Abstract
Plasma high density lipoprotein cholesterol (HDL) comprises a heterogeneous family of lipoprotein species, differing in surface charge, size and lipid and protein compositions. While HDL cholesterol (C) mass is a strong, graded and coherent biomarker of cardiovascular risk, genetic and clinical trial data suggest that the simple measurement of HDL-C may not be causal in preventing atherosclerosis nor reflect HDL functionality. Indeed, the measurement of HDL-C may be a biomarker of cardiovascular health. To assess the issue of HDL function as a potential therapeutic target, robust and simple analytical methods are required. The complex pleiotropic effects of HDL make the development of a single measurement challenging. Development of laboratory assays that accurately HDL function must be developed validated and brought to high-throughput for clinical purposes. This review discusses the limitations of current laboratory technologies for methods that separate and quantify HDL and potential application to predict CVD, with an emphasis on emergent approaches as potential biomarkers in clinical practice.
Abbreviations: 2D-PAGGE, two dimensional polyacrylamide gradient gel electrophoresis; ApoA-I, apolipoprotein A-I; CHD, coronary heart disease; CVD, cardiovascular disease; HDL, high density lipoprotein; HPLC, High Performance Liquid Chromatography; LCAT, lecithin–cholesterol acyltransferase; LDL, low density lipoprotein; MALDI, matrix-assisted laser desorption/ionization; MOP, myeloperoxidase; MS/MS, tandem-mass spectrometry; ND-PAGGE, non-denaturant polyacrylamide gradient gel electrophoresis; NMR, nuclear magnetic resonance; PEG, polyethylene glycol; PON1, paraoxonase 1; SELDI, surface enhanced laser desorption/ionization; TOF, time-of-flight; UTC, ultracentrifugation
Keywords: Atherosclerosis, Coronary artery disease, High density lipoproteins, Apolipoprotein A-I, Cellular cholesterol efflux, Vascular endothelial function, Biomarkers of cardiovascular risk
1. Introduction
Plasma levels of high density lipoprotein cholesterol (HDL-C) are strongly associated with atherosclerotic cardiovascular disease, especially coronary artery disease (CAD). This observation is strong, graded and coherent across the populations studied [1]. In post-hoc analysis of clinical trials, HDL-C remains a powerful predictor of residual risk, even at low LDL-C levels [2]. In recent years, Mendelian randomization experiments have casted doubt on the causal link between HDL-C and CAD [3]. Furthermore, drugs that increase HDL-C, including fibrates, niacin and the cholesteryl ester transfer protein inhibitors torcetrapib and dalcetrapib have failed to show improved cardiovascular outcomes. One possible explanation to explain the discrepancy between the epidemiological, genetic and clinical trial data is that the measurement of the cholesterol mass within HDL fails to capture the complexity of a highly dynamic process [4], [5]. HDL particles differ in size, ranging from 7 nm to 17 nm, shape (unfolded protein, discoidal and spherical), lipidome and proteome [6], [7]. The measurement of HDL-C has been standardized and current precipitation techniques achieve a high degree of accuracy for clinical purposes (Table 1). However, there is no accepted “gold standard” technique for the measurement of HDL particles. More refined techniques have been developed based on the physical and functional properties of HDL (Table 2, Table 3). In this review, we will address the techniques of HDL measurement, determine whether the information provided ads to our ability to predict CVD, and evaluate the limitations of these assays. The structural and composition (proteomic/lipidomic) of HDL may provide further insights on its function [8], [9]. HDL particles possess many pleiotropic properties that are unrelated to their cholesterol mass or the ability to transport it in the blood. These properties, observed in vitro, may be a better metric to determine CVD risk. These effects include HDL anti-inflammatory and anti-oxidant properties, vascular endothelial cell, nitric oxide (NO) production, expressions of inflammatory mediators, and endothelial progenitor cell proliferation [5], [10], [11], [12], [13]. Further, the structure and composition analysis of HDL particles (proteomic/lipidomic), which provide additional insight into the assessment of HDL particles with specific functions, are also discussed (Table 4, Table 5).
Table 1.
Direct measurement of HDL-C mass by precipitation.
Plasma or serum HDL-C concentration is commonly determined by precipitation methods using various reagents. Reagents involve polyanions such as heparin, dextran sulfate, and sodium phosphotungstate, which are used with a divalent cation, such as magnesium, heparin–manganese, or calcium.
Table 2.
Measurement of HDL by density and size.
The classic method for separation of lipoprotein subfractions is by density gradient ultracentrifugation. Ultimately, more convenient methods such as preparative ultracentrifugation or short sequence steps based on ultracentrifugation were developed. HDL subfractions can also be assessed based on size by ND-PAGGE or by charge and size with 2D-PAGGE. Fast liquid chromatography or high liquid chromatography (HPLC) is another method for classifying and quantifying lipoproteins according to particle size. Nuclear magnetic resonance (NMR) spectroscopy is another rapid method of assessing HDL subclasses that emits distinctive NMR signals arising from their unique physical structure.
| Separate HDL by ultracentrifugation | Figure |
|---|---|
| UTC separation | 1 |
| • Flotational analytical ultracentrifugation [47], [48] | |
| • Sequential ultracentrifugation: isopycnic equilibrium method [23] | |
| • Vertical auto profile: zonal ultracentrifugation [51], [53], [54] | |
| Separate HDL by charge | |
| • Capillary isotachophoresis [82], [83] | |
| Separate HDL by size | |
| Gel gradient electrophoresis separation | |
| • Electrophoresis one dimensional gel electrophoresis [72], [73] | 2 |
| • Electrophoresis 2D gel electrophoresis [6], [74] | 3 |
| Fast liquid chromatography | 4 |
| • Ion exchange chromatography [63], [64] | |
| • Gel filtration column [62] | |
| Nuclear magnetic resonance | 5 |
| • Proton NMR measurement [31], [92] | |
| • Diffusion ordered NMR spectroscopy (DOSY NMR) [94] |
Table 3.
HDL functional assay.
These tests explore HDL pleiotropic effects as a biomarker of HDL function as the measurement of HDL–LCAT enzyme activity within the plasma compartment. Another test is measurement of cholesterol efflux. RCT assays enable the measurement of cholesterol from macrophages to feces. Non-radioactive assays aim to quantify lipid poor apoA-I or cholesterol exchange to lipid poor apoA-I. Assays of antioxidant capacity of HDL involve: inflammatory index and monocyte chemotactic assay. Paraoxonase activity and HDL associated myeloperoxidase in vitro assays. Assays for the endothelial effects of HDL on endothelial NO and superoxide production and endothelial repair capacity were also discussed.
| HDL–LCAT functional assay | Figure |
|---|---|
| • LCAT mass: exogenous activity [65], [66], [119] | 6 |
| • LCAT fractional esterification rate: endogenous activity [113], [114] | 7 |
| Cholesterol efflux assays | |
| • Measure of cholesterol efflux [130] | 8 |
| • Fluorescence efflux assay using BODIPY-cholesterol [137], [138] | 8 |
| Non-radioactive assays for cholesterol exchange into lipid poor apoA-I | 12 |
| • Fluorescent apoA-I assay [198], [199] | |
| • TR-FRET version [140] | |
| • Spin-label electron magnetic resonance [142] | |
| Assays of HDL anti-inflammatory and functions of HDL | |
| Assays of anti-inflammatory functions of HDL | |
| • MCP1 production: inflammatory index [145], [146], [153] | 13 |
| • Monocyte chemotactic assay [144] | 14 |
| Assays of antioxidant functions of HDL | |
| • Cell free assay [151], [152], [153] | 15 |
| • HDL associated PON1 assays [146], [156], [157] | 16 |
| • HDL associated MPO assays [12], [141] | 17 |
| Vascular endothelial cell function and HDL | |
| • NO [10], [158], [169], [170], [171] | 18 |
| • eNOS [13], [168], [172] | 19 |
| • ICAM/VCAM [13] | 20 |
| • Endothelial cell [13] | 21 |
Table 4.
Determination of HDL components (proteomics and lipidomics).
Formal proteomic analyses of HDL based on the recognition that HDL contains many proteins, which are performed by using various MS techniques. Once HDL is purified, the wider mass spectrometric technologies that have been employed to directly mapping the HDL proteome include SELDI-TOF, MALDI-TOF, and ESI. Shut gun HDL lipidomic assays all used MS and involve direct/indirect infusion approach. Direct infusion of crude lipid extract into MS includes shotgun approaches: PSI-MS/MS, ESI-MS/MS, and most recently MALDI (QIT)-TOF-MS/MS. Indirect infusion assay separation of lipid species by LC–MS approaches uses two different LC strategies, LC coupled to ESI-MS (LC–MS) or MALDI-MS.
| Proteomic |
|---|
| Shut gun |
| • LC–MS/MS based MALDI-TOF [152] |
| • LC–MS/MS based SELDI-TOF [178] |
| Laser desorption ionization approaches |
| • LC–MS/MS ESI [200], [201] |
| Lipidomic |
| Shotgun: direct infusion |
| • LC–ESI-MS [196] |
| • ESI-MS/MS [192], [194] |
| Indirect infusion |
| • Maldi (QIT)-TOF-MS/MS [187], [196] |
| • Triple quadrupole-MRM-MS [8], [195] |
Table 5.
Summary.
Assays of HDL in human: advantages and limitations. The general principle and choice of isolation/fractionation procedure are listed. Effects and efficiencies of these various biomarkers are presented.
| Method | Subfractions based separation | Advantages | Limitations |
|---|---|---|---|
| Precipitation | ApoB depleted serum | Cost, clinical applicability and high throughput | Proteins and apoE fraction confounders in HDL supernatant |
| Density gradient UTC | Particle density | Gold standard for lipoprotein separation | High ionic strength and centrifugal force, shear forces and salt concentrations that may cause minimal structural disruption to the particles |
| Single step UTC: VAP assay | Particle density | Fast and use from whole plasma just one single predefined, narrow density ranges | Some HDL individual subpopulations cannot be isolated |
| Gradient gel ND-PAGGE | Size based separation | Sensitive approach for quantifying the size distribution of HDL subpopulations | — Non-preparative technique — Unable to separate preβ-2 populations, and applied in specialized laboratory (Fig. 1) — A few data use this technique to study HDL subclasses in predicting CVD — Precast 4% to 30% or 3% to 30% gels are not commercially available or are unreliable [73], [74] |
| 2D-PAGGE | Surface charge and mass | — Allows for the accurate diagnosis of disorders of HDL metabolism — Reproducible, standardized — Provided information about CVD |
— Consists of several variants in the protocol, and is applied in specialized laboratory — Provides little information on other HDL species as apoA-IV |
| Capillary isotachophoresis | Electrophoretic based charge separation | — Easy automation, one line monitoring and rapid separation [202] | — Expensive, limited high-throughput analysis [86] |
| HPLC | HDL particle size | Rapid, accurate, reproducible separation that does not affect lipoprotein composition | — Applied more in specialized or clinical laboratories — Albumin coelution with HDL fractions |
| NMR spectroscopy | NMR signal of purified HDL | — No prior sample manipulation — Suitable for use in high-workload — Efficiently quantifies HDL and lipoproteins |
— Unable to provide HDL chemical compositional information — All lipoprotein classes are not measured with the same degree of accuracy — It's not reported whether preβ-1 HDL subclass is detected — Limited evidence for CVD risk prediction beyond HDL-C |
| Cholesterol efflux | HDL cholesterol removing capacity from plasma/serum | — Gold standard [125] — Inversely associated with CAD and carotid atherosclerosis [126], [128] |
— Represent only a small fraction of macrophage RCT — Lack of standardization, and paradoxal association with CAD [132] — Low throughput and not able to assess the terminal components of the RCT pathway |
| LCAT assay | Fractional esterification rate | Rapid, cost and reproducible | — Require standardization and more larger studies are in need to provide CVD prediction — May not measure the initial esterification rate and may not reflect the turnover of cholesterol [113] |
| HDL inflammatory | In vivo analysis of HDL to suppress LDL-induced chemotaxis | Explore HDL anti-inflammatory function | Lack reproducibility and low throughput |
| HDL antioxidant | Assay of HDL antioxidant enzymes (PON1, MOP, cell free assay) | Explore HDL anti-oxidation function | Lack standardization and limited proof of concept |
| Endothelial assay | — Generation of NO, eNOS — ICAM/VCAM, MCP1, EPC |
Quantify protective HDL endothelial properties | Not available in routine |
| Proteomic | HDL protein content | Identify diversity of HDL proteins and peptides | — Lack technical standardization, and need external validation — Lack of coherence across analysis when compared to the same clinical state |
| Lipidomic | HDL lipid species content | Identify diversity of HDL lipidome | Limited by the available technologies |
2. Controversy surrounding the relationship between HDL-cholesterol measurement and CAD
Epidemiological studies have shown a consistent inverse association between HDL-C concentration and CAD [1]. Clinical trials aimed at raising HDL-C pharmacologically have failed to show clinical benefits in terms of CAD reduction [14], [15], [16], [17]. Moreover, Mendelian randomization studies do not support a causal role for HDL-C in the pathogenesis of CAD [3]. HDL-C level is a static measurement that likely represents a biomarker of cardiovascular health, rather than a risk factor. Recent clinical studies suggest that HDL-C is a helpful biomarker, but functional testing, such as the cholesterol efflux capacity of HDL improves discrimination, independently of HDL-C levels [18]. Despite the coherent epidemiological data suggesting a cardioprotective role for HDL-C, the antiatherogenic properties of different particles that constitute HDL are highly heterogeneous and have yet to be fully quantified and their roles properly evaluated. The cholesterol efflux capacity is likely more reflective of a biologically relevant pathway in the prevention of atherosclerosis and CAD [18].
