Abstract
Background
Calcifediol (25D) availability is crucial for calcitriol (1,25D) synthesis, but regulation of vitamin D hydroxylases is majorly responsible for 1,25D synthesis. The net efficiency of vitamin D hydroxylases might be informative. We assume that the ratio between calcitriol and calcifediol (25D/1,25D) serum concentrations could suggest the vitamin D hydroxylation efficiency.
Methods
We evaluated 25D/1,25D in different patient populations: hemodialysis (HD, n = 76), CKD stage 2–5 (n = 111), renal transplant (TX, n = 135), patients with no renal disease (No-CKD, n = 290), and primary hyperparathyroidism (PHP, n = 20).
Results
The geometric mean of 1,25D/25D (pg/ng) averaged 1.11 (HD), 1.36 (CKD), 1.77 (TX), 2.22 (No-CKD), and 4.11 (PHP), with a progressive increment from HD to PHP (p-value for the trend <0.001). Each clinical condition elicited a significant effect on 25D/1,25D (p < 0.0001) and adjusted multivariate analysis indicated levels of Cas, Ps, PTH, and 25D as predictors of 25D/1,25D. Both in vitamin D deficient and replete subjects (25D< or ≥20 ng/ml) 25D/1,25D associated with each clinical condition (p < 0.0001) and mean values increased progressively from HD to PHP (p-values for the trend <0.0001). Regression analysis between 25D (substrate) and 25D/1,25D (efficiency) revealed an exponential negative correlation in No-CKD (r2Exp = 0.53, p < 0.001) with sharp increments of 25D/1,25D when 25D values are <20 ng/ml. At variance, in CKD (r2lin = 0.19) and in TX (r2lin = 0.32) the regression was linear as if, in case of deficit, some inhibition of the system were operating.
Conclusion and General significance
In conclusion 1,25D/25D can reflect the efficiency of vitamin D hydroxylases more than separate evaluation of 25D and 1,25D and can facilitate the therapeutic choices in different patient populations.
Keywords: Vitamin D, Vitamin D hydroxylation, Calcitriol, Calcifediol, Chronic renal failure, Renal transplantation
Highlights
-
•
1,25D/25D ratio could represent an index of vitamin D hydroxylation efficiency.
-
•
1,25D/25D ratio progressively increases from HD to CKD, TX, No-CKD and PHP.
-
•
Each of these clinical conditions affected the value of the ratio.
-
•
In selected populations 1,25D/25D ratio could guide substitutive therapeutic choices.
1. Introduction
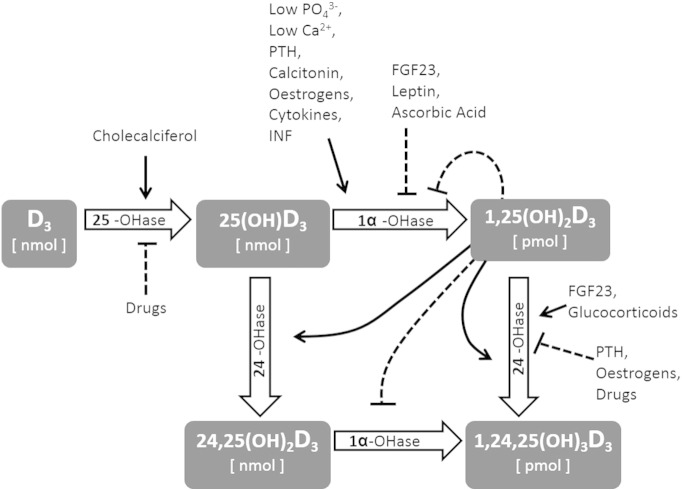
Circulating levels of 1,25-dihydroxyvitamin D (1,25D or calcitriol) are predominantly regulated within a very narrow, picomolar range by three enzymes of the cytochrome P450 family. The activity of hepatic 25-hydroxylase (CYP27A1, 25-OH-ase) is substrate dependent (i.e., induced by the availability of cholecalciferol) and is not inhibited by the nanomolar concentrations of its product, 25-hydroxycholecalciferol (25D or calcifediol) [1]. In contrast, the variable activity of the ubiquitously expressed 1-α-hydroxylase (CYP27-B1, 1-α-OH-ase) is substrate independent and tightly regulated by several hormones and substances, including calcitriol itself, the concentrations of which exert a negative feedback [2], [3]. The third enzyme, 24-hydroxylase (CYP24-A1, 24-OH-ase), which is similarly diffuse among numerous tissues, appears to be regulated by those same factors that activate 1-α-OH-ase but in the opposite direction. Thus, this enzyme represents a powerful catabolic pathway for both the active hormone and its precursor [4], [5]. In summary, it is the interplay among these three enzymes, which are regulated by several factors (Fig. 1), that determines the physiological fluctuations of calcitriol and calcitroic acid within the picomolar range and of the other metabolites within the nanomolar range. [1], [6], [7].
Fig. 1.
