Abstract
Intravesical Bacillus Calmette–Guerin (BCG) vaccine is the preferred first line treatment for non-muscle invasive bladder carcinoma (NMIBC) in order to prevent recurrence and progression of cancer. There is ongoing need for the rational selection of i) BCG dose, ii) frequency of BCG administration along with iii) synergistic adjuvant therapy and iv) a reliable set of biochemical markers relevant to tumor response. In this review we evaluate cellular and molecular markers pertinent to the immunological response triggered by the BCG instillation and respective mathematical models of the treatment. Specific examples of markers include diverse immune cells, genetic polymorphisms, miRNAs, epigenetics, immunohistochemistry and molecular biology ‘beacons’ as exemplified by cell surface proteins, cytokines, signaling proteins and enzymes. We identified tumor associated macrophages (TAMs), human leukocyte antigen (HLA) class I, a combination of Ki-67/CK20, IL-2, IL-8 and IL-6/IL-10 ratio as the most promising markers for both pre-BCG and post-BCG treatment suitable for the simulation studies.
The intricate and patient-specific nature of these data warrants the use of powerful multi-parametral mathematical methods in combination with molecular/cellular biology insight and clinical input.
Keywords: Bacillus Calmette–Guerin, Biological marker, Bladder cancer, Mathematical models
1. Introduction
Bladder cancer is the second most common cancer of the genitourinary tract worldwide after prostate neoplasms [1]. It accounts for about 7% and 2% of new cancer cases in men and women, respectively [2]. Bladder carcinoma (BC) is commonly diagnosed following the transurethral resection of bladder tumor (TURBT), determination of the tumor grade and the degree of tumor invasion [3]. However, prediction of the clinical behavior and optimized treatment regimen for BC are challenging up to this day. Approximately 70% of BCs are confined to layers above the muscularis propria and are termed non-muscle invasive bladder cancer (NMIBC) [4].
Multiple clinical reports suggest that intravesical Bacillus Calmette–Guerin (BCG) vaccine is the preferred 1st line treatment for the NMIBC in order to prevent the recurrence and progression of cancer [5]. Identification of the BCG response markers that are clinically significant, disease-relevant, reproducible and easily accessible in the clinical setting is of key importance in early treatment [6], [7]. Repeated administration of BCG therapy has been suggested for patients with intermediate and high risk of BC progression [8]. However, data from multiple randomized clinical trials lack clear guidelines and rationale for the maintenance schedule [9], [10].
There is ongoing need for the rational selection of i) dose, ii) frequency of BCG administration, iii) synergistic adjuvant therapy, and iv) biomarkers relevant to tumor dynamics. A multi-disciplinary approach involving clinical sciences, biology and mathematical modeling may yield a real opportunity to increase disease-free survival of patients with NMIBC. A similar strategy has been successfully applied to other areas of oncology [11] including pancreatic cancer [12] and lymphoma [13].
2. BCG: tentative mechanism of action
Activation of the innate immune system is a prerequisite for BCG-induced responses. The interaction of BCG with extracellular matrix glycoproteins of the BC cells including fibronectin and integrins is followed by their internalization, recognition and processing by the host cell's immune machinery. This is followed by production of cytokine and chemokine molecules as well as recruitment of leukocytes to the bladder wall. A cascade of proinflammatory events is triggered allowing for the ultimate detection of the resulting mediator molecules. Representative proteins include IL-1, IL-6, IL-8, IL-10, IL-12, IL-18, tumor necrosis factor (TNF)-α, granulocyte-macrophage colony stimulating factor (GM-CSF), macrophage inflammatory protein (MIP)-1α, macrophage-derived chemokine (MDC), and interferon-inducible protein (IP)-10 [14], [15]. The absence of standardization in BCG manufacturing and variation in treatment protocols cause notable differences in the phenotype, antigenicity and clinical characteristics of the numerous substrains of BCG used for the treatment of NMIBC [16].
BCG effect is mediated by multiple immune cells. BCG instillation is followed by an increased number of macrophages in bladder cancer infiltrates, the peri-tumoral bladder wall and urine [17], [18], [19]. BCG-stimulated macrophages could directly interact with and affect BC cells via the release of effector factors including TNF-α, IFN-γ and apoptotic signaling molecules [20]. TH1 family cytokines (e.g. IFN-γ) stimulate macrophage cytotoxicity whereas TH2 group molecules inhibit this effect. Neutrophils represent another important class of cells that mediate tissue response to BCG infection via TLR2, TLR4 receptors and adaptor protein MyD88. Neutrophil activation leads to the release of TNF-related apoptosis-inducing ligand (TRAIL). TRAIL selectively stimulates apoptosis in BC cells leading in part to the observed clinical effect. There is a correlation between increased urinary levels of TRAIL and BCG responsiveness in polymorphonuclear neutrophils (PMN) [21]. Release of macrophages and neutrophils activates NK, CD4 + T, CD8 + T and dendritic cells [22]. CD4 T cell analysis before each BCG instillation in NMIBC patients revealed a 5-fold increase in T cell count by week 2/3, and further increased 8-fold by week 4/5 [23]. Cytotoxicity of T and NK cells toward BC is mediated by the major histocompatibility complex (cytotoxic T lymphocytes (CTLs) or NK cells) [24]. Perforin, is a key cytolytic protein produced by CTLs and NK cells. It is implicated in selective pro-apoptotic elimination of the BC cells [25]. The highly specialized BCG-activated killer cell population neutralizes NK cell-resistant BC cells using a similar lytic mechanism [26].
3. Biological markers: general notes
A pre-/post-BCG treatment classification of biological markers has been suggested [27]. Pre-treatment markers of importance in clinical pathology include tumor size, multiplicity, stage/grade and history, additional carcinoma(s) in situ (CIS) and number of TURs before BCG. Whereas these parameters play a role in assessing the individual risk of tumor progression and its invasiveness, there are no universally applicable predictive markers of NMIBC. Several commercial tests relying on biomarkers have been introduced into the clinic [28]. These include BTA stat® (Bladder Tumor Antigen), BTA TRAK® (Human Complement Factor H), NMP22 (Nuclear Matrix Protein)/Bladder Chek,® ImmunoCyt™/uCyt™. Some of the assays are prone to yielding false positive results, especially in patients exhibiting renal or prostate inflammation. UroVysion™ test is aimed at analyzing aneuploidy in chromosomes 3, 7, 17 and 9p21 using fluorescence in situ hybridization technique. While being quite efficient in diagnosing NMIBC, UroVysion™ is expensive [29].
3.1. Clinical pathology: tumor size and age effect
Of the multiple factors including age, gender, CIS, stage, number of tumors, and tumor size for NMIBC patients treated with BCG, tumor size of > 3 cm in diameter was associated with BC recurrence, whereas tumor stage (Ta or T1) was associated with tumor progression [30]. In intermediate and high-risk NMIBC patients treated with BCG, older cohort exhibited unfavorable long-term prognosis [31].