Thus, a new paradigm states that we need to determine and measure the anti-atherogenic properties of HDL, rather than the cholesterol mass within HDL. Other methods for measuring HDL function, reflecting relevant causal pathways need to be established. Indeed, the cholesterol content of HDL does not represent many biologically important HDL properties that are relevant to CVD (Table 2, Table 3). Methods for measurement of HDL sub-fractions, as well as physiochemical (Table 2) and functional (Table 3) may be more effective in predicting CAD risk than HDL-C [19]. Thus, the concept that HDL-C does not necessarily reflect HDL function, and that HDL function may be a better biomarker of cardiovascular risk must be emphasized. Recently, various alternate HDL phenotypes are being examined as surrogates for the beneficial actions of HDL [5]. The functional heterogeneity of HDL particles makes the identification of effective clinical method to quantify HDL function an ongoing challenge [5], [20], [21]. The pleiotropic HDL biological activities (biomarker) have immediate relevance to understanding the key mechanisms implicated in the pathophysiology of atherosclerosis and thrombosis. Even though some HDL biomarkers, such as cholesterol efflux capacity look promising, it is too early to embrace these measurements in the clinical realm [22] (Table 5).
3. Methods of HDL measurement
In clinical practice, the standard measure of HDL is the cholesterol content in HDL particles after precipitation of apoB-containing lipoproteins (Table 1). More refined techniques to determine HDL-C in serum include ultracentrifugation (UTC) [23], electrophoresis [24], [25], high performance lipoprotein chromatography (HPLC) [26], [27], precipitation-based methods [28], direct measuring methods [29], [30], and nuclear magnetic resonance (NMR) [31] (Table 2).
3.1. Precipitation methods for the separation of HDL
HDL-C is first separated by precipitating apoB containing lipoproteins from serum by using a combination of polyanions, typically such as heparin–MnCl2, dextran sulfate–MgCl2 or phosphotungstate–MgCl2 [32], [33], and a divalent cation, such as magnesium, heparin–manganese, or calcium [34]. Subsequently, HDL is quantified as cholesterol in the supernatant [35]. Polyethylene glycol (PEG) although not a polyanion is also used to precipitate apoB-containing lipoproteins [36], [37]. This method is a convenient, reproducible, and rapid way to extract HDL from patient serum or plasma [38]. Incomplete precipitation of apoB lipoproteins [35] is a major drawback of this method [33], [39]. Supernatant turbidity, observed with hypertriglyceridemia, inflammatory conditions and cryopreservation [29], [40], [41] may lead to discordant results between methods [29], [35], [42]. Commercial immunoprecipitation reagent using specific antibodies directed against HDL particles could be effective in serum with elevated triglycerides [33]. Because of specificity of anti-apoB antibodies, HDL particles will not co-precipitate with apoB, which may be an issue with chemical precipitation methods [33]. Another limitation is that the experimental conditions (pH, ionic strength, temperature) may affect HDL-C measurement [43]. Precipitation methods are less expensive and time consuming than ultracentrifugation [29]. PEG precipitation preserves the integrity of pre-β HDL particles [37], [44]. However, this technique can be contaminated with albumin, and thus unsuitable for proteomic and lipidomic evaluation of HDL. Clinical data shows that chemical based-precipitation methods could generate inaccurate HDL-C results that were found to significantly compromise the accurate classification of CAD risk [45]. Thus underlying the limitations of these methods for measuring HDL-C [20], [46].
3.2. Ultracentrifugation methods
3.3. Density gradient fractionation of plasma lipoproteins
Analytical UTC with density gradient flotation using Schlieren optics was used over 70 years ago to characterize lipoproteins [47] (Table 2). With sequential flotation, lipoproteins could be separated into five major groups such as HDL, LDL, IDL, VLDL and chylomicrons. In this multi-step process plasma based on the initial hydrated density range (1.006 g/mL) is increased to 1.063 g/mL with neutral salts, such as NaBr or KBr [20], [48]. Density gradient UTC approach is based on the isopycnic equilibrium approach developed by Chapman et al. [23] (Table 2). This method that uses a swinging-rotor, plasma or serum is layered on the surface of a NaCl-KBr gradient, constructed by consecutive layering of 4 salt solution distinct densities at + 15 °C. The process involves a single ultra-centrifugal step, facilitating HDL isolation in a nondenatured and nonoxidized state, and avoids major contamination with the plasma proteins > 1.25 g/mL present at the bottom of the tube [20], [49]. The density gradient UTC method reduces centrifugation steps and preparation time [23] necessary for isolating lipoprotein subspecies [50]. An important advantage is that isopycnic UTC method allows isolating LDL subfractions simultaneously with those of HDL.
3.4. Vertical auto profile (VAP)
This assay, which involves a single vertical spin, is an inverted rate zonal density gradient UTC technique that sequentially measures the cholesterol content of all five lipoprotein classes [51], [52] (Table 2). The vertical rotor method or single vertical spin is a modification of the method for single spin separation and analysis of the major classes of lipoproteins [51], [53] described earlier. Unlike most other UTC methods, the VAP method separates all lipoproteins in less than 1 h at 65,000 rpm [52], [54], [55]. This assay is sensitive (requiring < 50 μL of plasma or serum) [48], economical and relatively widely available [20], and provides a reasonable degree of accuracy [52]. VAP was validated in the measurement of HDL2 and HDL3 [56], but limited studies have been performed to compare this method to lipoprotein subfraction measurement techniques, and show that NMR and ND-PAGGE agreed most frequently and VAP had a lesser degree of correlation with these methods [51].
3.5. Limitations of UTC
Shear forces generated by the ultracentrifugal field (57 × 107 g average/min) may strip off proteins associated with lipoproteins; these forces are reduced by the use of a swing rotor [48], [54]. Another biggest drawback of UTC lies in the fact that lipoproteins, especially HDL particles are subjected to high ionic strengths 5 to 20 times above those of human plasma and lymph [57], [58]. These conditions can alter the labile proteins on the HDL surface and cause minor structural disruption to the HDL particles. Accordingly, a proteomic approach showed that high salt can deplete the lipoproteins, especially HDL [58]. Isopycnic gradient UTC procedures in a buffer of deuterium oxide (D2O) and sucrose are suggested over salt [59]. Therefore, apolipoproteins, such as apoA-IV, can be significantly overestimated by this method [59]. Earlier studies showed that the loss of apoA-I from HDL during ultracentrifugal isolation is higher than other precipitation methods [60] by as much as 50% [61]. However, the loss of apolipoproteins from human HDL was not influenced by the effects of rotor configuration, centrifuge tube type, ionic strength or temperature [57]. Furthermore, the plethora of different types of equipment used in laboratories makes conditions extremely difficult to reproduce, and separations are highly dependent on the skills of the operator. While UTC is very useful in research and has been considered as a “gold standard technique” [20] for the separation of lipoproteins and HDL subpopulation, it is not considered practical for routine analytical measurement (Table 5).
3.6. High Performance Liquid Chromatography
HPLC is an analytical and preparative method for classifying and quantifying lipoproteins according to size [62] (Table 2). In this method, lipoproteins separated by permeation columns (exclusion chromatography), the lipid components (mainly cholesterol and triglycerides) are detected enzymatically. Various columns containing nonporous polymer-based gels are used for separation of major classes of human lipoproteins in serum and plasma [63]. For lipoprotein analysis, a Superose 6 column is most frequently used [36], [64], [65], [66]. Lipoprotein separation using HPLC is divided broadly into two categories: HPLC with a gel-filtration column and HPLC with an anion-exchange column (Table 2). Both methods can determine the lipid levels of fractionated serum lipoproteins of small amounts (~ 10 μL) within 30 min. The gel-filtration HPLC method determines the lipid levels of lipoprotein fractions by Gaussian approximation [67]. The HPLC method with an anion-exchange column elutes lipoproteins based on the ion intensity of the lipoprotein particle surface and its hydrophobic properties, and determines the cholesterol levels of separated lipoproteins without overlapping lipoprotein fractions. This method provides lipoprotein separation and larger-sized lipoproteins are eluted earlier (chylomicron, VLDL, IDL, LDL and HDL respectively). HPLC can be contaminated by plasma proteins that co-elute with HDL, especially albumin [26], [68]. Anion-exchange HPLC has been used to characterize various dyslipidemias and the small instrument variation in serum lipoprotein analysis confirms its clinical utility [69]. The rapidity of analysis and reproducible separation of lipoproteins [64] makes HPLC reliable for cholesterol determination in lipoproteins while not affecting lipoprotein composition [27], [70]. HPLC is also less tedious for large studies compared to UTC [57]. Combining HPLC with UTC for the separation of HDL or LDL subfractions is possible given the accuracy of HPLC [71]. This method was also successfully applied to the analysis of the plasma lipoproteins of patients with hyperlipidemia and showed more accuracy than sequential UTC [63] (Table 5).
3.7. Non-denaturing polyacrylamide gradient gel electrophoresis (ND-PAGGE)
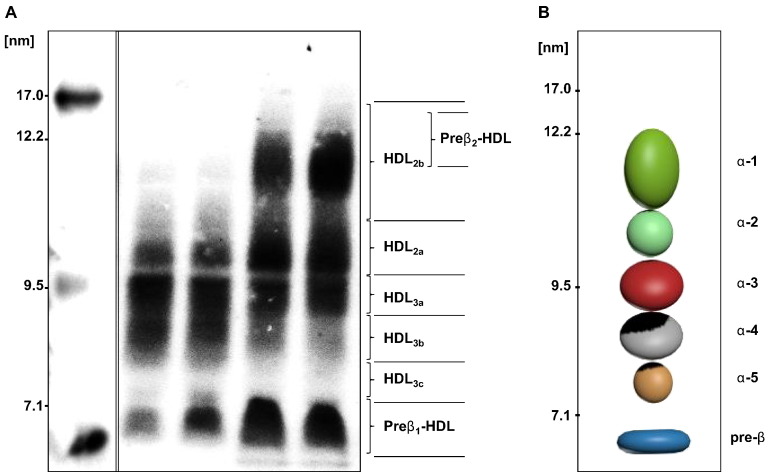
ND-PAGGE was the first method used for size-based separation of lipoprotein subfractions (Table 2) that has been used as a standard laboratory technique for the past three decades [48], [72]. This technique can identify various HDL subspecies separable on the basis of average diameter into 6 distinct subclasses [73]. Patterns of apoA-I containing HDL particles have been generated by ND-PAGGE and detected by apoA-I western blot or radioimmunodetection of apoA-I [20], [73]. Accordingly, a graphical representation of apoA-I containing HDL particles in the plasma is shown in Fig. 1B. Although ND-PAGGE is considered a sensitive and reproducible approach for quantifying the size distribution of HDL subpopulations in conjunction with automated density [74], [75], overall experience shows little additional benefit when compared with the more standard measurement of HDL-C [48]. Data for pre-β1 and HDL2 species from this method showed a significant linear correlation [73] when associated with immunodetection method of 2D-PAGGE. However, this method is labor intensive and standardization between laboratories is relatively poor, even when using commercially available pre-cast gels, limiting its broad application [76].
Fig. 1.
Separation of HDL-species by ND-PAGGE.
The left panel (A) shows the apoA-I containing HDL subpopulations separated by ND-PAGGE (5–35%) of a normolipidemic, healthy male subject (left) and healthy woman subject (right). Plasma samples were transferred to nitrocellulose membrane, and probed by radiolabeled-I125 apoA-I radio imaging. Molecular markers are indicated on the gel. Panel (B) is a schematic diagram of all the apoA-I containing α-HDL species.
3.8. Two-dimensional gradient gel electrophoresis (2D-PAGGE)
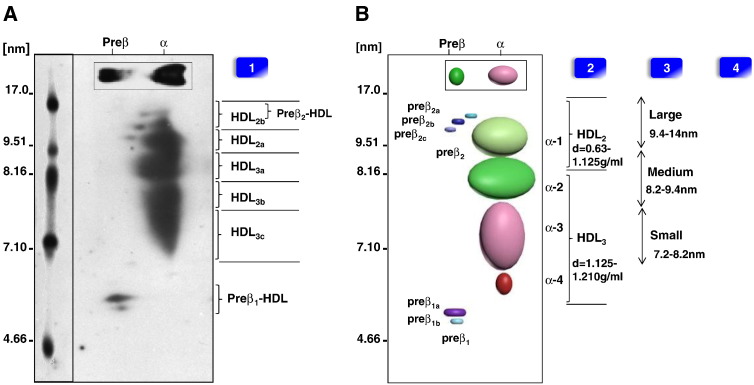
2D-PAGGE is another technique used to separate HDL based on their charge:mass ratio [74]. This method combines ND-PAGGE with agarose gel electrophoresis, which used surface charge density in the first dimension and particle size in the second dimension. Most human HDL particles in plasma are α-HDL observed as an α mobility by gel electrophoresis, or preβ-HDL having preβ electrophoretic mobility by gel electrophoresis [77]. Asztalos et al., by using antibodies to visualize individual protein migration pattern in a native 2D-PAGGE, found apoA-I in at least 11 distinct spots representing various species [74], [78]. Accordingly, a model of HDL subclasses was developed (Fig. 2). Analogous to ND-PAGGE, this method is able to quantify HDL populations in conjunction with automated densitometry for further analysis [75]. The two major HDL α-migrating species are usually described as HDL3 (8.0 nm) and HDL2 (9.2 nm) [73]. HDL subclasses have various nomenclatures, which depend on the HDL separation methods (Fig. 2). As a result, a new HDL nomenclature and classification based on size was recently proposed [20]. The 2D-PAGGE analytical assay describes several novel variants of HDL (Table 5). 2D-PAGGE quantifies preβ-1-HDL [22], [79], [80], but this technique does not correlate precisely with sandwich ELISA designed and may overestimate preβ-1 concentrations in plasma [81]. A variant of this method, using minigels and chemiluminescence has been recently described [6].
Fig. 2.
Separation of HDL-species by 2D-PAGGE and techniques for measurement.
