Abstract
Purpose
National reports on end-of-life symptom management reveal a gap in the evidence regarding symptoms other than pain and studies of diseases other than cancer. This study examines the frequency and severity of symptoms and quality of life (QOL) in persons with advanced cancer, amyotrophic lateral sclerosis (ALS), and congestive heart failure (CHF).
Methods
The present study is a cross-sectional examination of symptoms and QOL measured using the McGill QOL Questionnaire, among 147 participants.
Results
Forty one percent of participants had advanced cancer, 22% had ALS, and 37% had advanced CHF. A total of 266 symptoms were reported, with the common symptom categories being discomfort/pain, weakness/fatigue/sleep, and respiratory. Participants with CHF had the highest mean symptom severity and the lowest QOL.
Conclusion
Clinicians should be aware and attentive for symptoms other than pain in patients with advanced illness. Studies on diseases other than cancer, such as CHF and ALS, are important to improve symptom management in all disease groups.
Keywords: Chronic Disease, End of Life, Symptoms, Quality of Life
Introduction
During the last decade, several national reports have addressed problems with symptom management at the end of life in the U.S. In 2003, the National Cancer Policy Board in the Institute of Medicine issued a report, Describing Death in America: What We Need to Know (Lunney et al., 2003). In this report, authors concluded that while a great deal was known about where and when individuals die, insufficient information about the quality of life near death has limited the development of interventions to guide care at the end of life (Lunney et al., 2003). In 2004, the National Institute of Health (NIH) State-of-the-Science Conference Statement on Improving End-of-Life Care concluded that many Americans were dying in pain (National Institutes of Health, 2004). Although the literature on managing this symptom in persons with advanced cancer was growing, the report found that there is far less information on symptoms other than pain and on patients suffering from diseases other than cancer (National Institutes of Health, 2004). Likewise, the recent 2014 Institute of Medicine Report, Dying In America: Improving Quality and Honoring Individual Preferences Near the End of Life proposed the management of pain and other symptoms to be a core component of quality end-of-life care (Institute of Medicine, 2014). The 2011 NIH Report, Science of Compassion: Future Directions in End-of-Life and Palliative Care similarly recommended that persons with a variety of trajectories of illness be studied (National Institute of Nursing Research and Partners, 2011).
Background
Symptoms in advanced illness vary according to the particular disease. According to the American Cancer Society common symptoms among cancer patients include unexplained weight loss, fever, fatigue, pain, and skin changes (American Cancer Society, n.d.). The five most prevalent symptoms in the last year of life among patients with cancer are fatigue, weakness, pain, shortness of breath, and cough (Doorenbos et al., 2006). In the last month of life, in addition to pain and fatigue, the symptoms most commonly reported are a reduced sense of well-being, decreased appetite, and trouble sleeping (Semionov et al., 2012). By contrast, patients with amyotrophic lateral sclerosis (ALS) most commonly report symptoms of muscle weakness, twitching and muscle cramping, difficulty speaking, and shortness of breath (ALS Association, n.d.). At the end of life patients with ALS commonly experience symptoms of constipation, pain, cough, insomnia, dyspnea, and sialorrhea (hypersalivation) (Simmons, 2005). In patients with congestive heart failure (CHF), common symptoms include shortness of breath, persistent coughing, edema, fatigue, and impaired thinking (American Heart Association, n.d.). Similarly, patients with CHF at the end of life report symptoms of shortness of breath, weakness, fatigue, nausea, anorexia, constipation, edema, cough, altered mental status, anxiety, depression, pain, and trouble sleeping (Bekelman et al., 2009; Whellan et al., 2014).
