Background: RORγt is required for Th17 cell function and differentiation.
Results: TRAF5 stabilizes RORγt by ubiquitination and augments RORγt-mediated transcriptional expression of IL-17A.
Conclusion: TRAF5 is a positive regulator for RORγt.
Significance: TRAF5 could be a novel target for modulating RORγt-mediated inflammation and autoimmune diseases, including systemic lupus erythematosus.
Keywords: autoimmune disease, T helper cells, TNF receptor-associated factor (TRAF), transcription factor, ubiquitylation (ubiquitination), RORγt
Abstract
Retinoid-related orphan nuclear receptor γt (RORγt) is a key transcription factor for the development and function of Th17 cells. In this study, we show that tumor necrosis factor receptor-associated factor 5 (TRAF5), known as an E3 ubiquitin protein ligase and signal transducer, interacts with and ubiquitinates RORγt via Lys-63-linked polyubiquitination. TRAF5 stabilizes the RORγt protein level depending on its RING finger domain. Depletion of TRAF5 in Th17 cells destabilizes RORγt protein and down-regulates Th17-related genes, including IL-17A, an inflammatory cytokine involved in pathogenic mechanisms of several autoimmune diseases such as systemic lupus erythematosus. Moreover, up-regulation of the TRAF5 mRNA level was found in systemic lupus erythematosus patient CD4+ T cells. Our findings reveal a direct link between TRAF5-mediated ubiquitination and RORγt protein regulation, which may aggravate inflammatory progress and provide new therapeutic drug targets for autoimmune diseases.
Introduction
Th17 cells, which have attracted widespread attention as a subset of CD4+ T cells, contribute to the pathogenesis of multiple autoimmune and inflammatory diseases, such as systemic lupus erythematosus (SLE),4 inflammatory bowel disease, rheumatoid arthritis, and ankylosing spondylitis (1). Similar to Th1 and Th2 cells, Th17 cells express retinoic acid-related orphan nuclear receptor γt (RORγt) as the master regulator, orchestrating the differentiation, maintenance, and function of the Th17 lineage (2). RORγt is an isoform of RORγ with a distinctive N terminus and directs the transcription of the Th17-related cytokines IL-17A and IL-17F, which work as effector molecules of Th17 by inducing inflammatory cytokines and chemokines (2). Despite its significance for Th17 cells, the regulation of RORγt stability is still unclear.
Several E3 ubiquitin protein ligases and deubiquitinating enzymes have been confirmed to be important in the regulation of Th17 cell differentiation and function. Tumor necrosis factor receptor-associated factor 3 (TRAF3) is a negative regulator of IL-17R signaling (3), whereas TRAF5 and TRAF6 are essential for IL-17R signaling (4). The ubiquitin-specific protease USP18 plays a key role for T cells in the progres of differentiating into Th17 cells (5). USP25 could remove the Lys-63-linked ubiquitination in TRAF5 and TRAF6 mediated by Act1 and inhibit IL-17R signaling and inflammation (6).
However, these aforementioned studies did not focus on the ubiquitination and deubiquitination of RORγt, which is considered to be a meaningful posttranscriptional modification. Our previous research has revealed that both USP17 and USP4 could prevent RORγt degradation and modulate Th17 cell function through reducing Lys-48-linked polyubiquitination (7, 8).
Originally, TRAF5 was identified as a signaling adaptor involved in the CD40, nucleotide binding oligomerization domain-like receptor, retinoic-acid inducible gene I like receptor, and also IL-17 receptor (IL-17R) signaling pathways. In addition to its role as an adaptor protein, TRAF5 could also act as E3 ubiquitin ligase containing an N-terminal RING finger domain to exert catalytic activity (9).
In this paper, we investigate the role of TRAF5 in RORγt regulation. TRAF5 was identified as an E3 ubiquitin-protein ligase of RORγt by stabilizing and facilitating Lys-63-linked ubiquitination of RORγt via its RING finger domain. Furthermore, TRAF5 could interact with RORγt and promote RORγt-mediated IL-17A transcription.
Experimental Procedures
Plasmids, Antibodies, and Reagents
RORγt and TRAF5 and their corresponding truncations were amplified from HEK293T cells or human peripheral blood mononuclear cell cDNA and then cloned into the pIPFLAG2 or pIPMyc2 vectors. The shRNA expression vector pLKO.1 was purchased from Addgene. The plasmids Δ8.9 and vesicular stomatitis virus glycoprotein were gifts from Ke Lan (Institut Pasteur of Shanghai, Chinese Academy of Sciences). The antibodies used in this study included the following: anti-RORγ (catalog no. H-190, Santa Cruz Biotechnology), anti-TRAF5 (catalog no. H-257, Santa Cruz Biotechnology), anti-Ubiquitin (catalog no. P4D1, Santa Cruz Biotechnology), anti-HA (catalog no. F-7, Santa Cruz Biotechnology), anti-Myc (catalog no. 9E10, Santa Cruz Biotechnology), anti-FLAG (catalog no. M2, Sigma-Aldrich), and anti-β-actin (catalog no. C1213, Tianjin Sungene Biotech). Mouse IgG was from Millipore. Protein A/G Plus-agarose (catalog no. sc-2003) was purchased from Santa Cruz Biotechnology. MG132 (catalog no. Chemical Abstracts Service 133407-82-6) was from Merck. CD4-FITC, CD25-phycoerythrin, and CD45RA-allophycocyanin cycloheximide (catalog no. C7698-5G) was purchased from Sigma-Aldrich.
Cell Culture and Transfection
HEK293T cells were cultured in DMEM (Gibco) supplemented with 10% FBS (catalog no. 131212, ExCell Biology) and transfected with appropriate plasmids using PEI reagent (23966, Polysciences) according to the instructions of the manufacturer. Naïve CD4+ T cells and Th17 cells were cultured in X-VIVO15 medium (catalog no. 04-418Q, Lonza) supplemented with 10% human AB serum (Gibco), 1% sodium pyruvate, 1% GlutaMax, 1% non-essential amino acids, and 1% penicillin/streptomycin.