Panel (A) shows the apoA-I containing HDL subpopulations separated by 2D-PAGGE (3–24%) of a normolipidemic, healthy male subject. The plasma was subjected to 2-dimensional agarose/native PAGGE; samples were transferred to nitrocellulose membrane, and probed for radiolabeled-I125 apoA-I. Molecular markers are indicated on the gel. Panel (B) is a schematic diagram of all the apoA-I containing HDL particles. Nomenclatures of HDL subclasses determined by different methods are shown: [1] ND-PAGGE and 2D-PAGGE (mass: charge); [2] UTC (density) separation; and [3] NMR (size), [4] FPLC (size). The HDL particle images were created by using the Autodesk 3ds Max 2014 software.
3.9. Capillary isotachophoresis (cITP)
Analytical free flow capillary isotachophoresis (cITP) is a technique that separates plasma lipoproteins into subfractions according to their electrophoretic charge, and was originally developed by Bottcher et al. [82] and Schmitz et al. [83]. Capillary isotachophoresis is based on the specific staining of lipoproteins with the fluorescent lipophilic dye before separation [83]. This method can separate plasma lipoproteins into 3 major HDL subfractions (fast (f): only α-migrating HDL, intermediate (i): HDL particles rich in cholesterol, apoA-II, apoE and apoC, and slow migrating (s) HDL: consisted of both α and preβ-migrating HDL) according to their electrophoretic mobilities that can be determined as peak areas relative to an internal marker [83]. Also, this technique can separate charge based LDL subfractions in (fast, slow and minor) with a drop of plasma and within minutes [84]. This technique is reported in CAD clinical trial testing drugs that raise HDL-C levels [85], [86], or in evaluating mechanism of apoA-I mimetic peptides in plasma [87], [88] or also in some clinical laboratories as routine analytical assay [82]. Importantly, in patient with hypercholesterolemia the charge-modified LDL subfraction as determined by cITP (fLDL) subfraction is suggested as a possible potential useful biomarker for the risk of CAD [89]. Moreover, in lipid lowering drug therapy, fLDL was effectively reduced by low doses pravastatin and simvastatin [86]. More studies are needed to demonstrate whether cITP (fLDL) is related to clinical outcome and could be a target for therapy [90]. This method is highly sensitive and only a very small amount of sample is needed for the analysis. Unfortunately, the cITP technique has limited potential of quantification since the amount and fluorescence yield of the dye incorporated into lipoproteins is likely to vary with in-between lipoprotein subpopulations due to inter-individual variations in their lipid content [89]. In addition the cITP method determines not only the amount of total lipids but also the composition (mass) of the individual lipoproteins [91]. However, evidence is still lacking because cITP technique is limited in that the instruments for performing the cITP analysis are expensive and not readily available. Moreover, high-throughput analysis is limited by the nature of lipoproteins that they are susceptible to modification [86] (Table 5).
3.10. Nuclear magnetic resonance (NMR)
NMR employs the characteristic lipid methyl signal broadcast by HDL subclasses whose individual amplitude can be accurately measured. This technique uses proton (1H, 13C and 32P) spectroscopy to directly estimate the different sizes of lipoprotein subfractions rapidly [92]. NMR is widely used in specialty lipid reference laboratory [31], [92]. Using NMR, HDL has been classified into large (9.4–14 nm), medium (8.2–9.4 nm) and small (7.3–8.2 nm) HDL subclasses (Fig. 2) [20], [48], [74]. Current NMR methods allow for the separation of 26 subpopulations of HDL [9]. NMR analysis is precise for the determination and quantification of lipoprotein subclass [93]. Diffusion ordered NMR spectroscopy (DOSY) that was recently is used for the measurement of lipoprotein fractions to assess the risk of CVD [94]. However, NMR based techniques use mathematical assumptions that do not take into account variations in the protein and lipid cargoes of the particles [95] (Table 2). In addition, only a modest correlation of selective NMR HDL measures has been found with 2D gel parameters, and the clinical utility of NMR remains uncertain [96]. Whether preβ-1 HDL is detected by NMR [22] has not been reported. The most important limitation of this method is the requirement for specialized equipment not found in routine clinical laboratories.
4. HDL subclass measurement and CVD outcomes
Clinical and epidemiological studies have come to discordant conclusions on the prognostic value of HDL subclasses over the simple measurement of HDL-C to predict CVD risk [2], [13], [16], [47], [95], [97]. A key issue has been the identification of atheroprotective HDL. Multiple studies showed that the levels of HDL2, as assessed by UTC or gradient gel electrophoresis [24], [25], [72], are strongly associated with CHD independently of HDL-C. Mounting evidence suggests that HDL3 particles may constitute an equally valid measurement for assessing CVD risk [98], [99], [100], [101]. Overall, UTC-based methods report that HDL2 and HDL3 subfractions are independently related to CVD risk [102], [103]. Conflicting results between 2D-PAGGE and NMR for the prediction of CVD risk add to the controversy [25], [104]. Movva and Rader [48] attribute this discordance to ethnic variation, different assay methods used, or subfraction heterogeneity. Accumulating data using NMR methods highlighted the importance of taking into account treatment status when considering risk relationship of HDL species [94], [97], [105], [106]. Plasma levels of preβ-1 lipoproteins were found to be significantly higher in patients with ischemic heart disease [107], and an independent predictor of CHD beyond conventional risk factors [37], [108]. HDL-preβ-1 subclass is not found to be always atheroprotective [96], [107], [108]. Thus, considerable controversy remains as to the clinical usefulness of HDL sub-species for the prediction of CVD risk [109].
5. HDL functional assays
HDL function has become an area of interest, as descriptive tests of HDL particles have provided only and sometimes marginal incremental value over HDL-C measurement. This controversy has gained some support in CETP deficient subjects who, despite elevated HDL-C levels, may not be protected from atherosclerosis [110]. The well described apoAIMilano mutation appears to confer protection against atherosclerosis [111]. Despite extreme reduction in HDL-C, less than 50% of Tangier patients develop CAD before age 40 [112]. Thus, a better understanding of HDL functionality may shed light on the pleiotropic cardio-protective effects of HDL.
5.1. Lecithin:cholesterol acyltransferase (LCAT) assay
LCAT mediates the esterification of HDL cholesterol and favors the maintenance of a gradient of free cholesterol between the plasma membrane and HDL particles. An assay for endogenous LCAT activity:fractional esterification rate (FER) measures the esterification of radiolabeled free cholesterol that has been equilibrated with HDL (Table 3) [113], [114]. The FER is calculated as the ratio of radioactive unesterified to radioactive esterified cholesterol per unit of time and is expressed in (%/h) [115], [116]. A variant of this assay is based on the estimation of the radioactivity of free and esterified cholesterol in plasma depleted of apoB [73]. Although the FER reflects particle size distribution in HDL and LDL [48], [113], and is dependent on the metabolic milieu and distribution of lipoproteins [48]. The FER may not accurately reflect the turnover of cholesterol since the preincubation and equilibration phases may alter the substrate properties of the plasma and the radiolabeled exogenous cholesterol may not be in complete equilibrium with endogenous cholesterol [117]. The use of reconstituted HDL particles (proteoliposome) allows a more precise measure of LCAT activity [118] (Table 3). Using reconstituted HDL, LCAT activity is determined by the amount of cholesteryl ester (CE) incorporated into apoA-I-containing proteoliposomes, as a percent of CE divided by total cholesterol [65], [66]. This test correlated best with measures of LCAT cholesterol esterification rate [48], [117]. The proteoliposome substrate used in this assay is relatively easy to make and can be stored frozen for a prolonged period of time, without loss of LCAT activation [119]. Calabresi et al. showed that defective LCAT activity does not result in enhanced atherosclerosis, despite reduced HDL-C levels [120]. Adding to the controversy, LCAT activity from patients undergoing angiography was found to best predict the presence of coronary atherosclerotic lesions [114]. These findings challenge the notion that LCAT is required for effective atheroprotection and suggest that increasing LCAT activity may not be a promising strategy for reducing cardiovascular risk [121]. The controversy of the association between LCAT activity and protection against atherosclerosis remains [122], [123].
5.2. HDL cholesterol efflux assay
HDL removes cholesterol from cells through ABCA1, including macrophages, and this is considered to represent a major atheroprotective function of HDL particles as shown in vivo [124]. In humans, differences in macrophage-specific cholesterol efflux are predominantly due to ABCA1-mediated cholesterol efflux, not to the other transporters [37]. Thus, examination of ABCA1 mediated cholesterol efflux is a plausible target to quantify efflux capacity that could provide great relevance in assessing the risk of atherosclerosis. The mechanisms involved in efflux have attracted a great deal of investigation especially following the recent neutral clinical study data of the Treatment of HDL to Reduce the Incidence of Vascular Events (THRIVE) study [16]. Cholesterol efflux is regulated by various intracellular transporters, such as ATP binding cassette transporter proteins A1(ABCA1) and G1 (ABCG1) and scavenger receptor type B1 (SR-B1). The radioactive assay of cellular cholesterol efflux that explores ABCA1 function is proposed as a “gold standard” that measures the efflux of cholesterol to lipid poor apoA-I [125]. ABCA1 uses apoA-I as the initial cholesterol acceptor and represents the rate-limiting step in the reverse cholesterol transport pathway [99], [126], [127], [128], [129]. The cellular cholesterol efflux assay aims to quantify the rate of cholesterol efflux from cultured cells to an acceptor particle or to plasma (Table 3). Many variations exist and attempts are made to standardize this assay, by posting protocol on-line [130]. Protocols differ by the type of cell, acceptor milieu, efflux times, and the specificity of the transporter examined [96], [116], [126], [129]. Briefly, cholesterol efflux is calculated as the percentage of the cellular (3[H] or 14[C])-cholesterol that appears in the media onto an acceptor per unit of time [131]. A link between the in vitro efflux of cholesterol from macrophages and atherosclerosis has recently been established by clinical studies demonstrating a negative correlation between cholesterol efflux from J774 mouse cells and coronary artery disease (CAD) or carotid atherosclerosis independently of HDL-C mass [18], [126], [128], [129]. It remains to be determined if the HDL cholesterol efflux measurement correlates with clinical outcomes [132], [133]. A recent population-based cohort established inverse association between efflux capacity and the incidence of CAD that persisted after adjustment for traditional risk factors, HDL-C levels and HDL particle concentration [18]; these data should influence future study designs of HDL-modifying drugs.
In fact, the association between enhanced efflux and increase in the incidence of CVD is still fueling discussions as to the pertinence of this biomarker of HDL function. Regardless of the cellular model, it must be emphasized that the use of apoB-depleted serum neglects the contribution of apoB lipoproteins to the cholesterol efflux capacity [134]. Moreover, macrophages can efflux cholesterol not only onto lipid free apoA-I, but also to apoE, and onto nascent HDL particles via the ABCA1 transporter, or onto mature spherical HDL particles via the ABCG1 or SR-BI transporters. These confounders could strongly influence cholesterol efflux capacity of serum independently of HDL composition and functionality. However, it seems paradoxical that lipid free apoA-I would play a similar role in vivo, because lipid free apoA-I state is not usually present to a significant amount in plasma [79], [80]. One explanation is that lipid free apoA-I, generated in vivo through an apoA-I remodeling cycle is rapidly lipidated by ABCA1, or may be incorporated in preexisting plasma HDL [135]. It follows, that the ex-vivo cell-based efflux system may highly reflect total in vivo macrophage cholesterol efflux, and is quite useful for examining the first step in the RCT pathway [79], [135], [136] (Fig. 3). However, this approach does not lend itself easily to the development of a high-throughput assay that can efficiently screen large numbers of specimens [137].
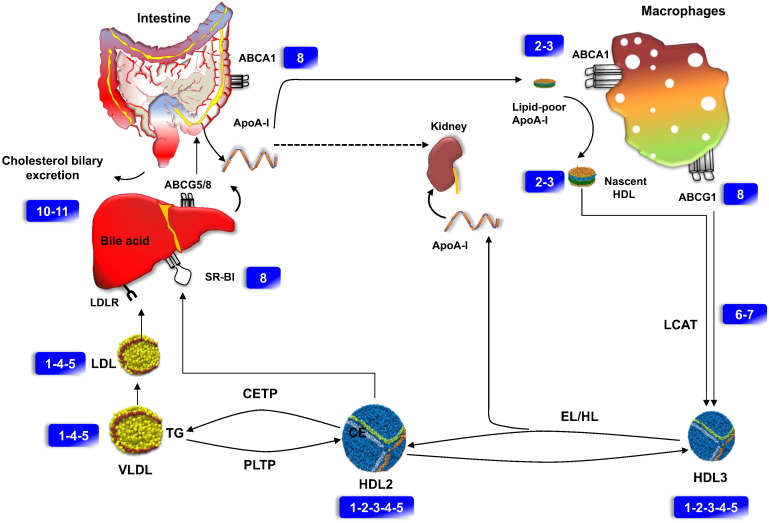
Fig. 3.
Schematic representation of HDL functional assays in RCT pathway. Hepatocytes, enterocytes and macrophages express ATP-binding cassette (ABC) transporter A1 (ABCA1), which effluxes phospholipids and cholesterol (assay 8) and thereby lipidates apoA-I extracellularly (assays 2–3). Effluxed (FC) is modified by the HDL enzyme (LCAT) into (CE) (assays 6–7). The initially smaller HDL3 (assays 1–2–3–4–5) particles grow in size by ongoing lipid efflux, and cholesterol esterification. The resulting HDL2 (assays 1–2–3–4–5) particles deliver lipids to the liver, either directly via SR-BI and indirectly via CETP mediated transfer of CE to VLDL and LDL (assays 1–4–5). The RCT is finalized by the biliary excretion of cholesterol from the liver into the intestine either directly via ABCG5 and ABCG8 to bile acids via the bile salt export pump ABCB11 (assays 10–11). The actions of hepatic lipase (HL), and endothelial lipase (EL) on HDL3, as well as of PLTP on HDL2, liberate lipid-free apoA-I (assays 2–3). Lipid free apoA-I is either used for de novo formation of mature HDL particles or is filtrated through the renal glomerulus for tubular uptake and degradation (dotted arrows). Numbers in rectangles refer to Table 2, Table 3.