Several factors affect the activity of Vitamin D hydroxylases, resulting in significantly different concentrations of circulating metabolites.
The role played by the regulation of this enzymatic machinery during pathological conditions can be appreciated with two clinical examples. First, during the earliest phase of chronic kidney disease (CKD) 1,25D levels start to rapidly decrease significantly prior to any crucial reduction of the nephron mass that might explain a quantitative reduction of 1-α-OH-ase availability [8], [9], [10]. Importantly, even substrate (calcifediol) levels decrease, but this drop occurs later, during more advanced stages of the disease [11]. In fact, the early reduction of 1,25D is now considered as a secondary event to the inhibition of 1-α-OH-ase and the stimulation of 24-OH-ase by fibroblast growth factor 23 (FGF23), the novel phosphate-regulating hormone, the levels of which increase prior to any reduction of 1,25D [12]. In contrast, in primary hyperparathyroidism (PHP), circulating calcitriol levels increase as a result of PTH-dependent stimulation of 1-α-OH-ase activity. It is interesting to consider that FGF23 levels also increase during PHP [13], [14], possibly as a secondary response of bone to limit increases in 1,25D [13], [15]. Even in this case, the relationship with the substrate, 25D, is weak [16].
These two examples illustrate how calcitriol levels are regulated by complex hormonal interplays (in the examples of FGF23 and PTH, with opposite effects on vitamin D hydroxylases) and how the final balance is only partially dependent on substrate availability. Accordingly, it is conceivable that in some patients, 25D levels are normal, but its conversion to calcitriol is impaired. In contrast, in other patients, 25D levels might be low, but the rate of conversion to 1,25D is increased. From a clinical perspective, patients with the first condition might be at increased risk for vitamin D deficiency compared to those with the second condition because even increased 25D levels may not guarantee adequate amounts of the active metabolite, calcitriol. Accordingly, the ability to accurately estimate the net efficiency of the vitamin D synthetic pathway might be informative in the context of various clinical conditions, such as those requiring a choice between active or inactive vitamin D supplements, those requiring evidence for the administration of drugs that interfere with the pathway in specific patient populations, or simply to evaluate a different approach at quantifying vitamin D status in epidemiological studies.
The aim of our study was to evaluate whether it is possible to describe and to estimate the efficiency of vitamin D hydroxylation by considering the ratio between the concentrations of circulating levels of calcitriol (the active hormone) and calcifediol (the substrate). The arbitrary unit obtained (pg/ng) can represent approximately how many picograms of calcitriol are produced per nanogram of available 25D. To verify this hypothesis, we calculated the ratio values and examined the behaviour of this ratio in different patient populations exhibiting hypothetically diverse efficiency.
2. Methods
2.1. Subjects
In this study, we enrolled eligible patients from the outpatient units of our hospitals. They were subjects who were referred for the possible presence of kidney disease or to control their suspected or known clinical conditions of isolated urinary abnormalities, hypertension, nephrolithiasis, CKD (either on conservative or on maintenance haemodialysis) or renal transplantation (TX). Inclusion criteria were designated as follows: the ability to give written informed consent; age 18–80 years; no evidence of acute underlying illness; and no previous therapy with active or natural vitamin D metabolites for at least three months. Exclusion criteria were designated as follows: active treatment with drugs known to interfere with vitamin D synthesis, with the exception of immunosuppressive drugs for TX patients. Fasting blood samples were obtained in all participants in order to measure serum levels of creatinine, Ca, P, PTH, 25D and 1,25D. To uniformly dilute the impact of 25D season-related shifts, blood samples were collected randomly over one year (between January and December 2010), according to casual ambulatory controls. The presence of proteinuria was evaluated by standard fasting urine tests. Clinical parameters and prescribed therapies were recorded for each patient. For the purpose of this study, we considered the following five different populations: patients on maintenance haemodialysis (HD); patients with CKD stage 2–5 (proteinuria > 150 mg/dl and eGFR < 90 ml/min/1.73 m2 confirmed in at least two measurements at a six-month interval); renal TX patients (TX = any level of eGFR or proteinuria plus stable clinical condition and transplant at least 6 months prior to enrolment); subjects with no evidence of chronic kidney damage or divalent ion abnormalities (No-CKD = no proteinuria or kidney structural abnormalities, eGFR > 90 ml/min/1.73 m2, corrected serum Ca < 10.2 mg/dl and PTH < 65 pg/ml); and patients with clinical and biochemical evidence of primary hyperparathyroidism (PHP = corrected serum Ca > 10.5 mg/dl, PTH levels > 70 pg/ml and history of nephrolithiasis).
