4 or Rtn-P4 and cultured for the indicated time.
3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
After transfection, the cellular ability of BV-2 microglial cells was measured by MTT assay. After the indicated time, the medium was changed to a fresh one containing no serum, and MTT reagent (0.05 mg/ml; Sigma-Aldrich) was added to the cells for 4 h at 37 °C. Medium was carefully removed, and 200 μl of DMSO was added to each well to solubilize the precipitated formazan product. The absorbance was determined at 595 nm. The survival rate of treated samples was expressed as a percentage of absorbance at 595 nm of the PBS control.
Western Blot Analysis
Microglia obtained from different treatments were lysed in ice-cold lysis buffer (50 mm Tris-HCl, 2 mm MgCl2, 1% Nonidet P-40, 10% glycerol, 100 mm NaCl), and 1% protease inhibitor mixture (Roche Applied Science) was added just before use. The lysates were cleared by centrifugation at 13,000 × g for 10 min at 4 °C. Supernatant protein (50 μg) was run in denaturing gels and transferred onto nitrocellulose membranes. The membranes were then blocked with 5% bovine serum albumin in TBST and incubated with specific primary antibody overnight at 4 °C. The following antibodies were used: goat anti-IL-1β (1:500; R&D Systems, AF-501-NA), rabbit anti-iNOS (1:800; Abcam, ab3523), goat anti-COX-2 (1:500; Santa Cruz Biotechnology, sc-1747), rabbit anti-NgR (1:1000; Merck Millipore, AB 15138), rabbit anti-phospho-NF-κB p65 at Ser-536 (1:1000; Cell Signaling Technology Inc., catalog no. 3033), rabbit anti-NF-κB p65 (1:1000; Cell Signaling Technology Inc., catalog no. 8242), rabbit anti-phospho-IKKβ (inhibitor of NF-κB kinase-β) at Ser-180 (1:1000; Cell Signaling Technology Inc., catalog no. 2697), rabbit anti-IKKβ (1:1000; Cell Signaling Technology Inc., catalog no. 8943), rabbit anti-phospho-IκBα (inhibitor of NF-κB-α) at Ser-32 (1:1000; Cell Signaling Technology Inc., catalog no. 2859), mouse anti-IκBα (1:1000; Cell Signaling Technology Inc., catalog no. 4814), rabbit anti-phospho-STAT3 at Tyr-705 (1:1000; Cell Signaling Technology Inc., catalog no. 9145), mouse anti-STAT3 (1:1000; Cell Signaling Technology Inc., catalog no. 9139), or β-actin (1:2000; Santa Cruz Biotechnology, sc-47778). After incubating with horseradish peroxidase-conjugated secondary antibodies, anti-mouse IgG (1:3000; Cell Signaling Technology Inc., catalog no. 7076), anti-goat IgG (1:500; R&D Systems, HAF019), or anti-rabbit IgG (1:3000; Cell Signaling Technology Inc., catalog no. 7074), immunoreactive bands were then visualized by chemiluminescence reagents (ECL; Thermo Fisher Scientific).
For blot densitometry assay, the images of protein bands were captured with a Bio-Rad Gel Doc XR documentation system. Semiquantitative densitometric analysis of the relative expressions of the protein were determined using Quantity One software by scanning the pixel density of the resultant blots.
NO Production
Concentrations of NO in the cell supernatants were determined by measuring nitrite, a major stable product of NO, using the Griess reagent (1% sulfanilamide, 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride, 2.5% H3PO4) (Invitrogen). 100 μl of each culture medium collected from different groups were mixed with an equal volume of Griess reagent. Absorbance was measured using a plate reader at 540 nm. The production of NO was calculated relative to that observed after control treatment.
Measurement of IL-1β, TNF-α, and PGE2 Release
Supernatants of various treatment groups were collected to detect the release level of IL-1β, TNF-α, and PGE2 by a commercial ELISA kit (R&D Systems, Minneapolis, MN) following the manufacturer's instructions. The absorbance at 450 nm was determined by a microplate reader.
Immunocytochemistry
For identification of the effect of Nogo-P4 on nuclear translocation of NF-κB, microglia cultured on coverslips were washed with PBS, fixed with 4% paraformaldehyde in PBS for 30 min, permeabilized with 0.1% Triton X-100 in PBS for 5 min, and then blocked with 10% normal goat serum in PBS and incubated overnight at 4 °C with primary polyclonal antibody to mouse anti-NF-κB (1:50; Santa Cruz Biotechnology, sc-8008) diluted in PBS containing 10% normal goat serum. Nuclei were labeled with Hoechst 33342 (10 μg/ml; Molecular Probes, H3570). After washing three times with PBS (pH 7.4), cells were incubated with Alexa Fluor® 594-conjugated goat anti-mouse secondary antibodies (1:300; Invitrogen, A11005) for 60 min at room temperature. The fluorescent imaging was visualized by using an Olympus fluorescence microscope (Japan).
Statistical Analysis
All present data represent the results of three or four independent experiments. Statistical analysis was performed using Student's t test or one-way analysis of variance (ANOVA) with post hoc Tukey's test using GraphPad Prism version 5 software (GraphPad Software Inc., La Jolla, CA). All data are presented as mean ± S.D. p values of p < 0.05 (indicated with an asterisk) were considered statistically significant.
Results
Stimulation with Nogo-P4 Increased Microglia Expressing iNOS and COX-2 as Well as Releasing IL-1β, TNF-α, NO, and PGE2
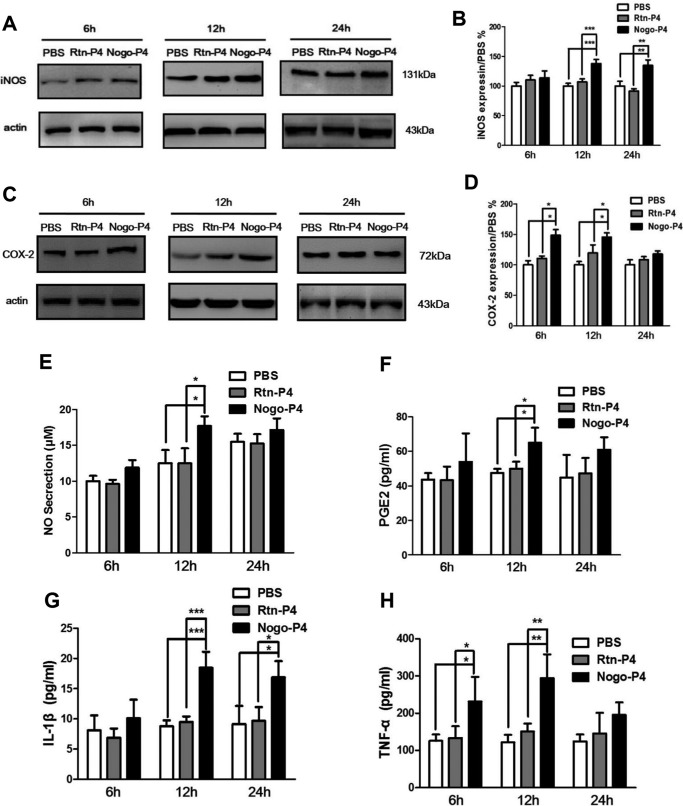
To investigate the effects of Nogo-P4 on the production of proinflammatory enzymes and cytokines in microglia, wells were precoated with Nogo-P4 (100 μg/ml) or Rtn-P4 (100 μg/ml), and then primary cultured microglia were added into the wells and cultured for 6, 12, or 24 h. At the end of stimulation, the cell lysates and supernatants were collected, and the expression levels of proinflammatory enzymes and cytokines were determined. Results in Fig. 1 showed that Nogo-P4 stimulation significantly promoted microglia to express iNOS (12 and 24 h) and COX-2 (6 and 12 h) and also raised the release of IL-1β (12 h), TNF-α (6 and 12 h), PGE2 (12 h), and NO (12 h) in microglia. However, Rtn-P4 had no effects. Thus, a 12 h stimulation time was used for the following study. Moreover, full-length Nogo-66 increased the expression of IL-1β and NO in microglia (data not shown). These results implied that Nogo peptide could directly participate in inflammation associated with microglia by increasing the expression of proinflammatory enzymes and cytokines in microglia.