Th17 Induction and Expansion
Naïve CD4+CD45RA+CD25− T cells were purified by FACS from human peripheral blood mononuclear cells. Peripheral blood mononuclear cells were isolated by Ficoll (Life Technologies) density gradient centrifugation, and FACS was performed to isolate the lineage: naïve CD4+T cells (CD4-FITC, CD25-phycoerythrin, and CD45RA-allophycocyanin). These cells were stimulated with CD3/CD28 beads (catalog no. 11132D, Invitrogen) in the medium indicated above. Th17 cells were polarized in the presence of 2.5 ng/ml of recombinant human (rh) TGF-β1 (catalog no. 240-B-002, R&D Systems), 50 ng/ml of rhIL-6 (catalog no. 206-IL-010, R&D Systems), 10 ng/ml of rhIL-1β (catalog no. 201-LB-005, R&D Systems), and 100 ng/ml of rhIL-23 (catalog no. 1290-IL-010, R&D Systems). After 7 days, Th17 cells were harvested for future assays.
Immunoprecipitation and Immunoblotting
Cells were harvested, washed with ice-cold PBS, and lysed on ice for 30 min in radioimmune precipitation assay buffer (20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 10% glycerol) containing protease inhibitor mixture (1:100, catalog no. P8340, Sigma-Aldrich), sodium fluoride (10 mm), PMSF (1 mm), and Na3VO4 (1 mm). Cell lysates were cleared by centrifugation, and supernatants were immunoprecipitated with appropriate antibodies for 1 h at 4 °C and then with protein A/G Plus-agarose for another hour at 4 °C. The immunocomplexes were then washed with radioimmune precipitation assay buffer four times. After washing, 2× sample loading buffer was added to the immunoprecipitates. Samples were then used for immunoblot analysis with the indicated antibodies.
Ubiquitin Pulldown Assay
His-ubiquitin and Myc-RORγt were co-transfected into HEK293T cells with or without FLAG-tagged TRAF5, and related cells were treated with 20 μm MG132 3 h before harvesting. Cells were washed with ice-cold PBS and then lysed in urea buffer (pH 8.0) (10 mm Tris (pH 8.0), 8 m urea, 100 mm Na2HPO4, 0.2% Triton X-100, and 10 mm imidazole) for 30 min. The lysates were incubated with nickel-nitrilotriacetic acid beads (catalog no. 30210, Qiagen) for 3 h at room temperature. The beads were washed three times in urea buffer (pH 8.0) before incubation. After 3 h of incubation, the beads were washed twice in urea buffer (pH 8.0), twice in urea buffer (pH 6.3) (10 mm Tris (pH 6.3), 8 m urea, 100 mm Na2HPO4, 0.2% Triton-100, and 10 mm imidazole), and once in wash buffer (20 mm Tris (pH 8.0), 100 mm NaCl, 20% glycerol, 1 mm dithiothreitol, and 10 mm imidazole). Beads were boiled in 2× loading buffer and analyzed by SDS-PAGE.
Luciferase Reporter Assay
The -600 to 0 region of the human IL-17a promoter with one RORγt binding site was cloned into the pGL3-Basic vector to generate the pGL3-IL-17a-Luc reporter construct. To analyze the effects of TRAFs on RORγt in terms of IL-17a promoter activity, the IL-17a luciferase reporter plasmid was co-transfected with a β-gal luciferase reporter into HEK293T cells. After 48 h, the cells were lysed, and luciferase reporter assays were performed using the Dual-Luciferase reporter kit (Promega).
Quantitative Real-time PCR
Total RNA was extracted from whole cells as well as CD4+ T cells from SLE patients and healthy controls using TRIzol reagent (Invitrogen) following the instructions of the manufacturer. cDNA was synthesized using a reverse transcriptase kit (TaKaRa), followed by quantitative RT-PCR analysis (SYBR Green, TaKaRa). The sequences of the used primers were as follows: IL-17A, 5′-accaatcccaaaaggtcctc-3′ (forward) and 5′-ggggacagagttcatgtggt-3′ (reverse); IL-17F, 5′-cctccccctggaattacact-3′ (forward) and 5′-accagcaccttctccaactg-3′ (reverse); IL-21, 5′-aggtcaagatcgccacatga-3′ (forward) and 5′-tgggccttctgaaaacagga-3′ (reverse); IL-23R, 5′-tcatcccagaacacaagcct-3′ (forward) and 5′-attgctgagatggcttccct-3′ (reverse); RORc, 5′-ctgctgagaaggacagggag-3′ (forward) and 5′-agttctgctgacgggtgc-3′ (reverse); TRAF5, 5′-aacctgaccccaatagcagc-3′ (forward) and 5′-tcagttaagtccacggccac-3′ (reverse); and β-actin, 5′-ctcttccagccttccttcct-3′ (forward) and 5′-cagggcagtgatctccttct-3′ (reverse).
Lentiviral Constructs and Infection
The shRNA oligos were cloned into the lentiviral vector pLKO.1 with a resistant of puromycin. Then shTRAF5, Δ8.9 and vesicular stomatitis virus glycoprotein were transfected into HEK293T cells. Viral supernatants were harvested after 48 h. Th17 cells were incubated with viral supernatants containing 8 μg/ml of Polybrene overnight. The viral supernatants were replaced with fresh medium on day 2. Puromycin was added to select the cells 2 days post-infection. The following shRNA sequences were used: shCK, 5′-caacaagatgaagagcaccaa-3′; shTRAF5-1, 5′-gatgtaatgccaaggttattc-3′; shTRAF5-2, 5′-ggctgtgctgtaacggataaa-3′; and shTRAF5-3, 5′-cagtgtctcgggcactaaa-3′.
