Abstract
Background
Long chain polyunsaturated fatty acids (LCPUFAs) are biologically active fatty acids which regulate placental angiogenesis, inflammation, and oxidative stress. Abnormalities in these aspects have been associated with preeclampsia (PE). Further, placenta has a heterogeneous structure with differential vascularization across different regions. We therefore hypothesize that the distribution of fatty acids in various regions of the placenta is altered in PE leading to poor fetal outcome.
Methods
In this cross-sectional study we recruited 69 normotensive control (NC) and 44 women with PE. PE women were further classified as those delivered preterm (PTPE, n = 24) and at term (TPE, n = 20). Fatty acid levels were analyzed from placental samples from four different regions (CF—central fetal, PF—peripheral fetal, CM—central maternal and PM—peripheral maternal).
Results
In the NC placenta, AA levels were lower (p < 0.05) in CM as compared with CF region. However, such differences were not seen in the TPE and PTPE. In contrast, the DHA levels varied between regions only in the PTPE placenta. Between groups, DHA levels were lower (p < 0.05 for both) in the CM and CF regions of the PTPE as compared with NC. The levels of DHA in TPE placenta were similar to NC. AA levels were lower (p < 0.05 for both) in CF region of TPE and PF region of PTPE placenta than NC.
Conclusions
There is differential pattern of LCPUFA distribution across various regions of the NC, TPE and PTPE placenta. This may have implications for placental growth and development as well as transfer of LCPUFA to the fetus.
Keywords: Preeclampsia, Regional placenta, Birth weight, Blood pressure, Polyunsaturated fatty acids
Graphical abstract
Highlights
-
•
There are regional differences in fatty acid levels in normal placenta.
-
•
Regional fatty acid distribution is further affected in preeclampsia.
-
•
Preterm preeclampsia placenta is more affected than term preeclampsia and control.
-
•
DHA of peripheral fetal region positively associated with baby weight in preeclampsia.
1. Introduction
Preeclampsia (PE) is a pregnancy induced hypertensive disorder which manifests after 20 weeks of gestation and till date its etiology is not well understood [1]. A number of placental abnormalities have been associated with PE pregnancies, such as insufficient spiral artery remodeling, shallow invasion, and reduced villous number, diameter and surface area. Placental function gets affected by these abnormalities, and ultimately deprives the developing fetus of the nutrients required for optimal growth [2], [3]. Nutrients like fatty acids are not only required by the developing fetus but also metabolized by the placenta for its growth and development [4].
Fatty acids especially long chain polyunsaturated fatty acids (LCPUFAs) are biologically active fatty acids which have been found to be associated with a number of developmental and functional aspects of the placenta. LCPUFAs are required by the placenta for membrane synthesis and to maintain fluidity for intercellular signaling [5]. Further, LCPUFAs act as ligands for transcription factors which regulate genes involved in trophoblast proliferation and differentiation [6]. Our earlier studies on women with PE report altered placental LCPUFA levels, disturbed angiogenesis and fatty acid transport in the maternal region of the placenta [7], [8]. It is well known that the morphological heterogeneity of the placenta comprises of both fetal and maternal tissues [9], [10], [11]. Differences in vascularisation across the placenta resulting in differences of oxygen availability are also reported within the placenta [12], [13]. Several studies have reported gradients in protein levels, gene expression and enzyme activities across the normal placenta [14], [15], [16], [17], [18]. Therefore, there could be functional specialization of different regions of the placenta which requires extensive research.
PE has also been associated with increased oxidative stress and inflammation originating from the placenta and affecting both mother and the fetus [19], [20]. LCPUFA can form active metabolites like eicosanoids, resolvins and protectins which regulates inflammation and it can also reduce oxidative damage in the trophoblast cells [21]. In the presence of reactive oxygen species, LCPUFA get per-oxidized into malondialdehyde (MDA) which is an oxidative stress marker [22]. Recently we have shown increased levels of MDA and decreased levels of catalase an antioxidant enzyme in a region specific manner in the PE placenta [23]. Further, studies have also reported regionwise alterations in the inflammatory cytokines, nitric oxide synthase enzyme and heat shock proteins in the PE placenta [20], [24], [25], [26].
It is likely that the LCPUFA metabolism may differ in the placenta depending upon the requirement by the trophoblast cells for different physiological processes. We therefore hypothesize that the regional distribution of fatty acids in the placenta is altered in preeclampsia leading to poor birth outcome. In the current study we examined the levels of fatty acid in various regions of the PE and normotensive control (NC) placenta and compared the levels within and between groups. Further, the associations of LCPUFA levels with birth weight and blood pressure were studied.