Unfortunately, the cholesterol efflux assay has a low throughput as it employs 3[H] cholesterol, has poor dynamic range, fails to measure phospholipid efflux by ABCA1, ignores measurement of hepatic HDL formation and includes limited data linking efflux capacity to coronary disease (Table 5) [137]. To avoid the use of radioisotopes, a fluorescent boron dipyrromethene difluoride cholesterol probe (BODIPY-cholesterol) was used as alternative to label cholesterol [137], [138]. This method showed moderate correlation (0.54) with measurement performed with radiolabeled cholesterol efflux [18]. Although this method provides efficient measurement of efflux when compared with the use of radiolabeled cholesterol, it still requires labeling of cellular sterols [139].
5.3. Non-radioactive assays for cholesterol exchange onto lipid poor apoA-I
These approaches focused on the conformational change in apoA-I protein in HDL biogenesis and remodeling. These techniques quantify HDL-apoA-I exchange between lipid associated and lipid free states, using time-resolved fluorescence resonance energy transfer (TR-FRET) or a discontinuous assay that uses the label-free Epic platform [140]. The TR-FRET assay employs HiLyte Fluor 647-labeled apoA-I with N-terminal biotin bound to streptavidin-terbium. When fluorescent apoA-I was incorporated into HDL, TR-FRET decreased proportionally to the increase in the ratio of lipids to apoA-I. In the Epic assay, biotinylated apoA-I was captured on a streptavidin-coated biosensor. Measured resonant wavelength shift was proportional to the amount of lipids associated with apoA-I, indicating that the assay senses apoA-I lipidation [140]. However, potential background florescence emission was observed when using fluorescent probe [140], [141]. The main disadvantage is that this assay appears to be less sensitive than the approach using FRET [140]. Moreover, the inherent fluorescence of blood plasma and serum limits the clinical utility of this approach to quantifying HDL-apoA-I exchange [140], [141]. A new assay that employs a site-directed spin-label electron paramagnetic resonance was recently described [142], or by sensing hydrophobicity change in POLARIC-labeled apoA-I [139]. These techniques show promise but remain to be validated in large population-based or clinical study samples.
5.4. HDL anti-inflammatory assay
The use of proteomics to probe the complex heterogeneity of HDL has helped uncover over 200 different proteins that reside on HDL particles. Of these, more than two dozens are related to the immune response [143] (Table 3). The anti-inflammatory properties of HDL have been described for some times and can be assessed by several techniques. The monocyte chemotaxis assay assesses the ability of HDL to inhibit LDL oxidation and monocyte chemoattractant protein 1 (MCP1) expression through the HDL inflammatory index in the presence or absence of HDL [144], [145], [146]. A new approach for quantifying the anti-inflammatory response to HDL involves measuring the cytokine response in lipopolysaccharide-activated macrophages [133], [147]. Some studies suggest that the HDL-inflammatory index may constitute an improved biomarker in assessing CVD risk over HDL-C levels [38], [145], [148]. The limited reproducibility of these assays casts doubt on their clinical utility. HDL also suppresses in vitro type I interferon response of macrophages induced by lipopolysaccharide [149]. To date, there has been limited application of these assays outside the research laboratory.
5.5. HDL antioxidant capacity assay
HDL particles contain several enzymes, including phospholipase A2, glutathione peroxidase, paraoxonase-1 (PON1) and myeloperoxidase (MPO) [5], [11], [20], [150]. These enzymes prevent or break down oxidized phospholipids, which prevent the formation of oxidized LDL particles [12], [98], [146]. Assays of HDL oxidation that are discussed include a variety of specific assays (Table 4).
-
1)
Cell free assays of HDL oxidation. The cell-free assay examines the effect of HDL on the production of reactive oxygen species after oxidation and conversion of dichlorodihydrofluorescein diacetate (DCF-DA) to fluorescent signal DCF (2′,7′-dichlorofluorescein) [151]. Direct measurement of ROS after exposure to HDL is reflected by the increased DCF fluorescence, which in turn reflects the oxidative properties of different types of HDL particles that vary in their capacity to engage intrinsic redox cycling [151]. Although this assay yielded data that correlated with a cell-based assay [151], [152], its use is limited by the short shelf life of DCF-DA and interference related to the assay's sensitivity to hemolysis and the presence of metal chelators [22]. A new fluorometric method based on the oxidation of dihydrorhodamine 123 (DHR) by HDL was developed [153]. This test assesses the intrinsic ability of HDL to be oxidized by measuring increasing fluorescence due to DHR oxidation over time. Based on this, the HDL oxidant/antioxidant index was used as the rate of DHR or rhodamine oxidation [154]. The direct comparison of this measurement correlated well with the results obtained by using a validated cell-based assay [152], [153]. However, this assay needs validation to large-scale clinical studies.
-
2)
Paraoxonase (PON1) measurement. PON1 activity can be measured by spectrophotometric methods that can be automated [155]. Serum arylesterase and paraoxonase activities are independently measured respectively by UV spectrophotometry using phenyl acetate (at 270 nm) or paraoxon (at 405 nm) as substrates [156]. Most assays are based on monitoring p-nitrophenol formation from the substrate paraoxon using phenyl acetate or paraoxon as substrates [157]. This assay is based on the chemiluminescence emitted by dichlorofluorescein that quantifies the antioxidation activity of HDL. Increasing evidence from both animal and human studies links low PON1 activity (but increased PON1 mass) to the increased likelihood of CVD [158], [159]. A PON1 activity method measurement has been proposed [146] as a biomarker of HDL functionality in experimental models and in therapeutic interventions in humans (Table 3). However, the correlation of PON1 activity with HDL-C levels in plasma is controversial [160]. Furthermore, PON1 activity was used in apoA-I mimetic drug studies as an indicator of the improvement of HDL antioxidant properties [153]. This enzyme is a promised biomarker of HDL function and cardiovascular risk independently of HDL-C levels. However, various factors should be considered when estimating PON1 activity, including its cardioprotective properties, as well as age, gender, lifestyle, medical conditions and pharmacological agent [161].
-
3)
Myeloperoxidase (MPO) activity measurement. MPO-mediated oxidation is proposed for measuring HDL oxidation (Table 3). Two MPO oxidation products, 3-chlorotyrosine and 3-nitrotyrosine, are quantified by tandem mass spectrometry in plasma and HDL [12]. By using this approach, phagocyte-derived MPO oxidation products might be useful indicators of the risk of CVD [162]. MPO activity can be analyzed by measuring MPO mass in plasma with an automated chemiluminescent microparticle immunoassay [118]. Recent data showed that both MPO and PON1 interact at the same site on HDL, which influences oxidant stress and lipid peroxidation during inflammation [11], [163]. Data among subjects with CVD shows that increased MPO activity was associated with a decrease in HDL functional measures [164]. In this context it is relevant that plasma MPO is elevated in ACS patients [118]. Increasing evidence suggests that MPO is causally linked to atherosclerosis and its measurement may improve CVD risk estimation [141], [150]. Recent studies highlighted the utility of the MPO assay as a biomarker of CVD risk in patients with systemic inflammation [164], [165]. Whether oxidized HDL-proteins by MPO activity are a good marker of dysfunctional HDL and CVD risk remain to be validated in large-scale studies.
5.6. Vascular endothelial eNOS assay
HDL particles and HDL-derived cholesterol have been shown to increase the expression and stability of nitric oxide synthase (eNOS) in vascular endothelial cells (Table 3). These properties of HDL can be examined by measuring NO production by fluorescence in cell culture systems [166]. HDL effects on eNOS activation can be measured by quantifying the ratio of ser1177/thr495 phosphorylation [166], [167], [168]. Data from this assay have consistently demonstrated the ability of HDL to modulate eNOS expression and NO production [10], [11], [158]. An automated method to measure NO production, peripheral tonometry (Endo-PAT), is available [169]. A close correlation exists between the Endo-PAT method and assay of HDL mediated eNOS phosphorylation in healthy children [167]. The validity of this approach is critically dependent on the timing of the measurement as NO dissipates rapidly [169]. Another approach is to measure brachial artery flow-mediated dilatation by using B-mode carotid ultrasound [170], [171]. A test based on electron spin resonance spectroscopy showed a reduced ability of HDL to stimulate endothelial NO production in patients with advanced CHD [13], [172]. This measure is difficult to apply routinely in the clinic because it is subject to high individual variation. The assessment of the endothelial function in the clinic is limited by the complex analytical methods and a high degree of inter- and intra-individual variability.
5.7. Endothelial ICAM/VCAM assay
The expression of intercellular adhesion molecules (ICAMs) and vascular cell adhesion molecule-1 (VCAM-1) that forms vascular endothelial cell in response to an inflammatory stimulus (via nuclear factor κB — NF-κB) can be modulated by HDL [13], [173] (Table 3). The peptides can be measured in plasma by ELISA or gel separation techniques [13], [168] and provide a biomarker of vascular inflammatory state. The effect of HDL on endothelial progenitor cells (EPC) had been examined in an in-vivo system of carotid denudation in the athymic nude mouse. In this system, injured (electrical endothelial denudation) carotid arteries are examined for EPC-mediated endothelial repair (Fig. 4) [174], [175]. The re-endothelialized area can be measured by computer-assisted morphometric analysis. This model has been shown to allow accurate quantification of re-endothelialization [13]. Clinical studies using this method have shown the restoration of endothelial-protective properties of HDL in patient with 2 diabetes mellitus under extended-release niacin therapy [13]. This is a fertile area of research of the biological effects on the vascular endothelium and provides a novel series of potential biomarkers of HDL function.
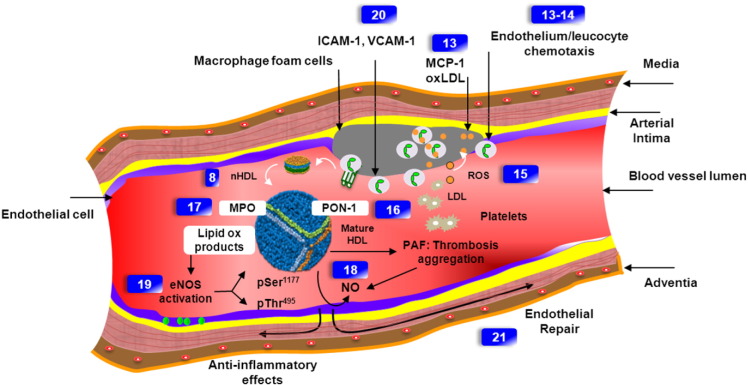
Fig. 4.
Mechanisms of vascular effects of normal HDL and associated functional assays. Circulating monocytes attach to endothelial cells by cell adhesion molecules (assay 20) that are induced in response to inflammatory signals, which is facilitated by endothelial adhesion molecules, including ICAM1/VCAM1 (assay 20). HDL causes membrane-initiated signaling, which stimulates eNOS activity (assay 19). Monocytes migrate through the endothelial layer into the intima, where they differentiate further into macrophages in response to locally produced factors such as monocyte colony-stimulating factor (assays 13–14). The recruited monocytes differentiate into macrophages or dendritic cells in the intima, where they interact with atherogenic lipoproteins (LDL) (assay 13). LDL penetrates into the artery wall where it can adhere to proteoglycans. These interactions are thought to trap the LDL particles and increase their susceptibility to oxidation. Enzymes contributing to LDL oxidation include lipoxygenases, MPO and eNOS that induce NO release in the endothelium (assays 17, 18, 19). HDL-associated PON1 (assay 16) inhibits macrophage cholesterol biosynthesis and enhances HDL-mediated cholesterol efflux. Numbers in rectangles refer to Table 3.
Figure inspired and modified from Bessler et al.
6. Proteomic analysis
Nearly 200 putative proteins have been identified in the HDL proteome, and more than half have been independently confirmed in separate laboratories [176]. Many proteins appear functionally associated with anti-inflammatory, antioxidant or anti-atherogenic properties of HDL [152], [177], suggesting that the HDL proteome offers a biomarker of HDL dysfunction in CVD or ACS due to oxidative stress [44], [173], [178], [179], [180] (Table 4, Table 5). Several techniques are used, including shotgun proteomics. In this approach, HDL proteins are first separated by electrophoresis, digested into peptides, and further separated by liquid chromatography and quantified by tandem mass spectrophotometry MS (LC–MS/MS) (Table 4). This method used electrospray ionization (ESI) [179]. LC–ESI-MS/MS is highly sensitive for the resolution of small peptides (6–20 amino acids long), down to the nano and picomole ranges [178], [181] showing additional protein group in HDL particles from patient with CHD [152], [172]. An additional benefit of ESI is the ability to use lower flow rates (3–6 μL/min) for faster evaporation time and longer measurement time. The LC–ESI-MS/MS has been the most frequently used technique when compared to other MS approaches, such as MALDI-TOF [44], [152], [177], [182], [183]. HDL shotgun proteomic analysis [22] is limited by its semi-quantitative nature, the dependence of the results on the MS technology used and the nature of the initial biological material (serum, plasma, or biologic extract). A degree of variation has been reported across studies with regard to the number and the identity of the proteins associated with HDL [176]. This is likely due to technical differences, experimental design and diagnostic relevance of the biomarker identified, which highlights the need for methodology standardization and external validation [184]. HDL proteomic studies are in an early stage of development and general conclusions about the HDL proteome as a biomarker for CVD are limited by the small number of published studies.
7. HDL lipidome assays
Recently, lipidomic approaches have provided insights into the HDL lipidome with identification of more than 200 individual molecular lipid species in HDL [9], [185]. Various approaches for the characterization of individual molecular species are discussed above (Table 4).
