Abstract
Background
The aim of this retrospective observational study was to describe thyroid function parameters (fT3, fT4 and TSH) in the course of normal pregnancies.
Methods
Data were obtained between 2006 and 2007 at the University Hospital in Innsbruck, Austria. The starting point was the identification of women who had had a normal birth as recorded in the birth registry of Tyrol. Thyroid function parameters were determined using methods implemented at the Department of Nuclear Medicine in Innsbruck.
Results
The fT3 and fT4 values were normally distributed. Grouping the results by trimester revealed the following values: 4.93 ± 0.59, 4.54 ± 0.48, and 4.27 ± 0.45 pmol/l for fT3; and 15.23 ± 2.43, 13.79 ± 1.99, and 13.32 ± 0.2.01 pmol/l for fT4, respectively. The values corresponding to the 10th-percentile were 3.9 pmol/l for fT3 and 11.3 pmol/l for fT4, respectively. TSH values showed a typical left skewed distribution, thus the mean values were calculated after log transformation of the data. The corresponding mean trimestral values for TSH were 1.46 ± 1.29, 1.68 ± 1.23, and 1.70 ± 2.22 mIU/l, respectively.
Conclusion
In an iodine sufficient population, thyroid function parameters in normal pregnancies do not differ from those in non-pregnant women. Our previously defined reference range for TSH of 0.3 to 3.5 mIU/l is equally valid for normal pregnancies.
General significance
The question of cognition and IQ development of children has been proposed to be associated with thyroid function. The addition of data regarding normal thyroid function during pregnancy will contribute to this research.
Abbreviations: TSH, thyroid stimulating hormone; fT3, free triiodothyronine; fT4, free thyroxine; hCG, human chorionic gonadotropin
Keywords: Thyroid function, TSH, fT4, fT3, Pregnancy
Highlights
-
•
TSH levels in normal pregnancies do not differ from levels seen in adults.
-
•
The 10th-percentile value for fT3 in pregnancy is 3.9 pmol/l.
-
•
The 10th-percentile value for fT4 in pregnancy is 11.3 pmol/l.
-
•
A drop of TSH levels early in pregnancy is not a general phenomenon.
1. Introduction
The role of thyroid function during pregnancy has been observed and described since many centuries [1]. Modern studies describe thyroid disease as the second most frequent endocrine disorder that can affect women in their reproductive age. When thyroid disease remains untreated in a pregnant woman some disorders can appear. These include risk of miscarriage, hypertension, growth restriction, and placental abruption [2]. One fundamental cause of thyroid dysfunction is iodine deficiency which will lead to the development of goiter [3]. When iodine deficiency is present together with obstetrical pathology the cognitive development of the offspring can be endangered [4]. Fierro-Benítez et al. added the factor of protein–caloric malnutrition in regulating mental development [5]. The putative negative effect of thyroid function on cognitive development, however, has not been confirmed in a recent study [6].
The regulation of thyroid function has been analyzed from a historical point of view by Toni [7]. Current descriptions place thyroid hormone action and regulation in the context of regulation of metabolism [8]. The cellular processes leading to thyroid hormone action require selenoproteins, the deiodinases [9], in order to convert the pro-hormone T4 to the active form T3 [10].
In the clinical setting the evaluation of thyroid function relies on morphological and laboratory methods. In the past years we have been involved in applying improved thyroid ultrasound techniques [11] as well as in evaluating thyroid hormone reference levels in children and adults [12], [13] under conditions of sufficient iodine supply. The aim of the present study was to analyze thyroid function parameters in normal pregnancies.
2. Materials and methods
2.1. Patients
This study was carried out at the University Hospital in Innsbruck, Austria between 2006 and 2007. The study was conceived by KH who had previously designed and implemented the Tyrolean Birth Registry (Registry). The investigation was conducted as a retrospective case study of women that had a normal pregnancy. The data regarding the uneventful course of the pregnancy were obtained from the Registry. Inclusion criteria were that all babies born were healthy with no obstetrical pathology (data not shown). After identifying the subjects, thyroid function parameters as well as pregnancy variables were retrieved from the hospital clinical database. The study was approved by the local Ethics Committee.
2.2. Laboratory methods
Thyroid function parameters were determined using routine methods implemented at the Department of Nuclear Medicine as described previously [14]. Reference values for TSH have been described by us before [12] and served as a control group. A total of 2028 data sets were available for TSH evaluation and 1822 for fT3 and fT4. Laboratory determinations were performed on an ADVIA Centaur XP Immunoassay System (Siemens, Erlangen, Germany). Assay performance characteristics are similar to those reported by Reix et al. [15].
2.3. Statistical methods
The same subject was studied up to 3 times during pregnancy. Data was grouped according to the week of pregnancy. At each week of pregnancy a descriptive data analysis for fT3, fT4, and TSH was done. Presentation of the results for fT3 and fT4 was done by depicting the confidence intervals. TSH values are shown in percentiles. Statistical analysis was done with IBM SPSS 22.