FIGURE 1.
Stimulation with Nogo-P4 increased the expression of inflammatory mediators in primary cultured microglia. Microglia separated from the cerebral cortex were seeded into wells precoated with PBS or Rtn-P4 (100 μg/ml) or Nogo-P4 (100 μg/ml) and stimulated for 6, 12, or 24 h. Cell lysates and supernatant were collected to detect the expression of inflammatory mediators. Western blot was used to determine the expression of iNOS (A and B) and COX-2 (C and D). Values are reported as mean ± S.D. (error bars), as a percentage of values determined in the PBS group (control, 100%). The Western blot data represent the results of four independent experiments. The Griess method was performed to detect NO release (E). The proportions of PGE2 (F), IL-1β (G), and TNF-α (H) secreted into the medium was assayed by ELISA kits. Values are reported as mean ± S.D. Griess and ELISA data represent the results of three independent experiments. Statistical analysis was performed using one-way ANOVA with post hoc Tukey's test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
NgR Was Responsible for the Effects of Nogo-P4 on Microglia Producing Proinflammatory Mediators
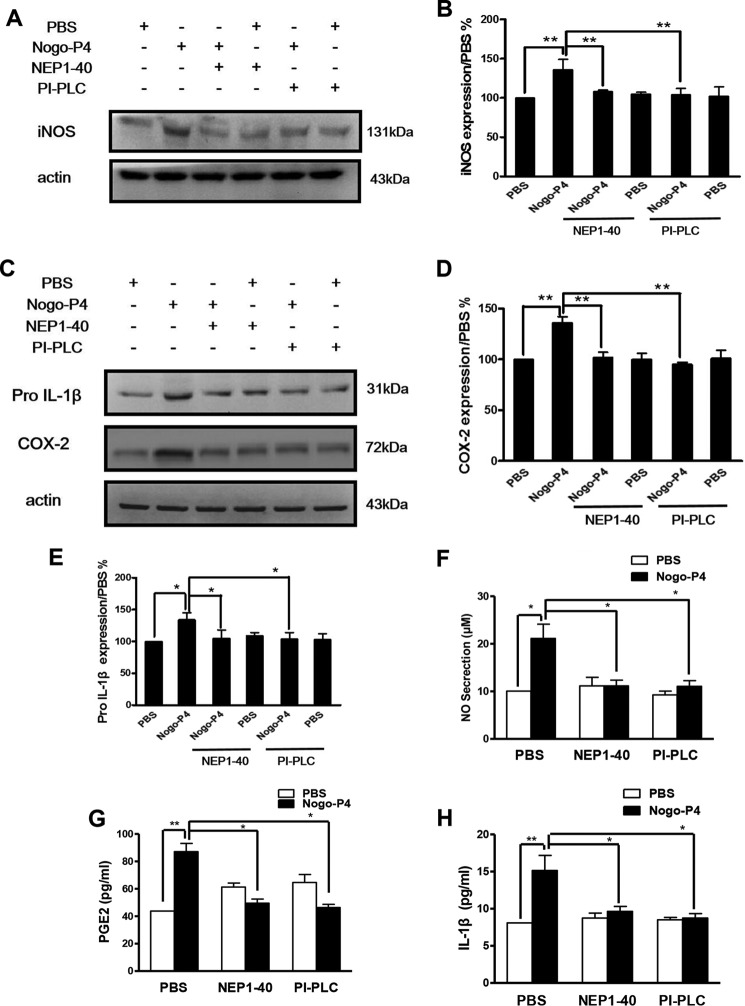
The glycosylphosphatidylinositol-linked cell surface NgR has been widely confirmed to be the Nogo-66 functional receptor to inhibit neurite growth (2) as well as neural cell migration (5, 22). To elucidate the possible signal pathway related to Nogo-P4, we assessed the mediation of NgR on Nogo-P4 facilitating proinflammatory cytokine production. The expression of NgR on primary cultured microglia has already been clearly identified in our previous study (5); thus, we pretreated microglia with NEP1–40 (10 μm) and the PI-PLC (0.3 units/ml) and then observed the effects of Nogo-P4 on expressing proinflammatory enzymes and cytokines. NEP1–40, a competitive antagonist peptide derived from the first 40 amino acids of the Nogo-66, promotes axonal outgrowth through blocking the binding of Nogo-66 to NgR in vitro and in vivo (23). PI-PLC is commonly used to release the glycosylphosphatidylinositol-anchored NgR from the cell surface (2). As seen in Fig. 2, the results showed that NEP1–40 and PI-PLC treatment significantly attenuated the expression of pro-IL-1β, iNOS, and COX-2 as well as the release of IL-1β, NO, and PGE2 in microglia after Nogo-P4 stimulation, indicating that NgR was necessary for the effects of Nogo-P4 on the production of proinflammatory mediators in microglia.
FIGURE 2.
NgR was responsible for the effects of Nogo-P4 on microglia expressing proinflammatory mediators. Before they were added to the protein-coated wells, microglia were pretreated with NEP1–40 (10 μm) or PI-PLC (0.3 units/ml) for 30 min to interrupt the function of NgR. The cells were then exposed to PBS or Nogo-P4 for 12 h. The proportions of iNOS (A and B), pro-IL-1β (C and D), and COX-2 (C and E) were determined by Western blot. Values are reported as mean ± S.D. (error bars), as a percentage of values determined in the PBS group (control, 100%). The release of NO was assayed by the Griess method (F). An ELISA was performed to detect the production of PGE2 (G) and IL-1β (H) in the supernatant. Values are reported as mean ± S.D. All data represent the results of three independent experiments. Statistical analysis was performed using Student's t test. *, p < 0.05; **, p < 0.01.