With the development of tandem MS, the analysis ability of MS has greatly improved by using analyzers including quadrupole time-of-flight (QqTOF) [44], [186], [187], or triple quadrupole instrument [101], [188] (Table 4). One of these applications is the ESI MS shotgun lipidomics [189]. Ionization technology ESI was showed to be more convenient for polar lipids and some other less-polar lipids [190], [191]. Shotgun ESI-MS/MS offer advantages, especially in terms of sample throughput, untargeted analysis and coverage of a broad spectrum of lipid species [192]. However the main drawbacks of direct infusion are potential low resolution [193]. For more accurate measurement, the HPLC separation before ESI-MS was introduced [192]. On the other hand, there are several detection modes in the triple quadrupole MS, precursor ion scan (PIN), neutral loss scan (NLS) [194] and multi-reaction monitor (MRM) [8]. The MRM approach demonstrates that 312 lipids could be monitored in 20 min [195]. Instrumental robustness, speed of analysis and sensitivities make targeted approaches more promising for high throughput lipid analysis of complex biological samples [196] (Table 4, Table 5).
8. Summary and perspectives
Various methodologies have been developed for isolation of HDL subfractions, without establishing uniform characterization of the subfraction of HDL (Table 5). The potential of “omics” approaches may provide additional insight into the assessment of HDL particles with specific functions, and might be important in unraveling the controversies surrounding HDL-based therapies. This review highlights the lack of agreement between methods, especially with specimens from patient with CVD. Each technique of HDL quantification raises inherent incompatibilities in the nomenclature of the separated HDL subclasses (Fig. 2). It is critical to develop new metrics to determine whether HDL is cardioprotective in humans. Attempts to harmonize the definition of HDL are an important first step [20]. Providing better biomarkers of HDL function would be important in understanding the current clinical equipoise and the neutrality of clinical benefit for many HDL-C raising therapies.
Transparency document
Transparency document.
Acknowledgments
Supported by grant MOP 15042 from the Canadian Institutes of Health Research. JG holds the McGill/Novartis Chair at McGill University. Anouar Hafiane is supported by a salary Award from the Fonds de Recherche du Québec – Santé.
Footnotes
The Transparency document associated with this article can be found, in the online version.
References
- 1.Boekholdt S.M., Arsenault B.J., Hovingh G.K., Mora S., Pedersen T.R., Larosa J.C., Welch K.M., Amarenco P., Demicco D.A., Tonkin A.M. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128:1504–1512. doi: 10.1161/CIRCULATIONAHA.113.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toth P.P., Barter P.J., Rosenson R.S., Boden W.E., Chapman M.J., Cuchel M., D'Agostino R.B., Sr., Davidson M.H., Davidson W.S., Heinecke J.W. High-density lipoproteins: a consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013;7:484–525. doi: 10.1016/j.jacl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Voight B.F., Peloso G.M., Orho-Melander M., Frikke-Schmidt R., Barbalic M., Jensen M.K., Hindy G., Holm H., Ding E.L., Johnson T. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rader D.J. Spotlight on HDL biology: new insights in metabolism, function, and translation. Cardiovasc. Res. 2014;103:337–340. doi: 10.1093/cvr/cvu164. [DOI] [PubMed] [Google Scholar]
- 5.Hafiane A., Genest J. HDL, atherosclerosis, and emerging therapies. Cholesterol. 2013;2013:891403. doi: 10.1155/2013/891403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman L.A. Native-native 2D gel electrophoresis for HDL subpopulation analysis. Methods Mol. Biol. 2013;1027:353–367. doi: 10.1007/978-1-60327-369-5_17. [DOI] [PubMed] [Google Scholar]
- 7.Sorci-Thomas M.G., Owen J.S., Fulp B., Bhat S., Zhu X., Parks J.S., Shah D., Jerome W.G., Gerelus M., Zabalawi M. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J. Lipid Res. 2012;53:1890–1909. doi: 10.1194/jlr.M026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yassine H., Borges C.R., Schaab M.R., Billheimer D., Stump C., Reaven P., Lau S.S., Nelson R. Mass spectrometric immunoassay and MRM as targeted MS-based quantitative approaches in biomarker development: potential applications to cardiovascular disease and diabetes. Proteomics Clin. Appl. 2013;7:528–540. doi: 10.1002/prca.201200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kontush A., Lhomme M., Chapman M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013;54:2950–2963. doi: 10.1194/jlr.R036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besler C., Luscher T.F., Landmesser U. Molecular mechanisms of vascular effects of high-density lipoprotein: alterations in cardiovascular disease. EMBO Mol. Med. 2012;4:251–268. doi: 10.1002/emmm.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riwanto M., Landmesser U. High density lipoproteins and endothelial functions: mechanistic insights and alterations in cardiovascular disease. J. Lipid Res. 2013;54:3227–3243. doi: 10.1194/jlr.R037762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y., Wu Z., Riwanto M., Gao S., Levison B.S., Gu X., Fu X., Wagner M.A., Besler C., Gerstenecker G. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J. Clin. Invest. 2013;123:3815–3828. doi: 10.1172/JCI67478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorrentino S.A., Besler C., Rohrer L., Meyer M., Heinrich K., Bahlmann F.H., Mueller M., Horvath T., Doerries C., Heinemann M. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121:110–122. doi: 10.1161/CIRCULATIONAHA.108.836346. [DOI] [PubMed] [Google Scholar]
- 14.Barter P. Lessons learned from the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Am. J. Cardiol. 2009;104:10E–15E. doi: 10.1016/j.amjcard.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 15.van der Steeg W.A., Holme I., Boekholdt S.M., Larsen M.L., Lindahl C., Stroes E.S., Tikkanen M.J., Wareham N.J., Faergeman O., Olsson A.G. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J. Am. Coll. Cardiol. 2008;51:634–642. doi: 10.1016/j.jacc.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 16.HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur. Heart J. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer E.J. Effects of cholesteryl ester transfer protein inhibitors on human lipoprotein metabolism: why have they failed in lowering coronary heart disease risk? Curr. Opin. Lipidol. 2013;24:259–264. doi: 10.1097/MOL.0b013e3283612454. [DOI] [PubMed] [Google Scholar]
- 18.Rohatgi A., Khera A., Berry J.D., Givens E.G., Ayers C.R., Wedin K.E., Neeland I.J., Yuhanna I.S., Rader D.R., de Lemos J.A. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenson R.S. Functional assessment of HDL: moving beyond static measures for risk assessment. Cardiovasc. Drugs Ther. 2010;24:71–75. doi: 10.1007/s10557-009-6214-3. [DOI] [PubMed] [Google Scholar]
- 20.Rosenson R.S., Brewer H.B., Jr., Chapman M.J., Fazio S., Hussain M.M., Kontush A., Krauss R.M., Otvos J.D., Remaley A.T., Schaefer E.J. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 2011;57:392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- 21.Degoma E.M., Rader D.J. Novel HDL-directed pharmacotherapeutic strategies. Nat. Rev. Cardiol. 2011;8:266–277. doi: 10.1038/nrcardio.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenson R.S., Brewer H.B., Jr., Ansell B., Barter P., Chapman M.J., Heinecke J.W., Kontush A., Tall A.R., Webb N.R. Translation of high-density lipoprotein function into clinical practice: current prospects and future challenges. Circulation. 2013;128:1256–1267. doi: 10.1161/CIRCULATIONAHA.113.000962. [DOI] [PubMed] [Google Scholar]
- 23.Chapman M.J., Goldstein S., Lagrange D., Laplaud P.M. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 1981;22:339–358. [PubMed] [Google Scholar]
- 24.Johansson J., Carlson L.A., Landou C., Hamsten A. High density lipoproteins and coronary atherosclerosis. A strong inverse relation with the largest particles is confined to normotriglyceridemic patients. Arterioscler. Thromb. 1991;11:174–182. doi: 10.1161/01.atv.11.1.174. [DOI] [PubMed] [Google Scholar]
- 25.Asztalos B.F., Collins D., Horvath K.V., Bloomfield H.E., Robins S.J., Schaefer E.J. Relation of gemfibrozil treatment and high-density lipoprotein subpopulation profile with cardiovascular events in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Metabolism. 2008;57:77–83. doi: 10.1016/j.metabol.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins L.A., Mirza S.P., Kissebah A.H., Olivier M. Integrated approach for the comprehensive characterization of lipoproteins from human plasma using FPLC and nano-HPLC-tandem mass spectrometry. Physiol. Genomics. 2010;40:208–215. doi: 10.1152/physiolgenomics.00136.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marz W., Siekmeier R., Scharnagl H., Seiffert U.B., Gross W. Fast lipoprotein chromatography: new method of analysis for plasma lipoproteins. Clin. Chem. 1993;39:2276–2281. [PubMed] [Google Scholar]
- 28.Warnick G.R., Albers J.J. A comprehensive evaluation of the heparin–manganese precipitation procedure for estimating high density lipoprotein cholesterol. J. Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 29.Warnick G.R., Nauck M., Rifai N. Evolution of methods for measurement of HDL-cholesterol: from ultracentrifugation to homogeneous assays. Clin. Chem. 2001;47:1579–1596. [PubMed] [Google Scholar]
- 30.Miida T., Nishimura K., Okamura T., Hirayama S., Ohmura H., Yoshida H., Miyashita Y., Ai M., Tanaka A., Sumino H. Validation of homogeneous assays for HDL-cholesterol using fresh samples from healthy and diseased subjects. Atherosclerosis. 2014;233:253–259. doi: 10.1016/j.atherosclerosis.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 31.Cole T.G., Contois J.H., Csako G., McConnell J.P., Remaley A.T., Devaraj S., Hoefner D.M., Mallory T., Sethi A.A., Warnick G.R. Association of apolipoprotein B and nuclear magnetic resonance spectroscopy-derived LDL particle number with outcomes in 25 clinical studies: assessment by the AACC Lipoprotein and Vascular Diseases Division Working Group on Best Practices. Clin. Chem. 2013;59:752–770. doi: 10.1373/clinchem.2012.196733. [DOI] [PubMed] [Google Scholar]
- 32.Warnick G.R., Benderson J., Albers J.J. Dextran sulfate–Mg2 + precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin. Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 33.Contois J.H., Albert A.L., Nguyen R.A. Immunoprecipitation of apolipoprotein B-containing lipoproteins for isolation of HDL particles. Clin. Chim. Acta. 2014;436C:348–350. doi: 10.1016/j.cca.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Kimberly M.M., Leary E.T., Cole T.G., Waymack P.P. Selection, validation, standardization, and performance of a designated comparison method for HDL-cholesterol for use in the cholesterol reference method laboratory network. Clin. Chem. 1999;45:1803–1812. [PubMed] [Google Scholar]
- 35.Bachorik P.S., Albers J.J. Precipitation methods for quantification of lipoproteins. Methods Enzymol. 1986;129:78–100. doi: 10.1016/0076-6879(86)29063-1. [DOI] [PubMed] [Google Scholar]
- 36.Jahani M., Huttash R.G., Lacko A.G. A novel chromatographic method for the preparation of high density lipoproteins. Prep. Biochem. 1980;10:431–444. doi: 10.1080/00327488008061741. [DOI] [PubMed] [Google Scholar]
- 37.de la Llera-Moya M., Drazul-Schrader D., Asztalos B.F., Cuchel M., Rader D.J., Rothblat G.H. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler. Thromb. Vasc. Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel P.J., Khera A.V., Jafri K., Wilensky R.L., Rader D.J. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J. Am. Coll. Cardiol. 2011;58:2068–2075. doi: 10.1016/j.jacc.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Warnick G.R., Nguyen T., Albers A.A. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin. Chem. 1985;31:217–222. [PubMed] [Google Scholar]
- 40.Nauck M., Warnick G.R., Rifai N. Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin. Chem. 2002;48:236–254. [PubMed] [Google Scholar]
- 41.Watanabe J., Grijalva V., Hama S., Barbour K., Berger F.G., Navab M., Fogelman A.M., Reddy S.T. Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J. Biol. Chem. 2009;284:18292–18301. doi: 10.1074/jbc.M109.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langlois M.R., Delanghe J.R., De Buyzere M., Rietzschel E., De Bacquer D. Unanswered questions in including HDL-cholesterol in the cardiovascular risk estimation. Is time still on our side? Atherosclerosis. 2013;226:296–298. doi: 10.1016/j.atherosclerosis.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 43.Contois J.H. A Critical Review of LDL Cholesterol and HDL Cholesterol Measurement. Sun Diagnostics, LLC. 2012. http://www.sundiagnostics.us/wp-content/uploads/2012/09/white-paper-a-critical-review-of-hdl-and-ldl-measurement.pdf
- 44.Alwaili K., Bailey D., Awan Z., Bailey S.D., Ruel I., Hafiane A., Krimbou L., Laboissiere S., Genest J. The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim. Biophys. Acta. 2012;1821:405–415. doi: 10.1016/j.bbalip.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Miller W.G., Myers G.L., Sakurabayashi I., Bachmann L.M., Caudill S.P., Dziekonski A., Edwards S., Kimberly M.M., Korzun W.J., Leary E.T. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin. Chem. 2010;56:977–986. doi: 10.1373/clinchem.2009.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaefer E.J., Otokozawa S., Ai M. Limitations of direct methods and the reference method for measuring HDL and LDL cholesterol. Clin. Chem. 2011;57:1081–1083. doi: 10.1373/clinchem.2010.159483. (author reply 1083) [DOI] [PubMed] [Google Scholar]