3.2. Cellular markers
Several studies suggested a marker of cellular proliferation Ki-67 to be predictive of post-BCG tumor recurrence [32], contradicting earlier data [33]. Further attempts to clarify this controversy were focused on analyzing combination of Ki-67 with additional biomolecules. In a recent study, CK20 expression was significantly correlated with recurrence-free survival (RFS). Ki-67 was the only marker significantly associated with progression-free survival (PFS). The combination of CK20 and Ki-67 was indicative of BC aggressiveness showing significantly worse PFS and cancer-specific survival (CSS) in tumors with high proliferation index [34].
The correlation between tumor associated macrophages (TAMs) infiltrating BC in situ and response to BCG therapy using anti-CD68 monoclonal antibody revealed that RFS was significantly better in patients with lower TAM count. Patients with lower cancer cell-to-lamina propria TAM ratio had higher RFS [35].
3.3. Genetic and epigenetic markers
Detailed analysis of literature dealing with gene polymorphisms and their link to BCG response in the NMIBC patients has been conducted [36]. Examples of gene polymorphisms that led to reduced RFS or increased recurrence risk post-BCG include XPA, XPC, XPD, XPG, XPF, ERCC1, ERCC2, ERCC6, XRCC1, XRCC4, APEX1, GSTM1, CCNB1, PON1, and SLCO1B1. As anticipated, gene polymorphisms of multiple cytokines were linked to the BCG treatment outcome, although RFS data were controversial, as exemplified by IL-6 [37]. More definitive outcome was reported for the IL-8 (-251 A/A) polymorphism which was associated with an increased RFS in BCG-treated patients and for the PPARγ SNP linked to a reduced recurrence risk [38]. Deregulation of FAS/FASL system, namely FASL-844 T/C was implicated in the immune escape affecting BCG therapy outcome [39].
Studies correlating NRAMP1 and hGPX1 gene polymorphisms to BCG response showed that the NRAMP1 D543N G:G genotype displayed decreased CSS. The hGPX1 CT genotype (Pro-Leu) exhibited decreased RFS time post-BCG, although the study cohort was small [40]. XRCC1 is a scaffolding protein in base excision repair. Variants in XRCC1 gene were suggested to alter protein structure/function or create alternatively spliced protein influencing repair efficiency. Genotyping for three polymorphic sites of XRCC1 at codons Arg194Trp (PvuII), Arg280His (RsaI) and Arg399Gln (MspI) yielded an association in heterozygous genotype of codons 280 and 399 with BC risk. The A/A genotype of codon 399 was associated with the high risk for recurrence in BCG treated patients showing reduced RFS [41]. The increased risk of BC was observed with the IL-1RN*2 and IFN-G + 874 A allele carriers in the post-BCG group of patients. TGF-B TT and IFN-G + 874 A carriers were associated with reduced and enhanced risk of recurrence post-BCG, respectively [42]. MicroRNAs (miRNAs) in serum and/or plasma have been introduced as non-invasive biomarkers of BC. A selection of 15 miRNAs was reported to be deregulated in different stage BC. Of these, 13 miRNAs were deregulated to the same extent in both NMIBC and muscle-invasive BC. Three specific miRNAs, namely miR-9, miR-182 and miR-200b were associated with tumor aggressiveness and RFS [43]. Changes in DNA methylation of tumor suppressor genes at the early stages of BC have been introduced as markers of tumor stage, aggressiveness and dynamics [44]. BC cell lines T24 and UM-UC-3 were treated with 5-aza-dC and 4-phenylbutyric acid (PBA) to mimic epigenetic silencing of miRNA genes followed by the analysis of epigenetic alteration in miRNA expression. miR-137, miR-124-2, miR-124-3, and miR-9-3 were frequently methylated in primary cancers suggestive of their potential utility as BC biomarkers [45]. The methylation analysis for 18 tumor suppressor genes in urine samples revealed that PRDM2, HLTF, ID4, DLC1, BNIP3, H2AFX, CACNA1G, TGIF and CACNA1A were methylated in BC. Of these, CACNA1A gene methylation was directly associated with tumor recurrence whereas PRDM2 and BNIP3 were linked to recurrence and disease specific survival, respectively [46]. The amounts of methylated DNA as well as the methylation frequencies were assessed in serum of BC patients and healthy subjects. TIMP3 was found to be most frequently methylated, followed by APC, RARB, and TIG1 genes. Both methylation levels for each gene site and the number of methylated genes were increased in BC compared to healthy individuals, however BCs at different stages of progression could not be differentiated from non-malignant disease [47]. Despite considerable progress in studies of genetic polymorphisms, miRNAs and epigenetic markers associated with both pre-BCG treatment and post-BCG treatment of NMIBC patients, more detailed, statistically empowered, ethnically diverse and tumor stage-related studies are needed to include these data into development of the individualized therapeutic regimen.
3.4. Molecular markers
3.4.1. Cell-surface proteins
BCs with high risk of recurrence/progression express the carbohydrate antigen sialyl-Tn (sTn). sTn and sTn-related antigen sialyl-6-T (s6T) protein levels were associated with BCG response and increased RFS [48]. Expression of podocalyxin-like anti-adhesive glycoprotein (PODXL) in patients with Ta and T1 tumors was an independent predictor of a reduced 2-year PFS [49]. Levels of tenascin-C (TN-C) in voided urine correlated with the BC grade showing ca. 22 times higher concentration in BC patients compared to healthy volunteers [50]. Human leukocyte antigen (HLA, MHC in humans) Class I plays a decisive role in the recognition and elimination of tumor cells. It is down-regulated in ca. 30% of BCs affecting presentation of a cancer antigen to the immune system (ex., CTLs) [48]. More profound alterations and a higher incidence of structural defects in HLA Class I expression were found in post-BCG-recurrent tumors. Also, HLA Class I down-regulation was a significant prognostic factor in patients undergoing BCG immunotherapy [51].
3.4.2. Cytokines
IL-2 cytokine secreted by activated T-cells (CD4 +) was introduced as an independent predictive parameter of BCG response [52]. High levels of IL-2 in urine post-BCG were directly associated with an increased RFS. A significant number of responders (70%) exhibited inducibility of IL-2 mRNA in peripheral blood mononuclear cells which was directly associated with an enhanced PFS. A time-dependent interplay between IL-2 and IL-10 levels has been noted following BCG induction and repeated booster vaccinations [53]. Based on these data, it was suggested that repeated BCG alone may not be beneficial for the general population of NMIBC patients [54].
The IL-6/IL-10 ratio post-BCG has been evaluated in NMIBC patients to reveal that subjects with the ratio value >.1 displayed higher RFS. Both multivariate analyses of the IL-6/IL-10 ratio and the number of lesions were identified as independent predictors of BCG response [55]. BCG-stimulated C57BL/6 macrophages exhibited reduced killing of BC MBT− 2 and MB49 cells and produced a high level of IL-10, which correlated with reduced production of TNF-α, IL-6 and NO. Macrophages from C57BL/6 IL-10−/− mice exhibited increased killing of MB49 cells suggesting that blockage of IL-10 could be beneficial clinically [56].