3. Results
The mean age of the subjects was 27.6 ± 6.2 years. Among the subjects studied, we observed 5 cases of hypothyroidism, 10 cases of hyperthyroidism, and 159 cases of latent hyperthyroidism. In 83 cases a latent hypothyroidism was detected having 95% CI values of TSH between 4.5 and 5.3 mIU/l. These cases were subsequently excluded from the analysis. In spite of thyroid dysfunction these women had a normal pregnancy and delivery.
The results of the study are presented in Table 1 showing the mean and S.D. for each parameter according to pregnancy week. These results are extracted giving the mean values for 3 periods of time: up to week 12, weeks 13 to 24, and weeks 25 to 36. The corresponding trimestral values for fT3 were 4.93 ± 0.59, 4.54 ± 0.48, and 4.27 ± 0.45 pmol/l, respectively. The corresponding trimestral values for fT4 were 15.23 ± 2.43, 13.79 ± 1.99, and 13.32 ± 2.01 pmol/l, respectively. Both parameters showed a continuous decline. The fT3 to fT4 ratio had a mean value of 0.32. The values corresponding to the 10th-percentile were 3.9 pmol/l for fT3 and 11.3 pmol/l for fT4, respectively. Fig. 1, Fig. 2 show the histograms depicting the normal distribution of fT3 and fT4; Fig. 3, Fig. 4 show the course of fT3 and fT4 during pregnancy.
Table 1.
Thyroid function reference values for fT3, fT4, TSH and fT3/fT4 ratio across pregnancy.
| Pregnancy |
fT3 pmol/l |
fT4 pmol/l |
TSH mIU/l |
fT3/fT4 |
|||
|---|---|---|---|---|---|---|---|
| Week | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean |
| 6 | 4.55 | 0.35 | 14.45 | 0.35 | 1.57 | 0.63 | 0.31 |
| 7 | 5.06 | 0.68 | 15.30 | 2.63 | 2.22 | 1.45 | 0.33 |
| 8 | 5.08 | 0.64 | 16.03 | 2.99 | 1.33 | 0.97 | 0.32 |
| 9 | 5.04 | 0.57 | 15.35 | 3.32 | 1.48 | 2.33 | 0.33 |
| 10 | 5.03 | 0.75 | 15.75 | 2.67 | 0.96 | 0.76 | 0.32 |
| 11 | 4.89 | 0.59 | 14.89 | 2.79 | 1.21 | 1.12 | 0.33 |
| 12 | 4.89 | 0.56 | 14.84 | 2.26 | 1.41 | 1.75 | 0.33 |
| 13 | 4.78 | 0.64 | 14.72 | 2.58 | 1.65 | 1.96 | 0.32 |
| 14 | 4.75 | 0.56 | 14.02 | 1.88 | 1.52 | 1.23 | 0.34 |
| 15 | 4.69 | 0.45 | 14.02 | 2.04 | 1.57 | 1.01 | 0.33 |
| 16 | 4.66 | 0.54 | 14.02 | 1.89 | 1.67 | 1.58 | 0.33 |
| 17 | 4.54 | 0.44 | 14.03 | 2.19 | 1.61 | 0.89 | 0.32 |
| 18 | 4.55 | 0.45 | 13.23 | 1.66 | 1.91 | 2.28 | 0.34 |
| 19 | 4.61 | 0.50 | 13.86 | 2.19 | 1.90 | 1.06 | 0.33 |
| 20 | 4.39 | 0.40 | 13.19 | 1.83 | 1.83 | 1.26 | 0.33 |
| 21 | 4.52 | 0.46 | 13.83 | 1.91 | 1.63 | 0.94 | 0.33 |
| 22 | 4.38 | 0.48 | 13.69 | 2.16 | 1.70 | 0.98 | 0.32 |
| 23 | 4.31 | 0.45 | 13.43 | 1.96 | 1.70 | 0.86 | 0.32 |
| 24 | 4.33 | 0.45 | 13.39 | 1.62 | 1.41 | 0.79 | 0.32 |
| 25 | 4.31 | 0.48 | 13.17 | 2.07 | 1.77 | 1.32 | 0.33 |
| 26 | 4.21 | 0.54 | 13.29 | 2.32 | 2.77 | 10.97 | 0.32 |
| 27 | 4.30 | 0.43 | 13.07 | 2.47 | 1.49 | 0.92 | 0.33 |
| 28 | 4.25 | 0.40 | 13.33 | 1.80 | 1.44 | 0.98 | 0.32 |
| 29 | 4.28 | 0.42 | 13.33 | 1.93 | 1.72 | 1.56 | 0.32 |
| 30 | 4.26 | 0.41 | 13.90 | 1.78 | 1.32 | 0.65 | 0.31 |
| 31 | 4.34 | 0.48 | 12.97 | 2.04 | 1.44 | 0.92 | 0.33 |
| 32 | 4.35 | 0.46 | 13.32 | 1.93 | 1.60 | 0.95 | 0.33 |
| 33 | 4.23 | 0.41 | 13.63 | 2.25 | 1.55 | 1.16 | 0.31 |
| 34 | 4.27 | 0.55 | 13.14 | 1.86 | 2.10 | 5.36 | 0.32 |
| 35 | 4.23 | 0.44 | 13.24 | 1.86 | 1.58 | 0.82 | 0.32 |
| 36 | 4.24 | 0.40 | 13.46 | 1.87 | 1.57 | 1.03 | 0.31 |
| 37 | 4.25 | 0.43 | 13.12 | 2.32 | 1.56 | 0.96 | 0.32 |
| 38 | 4.29 | 0.39 | 12.82 | 1.43 | 1.68 | 0.93 | 0.33 |
| 39 | 4.18 | 0.42 | 13.60 | 1.78 | 1.89 | 1.03 | 0.31 |
| 40 | 4.04 | 0.32 | 12.56 | 2.70 | 1.74 | 1.04 | 0.32 |
| 41 | 3.97 | 0.49 | 13.23 | 0.76 | 2.29 | 0.58 | 0.30 |
| Total mean | 4.47 | 0.48 | 13.81 | 2.06 | 1.66 | 1.53 | 0.32 |
Fig. 1.