What is not well known is whether the symptom severity and quality of life differ among patients who are in the advanced stages of GI/pancreatic cancer, ALS or CHF. The two-year mortality rate and expected disease trajectories differ among patients with one of these three diseases. In this sample the two-year mortality rate was 92% for persons with cancer, 81% for ALS, and 60% for CHF (Authors, 2007). The expected disease trajectories were a rapid, prolonged, or unpredictable decline in health status from diagnosis to death for persons with advanced cancer, ALS, and CHF respectively (Lynn & Adamson, 2003). Health professionals may assume that patients with advanced cancer who have the highest two-year mortality rate and the most rapid trajectory of illness, would have the most severe symptoms but we know of no studies that have examined this. Therefore, the purpose of this study was to analyze the differences in the symptom type and severity of persons in these three disease groups and the relationship of symptom severity to quality of life.
Methods
Design and Sample
A purposive sample of 147 patients with advanced cancer, ALS, and CHF who participated in a longitudinal, multi-site study was obtained. The purpose of the main study was to describe the natural history of health care decision making in participants from diagnosis with advanced disease until death (Authors, 2005; Authors, 2007). This cross-sectional analysis focuses specifically on symptoms and quality of life (QOL) using data collected from all patients at baseline (the first interview timepoint).
Inclusion criteria were: Spanish or English speaking adults aged 18 and older who were within eight weeks of being (1) diagnosed with non-resectable non-small cell lung cancer or stage III or IV gastrointestinal cancer, (2) hospitalized for New York Heart Association Class III or IV CHF, or (3) within six weeks of being diagnosed with ALS. The study was approved by the Johns Hopkins Hospital and Saint Vincent Hospital Institutional Review Boards.
Instrumentation
Symptoms and quality of life were measured at baseline with the McGill Quality of Life (QOL) Questionnaire, a 17-item, 10-point Likert scale (Cohen et al., 1995) that has been used with subjects who have cancer and ALS (Rousseau et al., 2011; Tang et al., 2014). The present study analyzed data from the McGill QOL Questionnaire – Part A question one, and Part B questions 1-3. Part A question one asked the following question that provided a global measure of QOL: “Considering all parts of my life – physical, emotional, social, spiritual, and financial – over the past two (2) days, the quality of my life has been…” on a 10 point-Likert scale, ranging from very bad (0) to excellent (10). Part B questions 1-3 asked participants to list up to three troublesome symptoms in their own words, and rate the severity of the symptoms on a 10 point-Likert scale ranging from no problem (0) to a tremendous problem (10). These three questions had a Cronbach's alpha of 0.85 (Cohen et al., 1996) in the present study.
For this analysis, the level of symptom severity was measured using the severity rating of the first identified troublesome symptom. All three symptom items were examined to determine the frequency and type of symptoms for the sample reported.
Procedures
Two of the authors classified all of the troublesome symptoms that were identified by participants into categories. Other study team members reviewed the classification, and revisions were made by the consensus of the entire study team.
Analysis
Using SPSS Version 16 (International Business Machines Corp (IBM), Armonk, New York), descriptive statistics were used to describe the sample characteristics and the number, type and severity of symptoms. One-way analysis of variance (ANOVA) was used to compare the mean QOL across the disease groups. ANOVA was also used to compare QOL across groups with different numbers of symptoms. Independent t-tests were used to examine the difference in mean QOL scores between males and females and between race categories dichotomized as Whites and non-Whites. The severity of symptoms was measured both as a continuous variable (0-10), and as a categorical variable: mild (0-3), moderate (4-7), and severe (8-10) (Abraham et al., 2006). Severity scores of 0 were excluded in the severity analysis, to avoid distortion of results. The Kruskal-Wallis test was used to compare distributions of maximum and average severity across disease groups.
A multivariate linear model was fit to assess the relationship between symptom severity, sex, race, diagnosis and number of symptoms on QOL. An interaction term was used to test whether the relationship between symptom severity and QOL differed by sex. All tests were performed at a p=0.05 statistical significance level.