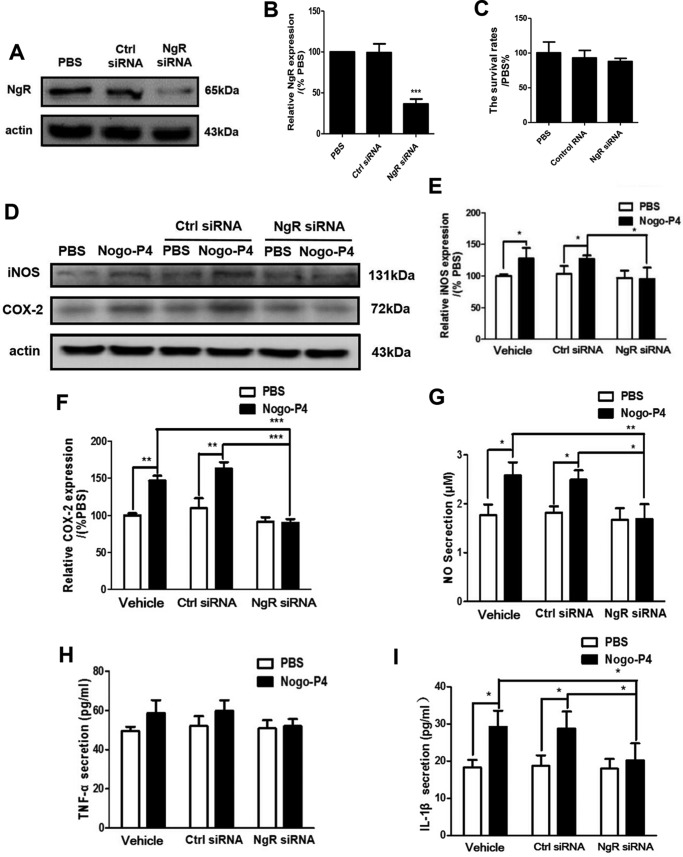
Furthermore, NgR was silenced by siRNA interference in BV-2 microglia, and the expression of proinflammatory mediators was analyzed. After transfection with NgR siRNA or control siRNA, the expression of NgR was determined by Western blot. Compared with control siRNA-treated cells, NgR siRNA-treated cells showed a significant decrease of the expression of NgR in BV-2 microglial cells (Fig. 3, A and B). Also, the transfection of control or NgR siRNA did not affect the cellular ability of BV-2 microglial cells (Fig. 3C). Results shown in Fig. 3, D–I, showed that NgR siRNA treatment significantly attenuated the expression of iNOS and COX-2 as well as the release of IL-1β, NO, and TNF-α in microglia after Nogo-P4 stimulation, indicating that NgR was responsible for the effects of Nogo-P4 on the expression of proinflammatory mediators in BV-2 microglial cells.
FIGURE 3.
Nogo-P4 increased the expression of inflammatory mediators in BV-2 microglia and NgR was responsible for the effects. A and B, the expression of NgR on BV-2 microglia was determined by Western blot after transfected with NgR siRNA or control siRNA. C, after BV-2 microglia treatment with NgR siRNA or control siRNA, the survival rate of microglia was determined by an MTT assay. Values are reported as mean ± S.D. (error bars), as a percentage of values determined in the PBS group (control, 100%). MTT data represent the results of four independent experiments and two repeats within each experiment. Before added to the protein-coated wells, BV-2 microglia was transfected with control or NgR siRNA to suppress the expression of NgR. The cells were then exposed to PBS or Nogo-P4 for 6 h. The proportions of iNOS (D and E) and COX-2 (D and F) were determined by Western blot. Values are reported as mean ± S.D., as a percentage of values determined in PBS group (control, 100%). The release of NO was assayed by the Griess method (G). An ELISA was performed to detect the production of TNF-α (H) and IL-1β (I) in the supernatant. Values are reported as mean ± S.D. MTT data represent the results of three independent experiments. Statistical analysis was performed using one-way ANOVA with post hoc Tukey's test. *, p < 0.05; **, p < 0.01.
Microglia Expressing Proinflammatory Mediators Induced by Nogo-P4 Was Mediated by NF-κB Pathway Activation via NgR
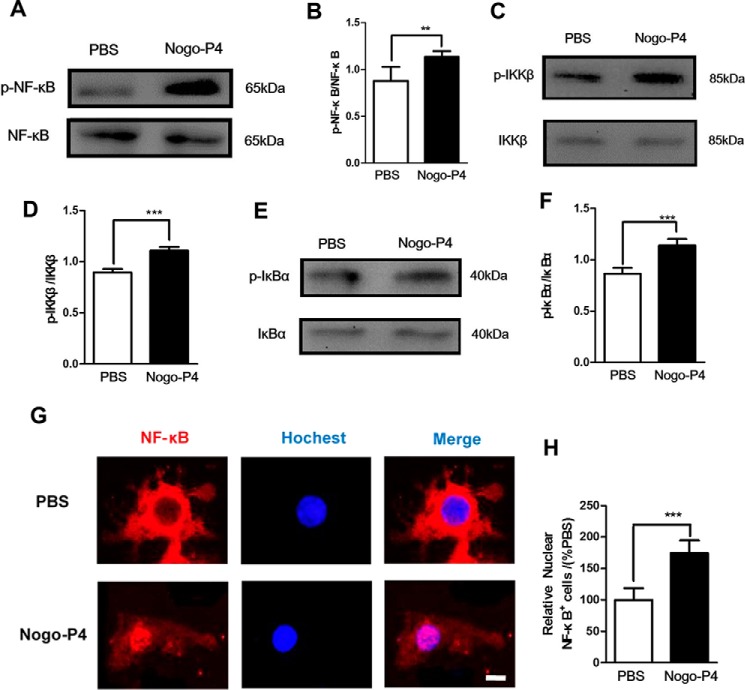
The nuclear factor NF-κB pathway has long been considered as a prototypical proinflammatory signal pathway (24). Thus, we investigated whether the NF-κB pathway was responsible for the effects of Nogo-P4 on the production of proinflammatory mediators in microglia. Microglia were seeded into the wells precoated with Nogo-P4, and then cell lyses were collected for Western blot. We observed a significant increase in the phosphorylation levels of NF-κB, IKKβ, and IκBα in microglia after stimulation by Nogo-P4 for 6 h (Fig. 4, A–F). In addition, Immunofluorescent staining analysis showed that Nogo-P4 induced NF-κB nuclear translocation in microglia (Fig. 4, G and H).
FIGURE 4.
Nogo-P4 induced NF-κB pathway activation in microglia. Microglia were incubated with PBS or Nogo-P4 for 6 h. The phosphorylation levels of NF-κB (A and B), IKKβ (C and D), and IκBα (E and F) were measured by Western blot. Nogo-P4 increased the phospho-NF-κB/NF-κB, phospho-IKKβ/IKKβ, and phospho- IκBα/IκBα levels. Values are reported as mean ± S.D. (error bars) Western blot data represent the results of four independent experiments. G and H, immunostaining of microglia with NF-κB and Hoechst. Scale bar, 10 μm. Nogo-P4 induced the NF-κB nuclear translocation. Values are reported as mean ± S.D. Immunostaining data represent the results of three independent experiments. Statistical analysis was performed using Student's t test. **, p < 0.01; ***, p < 0.001.