A neutrophil chemotactic factor (IL-8) is secreted by macrophages post-BCG inducing chemotaxis of primary neutrophils and other granulocytes [57]. Levels of IL-8, matrix metallopeptidase 9 (MMP-9) and syndecan in voided urine from BC patients were analyzed to suggest that all proteins were significantly elevated in BC subjects, however only IL-8 was an independent factor for the detection of BC [58] contradicting earlier studies [59]. High levels of IL-8 in the urine within the first 6 h after BCG administration were associated with an increased RFS [60]. More careful, statistically empowered and standardized studies are needed to address this controversy.
The level of IL-17 production and neutrophil count in BCG-treated bladder was reduced in γδ T-cells-deficient but not in CD4-cell-depleted mice. The survival of BC-inoculated γδ T-cell-deficient mice was not improved by BCG treatment. It was concluded that IL-17-producing γδ T-cells play a key role in the BCG-induced recruitment of neutrophils to the bladder [61]. In addition to IL-8, BCG-activated macrophages are reported to produce IFN-γ inducing factor (IL-18). Key responder cells activated by IL-18 include NK and CTLs triggering secretion of IFN-γ. Increased IL-18 levels in the urine measured within the first 12 h after BCG administration were significantly associated with an increased RFS [62]. Level of Gc-globulin (GC) in the urine of BC patients was 10-fold higher than in benign bladder conditions and normal controls [63].
3.4.3. Other proteins
Protein expression for p53, pRb, PTEN, Ki-67, p27, FGFR3, and CD9 has been examined in order to assess their predictive value in tumor recurrence and progression. Whereas increased p53 expression was associated with tumor progression after BCG, none of the markers correlated with RFS and PFS post-BCG [64]. In recent studies, CIS, gender and cancer sub-stage (T1m/T1e) were the most important variables for progression whereas FGFR3 gene mutation, Ki-67, P53 and P27 expression markers data were non-informative [65]. Histology data from the NMIBC patients post-BCG or BCG + IFN-α combo suggested that pRb expression was not associated with the outcome of BCG instillation as opposed to the combination therapy. Neither p53 expression nor p53 + pRb expression related to tumor response to BCG or BCG + IFN-α with respect to RFS and PFS [66].
Random peptide library of the circulating Ig's purified from a patient after BCG has been screened to identify the corresponding target antigens. Mycobacterium bovis heat-shock protein 65 (HSP-65) has been identified as a serological marker of the humoral response to the treatment. Increasing levels of IgA and IgG anti-HSP-65 titers directly correlated with a positive outcome in BC patients [67]. The effect of BCG on telomerase activity was examined in T24 and J82 BC cells. These were co-cultured with BCG for 5 days to yield significant decrease in telomerase activity as compared to the non-treated cells. The count of apoptotic cells was markedly increased suggesting that the reduction of telomerase activity is related to the BCG treatment [68]. Inducible nitric oxide synthase (iNOS) was found in the urothelial cells, macrophages and in the submucosa of post-BCG NMIBC patients. Endogenously formed NO was significantly increased including a ten-fold increase in mRNA expression for iNOS compared to healthy controls [69]. NMP-22 performed well as a marker of low-grade lesions compared to the cytology alone, however its levels were not affected by BCG therapy [70]. It was speculated that the urine NMP-22 assay was likely to measure the amount of cell turnover, including surface shedding from BCs and not necessary any specific BC tumor antigen [71]. In a recent development, a combination of cell cycle biomarker of aberrant growth, Mcm5 and NMP22 in urine identified 95% of potentially life threatening diagnoses [72]. HtrA1 is a secreted serine protease that processes IGF-binding proteins and regulates cell growth. Expression of native and autocatalytic forms of HtrA1 in human bladder tissue and urine has been analyzed via immunohistochemistry. Significantly higher amounts of both HtrA1 forms were found in urine from BC patients compared to both healthy subjects and patients with cystitis [73]. 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4) regulates intracellular levels of fructose 2,6-biphosphate, a key molecule in glycolysis. Expression levels of PFKFB4 mRNA in NMIBC tissue specimens were significantly higher in patients with high stage carcinoma and multiple tumors as compared to low stage and single tumors [74]. There was strong associations between lower UDP-glucuronosyltransferase 1A (UGT1A) expression and the risk of recurrence in high-grade NMIBC. In addition, the expression of UGT1A was positively and negatively correlated with those of estrogen receptor-α and estrogen receptor-β, respectively suggestive of its opposite regulation in normal bladder tissue vs. BCs [75].
4. Mathematical models of cancer therapy
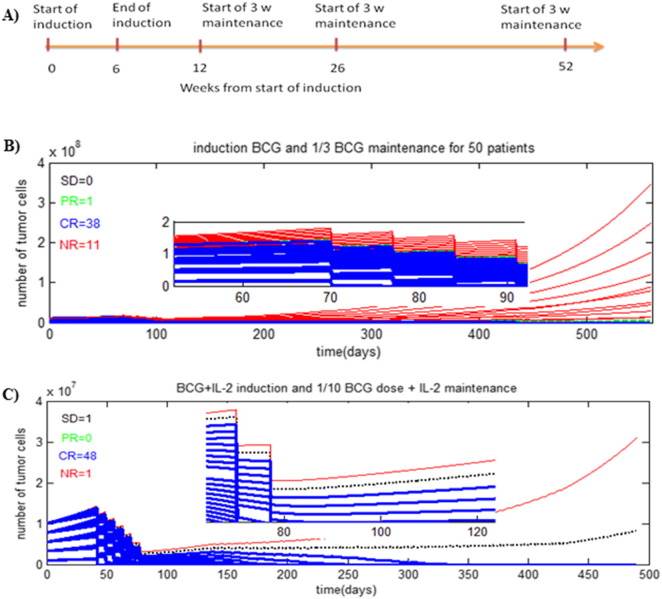
Several mathematical models of disease and respective therapeutic interventions aimed at optimization of dosing and treatment regimen have been introduced. Although this approach needs validation in oncology, there are multiple clinically relevant examples from other therapeutic areas [76]. A computational model of glioma growth reliably predicted the time to relapse using radiation alone and in combination with chemotherapy [77]. A mathematical simulation in breast cancer has been used to identify high-risk population, cancer screening strategies, predicted tumor growth and optimized cancer treatment [78]. The treatment outcome in orthotopic pancreatic tumor in vivo was described computationally using in vitro data [12] and further expanded to describe lymphoma [13]. Modeling approach to simulate tumor invasiveness and metastasis based on both in vivo and in vitro data has been introduced as well [79]. A comparison of experimental data for vaccination of HER-2/neu transgenic mouse and the respective mathematical model suggested that the simulation accurately captured i) favorable ‘intensive’ regimen of early vaccination, ii) age-related robustness of immune response in animals and iii) steady-state beneficial immune response maintained with fewer booster vaccinations [80]. Modeling studies aimed to optimize the efficacy of standard BCG protocol using estimated in vitro parameters have been reported [81], [82]. The model took into consideration biological interactions between BCG, immune cells and tumor cells post-BCG administration. Subsequent studies identified the dose of BCG and effective regimens that could be used to avoid undesirable vaccination side-effects. The model allowed simulating the effect of BCG pulses on the therapeutic outcome. Unfortunately, the parameters for the described model were taken from rather disparate in vitro, in vivo murine and human clinical studies vs. individual patients [83]. Simulation studies of NIMBC treatment with BCG or BCG + IL-2 combo used in situ data on tumor size, growth rate and immune response assessment from the clinical set of estimated parameters [84]. This model described the dynamic interactions of tumor cells within the bladder, the immune system, and the BCG immunotherapy [85], [86], [87]. Combinations of the initial tumor size and varying growth rates were tested using Lamm's maintenance protocol [88] (Fig. 2A) and varying regiments of BCG. In the simulation studies, 50 NMIBC patients were kept on the Lamm's maintenance schedule and treated with 1/3rd of the regular dose BCG as shown on Fig. 2B. The monotherapy resulted in 38 patients showing complete response, whereas 11 patients were refractive. Addition of IL-2 to BCG vaccine (standard dose for the induction followed by 1/10th of the standard dose in the maintenance treatment) yielded 48 complete responses, 1 partial response and 1 non-responder (Fig. 2C). This outcome suggested that a mathematical model could be of immediate clinical use to i) select a treatment protocol including both reduced BCG dosing and maintenance scheduling to minimize side effect(s) of vaccination; ii) predict the outcome and iii) assess the need for synergistic agents on an individual basis.