Results
Sample characteristics
Among the 147 participants, 41% (n=60) had advanced cancer, 22% (n=32) had ALS, and 37% (n=55) had advanced CHF. The sample was 65% White (n=94) and 63% male (n=93). The age of participants ranged from 27 to 89 years old with a mean of 62 years (SD = 12.5). Sixty-four percent (n=94) had equal to or less than high school education. Table 1 provides further detail on the characteristics of the sample. There were statistically significant differences in religious affiliation, educational attainment and race across the diagnostic groups (Table 1).
Table 1. Differences in Characteristics among Patients in the Three Disease Groups.
| Cancer N=60 | ALS N= 32 | CHF N=55 | P-value | |
|---|---|---|---|---|
|
| ||||
| Sex n (%) | 0.461 | |||
| Male | 37 (61.7) | 18 (56.3) | 38 (69.1) | |
|
| ||||
| Age (Years) | 0.977 | |||
| Mean (SD) | 62 (13) | 62 (11) | 62 (13) | |
|
| ||||
| Religion n (%)a | <0.0001 | |||
| Protestant | 7 (12.0) | 25 (78.1) | 25 (45.5) | |
| Catholic | 33 (56.0) | 3 (9.4) | 17 (30.9) | |
| Jewish | 3 (5.1) | 1 (3.1) | 1 (1.8) | |
| Other | 10 (17.0) | 1 (3.1) | 11 (20.0) | |
| None | 6 (10.2) | 2 (6.3) | 1 (1.8) | |
|
| ||||
| Education n (%) | 0.004 | |||
| < Grade school | 2 (3.3) | 0 (0.0) | 1 (1.8) | |
| grade school | 8 (13.3) | 2 (6.3) | 21 (38.2) | |
| high school | 25 (41.7) | 16 (50.0) | 20 (36.4) | |
| college | 18 (30.0) | 12 (37.5) | 7 (12.7) | |
| graduate/professional | 7 (11.7) | 2 (6.3) | 6 (10.9) | |
|
| ||||
| Race n (%)b | <0.0001 | |||
| White | 43 (71) | 30 (94) | 23 (42) | |
| Black | 10 (17) | 1 (3) | 23 (42) | |
| Hispanic | 5 (9) | 1 (3) | 4 (7) | |
| Asian | 1 (2) | - | 4 (7) | |
| Other | 1 (2) | - | 1 (2) | |
n=146. Religious affiliation is missing for one patient in the cancer group
n=145. Race is missing for two patients in the cancer group
Frequency & Severity of Symptoms
Overall 35% (n=51) of participants reported three troublesome symptoms; 27% (n=39), one symptom; 25% (n=37), two symptoms; and 14% (n=20), no symptoms. About one-third of participants in each disease group reported having three troublesome symptoms: 32% (n=19) in the cancer group, 36% (n=20) in the CHF group, and 38% (n=12) in the ALS group (see Table 2 for number of symptoms by disease group).
Table 2. Number of Symptoms by Disease Group.
| Total number of symptoms | Diagnosis | ||
|---|---|---|---|
| Cancer N=60 n (%) | ALS N=32 n (%) | CHF N=55 n (%) | |
| 0 | 6 (10.0) | 4 (12.5) | 10 (18.2) |
| 1 | 13 (21.7) | 10 (31.3) | 16 (29.1) |
| 2 | 22 (36.7) | 6 (18.8) | 9 (16.4) |
| 3 | 19 (31.7) | 12 (37.5) | 20 (36.4) |
The mean severity score of the first identified troublesome symptom among patients who reported at least one symptom (n=127) was 6.6 (median=7) on a scale that ranged from 0 no problem to 10 a tremendous problem. Participants with advanced CHF had the highest mean symptom severity score of 7.0 (SD=2.9, median = 8) compared with participants with cancer (mean=6.5, SD=2.4, median = 7), and participants with ALS (mean =6.4, SD=2.5, median = 7). Symptom severity, however, did not differ significantly across disease groups (Kruskal-Wallis chi-squared = 2.66 (df = 2), p-value = 0.265), by sex (Kruskal-Wallis chi-squared = 2.37 (df=1), p-value = 0.124), by race (Kruskal-Wallis chi-squared = 1.43 (df=2), p-value = 0.490), or by marital status (Kruskal-Wallis chi-squared = 3.35 (df=4), p-value = 0.502).