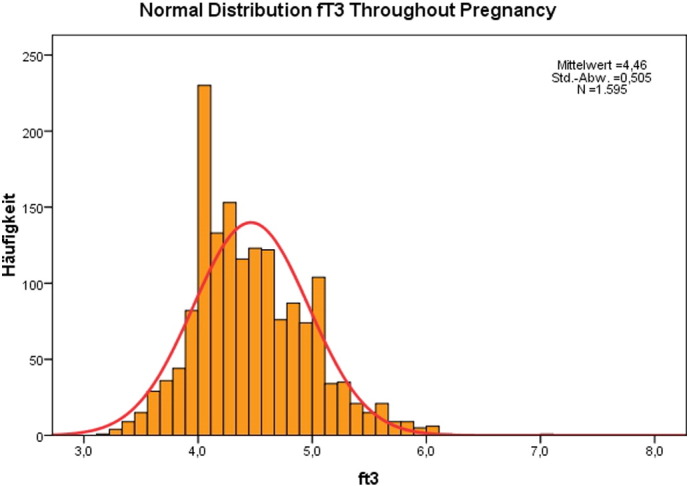
Normal distribution of fT3 values in normal pregnancies.
Fig. 2.
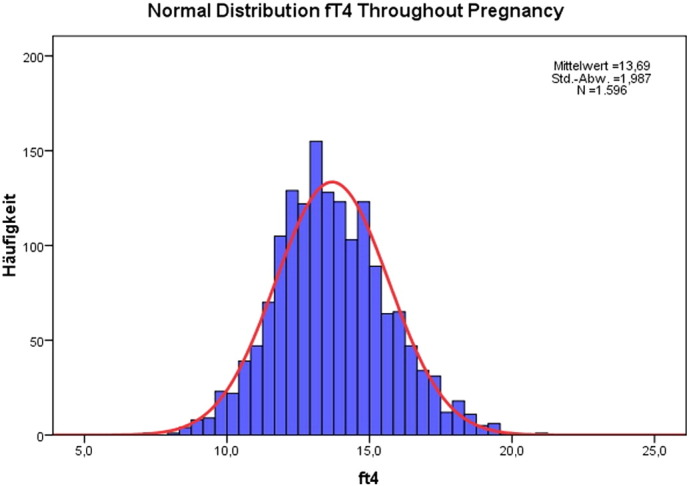
Normal distribution of fT4 values in normal pregnancies.
Fig. 3.
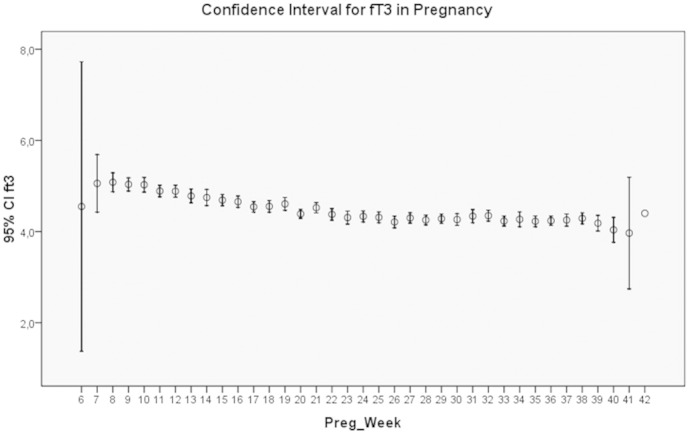
The course of fT3 levels through pregnancy.
Fig. 4.
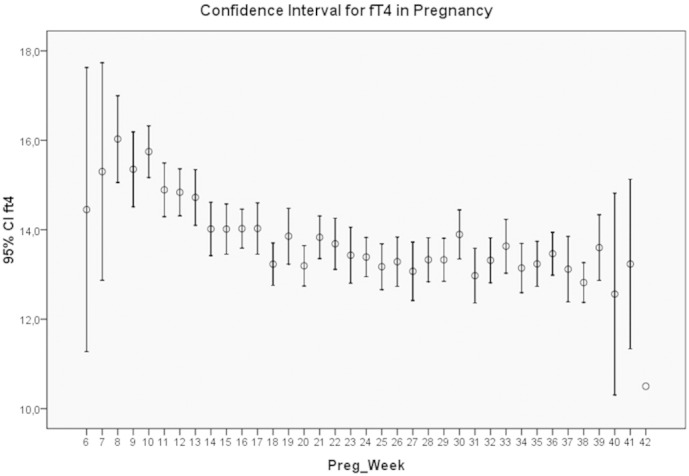
The course of fT4 levels through pregnancy.