Fig. 2.
A) Schedule of maintenance treatment plan (Lamm's protocol): six-weekly intravesical instillations of BCG (standard dose) and low dose of BCG (1/3 or 1/10 of the standard dose) for the 3 maintenance instillations until 52 weeks; B) simulation effects of a treatment regimen (BCG only) with maintenance of BCG instillations (1/3 of standard dose) for 50 people. Shown is the tumor cells count as a function of time (500 days during and after therapy); C) simulated effect of BCG + IL-2 (induction) and BCG + IL-2 (maintenance) on 50 virtual patients. Time evolution of tumor cells up to 500 days. Maintenance treatment was carried out with 1/10th of the standard BCG dose.
Red solid line — NR (non-responders to the treatment), blue solid line — CR (complete response), black solid line — PR (partial response), and green slashed line — SD (stable disease).
5. Experimental markers for the mathematical model of NMIBC
Despite significant advances in the identification of biological markers of NMIBC, very few of them hold promise as clinically relevant and accurate predictors of success in BCG treatment. Several key reasons include i) limited access to clinical data and patients; ii) lack of standardized bioanalytical procedures to evaluate biomarker levels; iii) ethnic, epigenetic, treatment backgrounds affecting gene polymorphism and epigenetic markers; iv) opportunistic urogenital conditions; v) longitudinal relationship between disease progression and markers panel, number of BCG installments, and adjuvant therapy; and vi) patient-specific ‘fingerprint’ of the disease.
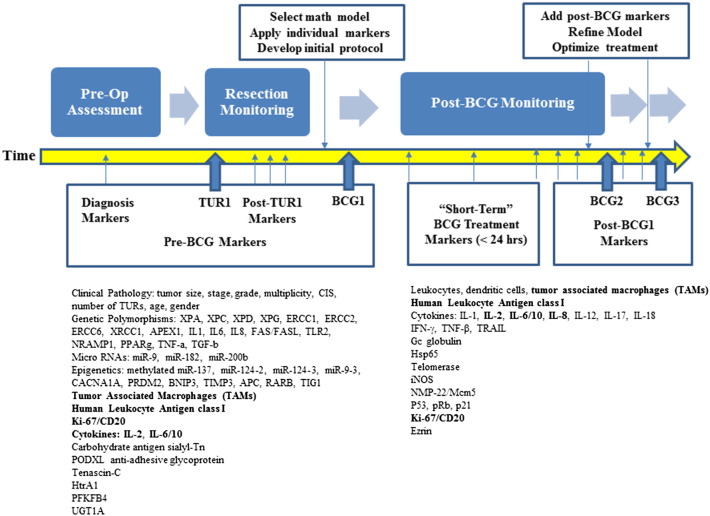
The pro-inflammatory cascade induced by NMIBC alone or in combination with the BCG instillation involves multiple cell types and molecules. In addition, time-resolved changes of these entities pre-/post-BCG need to be considered. We selected several markers that could be used as standalone parameters in the clinic and/or in the mathematical models to determine the optimized treatment regimen (Fig. 3, bold) using individual data from NMIBC patients.
Fig. 3.
A summary of pre- and post-BCG biological markers described in the text. The most promising predictive markers and/or their combination for BCG response are in bold.
Since the aforementioned cellular and molecular markers are likely to be deregulated regardless of their pre-/post-vaccination collection point, we recommend to analyze these as a panel throughout a patient's individual history. Elevated IL-2 and IL-8 levels in the urine are promising predictive markers of BCG response. Notably, high urinary levels of IL-8 within the first 6 h post-BCG were associated with an increased RFS. A direct interplay between cellular and molecular events occurring post-BCG is illustrated by the IL-6/IL-10 ratio being a prognostic marker of tumor recurrence (Fig. 1). As opposed to controversial data for Ki-67 alone, a combination of Ki-67/CK20 was reported to be a reliable indicator of BC aggressiveness. Down-regulation of HLA Class I in cancer cells is disadvantageous for presentation of a cancer antigen to the immune system. Low levels of HLA class I were suggested as a marker in patients undergoing BCG. The number of tumor associated macrophages (TAMs) infiltrating the cancer area is one of several cellular markers predictive of the treatment outcome pre-BCG. Once gene polymorphism, miRNAs and epigenetic markers become more standardized and mainstream in the clinic, they will be included in designing individual regiments for the treatment of NMIBC. It is anticipated that the pre-BCG clinical parameters will allow for the proper calibration of the mathematical model and customization of the treatment protocol. A post-BCG comparison of extrapolated and experimental outcome will enable further refinements of both simulation process and, more importantly, allow for the optimization of patient-specific therapeutic approach.