3. Assays
Serum creatinine (kinetic alkaline picrate method), Ca (cresolphthalein-complexone method), and P (ammonium molybdate method) levels were assayed by routine, standard colorimetric techniques using a routine analyser. Glomerular filtration rate (GFR) was estimated according to the Modification of Diet in Renal Disease (MDRD) Study formula. PTH was assayed using an immunoradiometric technique (DiaSorin, Stillwater, MN, USA) based on a double antibody probing of the intact molecule; our normal values were within the range of 10–55 pg/mL, with intra- and inter-assay variations of 6.5 and 9.8%, respectively. Levels of 25D were assayed using a commercial kit (DiaSorin, Stillwater, MN, USA) that included sample purification with acetonitrile followed by an 125I-based radioimmunoassay. Intra- and inter-assay coefficients of variation were 10.8 and 9.4%, respectively. Levels of 1,25D were measured using a radioimmunoassay according to the manufacturer's protocols (IDS Ltd, Boldon, UK), which included a monoclonal immune-extraction followed by quantitation using a standard 125I-based radioimmunoassay. Intra- and inter-assay coefficients of variation were < 12 and < 14%, respectively. The normal range observed in our laboratory was between 19.5 and 67.0 pg/mL. The 1,25D/25D ratio was calculated based on values of calcitriol (pg/ml) and calcifediol (ng/ml), which resulted in arbitrary units of pg/ng that should indicate how many pg of calcitriol are produced per ng of circulating calcifediol.
4. Statistical analysis
Statistical analysis was performed using R software version 2.15.3. The data are expressed as mean values ± SD. The data were log-transformed and consequently reported as geometric means (with 95% confidence intervals) when necessary. When the normality assumption was not tenable, F or Kruskal–Wallis statistics were used to test for any significant differences. When the F or Kruskal–Wallis statistic was significant, Bonferroni's adjusted pairwise comparisons were used to compare groups in pairs. The presence of trend effects was verified using the Jonckheere–Terpstra test. Multivariate regression analysis was applied to estimate the predictive parameters of the 1,25D/25D ratio and to account for the role of the different variables considered. The optimal multivariate regression model was selected using stepwise forward regression based on optimisation of the Akaike information criterion.
5. Results
5.1. Subject characteristics
Of the 632 total enrolled patients, 76 were on HD, 111 were CKD stage 2–5 on conservative therapy, 135 were TX with eGFR stage 1–5, 290 were No-CKD, and 20 presented with PHP. The main clinical and biochemical parameters of the five groups of subjects are reported in Table 1. On average, HD (64 ± 15 years old) and CKD (63 ± 15 years old.) patients were older than TX (51 ± 11 years old) and PHP (50 ± 17 years old) patients, whereas the No-CKD patients were the youngest (45 ± 13 years old). Male subjects were numerically more prevalent in all but the PHP group.
Table 1.
Main clinical and biochemical parameters of the five populations.
| HD | CKD 2–5 | TX | No-CKD | PHP | |
|---|---|---|---|---|---|
| N° | 76 | 111 | 135 | 290 | 20 |
| Age, y | 64 ± 15 | 63 ± 15 | 51 ± 11 | 45 ± 13.8 | 50 ± 17 |
| M/F | 43/34 | 72/39 | 79/56 | 146/141 | 9/11 |
| eGFR, ml/min | // | 36.4 ± 22 | 46.98 ± 19.17 | 112 ± 13.9 | 88 ± 34 |
| Time on HD, y | 4.32 ± 4.64 | // | // | // | // |
| Time on Tx, y | // | // | 5.7 ± 5.1 | // | // |
| Ca, mg/dl | 8.7 ± 0.8 | 9.2 ± 0.7 | 9.75 ± 0.86 | 9.4 ± 0.29 | 11 ± 0.52 |
| P, mg/dl | 4.86 ± 1.39 | 3.7 ± 1 | 3.3 ± 0.8 | 3.1 ± 0.54 | 3 ± 0.58 |
| PTH, pg/ml | 262 ± 286 | 98 ± 103 | 82.1 ± 113 | 439 ± 14.6 | 163 ± 82 |
| 25D, ng/ml | 12.26 ± 7.7 | 19.5 ± 13.3 | 25.4 ± 13.8 | 25.35 ± 13.3 | 17 ± 12 |
| 1,25D, pg/ml | 11.9 ± 4.9 | 27 ± 18 | 44.5 ± 24.6 | 50.4 ± 16.6 | 67 ± 33 |
Vitamin D status, as reflected by 25D levels, was more favourable in the No-CKD (25D = 25.35 ± 13.30 ng/ml) and TX (25D = 25.4 ± 13.8 ng/ml) patients and less favourable in the HD (12.26 ± 7.7 ng/ml) patients. The levels of 1,25D indicated, on average, a progressive increment from HD (11.9 ± 4.9 pg/ml) to CKD (27 ± 18), TX (44.5 ± 24.6), No-CKD (50.4 ± 16.6) and PHP (67 ± 33) patients.
Given the skewed distribution, we log-transformed the values of 25D and 1,25D for further statistical analysis and found that the two metabolites were positively correlated with each of the clinical conditions (Spearman correlation analysis: HD, r = 0.51, p < 0.001; CKD, r = 0.51, p < 0.001; No-CKD, r = 0.45, p < 0.001; TX, r = 0.25, p < 0.003; and PHP, r = 0.47, p < 0.038).