Type of Symptoms
Nine symptom categories were identified: cardiovascular, discomfort/pain, gastrointestinal (GI), musculoskeletal, neurological, psychosocial, respiratory, weakness/fatigue/sleep and no troubling symptoms (“none”). Examples of symptoms in each category are provided in Table 3. Each participant reported up to three symptoms, with a total of 266 symptoms reported. The most common symptom categories were discomfort/pain (21% n=55), weakness/fatigue/sleep (20%, n=54), and respiratory (17%, n=45). The least common symptom categories were psychosocial (9%, n=25), neurological (5%, n=14), and cardiovascular (3%, n=9). The most troublesome symptom categories listed as the first on the questionnaire were discomfort/pain (24%, n=31), respiratory (17%, n=22), and weakness/fatigue/sleep (17%, n=22), and differed between disease groups (p-value <0.001). The most troublesome symptom category for cancer patients was GI (32%, n=19), for ALS patients was weakness/fatigue/sleep (38%, n=12), and for CHF patients was respiratory (26%, n=14).
Table 3. Examples of symptoms provided by patients and categorization for analysis.
| Symptom Category | Examples | N = 266 (%) |
|---|---|---|
| Discomfort/Pain | Neck pain, headache, clogged ear, gout pain, drooling | 55 (20.7) |
| Weakness/Fatigue/Sleep | Lack of energy, nightmares, difficulty sleeping | 54 (20.3) |
| Respiratory | Shortness of breath, restricted chest, coughing | 45 (16.9) |
| Gastrointestinal | Lack of appetite, constipation, indigestion, vomiting, diarrhea | 33 (12.4) |
| Musculoskeletal | Trouble getting around, arthritis, trouble tying shoes, hand pain, difficulty moving hands | 31 (11.7) |
| Psychosocial | Nervousness, loneliness, anxiousness, depression | 25 (9.4) |
| Neurological | Dizziness, impaired balance, speech difficulty | 14 (5.3) |
| Cardiovascular | Edema, irregular heartbeat, chest pain | 9 (3.4) |
Quality of Life Score
The mean QOL score for the entire sample (n = 146) was 6.6 (SD = 2.6), with 0 representing very bad and 10 excellent. The QOL score differed significantly among the three disease groups [Kruskal-Wallis chi2-square=7.42, (df = 2), p = .025]. Participants with ALS reported the highest QOL (mean=7.53, SD=2.53, median = 8), and participants with CHF reported the lowest QOL (mean=5.98, SD=2.89, median = 6). The mean QOL score also differed significantly between men and women [p=0.034], with women reporting higher QOL (mean= 7.17, SD=2.15) compared with men (mean= 6.28, SD±2.84). There was no significant difference in QOL between Blacks and Whites (p-value = 0.933); the mean QOL score in Whites was 6.72 (SD=2.67), and in Blacks was 6.76 (SD=2.41). QOL decreased as the number of symptoms increased [p = 0.001]. Participants who reported no symptoms had the highest mean QOL score 7.75 (SD=2.43), while those who reported having three symptoms had the lowest 5.49 (SD=2.59).
After adjusting for race, number of symptoms, and diagnosis through a multivariate linear regression, symptom severity was negatively associated with QOL in men, but not in women (p-value for interaction = 0.018). For every 1 unit increase in symptom severity, QOL in men decreased by 0.36 units (95% CI: -0.57 to -0.16, p-value =0.001) (see Figure 1).
Figure 1. Relationship between QOL and most troublesome symptom severity by sex adjusted for race, number of symptoms and diagnosis.