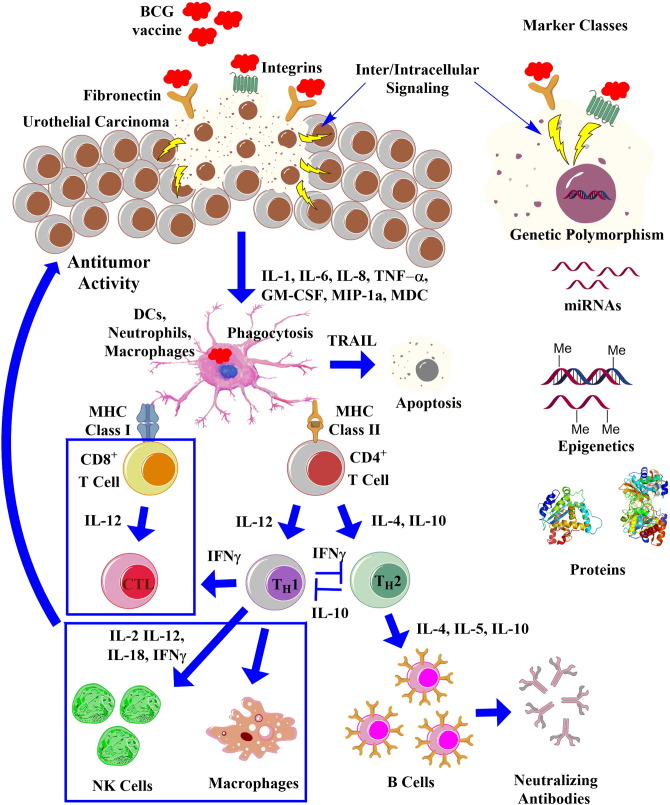
Fig. 1.
Tentative molecular cascade of immune response induced by intravesical BCG instillation. BCG is believed to cause tumor elimination by attachment of the BCG to specific receptors on the urothelium (ex., fibronectin, integrins) and initiation of inflammation reaction. This step leads to the release of multiple cytokines and chemokines (IL-1, IL-6, IL-8, etc.) from both tumor and normal cells to attract a variety of immune cells into the bladder wall (dendritic cells (DCs), neutrophils, macrophages; key effectors in the BCG response are marked with blue boxes). Internalization of BCG triggers phagocytosis, apoptotic death via the release of TNF-related apoptosis-inducing ligand (TRAIL), maturation and differentiation of naïve CD4 + T cells into TH1 and/or TH2 cells that direct immune responses toward cellular or humoral immunity, respectively. The therapeutic effect of BCG depends on the proper induction of TH1 immune responses. IL-10 inhibits TH1 immune responses whereas IFN-γ inhibits TH2 immune responses. Blocking IL-10 or inducing IFN-γ can lead to a TH1 dominated immunity that is essential for BCG-mediated bladder cancer destruction. Detection and quantification of these cells as well as additional biological markers pre-BCG treatment (ex., single nucleotide polymorphism, miRNAs, epigenetics, proteins) or after BCG installments in the clinical analytes is expected to provide better insight into cancer dynamics, its aggressiveness and optimize individual treatment options.
6. Conclusions
In this past decade, we witnessed a growing interest toward mathematical models of disease aimed at better understanding of i) key molecular targets suitable for intervention, ii) design and optimization of treatment protocols and iii) regimen-related toxicities. In the area of NMIBC, there is a real need for the rational selection of i) dose, ii) frequency of BCG administration along with iii) synergistic adjuvant therapy and iv) a reliable set of biochemical markers related to tumor response. Addressing these challenges via a multi-disciplinary approach involving simulation, molecular biology and clinical science may yield a real opportunity to increase disease-free and overall survival of patients. Specifically, integration of systems biology data with in situ clinical evidence and rationally designed treatment protocols are expected to result in much needed improvement(s) in the individual clinical response to BCG and other relevant BC vaccines [89].
Transparency document
Transparency document.
Footnotes
The Transparency document associated with this article can be found, in the online version.
References
- 1.Knowles M.A., Hurst C.D. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat. Rev. 2015;15:25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 2.Konety B.R., Carroll P.R. Urothelial carcinoma: cancers of the bladder, ureter, & renal pelvis. In: Tanagho E.A., JW M.A., editors. Smith's General Urology. 17th ed. The McGraw-Hill Companies; New York: 2008. pp. 308–320. [Google Scholar]
- 3.Vogeli T.A. The management of superficial transitional cell carcinoma of the bladder: a critical assessment of contemporary concepts and future perspectives. BJU Int. 2005;96:1171–1176. doi: 10.1111/j.1464-410X.2005.05928.x. [DOI] [PubMed] [Google Scholar]
- 4.Soloway M.S. Expectant treatment of small, recurrent, low-grade, noninvasive tumors of the urinary bladder. Urol. Oncol. 2006;24:58–61. doi: 10.1016/j.urolonc.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Luo Y., Askeland E.J., Newton M.R. In: Immunotherapy of Urinary Bladder Carcinoma: BCG and Beyond. Intech, editor. 2013. pp. 319–357. (Chapter 15) [Google Scholar]
- 6.Fernandez-Gomez J., Solsona E., Unda M. Prognostic factors in patients with nonmuscle-invasive bladder cancer treated with Bacillus Calmette–Guérin: multivariate analysis of data from four randomized CUETO trials. Eur. Urol. 2008;53:992–1002. doi: 10.1016/j.eururo.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Schrier B.P., Hollander M.P., van Rhijn B.W.G. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur. Urol. 2004;45:292–296. doi: 10.1016/j.eururo.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Babjuk M., Oosterlinck W., Sylvester R. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur. Urol. 2011;59:997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Lamm D.L., Blumenstein B.A., Crissman J.D. Maintenance bacillus Calmette–Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J. Urol. 2000;163:1124–1129. [PubMed] [Google Scholar]
- 10.Badalament R.A., Herr H.W., Wong G.Y. A prospective randomized trial of maintenance versus nonmaintenance intravesical bacillus Calmette–Guerin therapy of superficial bladder cancer. J. Clin. Oncol. 1987;5:441–449. doi: 10.1200/JCO.1987.5.3.441. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P., Brusic V. Mathematical modeling for novel cancer drug discovery and development. Expert Opin. Drug Discov. 2014;9:1133–1150. doi: 10.1517/17460441.2014.941351. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.J., Huang J., England C.G., McNally L.R., Frieboes H.B. Predictive modeling of in vivo response to gemcitabine in pancreatic cancer. PLoS Comput. Biol. 2013;9:e1003231. doi: 10.1371/journal.pcbi.1003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frieboes H.B., Smith B.R., Chuang Y.L., Ito K., Roettgers A.M. An integrated computational/experimental model of lymphoma growth. PLoS Comput. Biol. 2013;9:e1003008. doi: 10.1371/journal.pcbi.1003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becich M.J., Carroll S., Ratliff T.L. Internalization of bacille Calmette–Guerin by bladder tumor cells. J. Urol. 1991;145:1316–1324. doi: 10.1016/s0022-5347(17)38622-6. [DOI] [PubMed] [Google Scholar]
- 15.De Reijke T.M., De Boer E.C., Kurth K.H., Schamhart D.H. Urinary cytokines during intravesical bacillus Calmette–Guerin therapy for superficial bladder cancer: processing, stability and prognostic value. J. Urol. 1996;155:477–482. [PubMed] [Google Scholar]