- 47.De Lalla O.F., Gofman J.W. Ultracentrifugal analysis of serum lipoproteins. Methods Biochem. Anal. 1954;1:459–478. doi: 10.1002/9780470110171.ch16. [DOI] [PubMed] [Google Scholar]
- 48.Movva R., Rader D.J. Laboratory assessment of HDL heterogeneity and function. Clin. Chem. 2008;54:788–800. doi: 10.1373/clinchem.2007.101923. [DOI] [PubMed] [Google Scholar]
- 49.Kontush A., Chantepie S., Chapman M.J. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003;23:1881–1888. doi: 10.1161/01.ATV.0000091338.93223.E8. [DOI] [PubMed] [Google Scholar]
- 50.Guerin M., Lassel T.S., Le Goff W., Farnier M., Chapman M.J. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arterioscler. Thromb. Vasc. Biol. 2000;20:189–197. doi: 10.1161/01.atv.20.1.189. [DOI] [PubMed] [Google Scholar]
- 51.Chung B.H., Wilkinson T., Geer J.C., Segrest J.P. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J. Lipid Res. 1980;21:284–291. [PubMed] [Google Scholar]
- 52.Kulkarni K.R. Cholesterol profile measurement by vertical auto profile method. Clin. Lab. Med. 2006;26:787–802. doi: 10.1016/j.cll.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 53.May H.T., Nelson J.R., Kulkarni K.R., Anderson J.L., Horne B.D., Bair T.L., Muhlestein J.B. A new ratio for better predicting future death/myocardial infarction than standard lipid measurements in women > 50 years undergoing coronary angiography: the apolipoprotein A1 remnant ratio (Apo A1/[VLDL(3) + IDL]) Lipids Health Dis. 2013;12:55. doi: 10.1186/1476-511X-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung B.H., Segrest J.P., Ray M.J., Brunzell J.D., Hokanson J.E., Krauss R.M., Beaudrie K., Cone J.T. Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 1986;128:181–209. doi: 10.1016/0076-6879(86)28068-4. [DOI] [PubMed] [Google Scholar]
- 55.Kulkarni K.R., Marcovina S.M., Krauss R.M., Garber D.W., Glasscock A.M., Segrest J.P. Quantification of HDL2 and HDL3 cholesterol by the Vertical Auto Profile-II (VAP-II) methodology. J. Lipid Res. 1997;38:2353–2364. [PubMed] [Google Scholar]
- 56.Segrest J.P., Cheung M.C., Jones M.K. Volumetric determination of apolipoprotein stoichiometry of circulating HDL subspecies. J. Lipid Res. 2013;54:2733–2744. doi: 10.1194/jlr.M039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kunitake S.T., Kane J.P. Factors affecting the integrity of high density lipoproteins in the ultracentrifuge. J. Lipid Res. 1982;23:936–940. [PubMed] [Google Scholar]
- 58.Davidsson P., Hulthe J., Fagerberg B., Camejo G. Proteomics of apolipoproteins and associated proteins from plasma high-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2010;30:156–163. doi: 10.1161/ATVBAHA.108.179317. [DOI] [PubMed] [Google Scholar]
- 59.Stahlman M., Davidsson P., Kanmert I., Rosengren B., Boren J., Fagerberg B., Camejo G. Proteomics and lipids of lipoproteins isolated at low salt concentrations in D2O/sucrose or in KBr. J. Lipid Res. 2008;49:481–490. doi: 10.1194/jlr.D700025-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Rooke J.A., Skinner E.R. The dissociation of apolipoproteins from rat plasma lipoproteins during isolation by precipitation with polyanions. Int. J. Biochem. 1979;10:329–335. doi: 10.1016/0020-711x(79)90098-3. [DOI] [PubMed] [Google Scholar]
- 61.Fainaru M., Glangeaud M.C., Eisenberg S. Radioimmunoassay of human high density lipoprotein apo-protein A-1. Biochim. Biophys. Acta. 1975;386:432–443. doi: 10.1016/0005-2795(75)90286-x. [DOI] [PubMed] [Google Scholar]
- 62.Hara I., Okazaki M. High-performance liquid chromatography of serum lipoproteins. Methods Enzymol. 1986;129:57–78. doi: 10.1016/0076-6879(86)29062-x. [DOI] [PubMed] [Google Scholar]
- 63.Hirowatari Y., Yoshida H., Kurosawa H., Doumitu K.I., Tada N. Measurement of cholesterol of major serum lipoprotein classes by anion-exchange HPLC with perchlorate ion-containing eluent. J. Lipid Res. 2003;44:1404–1412. doi: 10.1194/jlr.D300003-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Anantharamaiah G.M., Jones J.L., Brouillette C.G., Schmidt C.F., Chung B.H., Hughes T.A., Bhown A.S., Segrest J.P. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J. Biol. Chem. 1985;260:10248–10255. [PubMed] [Google Scholar]
- 65.Awan Z., Bailey D., Hafiane A., Genest J. Acquired severe hypercholesterolemia and hypoalphalipoproteinemia. J. Clin. Lipidol. 2009;3:393–397. doi: 10.1016/j.jacl.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Diditchenko S., Gille A., Pragst I., Stadler D., Waelchli M., Hamilton R., Leis A., Wright S.D. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2013;33:2202–2211. doi: 10.1161/ATVBAHA.113.301981. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida H. Clinical significance of lipoprotein analysis method by HPLC. Rinsho Byori. 2010;58:1093–1098. [PubMed] [Google Scholar]
- 68.Wiesner P., Leidl K., Boettcher A., Schmitz G., Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009;50:574–585. doi: 10.1194/jlr.D800028-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Usui S., Nakamura M., Jitsukata K., Nara M., Hosaki S., Okazaki M. Assessment of between-instrument variations in a HPLC method for serum lipoproteins and its traceability to reference methods for total cholesterol and HDL-cholesterol. Clin. Chem. 2000;46:63–72. [PubMed] [Google Scholar]
- 70.Innis-Whitehouse W., Li X., Brown W.V., Le N.A. An efficient chromatographic system for lipoprotein fractionation using whole plasma. J. Lipid Res. 1998;39:679–690. [PubMed] [Google Scholar]
- 71.Dong J., Guo H., Yang R., Li H., Wang S., Zhang J., Zhou W., Chen W. A novel and precise method for simultaneous measurement of serum HDL and LDL subfractions and lipoprotein (a) cholesterol by ultracentrifugation and high-performance liquid chromatography. Clin. Chim. Acta. 2012;413:1071–1076. doi: 10.1016/j.cca.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 72.Nichols A.V., Krauss R.M., Musliner T.A. Nondenaturing polyacrylamide gradient gel electrophoresis. Methods Enzymol. 1986;128:417–431. doi: 10.1016/0076-6879(86)28084-2. [DOI] [PubMed] [Google Scholar]
- 73.Tian L., Long S., Li C., Liu Y., Chen Y., Zeng Z., Fu M. High-density lipoprotein subclass and particle size in coronary heart disease patients with or without diabetes. Lipids Health Dis. 2012;11:54. doi: 10.1186/1476-511X-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asztalos B.F., Tani M., Schaefer E.J. Metabolic and functional relevance of HDL subspecies. Curr. Opin. Lipidol. 2011;22:176–185. doi: 10.1097/MOL.0b013e3283468061. [DOI] [PubMed] [Google Scholar]
- 75.Bailey D., Jahagirdar R., Gordon A., Hafiane A., Campbell S., Chatur S., Wagner G.S., Hansen H.C., Chiacchia F.S., Johansson J. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J. Am. Coll. Cardiol. 2010;55:2580–2589. doi: 10.1016/j.jacc.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 76.Rainwater D.L., Andres D.W., Ford A.L., Lowe F., Blanche P.J., Krauss R.M. Production of polyacrylamide gradient gels for the electrophoretic resolution of lipoproteins. J. Lipid Res. 1992;33:1876–1881. [PubMed] [Google Scholar]
- 77.Kunitake S.T., La Sala K.J., Kane J.P. Apolipoprotein A-I-containing lipoproteins with pre-beta electrophoretic mobility. J. Lipid Res. 1985;26:549–555. [PubMed] [Google Scholar]
- 78.Asztalos B.F., Schaefer E.J. High-density lipoprotein subpopulations in pathologic conditions. Am. J. Cardiol. 2003;91:12E–17E. doi: 10.1016/s0002-9149(02)03383-0. [DOI] [PubMed] [Google Scholar]
- 79.Miyazaki O., Ogihara J., Fukamachi I., Kasumi T. Evidence for the presence of lipid-free monomolecular apolipoprotein A-1 in plasma. J. Lipid Res. 2014;55:214–225. doi: 10.1194/jlr.M041038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Asztalos B.F., Roheim P.S. Presence and formation of ‘free apolipoprotein A-I-like’ particles in human plasma. Arterioscler. Thromb. Vasc. Biol. 1995;15:1419–1423. doi: 10.1161/01.atv.15.9.1419. [DOI] [PubMed] [Google Scholar]
- 81.Miida T., Miyazaki O., Nakamura Y., Hirayama S., Hanyu O., Fukamachi I., Okada M. Analytical performance of a sandwich enzyme immunoassay for pre beta 1-HDL in stabilized plasma. J. Lipid Res. 2003;44:645–650. doi: 10.1194/jlr.D200025-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Bottcher A., Schlosser J., Kronenberg F., Dieplinger H., Knipping G., Lackner K.J., Schmitz G. Preparative free-solution isotachophoresis for separation of human plasma lipoproteins: apolipoprotein and lipid composition of HDL subfractions. J. Lipid Res. 2000;41:905–915. [PubMed] [Google Scholar]
- 83.Schmitz G., Mollers C., Richter V. Analytical capillary isotachophoresis of human serum lipoproteins. Electrophoresis. 1997;18:1807–1813. doi: 10.1002/elps.1150181015. [DOI] [PubMed] [Google Scholar]
- 84.Zhang B., Maeda N., Okada K., Tatsukawa M., Sawayama Y., Matsunaga A., Kumagai K., Miura S., Nagao T., Hayashi J. Association between fast-migrating low-density lipoprotein subfraction as characterized by capillary isotachophoresis and intima-media thickness of carotid artery. Atherosclerosis. 2006;187:205–212. doi: 10.1016/j.atherosclerosis.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Shimizu T., Miura S., Tanigawa H., Kuwano T., Zhang B., Uehara Y., Saku K. Rosuvastatin activates ATP-binding cassette transporter A1-dependent efflux ex vivo and promotes reverse cholesterol transport in macrophage cells in mice fed a high-fat diet. Arterioscler. Thromb. Vasc. Biol. 2014;34:2246–2253. doi: 10.1161/ATVBAHA.114.303715. [DOI] [PubMed] [Google Scholar]
- 86.Zhang B., Miura S., Yanagi D., Noda K., Nishikawa H., Matsunaga A., Shirai K., Iwata A., Yoshinaga K., Adachi H. Reduction of charge-modified LDL by statin therapy in patients with CHD or CHD risk factors and elevated LDL-C levels: the SPECIAL Study. Atherosclerosis. 2008;201:353–359. doi: 10.1016/j.atherosclerosis.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 87.Iwata A., Miura S., Zhang B., Imaizumi S., Uehara Y., Shiomi M., Saku K. Antiatherogenic effects of newly developed apolipoprotein A-I mimetic peptide/phospholipid complexes against aortic plaque burden in Watanabe-heritable hyperlipidemic rabbits. Atherosclerosis. 2011;218:300–307. doi: 10.1016/j.atherosclerosis.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 88.Zhang B., Miura S., Fan P., Kumagai K., Takeuchi K., Uehara Y., McMahon M., Rye K.A., Saku K. ApoA-I/phosphatidylcholine discs remodels fast-migrating HDL into slow-migrating HDL as characterized by capillary isotachophoresis. Atherosclerosis. 2006;188:95–101. doi: 10.1016/j.atherosclerosis.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 89.Zhang B., Bottcher A., Imaizumi S., Noda K., Schmitz G., Saku K. Relation between charge-based apolipoprotein B-containing lipoprotein subfractions and remnant-like particle cholesterol levels. Atherosclerosis. 2007;191:153–161. doi: 10.1016/j.atherosclerosis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 90.Zhang B., Matsunaga A., Rainwater D.L., Miura S., Noda K., Nishikawa H., Uehara Y., Shirai K., Ogawa M., Saku K. Effects of rosuvastatin on electronegative LDL as characterized by capillary isotachophoresis: the ROSARY Study. J. Lipid Res. 2009;50:1832–1841. doi: 10.1194/jlr.M800523-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schlenck A., Herbeth B., Siest G., Visvikis S. Characterization and quantification of serum lipoprotein subfractions by capillary isotachophoresis: relationships with lipid, apolipoprotein, and lipoprotein levels. J. Lipid Res. 1999;40:2125–2133. [PubMed] [Google Scholar]
- 92.Jeyarajah E.J., Cromwell W.C., Otvos J.D. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Ala-Korpela M., Lankinen N., Salminen A., Suna T., Soininen P., Laatikainen R., Ingman P., Jauhiainen M., Taskinen M.R., Heberger K. The inherent accuracy of 1H NMR spectroscopy to quantify plasma lipoproteins is subclass dependent. Atherosclerosis. 2007;190:352–358. doi: 10.1016/j.atherosclerosis.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 94.Mallol R., Rodriguez M.A., Heras M., Vinaixa M., Plana N., Masana L., Morris G.A., Correig X. Particle size measurement of lipoprotein fractions using diffusion-ordered NMR spectroscopy. Anal. Bioanal. Chem. 2012;402:2407–2415. doi: 10.1007/s00216-011-5705-9. [DOI] [PubMed] [Google Scholar]
- 95.Rye K.A., Barter P.J. Predictive value of different HDL particles for the protection against or risk of coronary heart disease. Biochim. Biophys. Acta. 2012;1821:473–480. doi: 10.1016/j.bbalip.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 96.de la Llera Moya M., McGillicuddy F.C., Hinkle C.C., Byrne M., Joshi M.R., Nguyen V., Tabita-Martinez J., Wolfe M.L., Badellino K., Pruscino L. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012;222:390–394. doi: 10.1016/j.atherosclerosis.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mackey R.H., Greenland P., Goff D.C., Jr., Lloyd-Jones D., Sibley C.T., Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis) J. Am. Coll. Cardiol. 2012;60:508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kontush A., Chapman M.J. Antiatherogenic function of HDL particle subpopulations: focus on antioxidative activities. Curr. Opin. Lipidol. 2010;21:312–318. doi: 10.1097/MOL.0b013e32833bcdc1. [DOI] [PubMed] [Google Scholar]