5.2. 1,25D/25D ratio
As illustrated in Fig. 2, the geometric mean (95% CI) of the 1,25D/25D ratio averaged 1.11 (0.96–1.28) pg/ng in HD, 1.36 (0.98–1.89) pg/ng in CKD, 1.77 (1.28–2.44) pg/ng in TX, 2.22 (1.64–3.00) pg/ng in No-CKD, and 4.11 (2.60–6.48) pg/ng in PHP patients, with a significant and progressive increment from HD to PHP (p-value for the trend < 0.001).
Fig. 2.
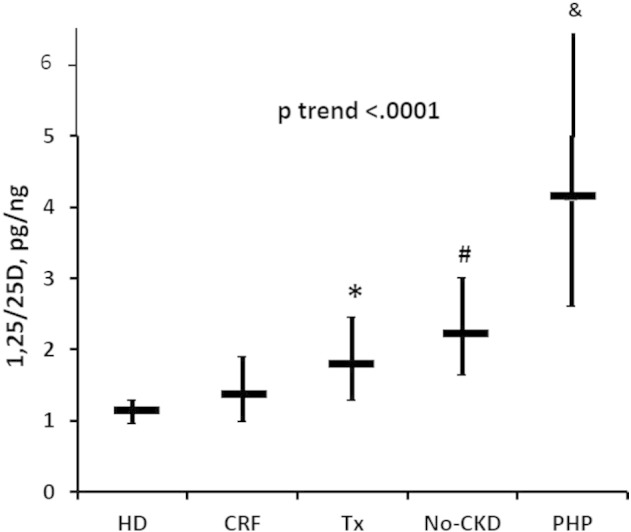
Progressive increase of the ratio (geometric means, 95% CI) in HD to PHP patients (p-value trend < 0.0001). HD and CKD had non-significantly different values. (*) = Increased ratio values in TX compared to HD (p < 0.0001) and CKD (p < 0.01) patients. (#) = Increased values of the ratio in No-CKD compared to HD (p < 0.0001), CKD (p < .0001) and TX (p < 0.007) patients. (&) = Increased values of the ratio in PHP compared to HD (p < 0.0001), CKD (p < 0.0001), TX (p < 0.0001) and No-CKD (p < 0.0003) patients.
In univariate analyses, each of the clinical conditions elicited a significant effect on the ratio (p < 0.0001), the mean geometric values of which did not differ between CKD and HD but were significantly increased in TX (vs HD, p < 0.0001; vs CKD, p < 0.01), No-CKD (vs HD, p < 0.0001; vs CKD, p < 0.0001; vs TX, p < 0.007), and in PHP (vs HD, p < 0.0001; vs CKD, p < 0.0001; vs Tx, p < 0.0001; vs No-CKD, p < 0.0003) patients (Fig. 2).
Adjusted multivariate analysis, performed without the eGFR parameter (due to its strict correlation with the different clinical conditions) indicated that the optimal estimate of the 1,25D/25D ratio was obtained by serum levels of Ca, P, PTH, and 25D levels in each of the clinical conditions (Table 2). Based on this analysis, we estimated using HD as the reference that after adjusting for the predictive variables (i.e., Ca, P, PTH and 25D), the ratio was increased by 20, 60, 100 and 135% in CKD (p < 0.018), TX (p < 0.0001), No-CKD (p < 0.0001), and PHP (p < 0.0001) patients, respectively.
Table 2.
Multivariate analysis performed to identify predictors of 1,25D/25D ratio.
| Coef | C.I. | p | |
|---|---|---|---|
| Ca, mg/dl | 1.183 | 1.112–1.258 | 0.000 |
| P, mg/dl | − 0.888 | 0.848–0.930 | 0.000 |
| PTH, pg/ml | − 0.968 | 0.938–0.999 | 0.042 |
| 25D, ng/ml | − 0.967 | 0.964–0.970 | 0.000 |
The role of eGFR on the ratio was next evaluated in a multi-regression analysis, which only included CKD and TX patients, given that they exhibited comparable ranges of renal function. After adjusting for sex, eGFR, Ca, P, and 25D, the TX patients exhibited significantly higher ratio values (p = 0.0044).
The role of PTH on the ratio was further analysed separately in patients with (HD, CRF, Tx) or without chronic renal failure (No-CKD). We found a positive correlation in No-CKD (r = 0.127; p = 0.030) with an inflection point of 0.915 in the PTH interval between 32 and 45 ng/ml. In chronic renal failure patients the correlation was negative (r = − 0.161; p = 0.0286) with an inflection point of 1.28 in the PTH interval between 77 and 95 pg/ml.