CD4+ T Cell Isolation
Human peripheral blood was collected from SLE patients who met the American College of Rheumatology criteria for SLE as well as from healthy controls. Patients were between 20 and 55 years old and were recruited from the Rheumatology Department of Huashan Hospital (Shanghai, China). The patients were divided into two groups according to the disease activity index (SLEDAI): an inactive group (SLEDAI < 8) and an active group (SLEDAI ≥ 8). CD4+ T cells were isolated from whole blood using a human CD4+ T cell enrichment mixture (StemCell Technologies).
Statistical Analyses
Data are presented as mean ± S.D. Student's t test was used for comparisons on GraphPad Prism 5.0, with p < 0.05 considered statistically significant.
Results
TRAF5 Up-regulates RORγt-mediated IL-17a Transcription
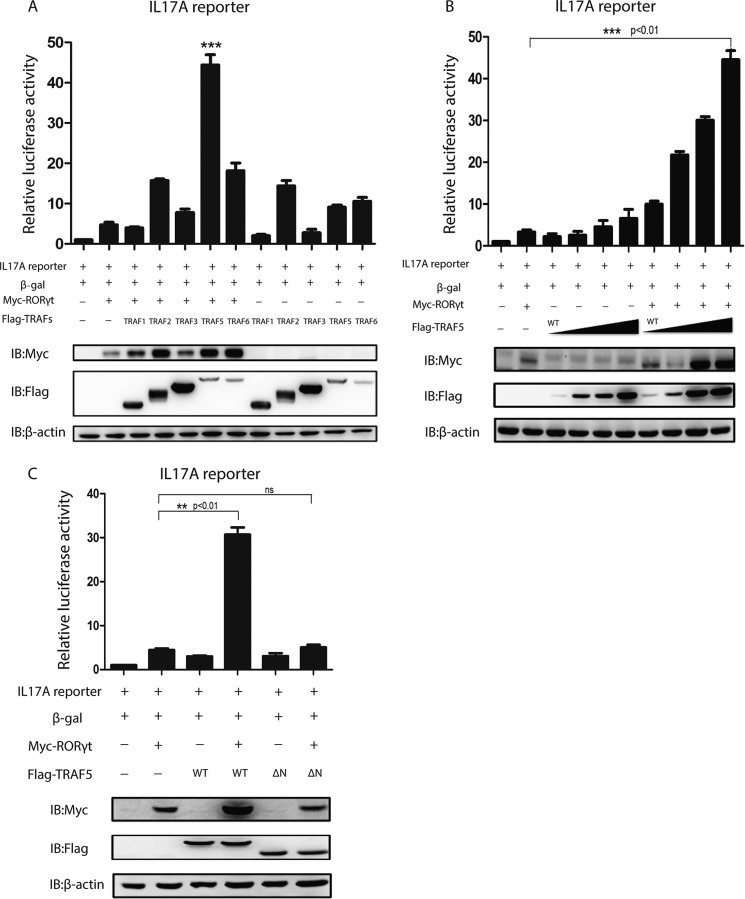
To explore whether any member of the TRAF family could regulate RORγt-mediated IL-17a transcriptional activity, the effects of five TRAF family members were tested by co-transfection of each individual FLAG-TRAF together with Myc-RORγt and the luciferase reporter construct containing the IL-17a promoter into HEK293T cells. TRAF5 significantly up-regulates RORγt-mediated IL-17a transcription compared with the control (Fig. 1A). The positive regulation of IL-17a transcription by TRAF5 was dose-dependent (Fig. 1B), whereas that of TRAF5ΔN90 (TRAF5ΔN) was not (Fig. 1C). TRAF5ΔN is a truncated form of TRAF5 lacking the N-terminal RING finger domain.
FIGURE 1.
TRAF5 up-regulates RORγt-mediated IL-17a promoter activation. A, the TRAF family members Myc-RORγt and IL-17a and control β-gal luciferase reporters were co-transfected into HEK293T cells. 48 h later, cells were harvested, and luciferase activities and protein levels were detected. IB, immunoblot. ***, p < 0.01. B, Myc-RORγt and FLAG-TRAF5 or its mutant TRAF5ΔN90 (0, 0.3, 0.6, and 0.9 μg) were co-transfected into HEK293T cells with IL-17a and β-gal luciferase reporters. Cells were treated the same as in A. C, HEK293T cells were transfected with luciferase reporters, increasing amounts of FLAG-TRAF5, and Myc-RORγt or control vector. Cells were treated the same as in A. **, p < 0.01. ns, not significant. Data represent at least three independent experiments, and error bars show mean ± S.D.