TSH values showed a typical left skewed distribution. The mean values were calculated after log transformation of the data. The corresponding mean trimestral values for TSH were 1.46 ± 1.29, 1.68 ± 1.23, and 1.70 ± 2.22 mIU/l, respectively. The maximal values reached levels of 3.5 mIU/l. Fig. 5 shows the TSH values at the percentile levels of 2.5, 5.0, 50.0, 95, and 97.5 across pregnancy. While TSH levels showed little variation, only those corresponding to the 2.5 and 5.0 percentiles decreased between weeks 6 and 8 of pregnancy.
Fig. 5.
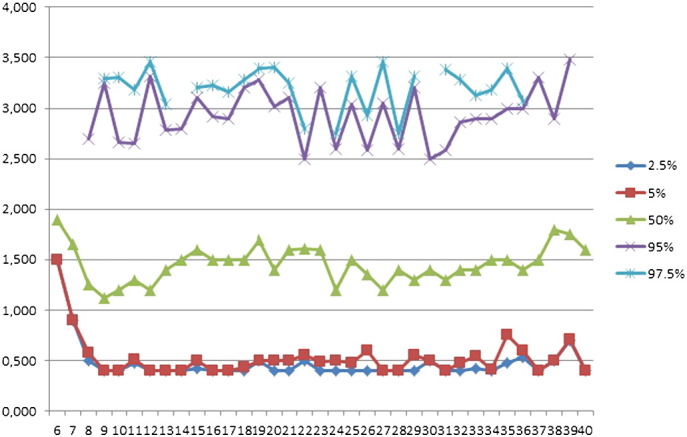
TSH values at the percentile levels of 2.5, 5.0, 50.0, 95, and 97.5 during pregnancy.
4. Discussion
This clinical observational study demonstrates that under conditions of sufficient iodine supply women that have a normal pregnancy present thyroid function parameters similar to those found in a normal adult population. The key message of our study is that TSH levels range between 0.3 and 3.5 mIU/l. These results indicate that it is not necessary to rely on so-called pregnancy-specific evaluations, nor on consensus recommendations.
4.1. Questioning the use of recommended reference values
The negative impact of relying on “recommendations” for the interpretation of thyroid laboratory results has been recently commented by us as: “lack of clinical congruence” [16]. By this we mean a misclassification of subjects by using an arbitrary cut-off value. This concern appears to be shared by other authors. Vila et al. discussed the impact of using an upper reference value for TSH of 2.5 mIU/ml and added that such a measure has: “…raised concern among clinicians and it has been argued that it would result in classifying healthy pregnant women as having a pathological disease” (page R25 in [17]). Moreover, a recent publication failed to show differences in the frequency of thyroid pathology found in pregnant women when these recommended values are used (Tables 3 and 4 in [18]). In relation to outcome of pregnancy, Hirsh et al. have recently shown that TSH values much higher than 2.5 mIU/ml do not appear to be related to altered outcome of pregnancies [19]. Similarly, Taylor et al. reported recently that TSH levels up to 4.5 mIU/l did not lead to the identification of patients with elevated miscarriage risk [20]. Similar observations have also been made within the field of in vitro fertilization. Reh and coworkers have also looked critically at this “desirable” TSH level [21]. They concluded that an increasing number of subjects would be falsely classified as being hypothyroid. In 2007 Spandorfer et al. reported a lack of difference in IVF outcome when TSH levels lower and higher than 4 mIU/l were taken as discriminators. The same result was observed when TSH levels lower or higher than 2.5 mIU/l were considered [22].
In daily practice, we (HM and RM) have had repeated referrals of pregnant and non-pregnant women who supposedly have high TSH values. This misinterpretation of thyroid function parameters originates from a publication in 2007 [23]. It is of utmost importance to note that the authors of the publication in question admitted that there is a poor level of evidence for their recommendation to consider an upper value of TSH of 2.5 mIU/l as a “desirable level”. In order to increase the level of evidence, the recommendations given by the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) Working Group [24] should be observed. One can read in this publication the following statement: “Recommendations must apply to specific settings and particular groups of patients whenever the benefits and harms differ across settings or patient groups” (page 1491 in [24]). It follows that in the field of thyroid diseases reference values have to be put first into the context of iodine supply, i.e. iodine deficient or iodine sufficient population, and then into the clinical context (age, sex, pregnancy, obstetrical findings).