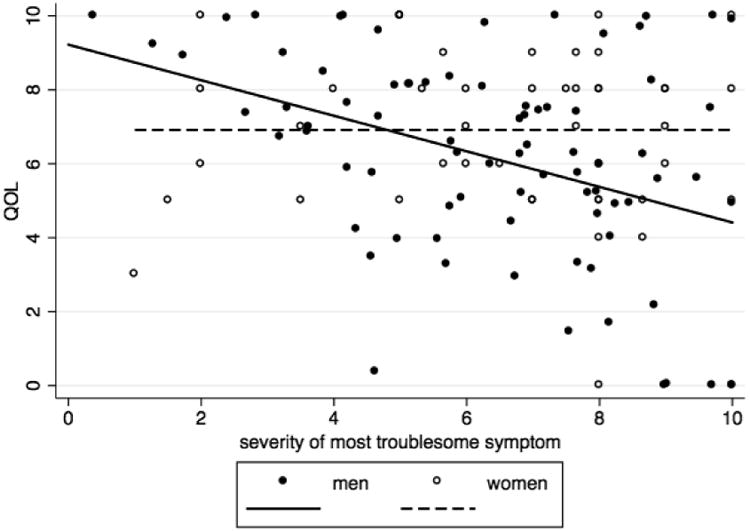
For symptom severity of the most severe symptom, QOL differed significantly among the symptom severity categories (mild, moderate, and severe) [p =0.049]. Participants who had the most severe symptoms reported the lowest mean QOL (5.95, SD=2.91), whereas participants who had mild symptoms reported the highest QOL (7.67, SD= 2.09).
Discussion
With an increasing prevalence of chronic illness and older adults, symptom management is an urgent public health concern (Deng et al., 2009) and the individualization of symptom management is critical to providing quality patient-centered care. This study examined the symptoms of persons diagnosed with one of three diseases with differing two year mortality rates and expected disease trajectories: advanced cancer, ALS and CHF. Symptom management in patients with terminal illness remains inadequate since one third of participants identified three troublesome symptoms and reported the most troublesome symptom to have a high level of severity. The majority of the participants identified troublesome symptoms other than pain, such as weakness and sleep disorders, supporting the 2011 NIH Science of Compassion report stressing that symptoms at the end of life are not limited to pain, nausea and dyspnea (National Institute of Nursing Research and Partners, 2011). Past studies on symptoms at the end life for cancer, ALS, and CHF patients similarly report a variety of symptoms, such as fatigue, weakness, and dyspnea (Bekelman et al., 2009; Doorenbos et al., 2006; Simmons, 2005).
Currently, the majority of end-of-life symptom management studies have narrowly focused on persons with cancer. This study adds to the literature about symptoms in patients with other diseases and revealed that patients with advanced CHF, a disease with a more favorable two-year mortality rate and less rapid decline to death, experienced higher symptom severity when compared to those of persons with advanced cancer. This suggests the need for further interventions to manage symptoms in patients with advanced CHF and further study of symptoms among other diseases. The growing number of studies on the use of palliative care among persons with ALS (Baxter et al., 2013; Maessen et al., 2009) and CHF (Kaasalainen et al., 2013; Lemond & Allen, 2011; Swetz et al., 2013) offer guidance on comprehensive approaches to symptom management. Further research is needed to examine the challenges in symptom management across transitions in care from the acute to long-term or home settings.
In this study, QOL was related to the number of symptoms experienced and symptom severity. Many patients had more than one symptom; therefore a comprehensive approach is needed to capture all symptoms. Palliative care instruments such as the Palliative Performance Scale (Anderson et al., 1996) can be one means to ensure an adequate symptom assessment. In this sample, symptom severity was negatively associated with QOL among men, but not women. Previous studies have found sex differences in how symptoms are experienced during chronic illness (Cheung et al., 2011) and which sociodemographic factors influence QOL (Cherepanov et al., 2010; Jayasinghe et al., 2013).