TRAF5 Interacts with and Stabilizes RORγt
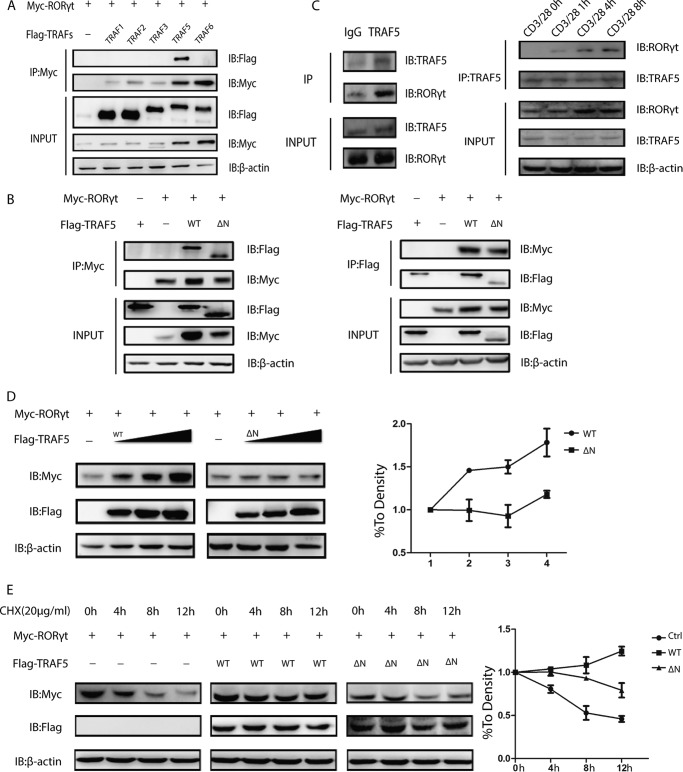
We next verified our hypothesis that the up-regulation of IL-17a transcription activity via TRAF5 may be on the basis of the interaction between TRAF5 and RORγt. First, HEK293T cells were introduced with FLAG-TRAFs and Myc-RORγt, followed by co-immunoprecipitation assay. The result revealed that TRAF5 could interact with RORγt, especially in the TRAF family (Fig. 2A). Second, expression vectors containing Myc-RORγt and FLAG-TRAF5 were co-transfected and ectopically expressed in HEK 293T cells for co-immunoprecipitation. A positive interaction was observed between TRAF5 and RORγt, and the RING finger domain of TRAF5 was dispensable for the interaction (Fig. 2B). In addition, the physical interaction was demonstrated by endogenous immunoprecipitation in human primary Th17 cells. We also tested different time points to check the interaction between TRAF5 and RORγt and found that a T cell receptor signal was necessary for the interaction.(Fig. 2C).
FIGURE 2.
TRAF5 interacts with and stabilizes RORγt. A, TRAF family members and Myc-RORγt were co-transfected into HEK293T cells. Cell lysates were immunoprecipitated (IP) with anti-Myc antibody. IB, immunoblot. B, Myc-RORγt and FLAG-TRAF5 or FLAG-TRAF5ΔN were co-transfected into HEK293T cells. Cell lysates were immunoprecipitated with either anti-Myc or anti-FLAG antibody. C, naïve CD4+ T cells were polarized under Th17 conditions, and then Th17 cells were harvested and lysed with radioimmune precipitation assay buffer. The cells lysate was immunoprecipitated with anti-TRAF5 antibody or a rabbit IgG control. Th17 cells were stimulated by anti-CD3 and anti-CD28 antibodies after resting for 1 day, and cells were collected at 0, 1, 4, and 8. D, HEK293T cells were co-transfected with Myc-RORγt and the indicated doses of FLAG-TRAF5 or its mutant (0, 0.4, 0.8, and 1.2 μg). The statistical curve was determined as shown. E, Myc-RORγt with or without FLAG-TRAF5 or TRAF5ΔN was transfected into HEK293T cells. The half-life of RORγt in cycloheximide (CHX)-treated cells was analyzed as shown. Ctrl, control. Data represent at least three independent experiments.
Considering the aforementioned results, we then assessed the effects of TRAF5 on RORγt stability by TRAF5. Myc-RORγt and FLAG-TRAF5 or a controlled FLAG vector were transfected into HEK293T cells. It was observed that the protein level of RORγt was up-regulated by TRAF5 in a dose-dependent manner whereas TRAF5ΔN was not (Fig. 2D). To confirm these results, we treated HEK293T cells introduced with or without TRAF5 or TRAF5ΔN with the protein synthesis inhibitor cycloheximide for the indicated times. The overexpression of TRAF5 effectively prolonged the half-life of RORγt protein but its TRAF5ΔN truncation did not (Fig. 2E). Taken together, these results showed that TRAF5 interacted with and stabilized RORγt.
TRAF5 Promotes the Ubiquitination of RORγt
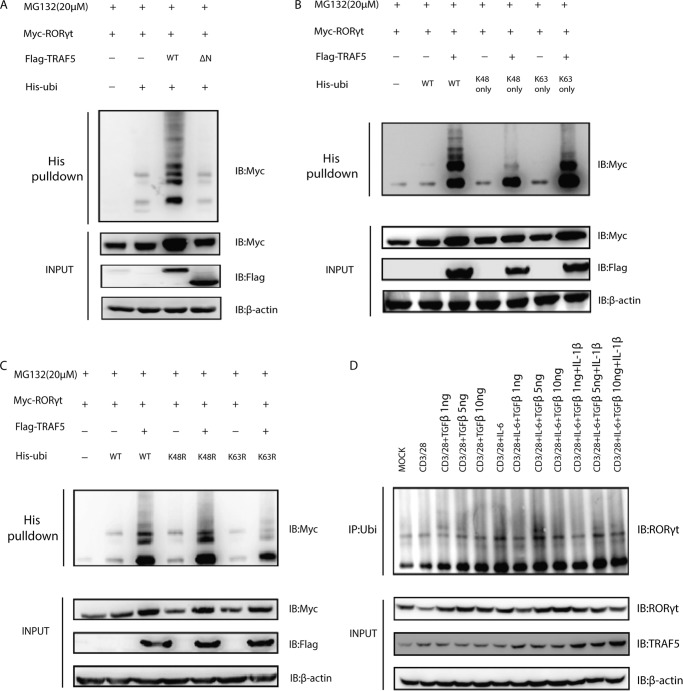
Because RORγt could be polyubiquitinated (6) and TRAF5 is a E3 ubiquitin protein ligase containing a RING finger domain, we reasoned that the function of TRAF5 toward RORγt might depend on its ubiquitin ligase activity. To further identify the role of TRAF5 in the regulation of RORγt, we co-transfected Myc-RORγt and His-ubiquitin into HEK293T cells with or without FLAG-TRAF5 or TRAF5ΔN. These cells were treated with the proteasome inhibitor MG132 for 3 h after 2 days to stabilize the ubiquitinated proteins before they were lysed under denaturing conditions. Our analysis revealed that the RORγt polyubiquitination was facilitated significantly by wild-type TRAF5 but not by the catalytically inactively truncated TRAF5ΔN (Fig. 3A).