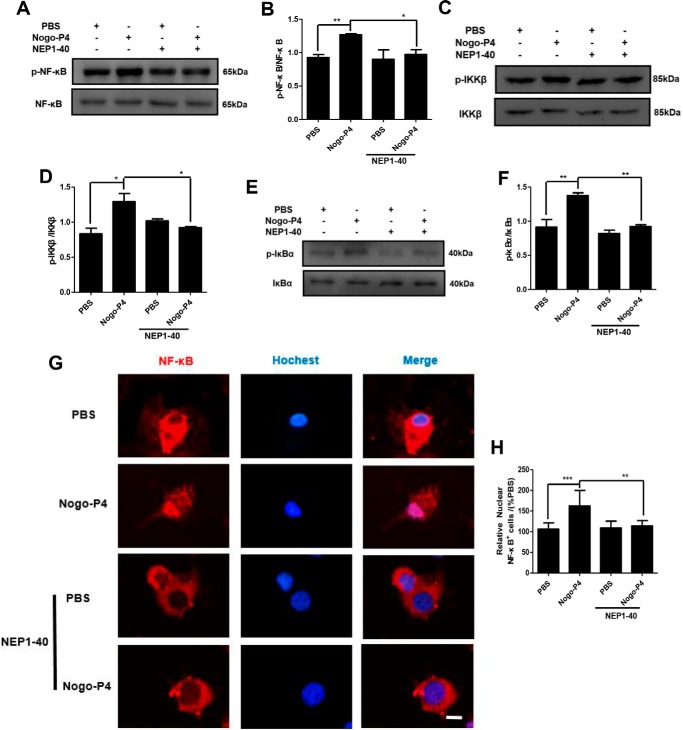
Furthermore, we determined whether NgR mediated NF-κB pathway activation induced by Nogo-P4. Before they were added to the protein-coated plate, microglia were pretreated with NEP1–40 (10 μm) for 30 min to interrupt the function of NgR. Results shown in Fig. 5, A–F, showed that NEP1–40 treatment significantly attenuated phosphorylation levels of NF-κB, IKKβ, and IκBα in microglia increased by Nogo-P4. Also, NF-κB nuclear translocation in microglia was significantly inhibited by NEP1–40. (Fig. 5, G and H). The results above indicated that Nogo-P4 bound to the NgR receptor and further triggered the activation of the NF-κB pathway.
FIGURE 5.
NgR was responsible for the activation of NF-κB pathway induced by Nogo-P4. Before being added to the protein-coated plate, microglia were pretreated with NEP1–40 (10 μm) for 30 min to interrupt the function of NgR. Microglia were incubated with PBS or Nogo-P4 for 6 h. The phosphorylation levels of NF-κB (A and B), IKKβ (C and D), and IκBα (E and F) were measured by Western blot. Activation of the NF-κB pathway in microglia induced by Nogo-P4 was mediated by NgR. Values are reported as mean ± S.D. (G and H). Shown is immunostaining of microglia with NF-κB and Hoechst. Scale bar, 10 μm. NF-κB nuclear translocation in microglia stimulated by via NgR. Values are reported as mean ± S.D. (error bars), as a percentage of values determined in PBS group (control, 100%). All data represent the results of three independent experiments. Statistical analysis was performed using one-way ANOVA with post hoc Tukey's test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
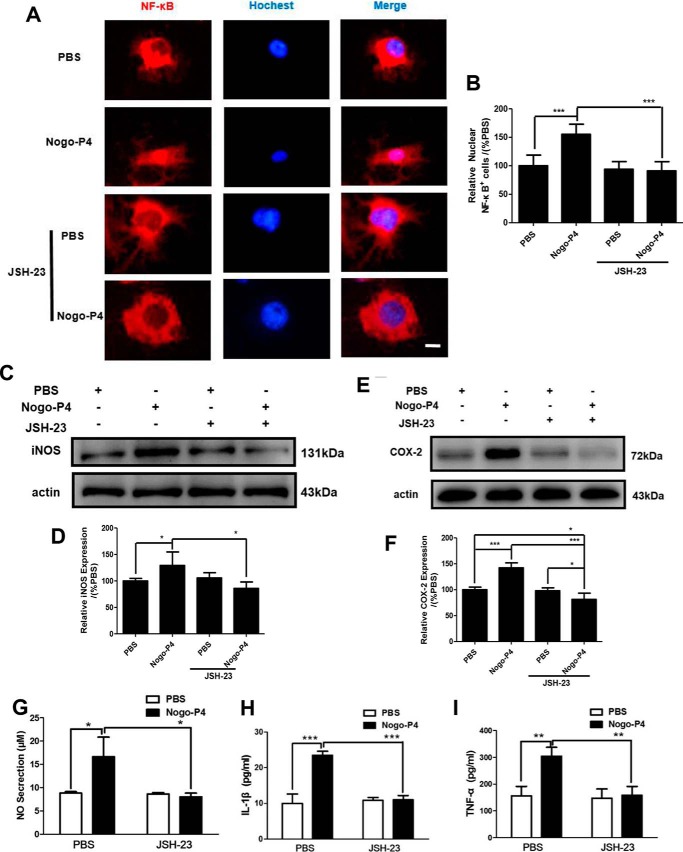
To validate whether the activation of NF-κB pathway was a response to the expression of proinflammatory cytokines induced by Nogo-P4, JSH-23 (an NF-κB activation inhibitor), which could inhibit nuclear translocation of NF-κB (25), was used to block the activity of the NF-κB signal pathway. We found that the nuclear translocation of NF-κB increased by Nogo-P4 was blocked in microglia pretreated with JSH-23 (10 μm) for 30 min (Fig. 6, A and B). Results presented in Fig. 6, C–I, indicated that promotion of Nogo-P4 upon microglial expression of iNOS and COX-2 as well as the release of IL-1β, TNF-α, NO, and PGE2 was attenuated by JSH-23 (10 μm) pretreatment, suggesting that the NF-κB pathway was responsible for the effects of the Nogo/NgR signal on microglia expressing proinflammatory mediators.
FIGURE 6.