Due to the unpredictable course of CHF characterized by acute exacerbations, (Goldstein & Lynn, 2006) persons with advanced CHF have had the greatest symptom severity and the lowest QOL. Because CHF is often marked by periods of seemingly adequate symptom management, healthcare providers may underestimate the symptoms experienced by those with this disease. This study suggests persons with advanced CHF suffered higher symptom severity than persons with cancer, which reinforces the need for future studies addressing end-of-life symptoms in diseases other than cancer.
This study used self-reported data on presence and severity of symptoms. Although we used validated scales to assess symptoms, it is possible that studies that examine concurrent physiologic signs of distress would provide a more comprehensive view of the symptom experience at the end of life. However, palliative care experts advocate managing symptoms from the patient's perspective, consistent with the approach taken in this research.
Study Limitations
In this study we were not able to examine trajectories of illness course and the evolution of bothersome symptoms over time. Future studies should consider examining the progression of symptoms and QOL at the end of life. The average participant in this sample was 62 years old with multiple chronic conditions. Therefore, it is possible the reported symptoms were not directly related to cancer, ALS, and CHF but another disease. Nevertheless, the findings deserve attention because persons with chronic diseases do not only experience symptoms from one disease. Demographic differences by religion, race, and education between the disease groups may have been a result of sampling bias. For instance, the participants with CHF were primarily recruited from an inner-city hospital in a predominately Black neighborhood. Although these demographic variables may influence the symptom experience, there is not enough information in this data set to draw conclusions. Future studies could examine the relationship between demographic variables and symptom severity and/or frequency.
Conclusion
This is one of few studies comparing symptoms among persons suffering from three diseases with different two-year mortality rates and expected disease trajectories using the same measure of QOL and symptom severity. Because findings from this study indicate that the symptom experience differs among persons suffering from advanced cancer, ALS, and CHF, clinicians should be more attentive to patients' symptoms in the setting of advanced illness. Further study of symptom management in diseases other than cancer should continue. Health professionals seeking to promote QOL and alleviation of symptoms should assess symptom severity and inquire about symptoms other than pain. Those with advanced CHF require the same attention to symptom management given to those with cancer and ALS. Providers should be aware that patients with advanced CHF may have greater symptom severity than those with advanced cancer, despite having a longer life expectancy. Further research should focus on developing interventions to reduce symptom severity of all disease groups.
Acknowledgments
Sources of funding: Past funding support from the following grant: Natural History of End of Life Decision Making, NINR, NIH, R01 NR005224
Current funding support from the following grant: Trial of Ascertaining Individual Preferences for Loved Ones' Role in End-of-life Decisions, NINR, NIH, 1R01 NR010733
Footnotes
Conflicts of interest: No conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jiayun Xu, Email: jxu32@jhu.edu.
Marie T. Nolan, Email: mnolan3@jhu.edu.
Katherine Heinze, Email: kgriff12@jhu.edu.
Gayane Yenokyan, Email: gyenoky1@jhu.edu.
Mark T. Hughes, Email: mthughes@jhmi.edu.
Julie Johnson, Email: jjohnso3@medicine.bsd.uchicago.edu.
Joan Kub, Email: jkub1@jhu.edu.
Carrie Tudor, Email: ctudor2@jhu.edu.
Daniel P. Sulmasy, Email: dsulmasy@bsd.uchicago.edu.
Lisa Soleymani Lehmann, Email: LLEHMANN1@PARTNERS.ORG.
Joseph J. Gallo, Email: jgallo@jhsph.edu.
Felicia Rockko, Email: felicia.rockko@gmail.com.