FIGURE 3.
TRAF5 promotes the ubiquitination of RORγt. A, HEK293T cells were transfected with Myc-RORγt, His-ubiquitin (His-ubi), FLAG-TRAF5, and FLAG-TRAF5ΔN. IB, immunoblot. B and C, His-ubiquitin and its mutants Lys-48 only, Lys-63 only, K48R, and K63R were co-transfected with Myc-RORγt and FLAG-TRAF5 to detect the ubiquitination type for TRAF5 against RORγt. D, human Th17 cells were stimulated with anti-CD3 and anti-CD28 antibodies overnight in fresh medium and then stimulated with different cytokines for 8 h. The cells were treated with MG132 (20 μm) for 4 h before being harvested, and immunoprecipitation (IP) was carried out with anti-ubiquitin antibody. Data represent at least three independent experiments.
Different types of polyubiquitin chains with definitive function could be made up by ubiquitin monomers because there were seven lysine residues in a ubiquitin molecule (Lys-6, Lys-11, Lys-27, Lys-29, Lys-33, Lys-48, and Lys-63). So far, the ubiquitination via Lys-48 or Lys-63 linkage has been well characterized. For example, Lys-63-linked polyubiquitination could signal in various proteasome-independent pathways, including ribosomal protein synthesis and DNA repair. On the contrary, Lys-48-linked polyubiquitination usually functions in the canonical signal for proteasomal degradation (10). In our study, RORγt was co-expressed with the Lys-63 only or Lys-48 only ubiquitin mutant, followed by nickel-nitrilotriacetic acid pulldown assay under denaturing conditions, and more TRAF5-mediated ubiquitination of RORγt was observed under the former condition (Fig. 3B). The result was consistent with the previous one, where the ubiquitin mutant was changed to K63R or K48R (Fig. 3C). Therefore, our data indicate that TRAF5 ubiquitinates RORγt mainly through Lys-63 linkage, which depends on its activity. To find out under what physiological condition RORγt is ubiquitinated, we carried out different stimulations of primary human Th17 cells after anti-CD3 and anti-CD28 Abs treatment overnight. Under the condition of IL-6 + TGF-β (5 ng), the ubiquitination of RORγt was the most, which suggested an important role of IL-6 and TGF-β in promoting ubiquitination of RORγt (Fig. 3D).
Knockdown of TRAF5 Decreases the Ubiquitination and Protein Level of RORγt
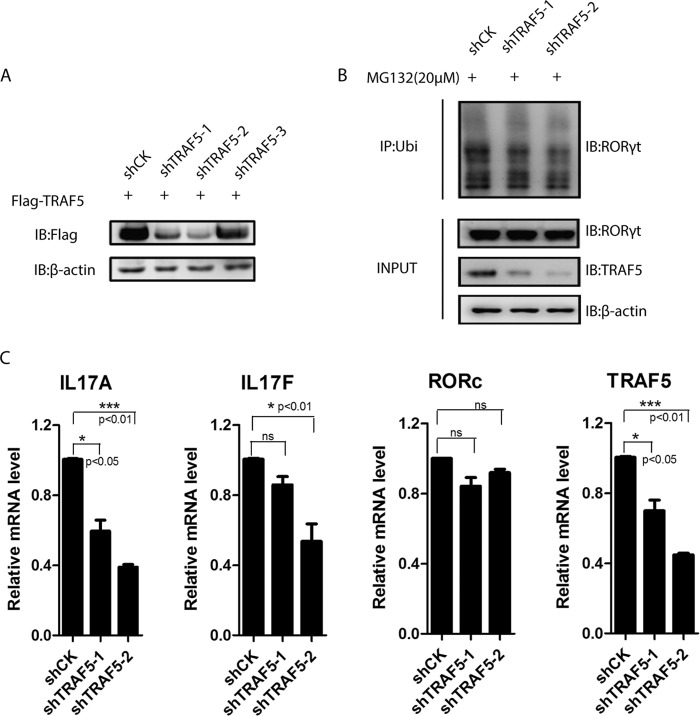
As mentioned above, TRAF5 could mediate the protein stabilization and function of RORγt, suggesting that TRAF5 contributes to the development of Th17 cells. To determine whether TRAF5 is involved in the regulation of Th17 function, three shTRAF5-expressing plasmids were constructed and tested by overexpression in HEK293T cells, and they could knock down TRAF5 effectively, except shTRAF5-3 (Fig. 4A). We found that the protein level of RORγt and Th17-related cytokines transcription levels were reduced in TRAF5-deficient human primary Th17 cells, whereas the RORγt mRNA levels did not (Fig. 4, B and C). In addition, the ubiquitination level of RORγt was decreased in the presence of shTRAF5 (Fig. 4B). These results indicate that TRAF5 is vital to the function of Th17 cells by mediating the stabilization and ubiquitination of RORγt.
FIGURE 4.
Knockdown of TRAF5 decreases RORγt protein levels and ubiquitination of RORγt. A, FLAG-TRAF5 and shTRAF5-1, -2, and -3 were co-transfected into HEK293T cells. IB, immunoblot. B, naïve CD4+ T cells were polarized under Th17 conditions. Th17 cells were transduced with a lentivirus containing shCK, shTRAF5-1, or shTRAF5-2. The cells were treated with MG132 for 4 h before being harvested. Immunoprecipitation (IP) was carried out with anti-ubiquitin (Ubi) antibody. C, TRAF5 was knocked down in Th17 cells using shRNA lentivirus containing a puromycin resistance cassette. IL-17A, IL-17F, IL-21, IL-23R, RORγt, and TRAF5 mRNA levels of puromycin-selected cells were analyzed by quantitative RT-PCR. ns, not significant. Data represent at least three independent experiments, and error bars show mean ± S.D.