Microglia expressing proinflammatory mediators induced by Nogo-P4 was mediated by the NF-κB pathway. Before they were added to the protein-coated plate, microglia were pretreated with JSH-23 (10 μm) for 30 min to interrupt the activation of NF-κB. Microglia were incubated with PBS or Nogo-P4 for 6 h. A and B, immunostaining of microglia with NF-κB and Hoechst. Scale bar, 10 μm. NF-κB activation inhibitor JSH-23 inhibited the NF-κB nuclear translocation induced by Nogo-P4. Immunostaining data represent the results of three independent experiments and two repeats within each experiment. Values are reported as mean ± S.D. (error bars), as a percentage of values determined in PBS group (control, 100%). After microglia were pretreated with JSH-23 (10 μm) for 30 min, microglia was incubated with PBS or Nogo-P4 for 12 h. The proportions of iNOS (C and D) and COX-2 (E and F) were determined by Western blot. Values are reported as mean ± S.D., as a percentage of values determined in the PBS group (control, 100%). Western blot data represent the results of four independent experiments. The release of NO was assayed by the Griess method (G). The proportions of IL-1β (H) and TNF-α (I) in the supernatant were detected by ELISA. Values are reported as mean ± S.D. Griess and ELISA data represent the results of three independent experiments. Statistical analysis was performed using one-way ANOVA with post hoc Tukey's test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
STAT3 Signal Pathway Involved in Microglia Expressing Proinflammatory Mediators Induced by Nogo-P4 via NgR
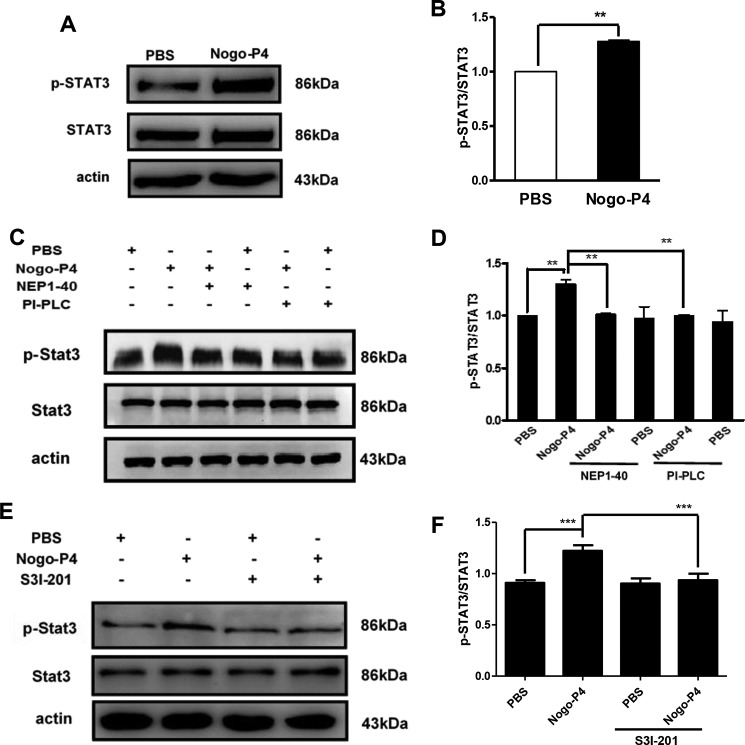
The STAT3 signal pathway has been shown to play roles in the inflammatory signaling cascades in response to several immune stimulations. Thus, we next investigated whether STAT3 signal pathway acted as the intracellular signal transducer to mediate the release of proinflammatory cytokines in microglia after Nogo-P4 stimulation. Microglia were seeded into the wells precoated with Nogo-P4, and then cells were collected for STAT3 activity detection. It was observed that Nogo-P4 significantly increased the STAT3 tyrosine phosphorylated at residue Tyr-705 (Fig. 7, A and B). Furthermore, the activation of the STAT3 pathway was greatly attenuated after the function block of NgR with NEP1–40 or PI-PLC (Fig. 7, C and D). The results above implied that Nogo-P4 bound to the NgR receptor and further triggered the phosphorylation of STAT3.
FIGURE 7.
Activation of STAT3 pathway by Nogo-P4 was blocked by S3I-201. A and B, Nogo-P4 induced the phosphorylation of STAT3. Microglia were incubated with PBS or Nogo-P4 for 6 h, and cell lysates were collected to detect the phosphorylation level of STAT3. Values are reported as mean ± S.D. (error bars) Statistical analysis was performed using Student's t test. C and D, activation of STAT3 pathway induced by Nogo-P4 was mediated by NgR. Microglia were pretreated with NEP1–40 (10 μm) or PI-PLC (0.3 units/ml) for 30 min in order to block the interaction of Nogo-P4 with NgR before the phosphorylation level of STAT3 was determined. Values are reported as mean ± S.D. E and F, S3I-201 blocked the phosphorylation of STAT3 activated by Nogo-P4. Microglia were pretreated with S3I-201 (20 μm) for 30 min before cells were cultured to determine the phosphorylation of STAT3. Values are reported as mean ± S.D. All data represent the results of three independent experiments. Statistical analysis was performed using one-way ANOVA with post hoc Tukey's test. **, p < 0.01; ***, p < 0.001.
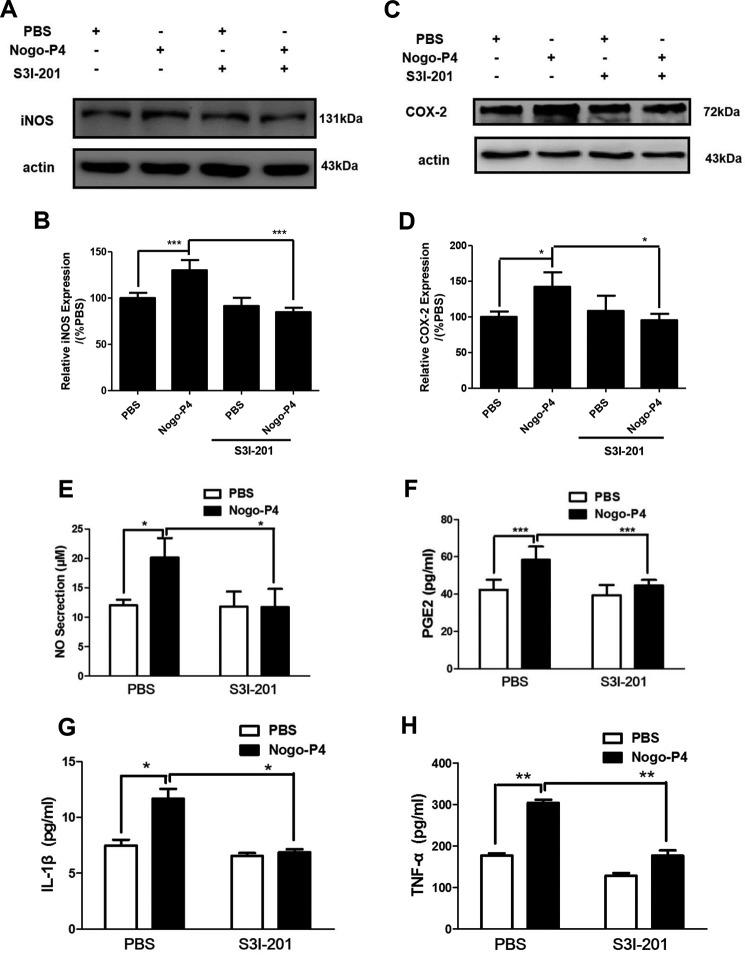
To validate whether the activation of STAT3 pathway was a response to the expression of proinflammatory cytokines induced by Nogo-P4, S3I-201, an inhibitor of STAT3 (26), was used to block the activity of the STAT3 signal pathway. S3I-201 (20 μm) lowered the phosphorylation of STAT3 induced by Nogo-P4 (Fig. 7, E and F). Results presented in Fig. 8 showed that S3I-201 (20 μm) pretreatment abolished the expression of iNOS and COX-2 as well as the release of IL-1β, TNF-α, NO, and PGE2 in microglia increased by Nogo-P4, suggesting that the STAT3 signal pathway might take part in microglia expressing proinflammatory cytokines in response to Nogo/NgR signal.
FIGURE 8.