- 16.Gan C., Mostafid H., Khan M.S., Lewis D.J.M. BCG immunotherapy for bladder cancer-the effects of substrain differences. Nat. Rev. Urol. 2013;10:580–588. doi: 10.1038/nrurol.2013.194. [DOI] [PubMed] [Google Scholar]
- 17.De Boer E.C., de Jong W.H., van der Meijden A.P. Leukocytes in the urine after intravesical BCG treatment for superficial bladder cancer. A flow cytofluorometric analysis. Urol. Res. 1991;19:45–50. doi: 10.1007/BF00294021. [DOI] [PubMed] [Google Scholar]
- 18.Saint F., Patard J.J., Maille P. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette–Guérin treatment for superficial bladder cancer. J. Urol. 2002;167:364–367. [PubMed] [Google Scholar]
- 19.Luo Y., Chen X., O'Donnell M.A. Mycobacterium bovis bacillus Calmette–Guérin (BCG) induces human CC- and CXC-chemokines in vitro and in vivo. Clin. Exp. Immunol. 2007;147:370–378. doi: 10.1111/j.1365-2249.2006.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godaly G., Young D.B. Mycobacterium bovis bacille Calmette Guerin infection of human neutrophils induces CXCL8 secretion by MyD88-dependent TLR2 and TLR4 activation. Cell. Microbiol. 2005;7:591–601. doi: 10.1111/j.1462-5822.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig A.T., Moore J.M., Luo Y. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for Bacillus Calmette–Guerin-induced antitumor activity. Cancer Res. 2004;64:3386–3390. doi: 10.1158/0008-5472.CAN-04-0374. [DOI] [PubMed] [Google Scholar]
- 22.Böhle A., Gerdes J., Ulmer A.J. Effects of local bacillus Calmette–Guerin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. J. Urol. 1990;144:53–58. doi: 10.1016/s0022-5347(17)39365-5. [DOI] [PubMed] [Google Scholar]
- 23.Elsaesser J., Janssen M.W., Becker F. Antigen-specific CD4 T cells are induced after intravesical BCG-instillation therapy in patients with BC and show similar cytokine profiles as in active tuberculosis. PLoS One. 2013;8:e69892. doi: 10.1371/journal.pone.0069892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suttmann H., Jacobsen M., Reiss K. Mechanisms of bacillus Calmette–Guerin mediated natural killer cell activation. J. Urol. 2004;172:1490–1495. doi: 10.1097/01.ju.0000131944.52354.63. [DOI] [PubMed] [Google Scholar]
- 25.Brandau S., Suttmann H., Riemensberger J. Perforin-mediated lysis of tumor cells by Mycobacterium bovis Bacillus Calmette–Guérin-activated killer cells. Clin. Cancer Res. 2000;6:3729–3738. [PubMed] [Google Scholar]
- 26.Brandau S., Riemensberger J., Jacobsen M. NK cells are essential for effective BCG immunotherapy. Int. J. Cancer. 2001;92:697–702. doi: 10.1002/1097-0215(20010601)92:5<697::aid-ijc1245>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.Zuiverloon T.C.M., Nieuweboer A.J.M., Vekony H. Markers predicting response to Bacillus Calmette–Guerin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur. Urol. 2012:128–145. doi: 10.1016/j.eururo.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Miremami J., Kiprianou N. The promise of novel molecular markers in bladder cancer. Int. J. Mol. Sci. 2014;15:23,897–23,908. doi: 10.3390/ijms151223897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol. Oncol. 2008;26:646–651. doi: 10.1016/j.urolonc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Zachos I., Tzortzis V., Mitrakas L. Tumor size and T stage correlate independently with recurrence and progression in high-risk non-muscle-invasive bladder cancer patients treated with adjuvant BCG. Tumor Biol. 2014;35:4185–4189. doi: 10.1007/s13277-013-1547-8. [DOI] [PubMed] [Google Scholar]
- 31.Oddens J.R., Sylvester R.J., Brausi M.A. The effect of age on the efficacy of maintenance Bacillus Calmette–Guérin relative to maintenance epirubicin in patients with stage Ta T1 urothelial bladder cancer: results from EORTC Genito-Urinary Group Study 30911. Eur. Urol. 2014;66:694–701. doi: 10.1016/j.eururo.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 32.Palou J., Algaba F., Vera I. Protein expression patterns of ezrin are predictors of progression in T1G3 bladder tumours treated with nonmaintenance bacillus Calmette–Guerin. Eur. Urol. 2009;56:829–836. doi: 10.1016/j.eururo.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 33.Zlotta A.R., Noel J.C., Fayt I. Correlation and prognostic significance of p53, p21WAF1/CIP1 and Ki-67 expression in patients with superficial bladder tumors treated with bacillus Calmette–Guérin intravesical therapy. J. Urol. 1999;161:792–798. [PubMed] [Google Scholar]
- 34.Bertz S., Otto W., Denzinger S. Combination of CK20 and Ki-67 immunostaining analysis predicts recurrence, progression, and cancer-specific survival in pT1 urothelial bladder cancer. Eur. Urol. 2014;65:218–226. doi: 10.1016/j.eururo.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 35.Takayama H., Nishimura K., Tsujimura A. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette–Guerin instillation. J. Urol. 2009;181:1894–1900. doi: 10.1016/j.juro.2008.11.090. [DOI] [PubMed] [Google Scholar]
- 36.Andrew A.S., Gui J., Hu T. Genetic polymorphisms modify bladder cancer recurrence and survival in a USA population-based prognostic study. BJU Int. 2015;115:238–247. doi: 10.1111/bju.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leibovici D., Grossman H.B., Dinney C.P. Polymorphisms in inflammation genes and bladder cancer: from initiation to recurrence, progression, and survival. J. Clin. Oncol. 2005;23:5746–5756. doi: 10.1200/JCO.2005.01.598. [DOI] [PubMed] [Google Scholar]
- 38.Ahirwar D.K., Mandhani A., Mittal R.D. IL-8 _251 T > A polymorphism is associated with bladder cancer susceptibility and outcome after BCG immunotherapy in a northern Indian cohort. Arch. Med. Res. 2010;41:97–103. doi: 10.1016/j.arcmed.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Lima L., Ferreira J.A., Tavares A. FASL polymorphism is associated with response to bacillus Calmette–Guerin immunotherapy in bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2014;32:44.e1–44.e7. doi: 10.1016/j.urolonc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Chiong E., Kesavan A., Mahendran R. NRAMP1 and hGPX1 gene polymorphism and response to Bacillus Calmette–Guerin therapy for bladder cancer. Eur. Urol. 2011;59:430–437. doi: 10.1016/j.eururo.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 41.Mittal R.D., Singh R., Manchanda P.K. XRCC1 codon 399 mutant allele: a risk factor for recurrence of urothelial bladder carcinoma in patients on BCG immunotherapy. Cancer Biol. Ther. 2008;7:645–650. doi: 10.4161/cbt.7.5.5763. [DOI] [PubMed] [Google Scholar]