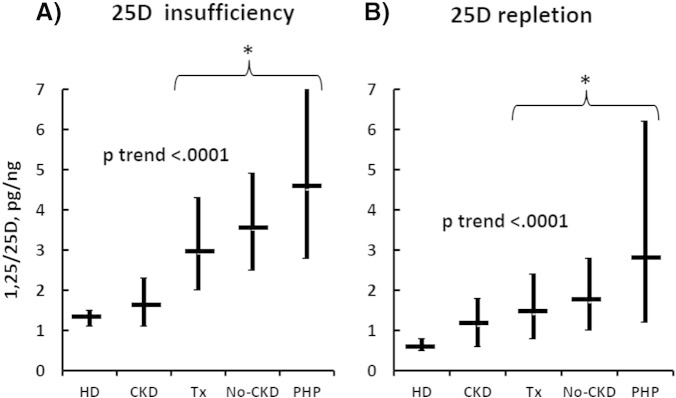
5.3. 1,25D/25D ratio and vitamin D status
To evaluate the effect of vitamin D status, we separately considered subjects with or without vitamin D deficiency (25D values < or ≥ 20 ng/ml).
In cases of vitamin D deficiency, univariate analysis confirmed the association between the ratio and each clinical condition (p < 0.0001), as well as a similar and progressive increment of mean values from HD to PHP (p-values for the trend < 0.0001), (Fig. 3A). The values did not differ between HD (1.3 pg/ng, 95% CI 1.1–1.5) and CKD (1.6 pg/ng, 95% CI 1.1–2.3) patients, whereas they were significantly increased in TX (2.9 pg/ng, 95% CI 2.0–4.3), No-CKD (3.5 pg/ng, 95% CI 2.5–4.9) and PHP (4.5 pg/ng, 95% CI 2.8–7.3) patients (p < 0.001). However, no differences were detected among the latter three populations (Fig. 3A). In vitamin D-replete (25D ≥ 20 ng/ml) subjects, the average ratio values in each population were invariably lower than that observed in the corresponding vitamin D-depleted subjects (Fig. 3, B). The clinical condition was associated with the ratio, which increased with a positive trend effect from HD to PHP (p < 0.0001, Fig. 3B). The mean values did not differ between HD (0.6 pg/ng, 95% CI 0.5–0.8) and CKD (1.1 pg/ng, 95% CI 0.6–1.8; p = n.s. vs HD) patients but increased significantly (p < 0.001) in TX (1.4 pg/ng, 95% CI 0.8–2.4), No-CKD (1.7 pg/ng, 95% CI 1.0–2.8) and PHP (2.8 pg/ng, 95% CI 1.2–6.2) patients, again with no difference among the latter three groups (Fig. 3B).
Fig. 3.
[A] Progressive increase of the ratio (geometric mean, 95% CI) in vitamin D-deficient HD to PHP (p trend < 0.0001) patients. Non-significantly different values between HD and CKD patients. (*) = Increased values of the ratio in TX, No-CKD and PHP (no differences among them) patients compared to HD (p < 0.001) and CKD (p < 0.001) patients. [B] Progressive increase of the ratio (geometric mean, 95% CI) in vitamin D-replete HD to PHP (p trend < 0.0001) patients. Non-significantly different values between HD and CKD patients. (*) = Increased values of the ratio in TX, No-CKD and PHP (no difference among them) compared to HD (p < 0.001) and CKD (p < 0.001) patients.
Adjusted multivariate analysis in vitamin D-deficient cases revealed a predictive role of each disease condition and of serum Ca, P, PTH and 25D levels, whereas for vitamin D-replete subjects, the role of age, Ca, P and 25D were predictive. During vitamin D deficiency, by taking HD as reference and after adjusting for the respective predictive variables, the ratio was marginally increased in CKD (+ 15%, p = n.s.) patients and significantly increased in TX (+ 80%; p < 0.0001), No-CKD (+ 120%; p < 0.0001) and PHP (+ 106%; p < 0.0001) patients (Table 3). However, in vitamin D-replete cases, the ratio was increased by 62, 95, 146, and 261% in CKD (p < 0.0001), TX (p < 0.0001), in No-CKD (p < 0.0001) and in PHP (p < 0.0001) patients (Table 3).
Table 3.
Percent increase of the ratio compared to HD patients (reference), in each of the separately studied populations in Vitamin D-depleted or -repleted cases. The increase observed in Vitamin D-depleted CKD patients did not significantly differ from that observed in HD patients.