STAT3 pathway was involved in Nogo-P4 triggered proinflammatory mediators expressing. Microglia were pretreated with S3I-201 (20 μm), an inhibitor of the activation of STAT3 pathway, for 30 min and then exposed to either PBS or Nogo-P4 for 12 h. The expression level of iNOS (A and B) and COX-2 (C and D) was determined by Western blot assay. Values are reported as mean ± S.D. (error bars), as a percentage of values determined in the PBS group (control, 100%). Western blot data represent the results of four independent experiments. Release of NO was assayed by the Griess method (E). ELISA was performed to detect the production of PGE2 (F), IL-1β (G), and TNF-α (H) in supernatant. Values are reported as mean ± S.D. Griess and ELISA data represent the results of three independent experiments. Statistical analysis was performed using one-way ANOVA with post hoc Tukey's test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
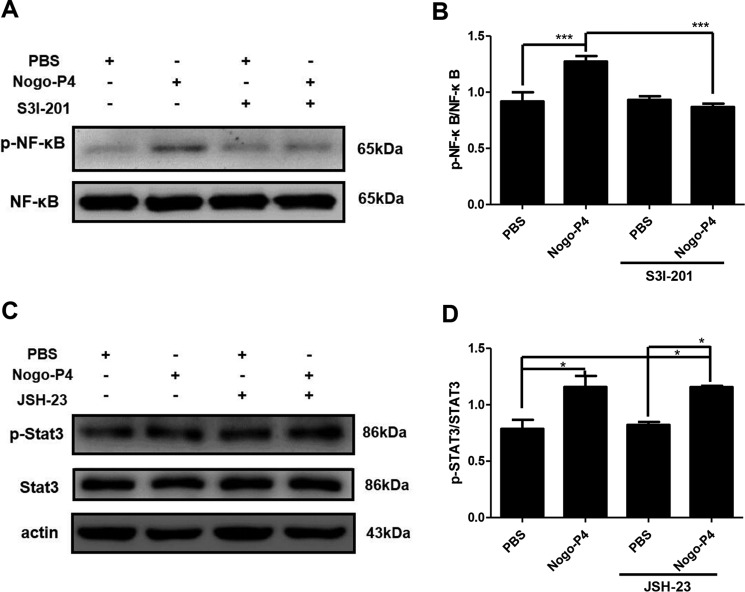
The Relationship between NF-κB and STAT3 Signal Pathway after Nogo-P4 Activated Microglia
We confirmed that both the NF-κB signal pathway and the STAT3 signal pathway were involved in the expression of proinflammatory mediators in microglia induced by Nogo-P4. But what is the relationship between the NF-κB signal pathway and the STAT3 signal pathway? We still do not know. Thus, we pretreated microglia with S3I-201 (20 μm; a STAT3 activation inhibitor) or JSH-23 (10 μm; an NF-κB activation inhibitor) for 30 min to investigate the relationship between the NF-κB pathway and the STAT3 pathway. The phosphorylation of NF-κB induced by Nogo-P4 was blocked after microglia were pretreated with S3I-201 (Fig. 9, A and B). However, the phosphorylation of STAT3 induced by Nogo-P4 was not affected after microglia were pretreated with JSH-23 (Fig. 9, C and D). These data suggested that the NF-κB pathway, possibly be downstream of the STAT3 pathway, mediated the effects of Nogo-P4 on the expression of proinflammatory mediators in microglia.
FIGURE 9.
Relationship between NF-κB and STAT3 signal pathway after Nogo-P4 activated microglia. Microglia were pretreated with S3I-201 (20 μm), an inhibitor of the STAT3 pathway, or JSH-23 (10 μm), an inhibitor of the NF-κB pathway, for 30 min and then exposed to either PBS or Nogo-P4 for 6 h; cell lysates were collected to detect the phosphorylation level of NF-κB (A and B) and STAT3 (C and D) by a Western blot assay. Values are reported as mean ± S.D. (error bars) Data represent the results of three independent experiments and two repeats within each experiment. Statistical analysis was performed using one-way ANOVA with post hoc Tukey's test. *, p < 0.05; ***, p < 0.001.
Discussion
Our results indicated that Nogo peptide elevated the production of proinflammatory cytokines of microglia in vitro, which was mediated by NF-κB and STAT3 signal pathways. From the results presented above, two points are particularly noteworthy. First, the results from our in vitro study confirmed that Nogo-P4 (the 25-amino acid inhibitory peptide sequence of Nogo-66) elevated the expression of proinflammatory cytokines in microglia. Nogo-P4, corresponding to residues 31–55 of the extracellular fragment of Nogo-66, significantly inhibits the neurite outgrowth and exhibits significant growth cone-collapsing activity in vitro (27–29). In many CNS disorders, such as multiple sclerosis (MS) and Alzheimer disease (AD), chronic inflammation is one of the main pathologic features (30). Chronic inflammatory processes in MS lead to neuronal injury as well as neurodegeneration (31). There is also evidence that the expression of Nogo-A is significantly up-regulated during MS (32), and Nogo-A could participate in the inflammation process of MS (33). In AD, chronic inflammatory is not a passive system activated by emerging senile plaques and neurofibrillary tangles but instead contributes more to pathogenesis than to plaques and tangles themselves (34). Moreover, Nogo-A is overexpressed by hippocampal neurons in AD and is associated with Aβ-amyloid deposits in senile plaques (35). Hence, might the potentiated effects of Nogo peptide contribute to chronic inflammatory processes and furthermore be involved in pathogenesis of chronic inflammatory diseases? Our results showed that Nogo-P4 could induce microglial expression of proinflammatory mediators, and blocking the binding of Nogo-P4 to NgR on microglia by NEP1–40 or PI-PLC pretreatment or silencing the expression of NgR by siRNA interference rescued the increased expression of proinflammatory mediators induced by Nogo-P4, indicating that NgR was necessary for the effects of Nogo-P4 on microglia expressing proinflammatory mediators.
Second, we also verified that the activation of the NF-κB pathway and STAT3 pathway was responsible for the effects of the Nogo/NgR signal on the expression of proinflammatory mediators in microglia. It is well established that NF-κB and STAT3 are transcription factors that participate in cytokine and growth factor actions in immune cells and transcription of a variety of genes involved in inflammation, cellular adaptation to stress, and cell death (36). Activated IKKβ phosphorylates IκB and triggers its degradation by proteasomes, and this process results in translocation of NF-κB to the nucleus and the induction of NF-κB-dependent transcription of a wide range of immune and inflammatory genes (37). Recently, research has found that the NF-κB signal pathway is central to many macrophage (25) and microglial functions, including phagocytosis and cytokine secretion. Moreover, strong microglial NF-κB pathway activation has been described following lipopolysaccharide (LPS) treatment in vitro (38) and in pathological situations that involve strong inflammatory processes and massive peripheral blood cell recruitment, such as MS (39), experimental autoimmune encephalitis (40), and spinal cord injury (41). STAT3 tyrosine phosphorylated at residue Tyr-705 translocates to the nucleus, where it binds to specific promoter sequences of several inflammation associated genes, thus playing a central role in regulating the expression of a series of proinflammatory cytokines. It is reported that the STAT3 pathway is involved in the function of Nogo/NgR on the fate decision of neural precursor differentiation (42). The present study demonstrated that the NF-κB and STAT3 pathways contributed to the inflammatory response by directly modifying the expression of inflammatory mediators in microglia after Nogo-P4 stimulation.