TRAF5 Expression Is Associated with Inflammation in Active Systemic Lupus Erythematosus
Considering the stimulative role played by Th17 cells in the pathogenesis of SLE, we detected TRAF5-, RORγt-, and Th17-related cytokine gene expression levels in CD4+ T cells from SLE patients and healthy donors. The mRNA levels of the Th17 cell transcription factor RORγt and cytokines such as IL-17A, IL-17F, IL-21, and the surface receptor IL-23R all increased in the patients. However, only the differences in IL-17A and IL-17F levels were statistical significant. The increased TRAF5 levels also showed a significant difference between SLE patients and healthy donors (Fig. 5A). Moreover, the elevated expression of TRAF5 was positively correlated with that of IL-17A (Fig. 5B), and TRAF5 was highly expressed in active patients compared with inactive patients and healthy donors. Our data show that TRAF5 might be related to the function of the Th17 lineage in SLE.
FIGURE 5.
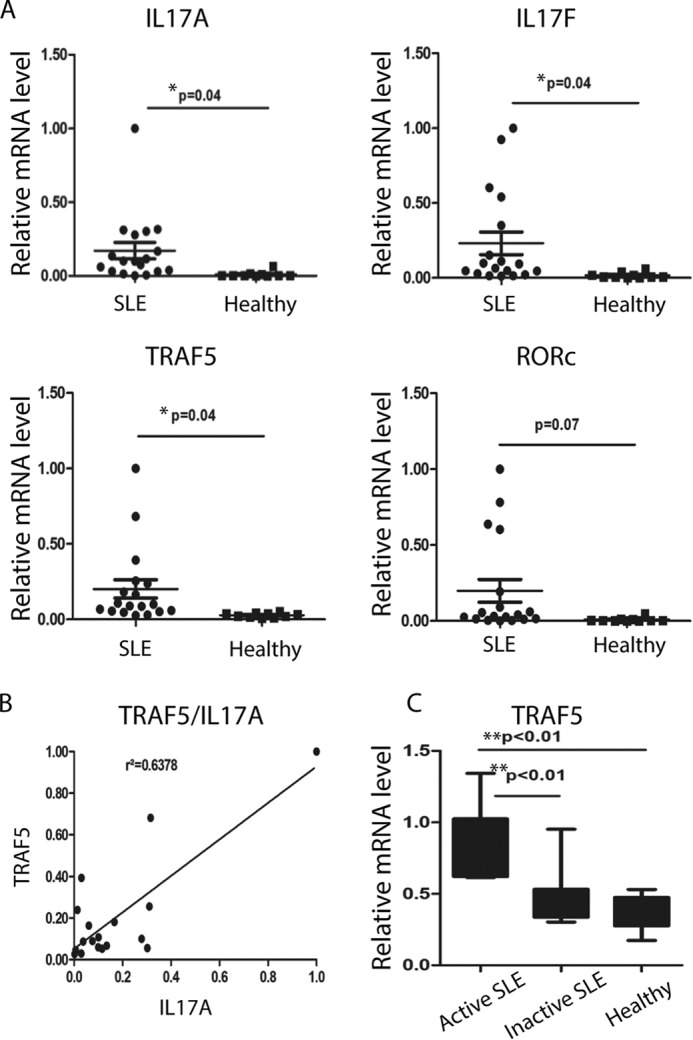
TRAF5 expression is associated with inflammation in active systemic lupus erythematosus. A, CD4+ T cells were isolated from the peripheral blood of SLE patients and healthy donors. IL-17A, IL-17F, IL-21, IL-23R, RORγt, and TRAF5 mRNA levels were detected via quantitative RT-PCR analysis normalized to the housekeeping gene β-actin. B, the correlation between TRAF5 and IL-17A was analyzed by GraphPad Prism on the basis of the data in A. C, the samples from SLE patients were divided into two groups on the basis of the SLEDAI. Data represent at least two independent experiments, and error bars show mean ± S.D.
Discussion
Although both RORγt and TRAF5 have been implicated in the pathogenesis of inflammation and autoimmune diseases, it has not been established whether there is a physical or functional link between the two. Our study shows that TRAF5, as a positive regulator of RORγt in the Th17 lineage, is an E3 ubiquitin protein ligase of RORγt.
RORγt, a major transcription factor in Th17 cells, promotes Th17 lineage development and exerts multifunctionality in the immune response. However, little attention has been paid to the posttranslational modification of RORγt. Our previous studies have identified USP17 and USP4 as the deubiquitinating enzymes of RORγt (7, 8). Furthermore, UBR5 and deubiquinating enzyme A have been reported as a pair of antagonistic E3 ligase and deubiquitinating enzyme regulating the expression of RORγt (11).
Here we revealed that TRAF5 could interact with and stabilize RORγt. TRAF5 may increase RORγt-dependent IL-17a promoter transcriptional activity. Then, as an E3 ubiquitin protein ligase, TRAF5 could facilitate Lys-63-linked polyubiquitination of RORγt. Our results suggest a new potential mechanism by which TRAF5 positively regulates RORγt in Th17 cells.
Since its discovery, the function of TRAF5 has remained elusive despite intensive studies of the subject. More and more studies have shown that TRAF5 has both inflammatory and proinflammatory effects. In the infected pathological state, the fact that CD8+ T cells from Traf−/− mice were unresponsive points out the important function of TRAF5 in enhancing T cell expansion (12). TRAF5 also has vital role in the glucocorticoid-induced TNFR signaling pathway, which would activate T cells (13). On the other hand, some studies contribute to the conclusion that TRAF5 can act as an anti-inflammatory factor. Researchers found that naïve CD4+ T cells from Traf−/− mice tend to differentiate toward Th17 cells (14), whereas B lymphocytes from Traf−/− mice could produce more cytokines, including IL-6, IL-10, and TNF-α, after Toll-like receptor stimulation (15).