4.2. Confounding factors related to thyroid function in pregnancy
Investigations performed at the end of the previous century did not meet the abovementioned criteria. Work which began in 1988 by Belgian researchers on thyroid function in pregnancy introduced bias by studying a population with iodine deficiency as well as subjects with obstetrical pathology [25]. While iodine supplementation programs have been initiated worldwide for many decades, the data presented by Vitti in 2003 demonstrated that iodine deficiency has still not been eliminated in Europe [26]. Even mild iodine deficiency led to thyroid disorders in 2012–2013 in countries including Belgium, France, and the U.K. [27], [28], [29]. Irrespective of historical descriptions that relate hypothyroidism during pregnancy to altered IQ of the offspring [30], investigation of thyroid function in pregnancy remains a matter of interest. In a study published in 2012 that investigated the cognitive development of the offspring, iodine deficiency was still a confounding variable [6], [31]. However, the authors were unable to demonstrate that treatment of hypothyroidism with thyroxine improved cognitive function of the children at age 3 years. Other investigators have proposed that additional mechanisms, i.e. prematurity, could play a role as determinants of intelligence [32]. Not only reports on TSH levels can be conflicting but also the evaluation of the percentile distribution of fT4 can show different results. In 1999 Pop et al. investigated an iodine sufficient population and calculated the 10th percentile for fT4 [33]. Their values are much lower than those obtained in the study herein. This difference implies that other factors influence thyroid economy. A similar observation was made when the ratio between fT3 and fT4 was evaluated. While the data obtained in the present study revealed quite constant values (mean 0.32) the results presented by Bassols et al. are quite different [34].
Another issue that is related to pregnancy and iodine deficiency is that of an apparent decrease in TSH levels during early pregnancy. This was originally described in a series of 8 women with iodine deficiency [35]. A similar finding was subsequently reported in the Belgian studies [25]. Some years later this hypothesis was published again and presented in a graphical form that depicted a decrease in TSH concentration whereas hCG values were high (Fig. 1 in [36]). This publication also reported an increase in fT4 during early pregnancy. A modern evaluation of the association between hCG and TSH concluded that in most pregnant women this relationship is weak [37]. However, the authors could identify a stronger relationship between hCG and TSH for TSH values in the lower centiles. Our results show a similar situation. Thus, the data indicate that a decrease in TSH levels during pregnancy is not a general phenomenon. In addition, the data presented herein do not support the notion that fT4 levels increase during early pregnancy as reported by Burrow [36]. Table 2, Table 3 summarize the results and methods of studies that have evaluated thyroid function in pregnant women.
Table 2.
Reference values from the literature for thyroid function across trimesters of pregnancy.
| Author | Year | Country | fT31 pmol/l |
fT32 pmol/l |
fT33 pmol/l |
fT41 pmol/l |
fT42 pmol/l |
fT43 pmol/l |
TSH1 mIU/l |
TSH2 mIU/l |
TSH3 mIU/l |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cotzias [43] | 2007 | U.K. | 3–7 | 3–5.5 | 2.5–5.5 | 10–16 | 9–15.5 | 8–14.5 | 0–5.5 | 0.5–3.5 | 0.5–4 |
| Soldin [44] | 2007 | USA | TT4: 141.35 nmol/l | TT4: 152.95 nmol/l | TT4: 142.65 nmol/l | 0.91 | 1.03 | 1.32 | |||
| Marwaha [45] | 2008 | India | 1.92–5.86 | 3.2–5.73 | 3.3–5.18 | 12–19.45 | 9.48–19.58 | 11.32–17.7 | 0.6–5.0 | 0.44–5.78 | 0.74–5.7 |
| Springer [46] | 2009 | Czech | 9.55–23.0 | 0.06–3.67 | |||||||
| Karakosta [47] | 2011 | Greece, Crete | 2.37–8.02 | 2.73–8.13 | 12.23–19.69 | 11.2–18.66 | 0.05–2.53 | 0.18–2.73 | |||
| Ekinci [48] | 2013 | Australia | 3.9–5.7 | 3.7–5.2 | 3.5–5.0 | 5.9–15.6 | 4.9–11.3 | 4.4–11.2 | 0.03–3.05 | 0.42–3.36 | 0.34–2.83 |
| Azizi [49] | 2013 | Iran | 8.5–19.0 (fT4I) | 9.7–21.0 (fT4I) | 8.7–20.4 (fT4I) | 0.2–3.9 | 0.5–4.1 | 0.6–4.1 | |||
| Moreno-Reyes [28] | 2013 | Belgium | 2.8–7.1 | 10.3–21.9 | 0.1–2.5 | 0.3–3.0 | |||||
| This study | 2007 | Austria | 4.93 ± 0.59 | 4.54 ± 0.48 | 4.27 ± 0.45 | 15.23 ± 2.43 | 13.79 ± 1.99 | 13.32 ± 2.01 | 1.46 ± 1.29 | 1.68 ± 1.23 | 1.70 ± 2.22 |
Statistical methods: Cotzias: median; Soldin: mean and median; Marwaha: fT3 + fT4: mean, median & percentile; TSH: log; Springer: mean; Karakosta: mean, median and TSH log; Ekinci: fT3 + fT4: mean; TSH: percentile and log; Azizi: TSH log; Moreno-Reyes: mean, median and log; and this Study: fT3, fT4 means, and log TSH means.
Table 3.