Both the STAT3 pathway and the NF-κB pathway are critical transcription factors involved in the inflammatory processes of many CNS diseases. The STAT3 pathway and NF-κB pathway regulate the expression of target genes, such as inflammatory genes, individually or cooperatively. For example, it has been reported that curcumin ameliorates the depressed MFG-E8 expression and the attenuated phagocytic ability of EMF-exposed N9 cells, which is attributable to the inhibition of the proinflammatory response through the NF-κB and STAT3 pathways (43). Moreover, hydroxysafflor yellow A protected Aβ(1–42)-induced AD model through inhibiting inflammatory response, which may involve the JAK2/STAT3/NF-κB pathway (44). In the present study, our results demonstrated that S3I-201, an inhibitor for STAT3, reversed the activation of the NF-κB pathway induced by Nogo-P4, suggesting that the NF-κB pathway might be downstream of the STAT3 pathway to mediate the promotion of microglia expressing proinflammatory mediators stimulated by Nogo-P4.
Conclusions
Our present study has demonstrated that exposing the Nogo peptide to microglia elevated the expression of proinflammatory enzymes and cytokines in vitro, which was mediated by the NF-κB and STAT3 pathways. These results imply that the interaction of Nogo peptide with NgR expressed on microglia might participate in diverse CNS diseases related to chronic neuroinflammation, such as neurodegenerative diseases.
Author Contributions
Y. Q. F, J. Y., C. H. L., L. M., H. L., L. Y. Z., and M. Y. conceived and designed the experiments. Y. Q. F., J. Y., L. M. Y., and X. Z. performed the experiments. Y. Q. F., J. Y., C. H. L., H. L., L. Y. Z., and T. P. analyzed and interpreted the data and wrote the paper. All authors analyzed the results and approved the final version of the manuscript.
This work was supported by National Natural Science Foundation of China Grants 81070967 and 81271338, Specialized Research Fund for the Doctoral Program of Higher Education of China Grant 20130096110011, and the Initial Fund of China Pharmaceutical University (to H. L.). The authors declare that they have no conflicts of interest with the contents of this article.
- NgR
- Nogo receptor
- iNOS
- inducible nitric-oxide synthase
- COX-2
- cyclooxygenase-2
- PGE2
- prostaglandin E2
- IKKβ
- inhibitor of NF-κB (IκB) kinase-β
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PI-PLC
- phosphatidylinositol-specific phospholipase C
- MS
- multiple sclerosis
- AD
- Alzheimer disease
- ANOVA
- analysis of variance.
References
- 1.He Z., and Koprivica V. (2004) The Nogo signaling pathway for regeneration block. Annu. Rev. Neurosci. 27, 341–368 [DOI] [PubMed] [Google Scholar]
- 2.Fournier A. E., GrandPre T., and Strittmatter S. M. (2001) Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409, 341–346 [DOI] [PubMed] [Google Scholar]
- 3.McDonald C. L., Bandtlow C., and Reindl M. (2011) Targeting the Nogo receptor complex in diseases of the central nervous system. Curr. Med. Chem. 18, 234–244 [DOI] [PubMed] [Google Scholar]
- 4.Fry E. J., Ho C., and David S. (2007) A role for Nogo receptor in macrophage clearance from injured peripheral nerve. Neuron 53, 649–662 [DOI] [PubMed] [Google Scholar]
- 5.Yan J., Zhou X., Guo J. J., Mao L., Wang Y. J., Sun J., Sun L. X., Zhang L. Y., Zhou X. F., and Liao H. (2012) Nogo-66 inhibits adhesion and migration of microglia via GTPase Rho pathway in vitro. J. Neurochem. 120, 721–731 [DOI] [PubMed] [Google Scholar]
- 6.McDonald C. L., Steinbach K., Kern F., Schweigreiter R., Martin R., Bandtlow C. E., and Reindl M. (2011) Nogo receptor is involved in the adhesion of dendritic cells to myelin. J. Neuroinflammation 8, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pool M., Niino M., Rambaldi I., Robson K., Bar-Or A., and Fournier A. E. (2009) Myelin regulates immune cell adhesion and motility. Exp. Neurol. 217, 371–377 [DOI] [PubMed] [Google Scholar]
- 8.Holmin S., Mathiesen T., Shetye J., and Biberfeld P. (1995) Intracerebral inflammatory response to experimental brain contusion. Acta Neurochir. 132, 110–119 [DOI] [PubMed] [Google Scholar]
- 9.Vila M., Jackson-Lewis V., Guégan C., Wu D. C., Teismann P., Choi D. K., Tieu K., and Przedborski S. (2001) The role of glial cells in Parkinson's disease. Curr. Opin. Neurol. 14, 483–489 [DOI] [PubMed] [Google Scholar]
- 10.Giulian D., and Vaca K. (1993) Inflammatory glia mediate delayed neuronal damage after ischemia in the central nervous system. Stroke 24, I84–I90 [PubMed] [Google Scholar]
- 11.González-Scarano F., and Baltuch G. (1999) Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 22, 219–240 [DOI] [PubMed] [Google Scholar]
- 12.Ryu J. K., Choi H. B., and McLarnon J. G. (2006) Combined minocycline plus pyruvate treatment enhances effects of each agent to inhibit inflammation, oxidative damage, and neuronal loss in an excitotoxic animal model of Huntington's disease. Neuroscience 141, 1835–1848 [DOI] [PubMed] [Google Scholar]
- 13.Weydt P., Weiss M. D., Möller T., and Carter G. T. (2002) Neuro-inflammation as a therapeutic target in amyotrophic lateral sclerosis. Curr. Opin. Investig. Drugs 3, 1720–1724 [PubMed] [Google Scholar]
- 14.Hanisch U. K., and Kettenmann H. (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394 [DOI] [PubMed] [Google Scholar]
- 15.Nam K. N., Koketsu M., and Lee E. H. (2008) 5-Chloroacetyl-2-amino-1,3-selenazoles attenuate microglial inflammatory responses through NF-κB inhibition. Eur. J. Pharmacol. 589, 53–57 [DOI] [PubMed] [Google Scholar]
- 16.Choi Y., Lee M. K., Lim S. Y., Sung S. H., and Kim Y. C. (2009) Inhibition of inducible NO synthase, cyclooxygenase-2 and interleukin-1β by torilin is mediated by mitogen-activated protein kinases in microglial BV2 cells. Br. J. Pharmacol. 156, 933–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi Y., Mizuno T., Maki Y., Jin S., Mizoguchi H., Ikeyama M., Doi M., Michikawa M., Takeuchi H., and Suzumura A. (2009) Microglia activated with the toll-like receptor 9 ligand CpG attenuate oligomeric amyloid β neurotoxicity in in vitro and in vivo models of Alzheimer's disease. Am. J. Pathol. 175, 2121–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Q., Li P., Lu J., Dheen S. T., Kaur C., and Ling E. A. (2010) Nuclear factor-κB/p65 responds to changes in the Notch signaling pathway in murine BV-2 cells and in amoeboid microglia in postnatal rats treated with the γ-secretase complex blocker DAPT. J. Neurosci. Res. 88, 2701–2714 [DOI] [PubMed] [Google Scholar]