It has been well established that TRAF5 is involved in the pathogenesis of several inflammatory and autoimmune diseases. The expression levels of TRAF5 increase in patients suffering from inflammatory bowel disease, and there is a weak correlation between TRAF5 and endoscopic disease activity index in patients with ulcerative colitis (16). Also, TRAF5 has been identified as a strong candidate for the rheumatoid arthritis susceptibility gene in the British population (17), and it is involved in the development of acute anterior uveitis (18), according to genetic polymorphism analysis in Han Chinese. In addition, cardiac hypertrophy and fibrosis are aggravated in Traf−/− mice compared with wild-type mice, suggesting that TRAF5 is a protective factor for the disease (19). We also confirmed a significant increase of TRAF5 in CD4+ T cells from patients with SLE, leading to the hypothesis that TRAF5 might drive the disease process by ubiquitinating RORγt under inflammatory conditions.
However, TRAF5 is involved in the OX40/OX40L pathway. OX40L, also called tumor necrosis factor superfamily member 4 (TNFSF4), is highly expressed in peripheral blood mononuclear cells from patients with systemic lupus erythematosus compared with healthy controls (20). TNFSF4 has been identified as a risk factor of SLE because upstream of it was a susceptibility locus in SLE (21). Furthermore, TNFSF4 is associated with renal disorder in Chinese SLE patients (22). Considering the interaction between TRAF5 and OX40 (23), we could not ignore the possibility that the overexpression of TRAF5 is partly attributable to the increase of OX40/OX40L in SLE.
Traditionally, it is well known that E3 ubiquitin protein ligases promote the degradation of target proteins. For example, Stub1 negatively modulates Foxp3 via promoting its Lys-48-linked polyubiquitination (24). Parkin mediates Lys-63-linked polyubiquitination of UCH-L1 and promotes UCH-L1 degradation by the autophagy-lysosome pathway (25). Unlike the conventional mechanism, our findings identify TRAF5 as a positive regulator of RORγt. The reason for stabilization is that TRAF5 might mediate more Lys-63-linked polyubiquitination than Lys-48-linked polyubiquitination of RORγt or that TRAF5 might simultaneously promote another lysine residue-linked ubiquitination of RORγt that we did not test.
In summary, our results suggest that TRAF5-mediated RORγt ubiquitination and stabilization could regulate immune disorder, especially in Th17-specific inflammation. Therefore, appropriately low TRAF5 levels may contribute to RORγt degradation and attenuated inflammation. Moreover, our study not only sheds light on the regulation of the Th17 lineage by ubiquitination but also provides a new explanation of the role of Th17 cells and potential therapeutic targets for autoimmune diseases.
Author Contributions
X. W. and J. Y. designed, performed, and analyzed the experiments shown in the figures. L. H. constructed the RORγt and IL-17a luciferase reporter plasmids. L. H. and K. Z. collected samples from SLE patients. Q. W., L. B., and Z. L. provided technical assistance and helped with the preparation of the manuscript. L. L. and B. L. conceived and coordinated the study, analyzed and interpreted data, and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank all anonymous reviewers for helpful suggestions.
Our research is supported by Chinese National Program on Key Basic Research Project Grants 2014CB541800, 2014CB541900, NSFC 81330072, 31300711, 31370863, 31170825, 31200646, 31200647, 81271835, and 81302532; Shanghai Key Grant 14JC1406100; and Shanghai Three-year Plan on Promoting TCM Development Grant ZY3-LCPT-2-1003. The authors declare that they have no conflicts of interest with the contents of this article.
- SLE
- systemic lupus erythematosus
- rh
- recombinant human
- TRAF
- tumor necrosis factor receptor-associated factor
- SLEDAI
- systemic lupus erythematosus disease activity index.
References
- 1.Han L., Yang J., Wang X., Li D., Lv L., and Li B. (2015) Th17 cells in autoimmune diseases. Front. Med. 9, 10–19 [DOI] [PubMed] [Google Scholar]
- 2.Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., and Littman D. R. (2006) The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 [DOI] [PubMed] [Google Scholar]
- 3.Zhu S., Pan W., Shi P., Gao H., Zhao F., Song X., Liu Y., Zhao L., Li X., Shi Y., and Qian Y. (2010) Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J. Exp. Med. 207, 2647–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu F., Gao H., Zhu S., Shi P., Zhang Y., Liu Y., Jallal B., Yao Y., Shi Y., and Qian Y. (2012) TRAF6-dependent Act1 phosphorylation by the IκB kinase-related kinases suppresses interleukin-17-induced NF-κB activation. Mol. Cell. Biol. 32, 3925–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X., Li H., Zhong B., Blonska M., Gorjestani S., Yan M., Tian Q., Zhang D. E., Lin X., and Dong C. (2013) USP18 inhibits NF-κB and NFAT activation during Th17 differentiation by deubiquitinating the TAK1-TAB1 complex. J. Exp. Med. 210, 1575–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong B., Liu X., Wang X., Chang S. H., Liu X., Wang A., Reynolds J. M., and Dong C. (2012) Negative regulation of IL-17-mediated signaling and inflammation by the ubiquitin-specific protease USP25. Nat. Immunol. 13, 1110–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han L., Yang J., Wang X., Wu Q., Yin S., Li Z., Zhang J., Xing Y., Chen Z., Tsun A., Li D., Piccioni M., Zhang Y., Guo Q., Jiang L., Bao L., Lv L., and Li B. (2014) The E3 deubiquitinase USP17 is a positive regulator of retinoic acid-related orphan nuclear receptor γt (RORγt) in Th17 cells. J. Biol. Chem. 289, 25546–25555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Xu P., Han L., Guo Z., Wang X., Chen Z., Nie J., Yin S., Piccioni M., Tsun A., Lv L., Ge S., and Li B. (2015) Cutting edge: ubiquitin-specific protease 4 promotes Th17 cell function under inflammation by deubiquitinating and stabilizing RORγt. J. Immunol. 194, 4094–4097 [DOI] [PubMed] [Google Scholar]