Methodologies used for the determination of thyroid function parameters in pregnancy.
| Author | fT3 | fT4 | TSH |
|---|---|---|---|
| Cotzias [43] | ADVIA Centaur competitive immunoassay | ADVIA Centaur competitive immunoassay | ADVIA Centaur two-side sandwich immunoassay |
| Soldin [44] | Immunoassay (ROCHE) | Chemiluminescence immunometric assay (Nichols Institute Diagnostics) | |
| Marwaha [45] | Electrochemiluminescence system — Elecsys 1010 Analyzer (ROCHE Diagnostics, Germany) | ||
| Springer [46] | Chemiluminometric immunoanalysis | Chemiluminometric immunoanalysis | |
| Karakosta [47] | Immunoassay system (IMMULITE 2000, Siemens) | ||
| Ekinci [48] | Chemiluminescent immunoassay using a Beckman DXL 800 Analyzer | ||
| Azizi [49] | Immunoassay system | Immunoassay system | |
| Moreno-Reyes [28] | Third generation chemiluminescence immunoassay (ROCHE) | ||
Recently (2014) we have observed a negative interaction arising from multivitamin preparations prescribed for pregnant women (n = 25). These preparations contain 200 to 220 μg iodine per tablet or capsule. The patients presented latent to overt hyperthyroidism although on ultrasound thyroid morphology was normal. We interpret this situation as an iodine-induced hyperthyroidism. After stopping the intake of these preparations thyroid function has returned to normal.
In summary, the lack of a validated system for laboratory results based on specific clinical traits damages the principles of evidence-based diagnostics [38]. Declaring that a euthyroid woman has thyroid disease or advocating treatment for a condition that most likely does not exist is not in keeping with good medical approach. An equivocal diagnosis only makes patients feel insecure, thus adding to psychological stress, which is already frequently observed in hypothyroidism [39], [40]. In times when unduly conduct is being reported every day in the media, we should remember that: “By not reporting poor care …. individuals clearly failed in their professional responsibility to protect patients” [41]. In view of faulty clinical work found in the scientific literature, Yong, Ledford, and Van have published a stimulating paper entitled: “Research ethics: 3 ways to blow the whistle” [42]. Considering that clinical work should be as accurate as possible, these recommendations are inspiring.
5. Conclusion
We conclude that the use of thyroid function reference values based on studies using different populations and different backgrounds can introduce bias in the evaluation of a local cohort. Three main factors should be considered: iodine supply, obstetrical pathology and the laboratory methodology. In our population, TSH values are within a range comparable to TSH values of normal adults determined using the same methodology [12], [13], [14]. We believe that our data are of direct relevance for clinicians working in a similar environment, i.e. iodine sufficiency and with a similar laboratory system.
Transparency document
Transparency document.
Conflict of interest
All authors declare no conflicts of interest.
Author contributions
RM coordinated the study between the Dept. of Obstetrics and Gynecology and the Thyroid outpatient unit of the Dept. of Nuclear Medicine, and carried out the statistical analyses. He also wrote the final manuscript.
BZ performed data mining of the Birth Registry as well as the hospital clinical system. She prepared the Excel data sheets containing all pertinent data and participated in the statistical analysis.
KH developed the Tyrol Birth Registry and conceived the study. He presented the project at the local Ethics Committee.
KO searched for newer publications on the topic, which appeared after 2007. She compared the methodology and results of similar recent studies.
HM was involved in clinical work with pregnancy patients.
Funding sources
This study received no external funding.
Footnotes
The Transparency document associated with this article can be found, in online version.
Contributor Information
Roy Moncayo, Email: Roy.Moncayo@i-med.ac.at.
Birgit Zanon, Email: birgit.zanon@klinik-ebe.de.
Kurt Heim, Email: heim_kurt@yahoo.com.
Karina Ortner, Email: Karina.Ortner@student.i-med.ac.at.
Helga Moncayo, Email: anmeldung@womed.at.