- 99.Alrasadi K., Awan Z., Alwaili K., Ruel I., Hafiane A., Krimbou L., Genest J. Comparison of treatment of severe high-density lipoprotein cholesterol deficiency in men with daily atorvastatin (20 mg) versus fenofibrate (200 mg) versus extended-release niacin (2 g) Am. J. Cardiol. 2008;102:1341–1347. doi: 10.1016/j.amjcard.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 100.Yu S., Yarnell J.W., Sweetnam P., Bolton C.H. High density lipoprotein subfractions and the risk of coronary heart disease: 9-years follow-up in the Caerphilly Study. Atherosclerosis. 2003;166:331–338. doi: 10.1016/s0021-9150(02)00361-1. [DOI] [PubMed] [Google Scholar]
- 101.Kim D.S., Burt A.A., Rosenthal E.A., Ranchalis J.E., Eintracht J.F., Hatsukami T.S., Furlong C.E., Marcovina S., Albers J.J., Jarvik G.P. HDL-3 is a superior predictor of carotid artery disease in a case–control cohort of 1725 participants. J. Am. Heart Assoc. 2014;3:e000902. doi: 10.1161/JAHA.114.000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Williams P.T., Feldman D.E. Prospective study of coronary heart disease vs. HDL2, HDL3, and other lipoproteins in Gofman's Livermore Cohort. Atherosclerosis. 2011;214:196–202. doi: 10.1016/j.atherosclerosis.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams P.T. Fifty-three year follow-up of coronary heart disease versus HDL2 and other lipoproteins in Gofman's Livermore Cohort. J. Lipid Res. 2012;53:266–272. doi: 10.1194/jlr.M019356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otvos J.D., Collins D., Freedman D.S., Shalaurova I., Schaefer E.J., McNamara J.R., Bloomfield H.E., Robins S.J. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 105.Ballantyne C.M., Miller M., Niesor E.J., Burgess T., Kallend D., Stein E.A. Effect of dalcetrapib plus pravastatin on lipoprotein metabolism and high-density lipoprotein composition and function in dyslipidemic patients: results of a phase IIb dose-ranging study. Am. Heart J. 2012;163:515–521. doi: 10.1016/j.ahj.2011.11.017. (521 e511-513) [DOI] [PubMed] [Google Scholar]
- 106.Swiger K.J., Martin S.S., Blaha M.J., Toth P.P., Nasir K., Michos E.D., Gerstenblith G., Blumenthal R.S., Jones S.R. Narrowing sex differences in lipoprotein cholesterol subclasses following mid-life: the very large database of lipids (VLDL-10B) J. Am. Heart Assoc. 2014;3:e000851. doi: 10.1161/JAHA.114.000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sethi A.A., Sampson M., Warnick R., Muniz N., Vaisman B., Nordestgaard B.G., Tybjaerg-Hansen A., Remaley A.T. High pre-beta1 HDL concentrations and low lecithin: cholesterol acyltransferase activities are strong positive risk markers for ischemic heart disease and independent of HDL-cholesterol. Clin. Chem. 2010;56:1128–1137. doi: 10.1373/clinchem.2009.139931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guey L.T., Pullinger C.R., Ishida B.Y., O'Connor P.M., Zellner C., Francone O.L., Laramie J.M., Naya-Vigne J.M., Siradze K.A., Deedwania P. Relation of increased prebeta-1 high-density lipoprotein levels to risk of coronary heart disease. Am. J. Cardiol. 2011;108:360–366. doi: 10.1016/j.amjcard.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 109.Superko H.R., Pendyala L., Williams P.T., Momary K.M., King S.B., III, Garrett B.C. High-density lipoprotein subclasses and their relationship to cardiovascular disease. J. Clin. Lipidol. 2012;6:496–523. doi: 10.1016/j.jacl.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 110.Chantepie S., Bochem A.E., Chapman M.J., Hovingh G.K., Kontush A. High-density lipoprotein (HDL) particle subpopulations in heterozygous cholesteryl ester transfer protein (CETP) deficiency: maintenance of antioxidative activity. PLoS ONE. 2012;7:e49336. doi: 10.1371/journal.pone.0049336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ibanez B., Giannarelli C., Cimmino G., Santos-Gallego C.G., Alique M., Pinero A., Vilahur G., Fuster V., Badimon L., Badimon J.J. Recombinant HDL(Milano) exerts greater anti-inflammatory and plaque stabilizing properties than HDL(wild-type) Atherosclerosis. 2012;220:72–77. doi: 10.1016/j.atherosclerosis.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 112.Serfaty-Lacrosniere C., Civeira F., Lanzberg A., Isaia P., Berg J., Janus E.D., Smith M.P., Jr., Pritchard P.H., Frohlich J., Lees R.S. Homozygous Tangier disease and cardiovascular disease. Atherosclerosis. 1994;107:85–98. doi: 10.1016/0021-9150(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 113.Dobiasova M., Frohlich J. Measurement of fractional esterification rate of cholesterol in plasma depleted of apoprotein B containing lipoprotein: methods and normal values. Physiol. Res. 1996;45:65–73. [PubMed] [Google Scholar]
- 114.Frohlich J., Dobiasova M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin. Chem. 2003;49:1873–1880. doi: 10.1373/clinchem.2003.022558. [DOI] [PubMed] [Google Scholar]
- 115.Dobiasova M., Adler L., Ohta T., Frohlich J. Effect of labeling of plasma lipoproteins with [(3)H]cholesterol on values of esterification rate of cholesterol in apolipoprotein B-depleted plasma. J. Lipid Res. 2000;41:1356–1357. [PubMed] [Google Scholar]
- 116.Hafiane A., Bielicki J.K., Johansson J.O., Genest J. Apolipoprotein E derived HDL mimetic peptide ATI-5261 promotes nascent HDL formation and reverse cholesterol transport in vitro. Biochim. Biophys. Acta. 2014;1842:1498–1512. doi: 10.1016/j.bbalip.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 117.Albers J.J., Chen C.H., Adolphson J.L. Lecithin:cholesterol acyltransferase (LCAT) mass; its relationship to LCAT activity and cholesterol esterification rate. J. Lipid Res. 1981;22:1206–1213. [PubMed] [Google Scholar]
- 118.Dullaart R.P., Tietge U.J., Kwakernaak A.J., Dikkeschei B.D., Perton F., Tio R.A. Alterations in plasma lecithin:cholesterol acyltransferase and myeloperoxidase in acute myocardial infarction: implications for cardiac outcome. Atherosclerosis. 2014;234:185–192. doi: 10.1016/j.atherosclerosis.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 119.Vaisman B.L., Remaley A.T. Measurement of lecithin–cholesterol acyltransferase activity with the use of a peptide–proteoliposome substrate. Methods Mol. Biol. 2013;1027:343–352. doi: 10.1007/978-1-60327-369-5_16. [DOI] [PubMed] [Google Scholar]
- 120.Calabresi L., Gomaraschi M., Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler. Thromb. Vasc. Biol. 2003;23:1724–1731. doi: 10.1161/01.ATV.0000094961.74697.54. [DOI] [PubMed] [Google Scholar]
- 121.Calabresi L., Franceschini G. Lecithin:cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc. Med. 2010;20:50–53. doi: 10.1016/j.tcm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 122.Kunnen S., Van Eck M. Lecithin:cholesterol acyltransferase: old friend or foe in atherosclerosis? J. Lipid Res. 2012;53:1783–1799. doi: 10.1194/jlr.R024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oldoni F., Sinke R.J., Kuivenhoven J.A. Mendelian disorders of high-density lipoprotein metabolism. Circ. Res. 2014;114:124–142. doi: 10.1161/CIRCRESAHA.113.300634. [DOI] [PubMed] [Google Scholar]
- 124.Rader D.J., Alexander E.T., Weibel G.L., Billheimer J., Rothblat G.H. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009;50:S189–194. doi: 10.1194/jlr.R800088-JLR200. (Suppl.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lawn R.M., Wade D.P., Garvin M.R., Wang X., Schwartz K., Porter J.G., Seilhamer J.J., Vaughan A.M., Oram J.F. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Invest. 1999;104:R25–31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khera A.V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M.F., Jafri K., French B.C., Phillips J.A., Mucksavage M.L., Wilensky R.L. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamamoto S., Yancey P.G., Ikizler T.A., Jerome W.G., Kaseda R., Cox B., Bian A., Shintani A., Fogo A.B., Linton M.F. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J. Am. Coll. Cardiol. 2012;60:2372–2379. doi: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Doonan R.J., Hafiane A., Lai C., Veinot J.P., Genest J., Daskalopoulou S.S. Cholesterol efflux capacity, carotid atherosclerosis, and cerebrovascular symptomatology. Arterioscler. Thromb. Vasc. Biol. 2014;34:921–926. doi: 10.1161/ATVBAHA.113.302590. [DOI] [PubMed] [Google Scholar]
- 129.Hafiane A., Jabor B., Ruel I., Ling J., Genest J. High-density lipoprotein mediated cellular cholesterol efflux in acute coronary syndromes. Am. J. Cardiol. 2014;113:249–255. doi: 10.1016/j.amjcard.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 130.Low H., Hoang A., Sviridov D. Cholesterol efflux assay. J. Vis. Exp. 2012:e3810. doi: 10.3791/3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rothblat G.H., de la Llera-Moya M., Favari E., Yancey P.G., Kellner-Weibel G. Cellular cholesterol flux studies: methodological considerations. Atherosclerosis. 2002;163:1–8. doi: 10.1016/s0021-9150(01)00713-4. [DOI] [PubMed] [Google Scholar]
- 132.Li X.M., Tang W.H., Mosior M.K., Huang Y., Wu Y., Matter W., Gao V., Schmitt D., Didonato J.A., Fisher E.A. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler. Thromb. Vasc. Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eckardstein A. Tachometer for reverse cholesterol transport? J. Am. Heart Assoc. 2012;1:e003723. doi: 10.1161/JAHA.112.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoang A., Drew B.G., Low H., Remaley A.T., Nestel P., Kingwell B.A., Sviridov D. Mechanism of cholesterol efflux in humans after infusion of reconstituted high-density lipoprotein. Eur. Heart J. 2012;33:657–665. doi: 10.1093/eurheartj/ehr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barrans A., Collet X., Barbaras R., Jaspard B., Manent J., Vieu C., Chap H., Perret B. Hepatic lipase induces the formation of pre-beta 1 high density lipoprotein (HDL) from triacylglycerol-rich HDL2. A study comparing liver perfusion to in vitro incubation with lipases. J. Biol. Chem. 1994;269:11572–11577. [PubMed] [Google Scholar]
- 136.Denis M., Haidar B., Marcil M., Bouvier M., Krimbou L., Genest J. Characterization of oligomeric human ATP binding cassette transporter A1. Potential implications for determining the structure of nascent high density lipoprotein particles. J. Biol. Chem. 2004;279:41529–41536. doi: 10.1074/jbc.M406881200. [DOI] [PubMed] [Google Scholar]
- 137.Sankaranarayanan S., Kellner-Weibel G., de la Llera-Moya M., Phillips M.C., Asztalos B.F., Bittman R., Rothblat G.H. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J. Lipid Res. 2011;52:2332–2340. doi: 10.1194/jlr.D018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Solanko L.M., Honigmann A., Midtiby H.S., Lund F.W., Brewer J.R., Dekaris V., Bittman R., Eggeling C., Wustner D. Membrane orientation and lateral diffusion of BODIPY-cholesterol as a function of probe structure. Biophys. J. 2013;105:2082–2092. doi: 10.1016/j.bpj.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Omura R., Nagao K., Kobayashi N., Ueda K., Saito H. Direct detection of ABCA1-dependent HDL formation based on lipidation-induced hydrophobicity change in apoA-I. J. Lipid Res. 2014;55:2423–2431. doi: 10.1194/jlr.D049445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Niedziela-Majka A., Lad L., Chisholm J.W., Lagpacan L., Schwartz K., Hung M., Jin D., Fung W., Brendza K.M., Liu X. Lipid-sensing high-throughput ApoA-I assays. J. Biomol. Screen. 2012;17:1050–1061. doi: 10.1177/1087057112451923. [DOI] [PubMed] [Google Scholar]
- 141.Cavigiolio G., Geier E.G., Shao B., Heinecke J.W., Oda M.N. Exchange of apolipoprotein A-I between lipid-associated and lipid-free states: a potential target for oxidative generation of dysfunctional high density lipoproteins. J. Biol. Chem. 2010;285:18847–18857. doi: 10.1074/jbc.M109.098434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Borja M.S., Zhao L., Hammerson B., Tang C., Yang R., Carson N., Fernando G., Liu X., Budamagunta M.S., Genest J. HDL-apoA-I exchange: rapid detection and association with atherosclerosis. PLoS ONE. 2013;8:e71541. doi: 10.1371/journal.pone.0071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vaisar T., Pennathur S., Green P.S., Gharib S.A., Hoofnagle A.N., Cheung M.C., Byun J., Vuletic S., Kassim S., Singh P. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Navab M., Hama S.Y., Anantharamaiah G.M., Hassan K., Hough G.P., Watson A.D., Reddy S.T., Sevanian A., Fonarow G.C., Fogelman A.M. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J. Lipid Res. 2000;41:1495–1508. [PubMed] [Google Scholar]
- 145.Ansell B.J., Navab M., Hama S., Kamranpour N., Fonarow G., Hough G., Rahmani S., Mottahedeh R., Dave R., Reddy S.T. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–2756. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 146.Breton C.V., Yin F., Wang X., Avol E., Gilliland F.D., Araujo J.A. HDL anti-oxidant function associates with LDL level in young adults. Atherosclerosis. 2014;232:165–170. doi: 10.1016/j.atherosclerosis.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.White C.R., Smythies L.E., Crossman D.K., Palgunachari M.N., Anantharamaiah G.M., Datta G. Regulation of pattern recognition receptors by the apolipoprotein A-I mimetic peptide 4F. Arterioscler. Thromb. Vasc. Biol. 2012;32:2631–2639. doi: 10.1161/ATVBAHA.112.300167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Navab M., Reddy S.T., Van Lenten B.J., Fogelman A.M. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011;8:222–232. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 149.Suzuki M., Pritchard D.K., Becker L., Hoofnagle A.N., Tanimura N., Bammler T.K., Beyer R.P., Bumgarner R., Vaisar T., de Beer M.C. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation. 2010;122:1919–1927. doi: 10.1161/CIRCULATIONAHA.110.961193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schindhelm R.K., van der Zwan L.P., Teerlink T., Scheffer P.G. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin. Chem. 2009;55:1462–1470. doi: 10.1373/clinchem.2009.126029. [DOI] [PubMed] [Google Scholar]