- 42.Ahirwar D.K., Agrahari A., Mandhani A., Mittal R.D. Cytokine gene polymorphisms are associated with risk of urinary BC and recurrence after BCG immunotherapy. Biomarkers. 2009;14:213–218. doi: 10.1080/13547500902818246. [DOI] [PubMed] [Google Scholar]
- 43.Pignot G., Cizeron-Clairac G., Vacher S. microRNA expression profile in a large series of bladder tumors: Identification of a 3-miRNA signature associated with aggressiveness of muscle-invasive bladder cancers. Int. J. Cancer. 2013;132:2479–2491. doi: 10.1002/ijc.27949. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira A.I., Jeronimo C., Henrique R. Moving forward in bladder cancer detection and diagnosis: the role of epigenetic biomarkers. Expert Rev. Mol. Diagn. 2012;12:871–878. doi: 10.1586/erm.12.114. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu T., Suzuki H., Nojima M. Methylation of a panel of microRNA genes is a novel biomarker for detection of bladder cancer. Eur. Urol. 2013;63:1091–1100. doi: 10.1016/j.eururo.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Baquero R., Puerta P., Beltran M. Methylation of a novel panel of tumor suppressor genes in urine moves forward noninvasive diagnosis and prognosis of bladder cancer: a 2-center prospective study. J. Urol. 2013;190:723–730. doi: 10.1016/j.juro.2013.01.105. [DOI] [PubMed] [Google Scholar]
- 47.Hauser S., Kogej M., Fechner G. Serum DNA hypermethylation in patients with bladder cancer: results of a prospective multicenter study. Anticancer Res. 2013;33:779–784. [PubMed] [Google Scholar]
- 48.Lima L., Severino P.F., Silva M. In vitro studies demonstrated higher adhesion and internalization of the bacillus to cells expressing sTn, promoting cell death. Br. J. Cancer. 2013;109:2106–2114. doi: 10.1038/bjc.2013.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boman K., Larsson A.H., Segersten U. Membranous expression of podocalyxin-like protein is an independent factor of poor prognosis in urothelial bladder cancer. Br. J. Cancer. 2013;108:2321–2328. doi: 10.1038/bjc.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guan Z., Zeng J., Wang Z. Urine tenascin-C is an independent risk factor for bladder cancer patients. Mol. Med. Rep. 2014;9:961–966. doi: 10.3892/mmr.2013.1873. [DOI] [PubMed] [Google Scholar]
- 51.Carretero R., Cabrera T., Gil H. Bacillus Calmette–Guerin immunotherapy of bladder cancer induces selection of human leukocyte antigen class I-deficient tumor cells. Int. J. Cancer. 2011;129:839–846. doi: 10.1002/ijc.25733. [DOI] [PubMed] [Google Scholar]
- 52.Kaempfer R., Gerez L., Farbstein H. Prediction of response to treatment in superficial bladder carcinoma through pattern of interleukin-2 gene expression. J. Clin. Oncol. 1996;14:1778–1786. doi: 10.1200/JCO.1996.14.6.1778. [DOI] [PubMed] [Google Scholar]
- 53.Saint F., Patard J.J., Maille P. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette–Guerin treatment for superficial bladder cancer. J. Urol. 2002;167:364–367. [PubMed] [Google Scholar]
- 54.Saint F., Kurth N., Maille P. Urinary IL-2 assay for monitoring intravesical bacillus Calmette–Guerin response of superficial bladder cancer during induction course and maintenance therapy. Int. J. Cancer. 2003;107:434–440. doi: 10.1002/ijc.11352. [DOI] [PubMed] [Google Scholar]
- 55.Tommaso C., Nesi G., Mazzoli S. Prediction of response to bacillus Calmette–Guerin treatment in non-muscle invasive bladder cancer patients through interleukin-6 and interleukin-10 ratio. Exp. Ther. Med. 2012;4:459–464. doi: 10.3892/etm.2012.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo Y., Han R., Evanoff D.P., Chen X. Interleukin-10 inhibits Mycobacterium bovis bacillus Calmette–Guerin (BCG)-induced macrophage cytotoxicity against bladder cancer cells. Clin. Exp. Immunol. 2010;160:359–368. doi: 10.1111/j.1365-2249.2010.04105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thalmann G.N., Dewald B., Baggiolini M., Studer U.E. Interleukin-8 expression in the urine after bacillus Calmette–Guerin therapy: a potential prognostic factor of tumor recurrence and progression. J. Urol. 1997;158:1340–1344. [PubMed] [Google Scholar]
- 58.Urquidi V., Chang M., Dai Y. IL-8 as a urinary biomarker for the detection of bladder cancer. BMC Urol. 2012;12:12. doi: 10.1186/1471-2490-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabinowitz R., Smith D.S., Tiemann D.D., Hudson M.A. Urinary interleukin-8/creatinine level as a predictor of response to intravesical bacillus Calmette–Guerin therapy in bladder tumor patients. J. Urol. 1997;158:1728–1731. doi: 10.1016/s0022-5347(01)64111-9. (1731–2) [DOI] [PubMed] [Google Scholar]
- 60.Sagnak L., Ersoy H., Ozok U. Predictive value of urinary interleukin-8 cutoff point for recurrences after transurethral resection plus induction bacillus Calmette–Guerin treatment in non-muscle invasive bladder tumors. Clin. Genitourin. Cancer. 2009;7:E16–E23. doi: 10.3816/CGC.2009.n.016. [DOI] [PubMed] [Google Scholar]
- 61.Takeuchi A., Dejima T., Yamada H. IL-17 production by γδ T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette–Guerin treatment against bladder cancer. Eur. J. Immunol. 2011;41:246–251. doi: 10.1002/eji.201040773. [DOI] [PubMed] [Google Scholar]
- 62.Thalmann G.N., Sermier A., Rentsch C. Urinary interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette–Guerin. J. Urol. 2000;164:2129–2133. [PubMed] [Google Scholar]
- 63.Li F., Chen D., He C. Identification of urinary Gc-globulin as a novel biomarker for bladder cancer by two-dimensional fluorescent differential gel electrophoresis 2D-DIGE. J. Proteomics. 2012;77:225–236. doi: 10.1016/j.jprot.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Park J., Song C., Shin E. Do molecular biomarkers have prognostic value in primary T1G3 BC treated with bacillus Calmette–Guerin intravesical therapy? Urol. Oncol. Semin. Orig. Investig. 2013;31:849–856. doi: 10.1016/j.urolonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 65.van Rhijn B.W.G., Liu L., Vis A.N. Prognostic value of molecular markers, sub-stage and European Organisation for the Research and Treatment of cancer risk scores in primary T1 bladder cancer. BJU Int. 2012;110:1169–1176. doi: 10.1111/j.1464-410X.2012.10996.x. [DOI] [PubMed] [Google Scholar]