| Subjects with 25D < 20 ng/ml | Subjects with 25D > 20 ng/ml | |
|---|---|---|
| CKD 2–5 | + 15 | + 62 |
| TX | + 80 | + 95 |
| No-CKD | + 120 | + 146 |
| PHP | + 103 | + 261 |
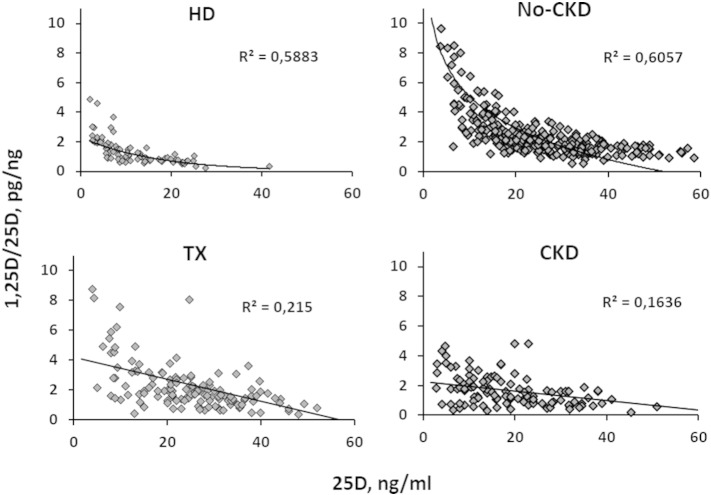
Lastly, as illustrated in Fig. 4, regression analysis revealed a significant negative correlation between 25D (substrate) and the ratio (efficiency) in each clinical condition considered (in this analysis, we excluded PHP due to the low number of cases). Importantly, the goodness of fit test indicated an exponential shape in HD (r2lin = 0.41 vs r2Exp = 0.59; p < 0.001) and No-CKD (r2lin = 0.40 vs r2Exp = 0.53, p < 0.001) patients and a linear shape in CKD (r2lin = 0.19 vs r2Exp = 0.18, p = n.s.) and in TX (r2lin = 0.32 vs r2Exp = 0.33, p = n.s.) patients.
Fig. 4.
Exponential negative relationship between 25D and the ratio in HD and No-CKD patients, compared to the linear regression observed in CKD and TX patients.
6. Discussion
Numerous epidemiological observations among different pathological conditions [17], [18], [19], [20] and demographics [21], [22], [23], [24] describe the association between Vitamin D deficiency and increased mortality. The plausibility of this association is based on the fact that the Vitamin D receptor exhibits a widespread expression pattern in the human body and that active vitamin D compounds can induce important biological effects on several cellular signalling pathways. In these epidemiological studies, Vitamin D status has been predominantly evaluated by levels of 25D, or less frequently, 1,25D. However, calcitriol and calcifediol are characterised by different biological properties, and their concentrations are only weakly correlated [8], [25]. Moreover, the physiological complexity of the synthetic pathway of this vitamin suggests that the efficient regulation of vitamin D hydroxylation might be more crucial than the concentration of any D metabolite alone.
Data from our study indicate that the 1,25D/25D ratio, which theoretically represents how many picograms of calcitriol are produced per nanogram of circulating calcifediol, can be indicative of the efficiency of vitamin D hydroxylation. Indeed, the 1,25D/25D ratios were quite different among patient populations exhibiting anticipated differences in the efficiency of hydroxylation and, as expected, increased progressively from HD to CKD, TX and No-CKD, with peak values in patients with PHP. In multivariate analysis PTH was a powerful predictor of the ratio. Interestingly, in separate populations, the correlation between PTH and 1,25D/25D ratio was positive in No-CKD patients and negative in those with any type of CRF (HD, TX, CRF). Thus, in No-CKD the positive correlation reflects the expected stimulus of PTH on hydroxylase efficiency, while in renal failure PTH increases to tentatively restore the declined efficiency of hydroxylases. Furthermore, vitamin D status affected the ratio in the most anticipated manner. In fact, compared to vitamin D deficiency, vitamin D repletion resulted in lower values, invariably in each of the clinical conditions considered. Notably, the progressive increment of the ratio persisted even in case of substrate deficit clearly underlining the role of different hydroxylation efficiency in these sub-populations. The lowest absolute values were observed, at first glance surprisingly, in the absence of deficiency in HD patients but these patients cannot be expected to substantially increase circulating levels of calcitriol from damaged kidneys simply because of substrate availability. In comparison, replete CKD patients exhibited a hydroxylation efficiency that, on average, did not significantly differ from HD patients, suggesting that the system is blunted in spite of some residual renal function. Accordingly, we would anticipate limited efficacy of supplemental therapies with inactive products in these two populations. Interestingly, our analysis, which adjusted for eGFR, indicated that vitamin D hydroxylation is less blunted in TX compared to CKD patients, a finding possibly explained either by the different types of secondary hyperparathyroidism in these populations or by the effects of immunosuppressive therapy on the TX patients. Consistent with our results, a recent study on dialysis patients found no change in the intestinal absorption of Ca after 3 months of 20,000 IU/week of cholecalciferol therapy before the normalisation of 25D and improvement of 1,25D levels [26]. In further studies, no effect on bone metabolism parameters was evident following cholecalciferol supplementation of dialysis patients [27], whereas in renal transplant patients, replacement therapy with cholecalciferol reduced PTH levels [28].