- 151.Navab M., Hama S.Y., Hough G.P., Subbanagounder G., Reddy S.T., Fogelman A.M. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J. Lipid Res. 2001;42:1308–1317. [PubMed] [Google Scholar]
- 152.Kelesidis T., Currier J.S., Huynh D., Meriwether D., Charles-Schoeman C., Reddy S.T., Fogelman A.M., Navab M., Yang O.O. A biochemical fluorometric method for assessing the oxidative properties of HDL. J. Lipid Res. 2011;52:2341–2351. doi: 10.1194/jlr.D018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Watson C.E., Weissbach N., Kjems L., Ayalasomayajula S., Zhang Y., Chang I., Navab M., Hama S., Hough G., Reddy S.T. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 2011;52:361–373. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kelesidis T., Reddy S.T., Huynh D., Meriwether D., Fogelman A.M., Navab M., Yang O.O. Effects of lipid–probe interactions in biochemical fluorometric methods that assess HDL redox activity. Lipids Health Dis. 2012;11:87. doi: 10.1186/1476-511X-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Browne R.W., Koury S.T., Marion S., Wilding G., Muti P., Trevisan M. Accuracy and biological variation of human serum paraoxonase 1 activity and polymorphism (Q192R) by kinetic enzyme assay. Clin. Chem. 2007;53:310–317. doi: 10.1373/clinchem.2006.074559. [DOI] [PubMed] [Google Scholar]
- 156.Bhattacharyya T., Nicholls S.J., Topol E.J., Zhang R., Yang X., Schmitt D., Fu X., Shao M., Brennan D.M., Ellis S.G. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yin F., Lawal A., Ricks J., Fox J.R., Larson T., Navab M., Fogelman A.M., Rosenfeld M.E., Araujo J.A. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2013;33:1153–1161. doi: 10.1161/ATVBAHA.112.300552. [DOI] [PubMed] [Google Scholar]
- 158.Besler C., Heinrich K., Rohrer L., Doerries C., Riwanto M., Shih D.M., Chroni A., Yonekawa K., Stein S., Schaefer N. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Invest. 2011;121:2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gugliucci A., Menini T. Paraoxonase 1 and HDL maturation. Clin Chim Acta. 2015;439:5–13. doi: 10.1016/j.cca.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 160.Razavi A.E., Ani M., Pourfarzam M., Naderi G.A. Associations between high density lipoprotein mean particle size and serum paraoxonase-1 activity. J. Res. Med. Sci. 2012;17:1020–1026. [PMC free article] [PubMed] [Google Scholar]
- 161.Soran H., Younis N.N., Charlton-Menys V., Durrington P. Variation in paraoxonase-1 activity and atherosclerosis. Curr. Opin. Lipidol. 2009;20:265–274. doi: 10.1097/MOL.0b013e32832ec141. [DOI] [PubMed] [Google Scholar]
- 162.Shao B., Tang C., Sinha A., Mayer P.S., Davenport G.D., Brot N., Oda M.N., Zhao X.Q., Heinecke J.W. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ. Res. 2014;114:1733–1742. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McMillen T.S., Heinecke J.W., LeBoeuf R.C. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111:2798–2804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 164.Charles-Schoeman C., Lee Y.Y., Grijalva V., Amjadi S., FitzGerald J., Ranganath V.K., Taylor M., McMahon M., Paulus H.E., Reddy S.T. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2012;71:1157–1162. doi: 10.1136/annrheumdis-2011-200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shao B., Pennathur S., Heinecke J.W. Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J. Biol. Chem. 2012;287:6375–6386. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gomaraschi M., Ossoli A., Pozzi S., Nilsson P., Cefalu A.B., Averna M., Kuivenhoven J.A., Hovingh G.K., Veglia F., Franceschini G. eNOS activation by HDL is impaired in genetic CETP deficiency. PLoS ONE. 2014;9:e95925. doi: 10.1371/journal.pone.0095925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Muller U., Matsuo Y., Lauber M., Walther C., Oberbach A., Schuler G., Adams V. Correlation between endothelial function measured by finger plethysmography in children and HDL-mediated eNOS activation — a preliminary study. Metabolism. 2013;62:634–637. doi: 10.1016/j.metabol.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 168.Morawietz H., Goettsch W., Brux M., Reimann M., Bornstein S.R., Julius U., Ziemssen T. Lipoprotein apheresis of hypercholesterolemic patients mediates vasoprotective gene expression in human endothelial cells. Atheroscler. Suppl. 2013;14:107–113. doi: 10.1016/j.atherosclerosissup.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 169.Bruyndonckx L., Radtke T., Eser P., Vrints C.J., Ramet J., Wilhelm M., Conraads V.M. Methodological considerations and practical recommendations for the application of peripheral arterial tonometry in children and adolescents. Int. J. Cardiol. 2013;168:3183–3190. doi: 10.1016/j.ijcard.2013.07.236. [DOI] [PubMed] [Google Scholar]
- 170.Kastelein J.J., Duivenvoorden R., Deanfield J., de Groot E., Jukema J.W., Kaski J.C., Munzel T., Taddei S., Lehnert V., Burgess T. Rationale and design of dal-VESSEL: a study to assess the safety and efficacy of dalcetrapib on endothelial function using brachial artery flow-mediated vasodilatation. Curr. Med. Res. Opin. 2011;27:141–150. doi: 10.1185/03007995.2010.536207. [DOI] [PubMed] [Google Scholar]
- 171.Luscher T.F., Taddei S., Kaski J.C., Jukema J.W., Kallend D., Munzel T., Kastelein J.J., Deanfield J.E. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur. Heart J. 2012;33:857–865. doi: 10.1093/eurheartj/ehs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Adams V., Besler C., Fischer T., Riwanto M., Noack F., Hollriegel R., Oberbach A., Jehmlich N., Volker U., Winzer E.B. Exercise training in patients with chronic heart failure promotes restoration of high-density lipoprotein functional properties. Circ. Res. 2013;113:1345–1355. doi: 10.1161/CIRCRESAHA.113.301684. [DOI] [PubMed] [Google Scholar]
- 173.Riwanto M., Rohrer L., Roschitzki B., Besler C., Mocharla P., Mueller M., Perisa D., Heinrich K., Altwegg L., von Eckardstein A. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein–proteome remodeling. Circulation. 2013;127:891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 174.Brouchet L., Krust A., Dupont S., Chambon P., Bayard F., Arnal J.F. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103:423–428. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- 175.Lindner V., Fingerle J., Reidy M.A. Mouse model of arterial injury. Circ. Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- 176.Shah A.S., Tan L., Long J.L., Davidson W.S. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 2013;54:2575–2585. doi: 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Davidson W.S., Silva R.A., Chantepie S., Lagor W.R., Chapman M.J., Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 2009;29:870–876. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Heinecke J.W. The HDL proteome: a marker–and perhaps mediator–of coronary artery disease. J. Lipid Res. 2009;50:S167–171. doi: 10.1194/jlr.R800097-JLR200. (Suppl.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vaisar T. Proteomics investigations of HDL: challenges and promise. Curr. Vasc. Pharmacol. 2012;10:410–421. doi: 10.2174/157016112800812755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vaisar T., Mayer P., Nilsson E., Zhao X.Q., Knopp R., Prazen B.J. HDL in humans with cardiovascular disease exhibits a proteomic signature. Clin. Chim. Acta. 2010;411:972–979. doi: 10.1016/j.cca.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shevchenko A., Tomas H., Havlis J., Olsen J.V., Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 182.Gordon S.M., Deng J., Lu L.J., Davidson W.S. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J. Proteome Res. 2010;9:5239–5249. doi: 10.1021/pr100520x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Holzer M., Wolf P., Curcic S., Birner-Gruenberger R., Weger W., Inzinger M., El-Gamal D., Wadsack C., Heinemann A., Marsche G. Psoriasis alters HDL composition and cholesterol efflux capacity. J. Lipid Res. 2012;53:1618–1624. doi: 10.1194/jlr.M027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Burillo E., Vazquez J., Jorge I. Quantitative proteomics analysis of high-density lipoproteins by stable 18O-isotope labeling. Methods Mol. Biol. 2013;1000:139–156. doi: 10.1007/978-1-62703-405-0_11. [DOI] [PubMed] [Google Scholar]
- 185.Yetukuri L., Soderlund S., Koivuniemi A., Seppanen-Laakso T., Niemela P.S., Hyvonen M., Taskinen M.R., Vattulainen I., Jauhiainen M., Oresic M. Composition and lipid spatial distribution of HDL particles in subjects with low and high HDL-cholesterol. J. Lipid Res. 2010;51:2341–2351. doi: 10.1194/jlr.M006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pluskal T., Castillo S., Villar-Briones A., Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinf. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Koy C., Mikkat S., Raptakis E., Sutton C., Resch M., Tanaka K., Glocker M.O. Matrix-assisted laser desorption/ionization- quadrupole ion trap-time of flight mass spectrometry sequencing resolves structures of unidentified peptides obtained by in-gel tryptic digestion of haptoglobin derivatives from human plasma proteomes. Proteomics. 2003;3:851–858. doi: 10.1002/pmic.200300381. [DOI] [PubMed] [Google Scholar]
- 188.Simons B., Kauhanen D., Sylvänne T., Tarasov K., Duchoslav E., Ekroos K. Shotgun lipidomics by sequential precursor ion fragmentation on a hybrid quadrupole time-of-flight mass spectrometer. Metabolites. 2012;2:195–213. doi: 10.3390/metabo2010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li L., Han J., Wang Z., Liu J., Wei J., Xiong S., Zhao Z. Mass spectrometry methodology in lipid analysis. Int. J. Mol. Sci. 2014;15:10492–10507. doi: 10.3390/ijms150610492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Han X., Gross R.W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J. Lipid Res. 2003;44:1071–1079. doi: 10.1194/jlr.R300004-JLR200. [DOI] [PubMed] [Google Scholar]
- 191.Blanksby S.J., Mitchell T.W. Advances in mass spectrometry for lipidomics. Annu Rev Anal Chem (Palo Alto, Calif) 2010;3:433–465. doi: 10.1146/annurev.anchem.111808.073705. [DOI] [PubMed] [Google Scholar]
- 192.Scherer M., Bottcher A., Liebisch G. Lipid profiling of lipoproteins by electrospray ionization tandem mass spectrometry. Biochim. Biophys. Acta. 2011;1811:918–924. doi: 10.1016/j.bbalip.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 193.Retra K., Bleijerveld O.B., van Gestel R.A., Tielens A.G., van Hellemond J.J., Brouwers J.F. A simple and universal method for the separation and identification of phospholipid molecular species. Rapid Commun. Mass Spectrom. 2008;22:1853–1862. doi: 10.1002/rcm.3562. [DOI] [PubMed] [Google Scholar]
- 194.Isaac G. Electrospray ionization tandem mass spectrometry (ESI-MS/MS)-based shotgun lipidomics. Methods Mol. Biol. 2011;708:259–275. doi: 10.1007/978-1-61737-985-7_16. [DOI] [PubMed] [Google Scholar]
- 195.Weir J.M., Wong G., Barlow C.K., Greeve M.A., Kowalczyk A., Almasy L., Comuzzie A.G., Mahaney M.C., Jowett J.B., Shaw J. Plasma lipid profiling in a large population-based cohort. J. Lipid Res. 2013;54:2898–2908. doi: 10.1194/jlr.P035808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stubiger G., Aldover-Macasaet E., Bicker W., Sobal G., Willfort-Ehringer A., Pock K., Bochkov V., Widhalm K., Belgacem O. Targeted profiling of atherogenic phospholipids in human plasma and lipoproteins of hyperlipidemic patients using MALDI-QIT-TOF-MS/MS. Atherosclerosis. 2012;224:177–186. doi: 10.1016/j.atherosclerosis.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 197.Lackner K.J., Schmitz G. beta-VLDL of patients with type III hyperlipoproteinemia interferes with homogeneous determination of HDL-cholesterol based on polyethylene glycol-modified enzymes. Clin. Chem. 1998;44:2546–2548. [PubMed] [Google Scholar]
- 198.Degorce F., Card A., Soh S., Trinquet E., Knapik G.P., Xie B. HTRF: a technology tailored for drug discovery — a review of theoretical aspects and recent applications. Curr. Chem. Genomics. 2009;3:22–32. doi: 10.2174/1875397300903010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jia Y., Quinn C.M., Gagnon A.I., Talanian R. Homogeneous time-resolved fluorescence and its applications for kinase assays in drug discovery. Anal. Biochem. 2006;356:273–281. doi: 10.1016/j.ab.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 200.Fournier M.L., Gilmore J.M., Martin-Brown S.A., Washburn M.P. Multidimensional separations-based shotgun proteomics. Chem. Rev. 2007;107:3654–3686. doi: 10.1021/cr068279a. [DOI] [PubMed] [Google Scholar]
- 201.Lee C.Y., Lesimple A., Larsen A., Mamer O., Genest J. ESI-MS quantitation of increased sphingomyelin in Niemann–Pick disease type B HDL. J. Lipid Res. 2005;46:1213–1228. doi: 10.1194/jlr.M500011-JLR200. [DOI] [PubMed] [Google Scholar]
- 202.Yamaguchi Y., Kunitomo M., Haginaka J. Assay methods of modified lipoproteins in plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002;781:313–330. doi: 10.1016/s1570-0232(02)00433-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.