References
- Authors. When patients lack capacity: the roles that patients with terminal diagnoses would choose for their physicians and loved ones in making medical decisions. J Pain Symptom Manage. 2005;30(4):342–53. doi: 10.1016/j.jpainsymman.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authors. How would terminally ill patients have others make decisions for them in the event of decisional incapacity? A longitudinal study. J Am Geriatr Soc. 2007;55(12):1981–8. doi: 10.1111/j.1532-5415.2007.01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Kutner JS, Beaty B. Suffering at the end of life in the setting of low physical symptom distress. J Palliat Med. 2006;9(3):658–65. doi: 10.1089/jpm.2006.9.658. [DOI] [PubMed] [Google Scholar]
- ALS Association Symptoms. ALS Association. [Accessed July 22, 2014]; http://www.alsa.org/about-als/symptoms.html.
- American Cancer Society. American Cancer Society; [Accessed July 22, 2014]. Signs and Symptoms of Cancer. http://www.cancer.org/cancer/cancerbasics/signs-and-symptoms-of-cancer. [Google Scholar]
- American Heart Association. American Heart Association; [Accessed July 22, 2014]. Symptoms & Diagnosis of Heart Failure. http://www.heart.org/HEARTORG/Conditions/HeartFailure/SymptomsDiagnosisofHeartFailure/Symptoms-Diagnosis-of-Heart-Failure_UCM_002047_Article.jsp. [Google Scholar]
- Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): anew tool. [Accessed December 12, 2012];J Palliat Care. 1996 12(1):5–11. http://www.ncbi.nlm.nih.gov/pubmed/8857241. [PubMed] [Google Scholar]
- Baxter SK, Baird WO, Thompson S, et al. The use of non-invasive ventilation at end of life in patients with motor neurone disease: a qualitative exploration of family carer and health professional experiences. Palliat Med. 2013;27(6):516–23. doi: 10.1177/0269216313478449. [DOI] [PubMed] [Google Scholar]
- Bekelman DB, Rumsfeld JS, Havranek EP, et al. Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. J Gen Intern Med. 2009;24(5):592–8. doi: 10.1007/s11606-009-0931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanov D, Palta M, Fryback DG, Robert SA. Gender differences in health-related quality-of-life are partly explained by sociodemographic and socioeconomic variation between adult men and women in the US: evidence from four US nationally representative data sets. Qual Life Res. 2010;19(8):1115–24. doi: 10.1007/s11136-010-9673-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WY, Le LW, Gagliese L, Zimmermann C. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer. 2011;19(3):417–23. doi: 10.1007/s00520-010-0865-2. [DOI] [PubMed] [Google Scholar]
- Cohen SR, Mount BM, Strobel MG, Bui F. The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. [Accessed December 12, 2012];Palliat Med. 1995 9(3):207–19. doi: 10.1177/026921639500900306. http://www.ncbi.nlm.nih.gov/pubmed/7582177. [DOI] [PubMed] [Google Scholar]
- Cohen SR, Mount BM, Tomas JJ, Mount LF. Existential well-being is an important determinant of quality of life. Evidence from the McGill Quality of Life Questionnaire. Cancer. 1996;77(3):576–86. doi: 10.1002/(SICI)1097-0142(19960201)77:3<576∷AID-CNCR22>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Deng G, Weber W, Sood A, Kemper K. Integrative Medicine Research: Context and Priorities. [Accessed July 22, 2014];2009 http://www.iom.edu/∼/media/Files/ActivityFiles/Quality/IntegrativeMed/IntegrativeMedicineResearch--ContextandPriorities.pdf.
- Doorenbos AZ, Given CW, Given B, Verbitsky N. Symptom experience in the last year of life among individuals with cancer. J Pain Symptom Manage. 2006;32(5):403–12. doi: 10.1016/j.jpainsymman.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein NE, Lynn J. Trajectory of end-stage heart failure: the influence of technology and implications for policy change. Perspect Biol Med. 2006;49(1):10–8. doi: 10.1353/pbm.2006.0008. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. [Accessed December 27, 2014]; http://www.nap.edu/openbook.php?record_id=18748. Published 2014.