References
- 1.Crooks J. The incidence of goitre during pregnancy. Lancet. 1964;2:334–336. doi: 10.1016/s0140-6736(64)90277-6. (PM:14172331) [DOI] [PubMed] [Google Scholar]
- 2.Carney L.A. Thyroid disease in pregnancy. Am. Fam. Physician. 2014;89:273–278. (PM:24695447) [PubMed] [Google Scholar]
- 3.McCarrison R. Observations on endemic goitre in the Chitral and Gilgit valleys. Med. Chir. Trans. 1906;89:437–470. (PM:20897060) [PMC free article] [PubMed] [Google Scholar]
- 4.Man E.B. Thyroid function in pregnancy and infancy. Maternal hypothyroxinemia and retardation of progeny. CRC Crit. Rev. Clin. Lab. Sci. 1972;3:203–225. doi: 10.3109/10408367209151327. (PM:4115124) [DOI] [PubMed] [Google Scholar]
- 5.Fierro-Benítez R. Effect of iodine correction early in fetal life on intelligence quotient. A preliminary report. Adv. Exp. Med. Biol. 1972;30:239–247. (PM:4350936) [PubMed] [Google Scholar]
- 6.Lazarus J.H. Antenatal thyroid screening and childhood cognitive function. N. Engl. J. Med. 2012;366:493–501. doi: 10.1056/NEJMoa1106104. (PM:22316443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toni R. Ancient views on the hypothalamic–pituitary–thyroid axis: an historical and epistemological perspective. Pituitary. 2000;3:83–95. doi: 10.1023/a:1009953723963. (PM:11141700) [DOI] [PubMed] [Google Scholar]
- 8.Mullur R. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. (PM:24692351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvatore D. Deiodinases: keeping the thyroid hormone supply in balance. J. Endocrinol. 2011;209:259–260. doi: 10.1530/JOE-11-0058. (PM:21610254) [DOI] [PubMed] [Google Scholar]
- 10.Fliers E. Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol. Cell. Endocrinol. 2006;251:1–8. doi: 10.1016/j.mce.2006.03.042. (PM:16707210) [DOI] [PubMed] [Google Scholar]
- 11.Moncayo R., Moncayo H. Advanced 3D sonography of the thyroid: focus on vascularity. In: Thoirs K., editor. Sonography, Intech. 2012. pp. 273–292. [Google Scholar]
- 12.Moncayo H. Diagnostic accuracy of basal TSH determinations based on the intravenous TRH stimulation test: an evaluation of 2570 tests and comparison with the literature. BMC Endocr. Disord. 2007;7:5. doi: 10.1186/1472-6823-7-5. (PM:17678551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapelari K. Pediatric reference intervals for thyroid hormone levels from birth to adulthood: a retrospective study. BMC Endocr. Disord. 2008;8:15. doi: 10.1186/1472-6823-8-15. (PM:19036169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moncayo R. Reference values for thyrotropin. Thyroid. 2005;15:1204–1205. (PM:16425442) [PubMed] [Google Scholar]
- 15.Reix N. Thyroid-stimulating hormone and free thyroxine on the ADVIA Centaur immunoassay system: a multicenter assessment of analytical performance. Clin. Biochem. 2013;46:1305–1308. doi: 10.1016/j.clinbiochem.2013.04.015. (PM:23628596) [DOI] [PubMed] [Google Scholar]
- 16.Moncayo H., Moncayo R. The lack of clinical congruence in diagnosis and research in relation to subclinical hypothyroidism. Fertil. Steril. 2014;101:e30. doi: 10.1016/j.fertnstert.2014.01.010. (PM:24534287) [DOI] [PubMed] [Google Scholar]
- 17.Vila L. On the need for universal thyroid screening in pregnant women. Eur. J. Endocrinol. 2014;170:R17–R30. doi: 10.1530/EJE-13-0561. (PM:24128429) [DOI] [PubMed] [Google Scholar]
- 18.Ahmed I.Z. Comparison of universal and targeted screening for thyroid dysfunction in pregnant Egyptian women. Eur. J. Endocrinol. 2014;171:285–291. doi: 10.1530/EJE-14-0100. (PM:24842727) [DOI] [PubMed] [Google Scholar]
- 19.Hirsch D. Pregnancy outcomes in women with severe hypothyroidism. Eur. J. Endocrinol. 2013;169:313–320. doi: 10.1530/EJE-13-0228. (PM:23811188) [DOI] [PubMed] [Google Scholar]
- 20.Taylor P.N. TSH levels and risk of miscarriage in women on long-term levothyroxine: a community-based study. J. Clin. Endocrinol. Metab. 2014;99:3895–3902. doi: 10.1210/jc.2014-1954. (PM:25057882) [DOI] [PubMed] [Google Scholar]
- 21.Reh A. What is a normal thyroid-stimulating hormone (TSH) level? Effects of stricter TSH thresholds on pregnancy outcomes after in vitro fertilization. Fertil. Steril. 2010;94:2920–2922. doi: 10.1016/j.fertnstert.2010.06.041. (PM:20655528) [DOI] [PubMed] [Google Scholar]
- 22.Spandorfer S.D. Serum TSH: is there a link between subclinical hypothyroidism and IVF outcome. Fertil. Steril. 2007;88(Suppl. 1):S189–S190. ( http://www.sciencedirect.com/science/article/pii/S0015028207023072) [Google Scholar]
- 23.Abalovich M. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2007;92(8 Suppl.):s1–s47. doi: 10.1210/jc.2007-0141. (PM:17948378) [DOI] [PubMed] [Google Scholar]
- 24.Atkins D. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. (PM:15205295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glinoer D. Regulation of maternal thyroid during pregnancy. J. Clin. Endocrinol. Metab. 1990;71(2):276–287. doi: 10.1210/jcem-71-2-276. (PM:2116437) [DOI] [PubMed] [Google Scholar]
- 26.Vitti P. Europe is iodine deficient. Lancet. 2003;361:1226. doi: 10.1016/S0140-6736(03)12935-2. (PM:12686067) [DOI] [PubMed] [Google Scholar]