- 9.Xie P. (2013) TRAF molecules in cell signaling and in human diseases. J. Mol. Signal. 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickart C. M., and Fushman D. (2004) Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 8, 610–616 [DOI] [PubMed] [Google Scholar]
- 11.Rutz S., Kayagaki N., Phung Q. T., Eidenschenk C., Noubade R., Wang X., Lesch J., Lu R., Newton K., Huang O. W., Cochran A. G., Vasser M., Fauber B. P., DeVoss J., Webster J., Diehl L., Modrusan Z., Kirkpatrick D. S., Lill J. R., Ouyang W., and Dixit V. M. (2015) Deubiquitinase DUBA is a post-translational brake on interleukin-17 production in T cells. Nature 518, 417–421 [DOI] [PubMed] [Google Scholar]
- 12.Kraus Z. J., Haring J. S., and Bishop G. A. (2008) TNF receptor-associated factor 5 is required for optimal T cell expansion and survival in response to infection. J. Immunol. 181, 7800–7809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esparza E. M., Lindsten T., Stockhausen J. M., and Arch R. H. (2006) Tumor necrosis factor receptor (TNFR)-associated factor 5 is a critical intermediate of costimulatory signaling pathways triggered by glucocorticoid-induced TNFR in T cells. J. Biol. Chem. 281, 8559–8564 [DOI] [PubMed] [Google Scholar]
- 14.Nagashima H., Okuyama Y., Asao A., Kawabe T., Yamaki S., Nakano H., Croft M., Ishii N., and So T. (2014) The adaptor TRAF5 limits the differentiation of inflammatory CD4(+) T cells by antagonizing signaling via the receptor for IL-6. Nat. Immunol. 15, 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchta C. M., and Bishop G. A. (2014) TRAF5 negatively regulates TLR signaling in B lymphocytes. J. Immunol. 192, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen J., Qiao Y. Q., Ran Z. H., and Wang T. R. (2013) Up-regulation and pre-activation of TRAF3 and TRAF5 in inflammatory bowel disease. Int. J. Med. Sci. 10, 156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter C., Eyre S., Cope A., Worthington J., and Barton A. (2007) Investigation of association between the TRAF family genes and RA susceptibility. Ann. Rheum. Dis. 66, 1322–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang Q., Chen L., Fang J., Hou S., Wei L., Bai L., Liu Y., Zhou Y., Kijlstra A., and Yang P. (2013) TNF receptor-associated factor 5 gene confers genetic predisposition to acute anterior uveitis and pediatric uveitis. Arthritis Res. Ther. 15, R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bian Z., Dai J., Hiroyasu N., Guan H., Yuan Y., Gan L., Zhou H., Zong J., Zhang Y., Li F., Yan L., Shen D., Li H., and Tang Q. (2014) Disruption of tumor necrosis factor receptor associated factor 5 exacerbates pressure overload cardiac hypertrophy and fibrosis. J. Cell. Biochem. 115, 349–358 [DOI] [PubMed] [Google Scholar]
- 20.Rajabi P., Alaee M., Mousavizadeh K., and Samadikuchaksaraei A. (2012) Altered expression of TNFSF4 and TRAF2 mRNAs in peripheral blood mononuclear cells in patients with systemic lupus erythematosus: association with atherosclerotic symptoms and lupus nephritis. Inflamm. Res. 61, 1347–1354 [DOI] [PubMed] [Google Scholar]
- 21.Manku H., Langefeld C. D., Guerra S. G., Malik T. H., Alarcon-Riquelme M., Anaya J. M., Bae S. C., Boackle S. A., Brown E. E., Criswell L. A., Freedman B. I., Gaffney P. M., Gregersen P. A., Guthridge J. M., Han S. H., Harley J. B., Jacob C. O., James J. A., Kamen D. L., Kaufman K. M., Kelly J. A., Martin J., Merrill J. T., Moser K. L., Niewold T. B., Park S. Y., Pons-Estel B. A., Sawalha A. H., Scofield R. H., Shen N., Stevens A. M., Sun C., Gilkeson G. S., Edberg J. C., Kimberly R. P., Nath S. K., Tsao B. P., and Vyse T. J. (2013) Trans-ancestral studies fine map the SLE-susceptibility locus TNFSF4. PLoS Genet. 9, e1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X. J., Cheng F. J., Qi Y. Y., Zhao M. H., and Zhang H. (2013) A replication study from Chinese supports association between lupus-risk allele in TNFSF4 and renal disorder. BioMed Res. Int. 2013, 597921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamata S., Hori T., Imura A., Takaori-Kondo A., and Uchiyama T. (1998) Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-κB activation. J. Biol. Chem. 273, 5808–5814 [DOI] [PubMed] [Google Scholar]
- 24.Chen Z., Barbi J., Bu S., Yang H.-Y., Li Z., Gao Y., Jinasena D., Fu J., Lin F., Chen C., Zhang J., Yu N., Li X., Shan Z., Nie J., Gao Z., Tian H., Li Y., Yao Z., Zheng Y., Park B. V., Pan Z., Zhang J., Dang E., Li Z., Wang H., Luo W., Li L., Semenza G. L., Zheng S.-G., Loser K., Tsun A., Greene M. I., Pardoll D. M., Pan F., and Li B. (2013) The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKeon J. E., Sha D., Li L., and Chin L.-S. (2015) Parkin-mediated K63-polyubiquitination targets ubiquitin C-terminal hydrolase L1 for degradation by the autophagy-lysosome system. Cell. Mol. Life Sci. 72, 1811–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]