- 66.Esuvaranathan K., Chiong E., Thamboo T.P. Predictive value of p53 and pRb expression in superficial bladder cancer patients treated with BCG and interferon-alpha. HLA class I antigen abnormalities and immune escape by malignant cells. Semin. Cancer Biol. 2002;12:3–13. [Google Scholar]
- 67.Ardelt P.U., Kneitz B., Adam P. Reactive antibodies against bacillus Calmette–Guerin heat-shock protein-65 potentially predict the outcome of immunotherapy for high-grade transitional cell carcinoma of the bladder. Cancer. 2010;116:600–609. doi: 10.1002/cncr.24770. [DOI] [PubMed] [Google Scholar]
- 68.Saitoh H., Mori K., Kudoh S. BCG effects on telomerase activity in bladder cancer cell lines. Int. J. Clin. Oncol. 2002;7:165–170. doi: 10.1007/s101470200024. [DOI] [PubMed] [Google Scholar]
- 69.Koskela L.R., Poljakovic M., Ehren I. Localization and expression of inducible nitric oxide synthase in patients after BCG treatment for bladder cancer. Nitric Oxide. 2012;27:185–191. doi: 10.1016/j.niox.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Mansoor I., Calam R.R., Al-Khafaji B. Role of urinary NMP-22 combined with urine cytology in follow-up surveillance of recurring superficial bladder urothelial carcinoma. Anal. Quant. Cytol. Histol. 2008;30:25–32. [PubMed] [Google Scholar]
- 71.Miyake M., Goodison S., Giacoia E.G. Influencing factors on the NMP-22 urine assay: an experimental model. BMC Urol. 2012;12:23. doi: 10.1186/1471-2490-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly J.D., Dudderidge T.J., Wollenschlaeger A. Bladder cancer diagnosis and identification of clinically significant disease by combined urinary detection of Mcm5 and nuclear matrix protein 22. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040305. e40305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lorenzi T., Lorenzi M., Altobelli E. HtrA1 in human urothelial bladder cancer: a secreted protein and a potential novel biomarker. Int. J. Cancer. 2013;133:2650–2661. doi: 10.1002/ijc.28280. [DOI] [PubMed] [Google Scholar]
- 74.Yun S.J., Jo S.-W., Ha Y.-S. PFKFB4 as a prognostic marker in non-muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2012;30:893–899. doi: 10.1016/j.urolonc.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 75.Izumi K., Li Y., Ishiguro H. Expression of UDP-glucuronosyltransferase 1A in bladder cancer: Association with prognosis and regulation by estrogen. Mol. Carcinog. 2014;53:314–324. doi: 10.1002/mc.21978. [DOI] [PubMed] [Google Scholar]
- 76.Mirams G.R., Davies M.R., Cui Y. Application of cardiac electrophysiology simulations to pro-arrhythmic safety testing. Br. J. Pharmacol. 2012;167:932–945. doi: 10.1111/j.1476-5381.2012.02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neal M.L., Trister A.D., Ahn S. Response classification based on a minimal model of glioblastoma growth is prognostic for clinical outcomes and distinguishes progression from pseudoprogression. Cancer Res. 2013;73:2976–2986. doi: 10.1158/0008-5472.CAN-12-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frieboes H.B., Edgerton M.E., Fruehauf J.P. Prediction of drug response in breast cancer using integrative experimental/computational modelling. Cancer Res. 2009;69:4484–4492. doi: 10.1158/0008-5472.CAN-08-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pham K., Frieboes H.B., Cristini V., Lowengrub J. Predictions of tumour morphological stability and evaluation against experimental observations. J. R. Soc. Interface. 2011;8:16–29. doi: 10.1098/rsif.2010.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palladini A., Nicoletti G., Pappalardo F. In silico modeling and in vivo efficacy of cancer-preventive vaccinations. Cancer Res. 2010;70:7755–7763. doi: 10.1158/0008-5472.CAN-10-0701. [DOI] [PubMed] [Google Scholar]
- 81.Bunimovich-Mendrazitsky S., Shochat E., Stone L. Mathematical model of BCG immunotherapy in superficial bladder cancer. Bull. Math. Biol. 2007;69:1847–1870. doi: 10.1007/s11538-007-9195-z. [DOI] [PubMed] [Google Scholar]
- 82.Bunimovich-Mendrazitsky S., Byrne H.M., Stone L. Mathematical model of pulsed immunotherapy for superficial bladder cancer. Bull. Math. Biol. 2008;70:2055–2276. doi: 10.1007/s11538-008-9344-z. [DOI] [PubMed] [Google Scholar]
- 83.Bunimovich-Mendrazitsky S., Chaskalovic J., Gluckman J.-C. A mathematical model of combined Bacillus Calmette–Guerin (BCG) and interleukin (IL)-2 immunotherapy of superficial bladder cancer. J. Theor. Biol. 2011;277:27–40. doi: 10.1016/j.jtbi.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 84.Bunimovich-Mendrazitsky S., Halachmi S., Kalev N. Improving Bacillus Calmette Guerin (BCG) immunotherapy for bladder cancer by adding interleukin-2 (IL-2): a mathematical model. Math. Med. Biol. 2015:1–30. doi: 10.1093/imammb/dqv007. [DOI] [PubMed] [Google Scholar]
- 85.Kirschner D., Panetta J. Modelling immunotherapy of the tumor-immune interaction. J. Math. Biol. 1998;37(3):235–252. doi: 10.1007/s002850050127. [DOI] [PubMed] [Google Scholar]
- 86.Kuznetsov V.A., Makalkin I.A., Taylor M.A., Perelson A.S. Nonlinear dynamics of immunogenic tumours: parameter estimation and global bifurcation analysis. Bull. Math. Biol. 1994;56:295–321. doi: 10.1007/BF02460644. [DOI] [PubMed] [Google Scholar]
- 87.De Pillis L.G., Radunskaya A.E., Wiseman C.L. A validated mathematical model of cell-mediated immune response to tumor growth. Cancer Res. 2005;65(17):7950–7958. doi: 10.1158/0008-5472.CAN-05-0564. [DOI] [PubMed] [Google Scholar]
- 88.Lamm D.L., Blunemstein B.A., Crissman J.D. Maintenance BCG immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J. Urol. 2000;163:1124–1129. [PubMed] [Google Scholar]
- 89.Wang Z., Deisboeck T.S. Mathematical modeling in cancer drug discovery. Drug Discov. Today. 2013;19:145–150. doi: 10.1016/j.drudis.2013.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.