- Jayasinghe UW, Harris MF, Taggart J, Christl B, Black DA. Gender differences in health-related quality of life of Australian chronically-ill adults: patient and physician characteristics do matter. Health Qual Life Outcomes. 2013;11:102. doi: 10.1186/1477-7525-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasalainen S, Strachan PH, Heckman GA, et al. Living and dying with heart failure in long-term care: experiences of residents and their family members. [Accessed July 22, 2014];Int J Palliat Nurs. 2013 19(8):375–82. doi: 10.12968/ijpn.2013.19.8.375. http://www.ncbi.nlm.nih.gov/pubmed/23970293. [DOI] [PubMed] [Google Scholar]
- Lemond L, Allen LA. Palliative care and hospice in advanced heart failure. Prog Cardiovasc Dis. 2011;54(2):168–78. doi: 10.1016/j.pcad.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney JR, Foley KM, Smith TJ, Gelband H, editors. Washington, D.C: The National Academies Press; 2003. [Accessed July 22, 2014]. Describing Death in America: What We Need to Know. http://www.nap.edu/openbook.php?isbn=0309087252. [PubMed] [Google Scholar]
- Lynn J, Adamson DM. Living Well at the End of Life. Arlington, VA: RAND; 2003. [Google Scholar]
- Maessen M, Veldink JH, Onwuteaka-Philipsen BD, et al. Trends and determinants of end-of-life practices in ALS in the Netherlands. Neurology. 2009;73(12):954–61. doi: 10.1212/WNL.0b013e3181b87983. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. National Institutes of Health State-of-the-Science Conference Statement on Improving End-of-Life Care. [Accessed July 22, 2014]; http://consensus.nih.gov/2004/2004EndofLifeCareSOS024html.htm. Published December 8, 2004.
- National Institute of Nursing Research and Partners. The Science of Compassion: FutureDirections in Improving End-of-Life Care. [Accessed July 22, 2014]; https://www.ninr.nih.gov/sites/www.ninr.nih.gov/files/science-of-compassion-executive-summary.pdf. Published 2011.
- Rousseau MC, Pietra S, Blaya J, Catala A. Quality of life of ALS and LIS patients with and without invasive mechanical ventilation. J Neurol. 2011;258(10):1801–4. doi: 10.1007/s00415-011-6018-9. [DOI] [PubMed] [Google Scholar]
- Semionov V, Singer Y, Shvartzman P. Prevalence and management of symptoms during the last month of life. [Accessed July 22, 2014];Isr Med Assoc J. 2012 14(2):96–9. http://www.ncbi.nlm.nih.gov/pubmed/22693789. [PubMed] [Google Scholar]
- Simmons Z. Management strategies for patients with amyotrophic lateral sclerosis from diagnosis through death. [Accessed July 22, 2014];Neurologist. 2005 11(5):257–70. doi: 10.1097/01.nrl.0000178758.30374.34. http://www.ncbi.nlm.nih.gov/pubmed/16148733. [DOI] [PubMed] [Google Scholar]
- Swetz KM, Cook KE, Ottenberg AL, Chang N, Mueller PS. Clinicians' attitudes regarding withdrawal of left ventricular assist devices in patients approaching the end of life. Eur J Heart Fail. 2013;15(11):1262–6. doi: 10.1093/eurjhf/hft094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ST, Liu LN, Lin KC, et al. Trajectories of the multidimensional dying experience for terminally ill cancer patients. J Pain Symptom Manage. 2014 doi: 10.1016/j.jpainsymman.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Whellan DJ, Goodlin SJ, Dickinson MG, et al. End-of-life care in patients with heart failure. J Card Fail. 2014;20(2):121–34. doi: 10.1016/j.cardfail.2013.12.003. [DOI] [PubMed] [Google Scholar]