Moving from quantitative data to qualitative behaviour, regression analysis between 25D levels and the 1,25D/25D ratio can theoretically describe the response of hydroxylation to progressive vitamin D depletion; this analysis indicated a negative linear trend in CKD and TX patients and a negative but exponential regression in No-CKD and, unexpectedly, in HD patients. If we consider the behaviour in the No-CKD population as a reference, it is evident from our data that the exponential increment begins when 25D levels decline below 20 ng/ml, consistent with the most commonly adopted threshold for deficiency. In CKD and TX patients, the qualitative response is hampered, consistent with ensuing or persistent SH conditions, respectively. As per the unexpected results suggesting that HD patients behave as No-CKD patients, it is evident from the data that the ratio invariably averages significantly lower values in HD patients compared to any other group and that the exponential increment is observed only when 25D values are approximately undetectable (< 5 ng/ml). Assuming that the contribution of renal hydroxylation is trivial in HD patients, we can hypothesise that in this case, the ratio is reflective of the efficiency of extra-renal calcitriol synthesis. It would be interesting to confirm this last observation in anephric patients in whom the circulating calcitriol levels are, by definition, of exclusive extra-renal origin.
Taken together, we propose that the ratio of 1,25D/25D can be considered as representative of vitamin D hydroxylation efficiency and, as such, can be useful as a different approach at evaluating vitamin D status in various populations of patients, including those not examined in this study. For example, for patients with systemic inflammatory diseases, such as lupus nephritis, who have been reported to experience worse prognosis in the presence of low 25D levels [20] and who are suggested to benefit from vitamin D replacement therapy, the ratio values might help to guide reasoned choices between natural or active metabolites. In anephric patients, as mentioned above, the ratio might reflect the efficiency of the extra-renal synthetic pathway independently of actual circulating calcitriol levels (undoubtedly a topic of investigative interest). In transplant patients, the ratio might be helpful to evaluate whether different immunosuppressive regimens elicit diverse effects on vitamin D metabolism.
We acknowledge that our study has several limitations. First, it is purely speculative. However, the potential clinical implications are intriguing, and it would be interesting to verify our findings with further studies in different patient populations. Second, we did not assay other vitamin D metabolites, in particular the catabolic 24-hydroxylated compounds, although this could have added precision to our evaluation. However, experience with the assessment of 24-hydroxylated metabolites remains limited to few research centres. Third, we performed a single sampling; it would have been important to confirm the data in more than one assay in the same subject to discern the extent of variability. Overall, however, the number of patients that we enrolled cannot be regarded as low, and the results described are consistent with current pathophysiological concepts. Fourth, we did not include anephric patients to verify the occurrence of the lowest values of the ratio. Fifth, we did not evaluate the correlation between the ratio and other parameters like intestinal calcium absorption, bone mineral density and mortality.
In conclusion, our data indicate that the ratio of 1,25D/25D can reflect the efficiency of vitamin D hydroxylation. Determination of the ratio is more optimal than the separate evaluation of the two most commonly assayed metabolites and could facilitate the estimation of how efficiently the vitamin D system is working to maintain the picomolar amounts of active metabolite required for the expected biological effects. This helpful information could facilitate the elucidation of specific treatment parameters or rational therapeutic choices in different patient populations.
Conflict of interest declaration
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Transparency document
Transparency document.
Footnotes
The Transparency document associated with this article can be found, in the online version.
References
- 1.Jones G., Prosser D.E. The Activating Enzymes of Vitamin D Metabolism (25- and 1 alpha-Hydroxylases) In: Feldman D., Pike J.W., Adams S.J., editors. Vitamin D. 3rd ed. Academic Press; 2011. pp. 23–42. [Google Scholar]
- 2.Prosser D.E., Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem. Sci. 2004 December;29(12):664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Takeyama K., Kato S. The vitamin D3 1alpha-hydroxylase gene and its regulation by active vitamin D3. Biosci. Biotechnol. Biochem. 2011;75(2):208–213. doi: 10.1271/bbb.100684. [DOI] [PubMed] [Google Scholar]
- 4.St-Arnaud R. CYP24A1: Structure, Function, and Physiological Role. In: Feldman D., Pike J.W., Adams S.J., editors. Vitamin D. 3rd ed. Academic Press; 2011. pp. 43–56. [Google Scholar]