- 27.Raverot V. Pregnant French women living in the Lyon area are iodine deficient and have elevated serum thyroglobulin concentrations. Thyroid. 2012;22:522–528. doi: 10.1089/thy.2011.0184. (PM:22468941) [DOI] [PubMed] [Google Scholar]
- 28.Moreno-Reyes R. High prevalence of thyroid disorders in pregnant women in a mildly iodine-deficient country: a population-based study. J. Clin. Endocrinol. Metab. 2013;98:3694–3701. doi: 10.1210/jc.2013-2149. (PM:23846819) [DOI] [PubMed] [Google Scholar]
- 29.Bath S.C. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC) Lancet. 2013;382:331–337. doi: 10.1016/S0140-6736(13)60436-5. (PM:23706508) [DOI] [PubMed] [Google Scholar]
- 30.Man E.B. Thyroid function in human pregnancy. 8. Retardation of progeny aged 7 years; relationships to maternal age and maternal thyroid function. Am. J. Obstet. Gynecol. 1971;111:905–916. (PM:4107381) [PubMed] [Google Scholar]
- 31.Bath S.C., Rayman M.P. Antenatal thyroid screening and childhood cognitive function. N. Engl. J. Med. 2012;366:1640–1641. doi: 10.1056/NEJMc1202720. (PM:22533585) [DOI] [PubMed] [Google Scholar]
- 32.Casey B.M. Subclinical hypothyroidism and pregnancy outcomes. Obstet. Gynecol. 2005;105:239–245. doi: 10.1097/01.AOG.0000152345.99421.22. (PM:15684146) [DOI] [PubMed] [Google Scholar]
- 33.Pop V.J. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin. Endocrinol. 1999;50:149–155. doi: 10.1046/j.1365-2265.1999.00639.x. (PM:10396355) [DOI] [PubMed] [Google Scholar]
- 34.Bassols J. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J. Clin. Endocrinol. Metab. 2011;96:3717–3723. doi: 10.1210/jc.2011-1784. (PM:21917863) [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto T. Longitudinal study or serum thyroid hormones, chorionic gonadotrophin and thyrotrophin during and after normal pregnancy. Clin. Endocrinol. 1979;10:459–468. doi: 10.1111/j.1365-2265.1979.tb02102.x. (PM:113141) [DOI] [PubMed] [Google Scholar]
- 36.Burrow G.N. Maternal and fetal thyroid function. N. Engl. J. Med. 1994;331:1072–1078. doi: 10.1056/NEJM199410203311608. (PM:8090169) [DOI] [PubMed] [Google Scholar]
- 37.Haddow J.E. Variability in thyroid-stimulating hormone suppression by human chorionic [corrected] gonadotropin during early pregnancy. J. Clin. Endocrinol. Metab. 2008;93:3341–3347. doi: 10.1210/jc.2008-0568. (PM:18544616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gluud C., Gluud L.L. Evidence based diagnostics. BMJ. 2005;330:724–726. doi: 10.1136/bmj.330.7493.724. (PM:15790646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moncayo R., Moncayo H. Exploring the aspect of psychosomatics in hypothyroidism: the WOMED model of body–mind interactions based on musculoskeletal changes, psychological stressors, and low levels of magnesium. Woman. 2014;1:1–11. ( http://www.sciencedirect.com/science/article/pii/S2213560X14000022) [Google Scholar]
- 40.Freeman E.W. Emotional distress patterns among women having first or repeat abortions. Obstet. Gynecol. 1980;55:630–636. (PM:7366922) [PubMed] [Google Scholar]
- 41.Blowing the whistle on intimidation of NHS whistleblowers. Lancet. 2011;378:458. doi: 10.1016/S0140-6736(11)61233-6. (PM:21821171) [DOI] [PubMed] [Google Scholar]
- 42.Yong E. Research ethics: 3 ways to blow the whistle. Nature. 2013;503:454–457. doi: 10.1038/503454a. (PM:24284713) [DOI] [PubMed] [Google Scholar]
- 43.Cotzias C. A study to establish gestation-specific reference intervals for thyroid function tests in normal singleton pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008;137:61–66. doi: 10.1016/j.ejogrb.2007.10.007. (PM:18093719) [DOI] [PubMed] [Google Scholar]
- 44.Soldin O.P. Gestation-specific thyroxine and thyroid stimulating hormone levels in the United States and worldwide. Ther. Drug Monit. 2007;29:553–559. doi: 10.1097/FTD.0b013e31815709ac. (PM:17898643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marwaha R.K. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG. 2008;115:602–606. doi: 10.1111/j.1471-0528.2008.01673.x. (PM:18333941) [DOI] [PubMed] [Google Scholar]
- 46.Springer D. Reference intervals in evaluation of maternal thyroid function during the first trimester of pregnancy. Eur. J. Endocrinol. 2009;160:791–797. doi: 10.1530/EJE-08-0890. (PM:19228824) [DOI] [PubMed] [Google Scholar]
- 47.Karakosta P. First- and second-trimester reference intervals for thyroid hormones during pregnancy in “Rhea” Mother–Child Cohort, Crete, Greece. J. Thyroid. Res. 2011;2011:490783. doi: 10.4061/2011/490783. (PM:22175032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekinci E.I. Longitudinal assessment of thyroid function in pregnancy. Ann. Clin. Biochem. 2013;50:595–602. doi: 10.1177/0004563213486450. (PM:23873872) [DOI] [PubMed] [Google Scholar]
- 49.Azizi F. Establishment of the trimester-specific reference range for free thyroxine index. Thyroid. 2013;23:354–359. doi: 10.1089/thy.2012.0407. (PM:23167270) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.