- 19.GrandPré T., Nakamura F., Vartanian T., and Strittmatter S. M. (2000) Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403, 439–444 [DOI] [PubMed] [Google Scholar]
- 20.Oertle T., van der Haar M. E., Bandtlow C. E., Robeva A., Burfeind P., Buss A., Huber A. B., Simonen M., Schnell L., Brösamle C., Kaupmann K., Vallon R., and Schwab M. E. (2003) Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J. Neurosci. 23, 5393–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M. S., Huber A. B., van der Haar M. E., Frank M., Schnell L., Spillmann A. A., Christ F., and Schwab M. E. (2000) Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403, 434–439 [DOI] [PubMed] [Google Scholar]
- 22.Su Z., Cao L., Zhu Y., Liu X., Huang Z., Huang A., and He C. (2007) Nogo enhances the adhesion of olfactory ensheathing cells and inhibits their migration. J. Cell Sci. 120, 1877–1887 [DOI] [PubMed] [Google Scholar]
- 23.GrandPré T., Li S., and Strittmatter S. M. (2002) Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 417, 547–551 [DOI] [PubMed] [Google Scholar]
- 24.Lawrence T. (2009) The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1, a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren S., Zhang S., Li M., Huang C., Liang R., Jiang A., Guo Y., Pu Y., Huang N., Yang J., and Li Z. (2013) NF-κB p65 and c-Rel subunits promote phagocytosis and cytokine secretion by splenic macrophages in cirrhotic patients with hypersplenism. Int. J. Biochem. Cell Biol. 45, 335–343 [DOI] [PubMed] [Google Scholar]
- 26.Siddiquee K., Zhang S., Guida W. C., Blaskovich M. A., Greedy B., Lawrence H. R., Yip M. L., Jove R., McLaughlin M. M., Lawrence N. J., Sebti S. M., and Turkson J. (2007) Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc. Natl. Acad. Sci. U.S.A. 104, 7391–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita T., and Tohyama M. (2003) The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat. Neurosci. 6, 461–467 [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa Y., Fujitani M., Hata K., Tohyama M., Yamagishi S., and Yamashita T. (2004) Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J. Neurosci. 24, 6826–6832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolando C., Parolisi R., Boda E., Schwab M. E., Rossi F., and Buffo A. (2012) Distinct roles of Nogo-a and Nogo receptor 1 in the homeostatic regulation of adult neural stem cell function and neuroblast migration. J. Neurosci. 32, 17788–17799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas S. M., Rothwell N. J., and Gibson R. M. (2006) The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 147, S232–S240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zindler E., and Zipp F. (2010) Neuronal injury in chronic CNS inflammation. Best Pract. Res. Clin. Anaesthesiol. 24, 551–562 [DOI] [PubMed] [Google Scholar]
- 32.Satoh J., Onoue H., Arima K., and Yamamura T. (2005) Nogo-A and nogo receptor expression in demyelinating lesions of multiple sclerosis. J. Neuropathol. Exp. Neurol. 64, 129–138 [DOI] [PubMed] [Google Scholar]
- 33.Fontoura P., Ho P. P., DeVoss J., Zheng B., Lee B. J., Kidd B. A., Garren H., Sobel R. A., Robinson W. H., Tessier-Lavigne M., and Steinman L. (2004) Immunity to the extracellular domain of Nogo-A modulates experimental autoimmune encephalomyelitis. J. Immunol. 173, 6981–6992 [DOI] [PubMed] [Google Scholar]
- 34.Heneka M. T., Carson M. J., El Khoury J., Landreth G. E., Brosseron F., Feinstein D. L., Jacobs A. H., Wyss-Coray T., Vitorica J., Ransohoff R. M., Herrup K., Frautschy S. A., Finsen B., Brown G. C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G. C., Town T., Morgan D., Shinohara M. L., Perry V. H., Holmes C., Bazan N. G., Brooks D. J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C. A., Breitner J. C., Cole G. M., Golenbock D. T., and Kummer M. P. (2015) Neuroinflammation in Alzheimer's disease. Lancet Neurol. 14, 388–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gil V., Nicolas O., Mingorance A., Ureña J. M., Tang B. L., Hirata T., Sáez-Valero J., Ferrer I., Soriano E., and del Río J. A. (2006) Nogo-A expression in the human hippocampus in normal aging and in Alzheimer disease. J. Neuropathol. Exp. Neurol. 65, 433–444 [DOI] [PubMed] [Google Scholar]
- 36.Acarin L., González B., and Castellano B. (2000) STAT3 and NFκB activation precedes glial reactivity in the excitotoxically injured young cortex but not in the corresponding distal thalamic nuclei. J. Neuropathol. Exp. Neurol. 59, 151–163 [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Liu X., Hao W., Decker Y., Schomburg R., Fülöp L., Pasparakis M., Menger M. D., and Fassbender K. (2014) IKKβ deficiency in myeloid cells ameliorates Alzheimer's disease-related symptoms and pathology. J. Neurosci. 34, 12982–12999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heese K., Fiebich B. L., Bauer J., and Otten U. (1998) NF-κB modulates lipopolysaccharide-induced microglial nerve growth factor expression. Glia 22, 401–407 [DOI] [PubMed] [Google Scholar]
- 39.Gveric D., Kaltschmidt C., Cuzner M. L., and Newcombe J. (1998) Transcription factor NF-κB and inhibitor IκBα are localized in macrophages in active multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 57, 168–178 [DOI] [PubMed] [Google Scholar]
- 40.Kaltschmidt C., Kaltschmidt B., Lannes-Vieira J., Kreutzberg G. W., Wekerle H., Baeuerle P. A., and Gehrmann J. (1994) Transcription factor NF-κB is activated in microglia during experimental autoimmune encephalomyelitis. J. Neuroimmunol. 55, 99–106 [DOI] [PubMed] [Google Scholar]
- 41.Bethea J., Castro M., Keane R. W., Lee T. T., Dietrich W. D., and Yezierski R. P. (1998) Traumatic spinal cord injury induces nuclear factor-κB activation. J. Neurosci. 18, 3251–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang B., Xiao Z., Chen B., Han J., Gao Y., Zhang J., Zhao W., Wang X., and Dai J. (2008) Nogo-66 promotes the differentiation of neural progenitors into astroglial lineage cells through mTOR-STAT3 pathway. PLoS One 3, e1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He G. L., Liu Y., Li M., Chen C. H., Gao P., Yu Z. P., and Yang X. S. (2014) The amelioration of phagocytic ability in microglial cells by curcumin through the inhibition of EMF-induced pro-inflammatory responses. J. Neuroinflammation 11, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z. H., Yu L. J., Hui X. C., Wu Z. Z., Yin K. L., Yang H., and Xu Y. (2014) Hydroxy-safflor yellow A attenuates Aβ1–42-induced inflammation by modulating the JAK2/STAT3/NF-κB pathway. Brain Res. 1563, 72–80 [DOI] [PubMed] [Google Scholar]