- 5.Schuster I., Egger H., Nussbaumer P., Kroemer R.T. Inhibitors of vitamin D hydroxylases: structure–activity relationships. J. Cell. Biochem. 2003 February 1;88(2):372–380. doi: 10.1002/jcb.10365. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein M.B. Drug and Hormone Effects on Vitamin D Metabolism. In: Feldman D., Pike J.W., Adams S.J., editors. Vitamin D. Third ed. Academic Press; 2011. pp. 43–56. [Google Scholar]
- 7.Clemens T.L., Fraher L.J., Sandler L.M., O'Rioedan J.L. Demonstration of circulating 1,24,25-trihydroxyvitamin D3 in man by radioimmunoassay. Clin. Endocrinol. 1982 Apr;16(4):337–343. doi: 10.1111/j.1365-2265.1982.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 8.Holick M.F. Vitamin D for health and in chronic kidney disease. Semin. Dial. 2005 July;18(4):266–275. doi: 10.1111/j.1525-139X.2005.18402.x. [DOI] [PubMed] [Google Scholar]
- 9.Dusso A.S., Gonzalez E.A., Martin K.J. Vitamin D. Best Pract. Res. Clin. Endocrinol. Metab. 2011 Aug;25(4):647–655. doi: 10.1016/j.beem.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Adams J.S., Hewison M. Update in vitamin D. J. Clin. Endocrinol. Metab. 2010 February;95(2):471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin A., Bakris G.L., Molitch M., Smulders M., Tian J., Williams L.A. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007 January;71(1):31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 12.Quarles L.D. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp. Cell Res. 2012 May;318(9):1040–1048. doi: 10.1016/j.yexcr.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawata T., Imanishi Y., Kobayashi K., Miki T., Arnold A., Inaba M. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J. Am. Soc. Nephrol. 2007 October;18(10):2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi K., Imanishi Y., Miyauchi A., Onoda N., Kawata T., Tahara H. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur. J. Endocrinol. 2006 January;154(1):93–99. doi: 10.1530/eje.1.02053. [DOI] [PubMed] [Google Scholar]
- 15.Witteveen J.E., van Lierop A.H., Papapoulos S.E., Hamdy N.A. Increased circulating levels of FGF23: an adaptive response in primary hyperparathyroidism? Eur. J. Endocrinol. 2012 January;166(1):55–60. doi: 10.1530/EJE-11-0523. [DOI] [PubMed] [Google Scholar]
- 16.Moosgaard B., Vestergaard P., Heickendorff L., Mosekilde L. Plasma 1,25-dihydroxyvitamin D levels in primary hyperparathyroidism depend on sex, body mass index, plasma phosphate and renal function. Clin. Endocrinol.(Oxf) 2007 January;66(1):35–42. doi: 10.1111/j.1365-2265.2006.02680.x. [DOI] [PubMed] [Google Scholar]
- 17.Aygencel G., Turkoglu M., Tuncel A.F., Candir B.A., Bildaci Y.D., Pasaoglu H. Is vitamin D insufficiency associated with mortality of critically ill patients? Crit. Care Res. Pract. 2013;2013:856747. doi: 10.1155/2013/856747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villasenor A., Ballard-Barbash R., Ambs A., Bernstein L., Baumgartner K., Baumgartner R. Associations of serum 25-hydroxyvitamin D with overall and breast cancer-specific mortality in a multiethnic cohort of breast cancer survivors. Cancer Causes Control. 2013 April;24(4):759–767. doi: 10.1007/s10552-013-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobnig H., Pilz S., Scharnagl H., Renner W., Seelhorst U., Wellnitz B. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch. Intern. Med. 2008 June 23;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 20.Wu P.W., Rhew E.Y., Dyer A.R., Dunlop D.D., Langman C.B., Price H. 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Rheum. 2009 October 15;61(10):1387–1395. doi: 10.1002/art.24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomson J., Emberson J., Hill M., Gordon A., Armitage J., Shipley M. Vitamin D and risk of death from vascular and non-vascular causes in the Whitehall study and meta-analyses of 12,000 deaths. Eur. Heart J. 2013 May;34(18):1365–1374. doi: 10.1093/eurheartj/ehs426. [DOI] [PubMed] [Google Scholar]
- 22.Brondum-Jacobsen P., Benn M., Jensen G.B., Nordestgaard B.G. 25-hydroxyvitamin D levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler. Thromb. Vasc. Biol. 2012 November;32(11):2794–2802. doi: 10.1161/ATVBAHA.112.248039. [DOI] [PubMed] [Google Scholar]
- 23.Pilz S., Dobnig H., Nijpels G., Heine R.J., Stehouwer C.D., Snijder M.B. Vitamin D and mortality in older men and women. Clin. Endocrinol. (Oxf) 2009 November;71(5):666–672. doi: 10.1111/j.1365-2265.2009.03548.x. [DOI] [PubMed] [Google Scholar]
- 24.Melamed M.L., Michos E.D., Post W., Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch. Intern. Med. 2008 August 11;168(15):1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wacker M., Holick M.F. Sunlight and Vitamin D: a global perspective for health. Dermatoendocrinol. 2013 Jan;5(1):51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armas L.A., Zena M., Lund R., Heaney R.P. Calcium absorption response to cholecalciferol supplementation in hemodialysis. Clin. J. Am. Soc. Nephrol. 2013 June;8(6):1003–1008. doi: 10.2215/CJN.08610812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewitt N.A., O'Connor A.A., O'Shaughnessy D.V., Elder G.J. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013 July;8(7):1143–1149. doi: 10.2215/CJN.02840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courbebaisse M., Thervet E., Souberbielle J.C., Zuber J., Eladari D., Martinez F. Effects of vitamin D supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int. 2009 March;75(6):646–651. doi: 10.1038/ki.2008.549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.