2. Materials and methods
2.1. Study participants
This is a cross-sectional study where pregnant women were recruited at Department of Obstetrics and Gynecology of Bharati Hospital, Pune, India. This study was ethically approved by the Bharati Vidyapeeth Medical College Institutional Ethical Committee, Pune, India. Written informed consents were taken from each participant. Maternal weight and height were taken at the time of recruitment for the calculate body mass index (BMI). A total of 69 normotensive pregnant women delivering at term (gestation ≥ 37 weeks, baby weight ≥ 2.5 kg) and 44 pregnant women with PE were recruited for the study. PE was defined by systolic BP and diastolic BP > 140 and 90 mm Hg (repeated measures by Mercury sphygmomanometer) respectively and the presence of proteinuria (> 1 + or 300 mg per 24 h, by Dipstick test). The detailed exclusion and inclusion criteria were as described by us earlier [23]. The PE cases were further divided into 24 preterm PE (PTPE) and 20 term PE (TPE) after delivery. Baby birth weight was measured using a digital weighing scale (Zeal Medical Private Limited, India) with 10 g accuracy. Baby length was measured using a portable infantometer.
2.2. Placental sampling
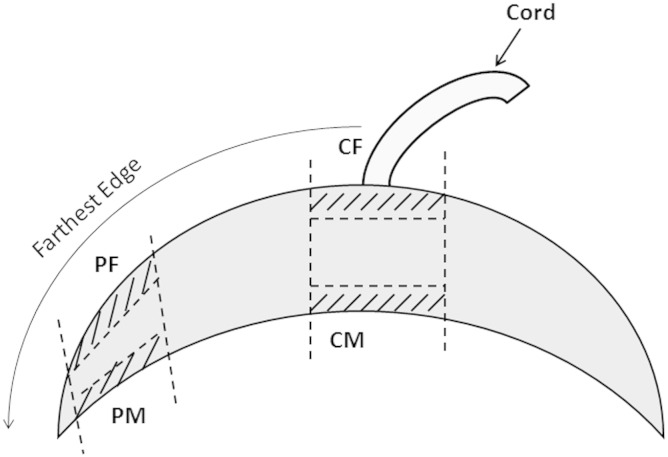
Placenta was collected in 1X phosphate buffer saline (PBS) immediately following delivery. Small pieces of fresh tissues were collected from four different sites of the placenta after removing the fetal membrane as described in our recent study [23]. Velamentous cord insertion cases were excluded from the study. Briefly, four sampling sites were selected 1) CM (central maternal) — small pieces (~ 1 cm2) of basal plate villous tissue were cut out from the normal appearing cotyledons around the cord insertion avoiding blood clots and infarcts, 2) PM (peripheral maternal) — from peripheral cotyledons of the basal plate farthest from the cord insertion and 1 cm away from the lateral edge of the placental disk, similarly 3) CF (central fetal) — from central cotyledons of the chorionic plate and 4) PF (peripheral fetal) — from peripheral cotyledons of the chorionic plate (Fig. 1). Excess blood was washed off with 1X PBS and then the samples were stored at − 80 °C till further analyzed.
Fig. 1.
Sampling sites of the placenta.
CM — central maternal region, PM — peripheral maternal region, CF — central fetal region, PF — peripheral fetal region.
2.3. Fatty acid analysis
The fatty acid estimation procedure followed for analysis was as reported in series of our earlier studies [7], [27]. Briefly, placental tissue was homogenized with chilled lysis buffer and ultra-centrifuged. Transesterification of fatty acids present in the cell membrane fraction was done using methanolic–hydrochloric acid. These fatty acid methyl esters (FAMEs) were analyzed using a Perkin Elmer Gas Chromatograph at our standardized settings (SP 2330, 30 m capillary Supelco column). A mixture of standard fatty acid methyl esters (Sigma) was used to identify 15 important fatty acids by retention time. The relative FAME amounts were expressed as g/100 g fatty acid (% total fatty acids). Fatty acids were categorized into total omega 6 fatty acids (omega 6): summation of linoleic acid (LA), gamma linolenic acid, dihomo gamma linolenic acid, docosapentaenoic acid and arachidonic acid (AA); total omega 3 fatty acids (omega 3): summation of alpha linolenic acid (ALA), eicosapentaenoic acid and docosahexaenoic acid (DHA); saturated fatty acids (SFAs): summation of myristic acid, palmitic acid and stearic acid; and monounsaturated fatty acids (MUFAs): summation of myristoleic acid, palmitoleic acid, oleic acid and nervonic acid.
2.4. Statistical analysis
The sample size was calculated based on our earlier study taking the mean difference of placental DHA levels between the case and the control [7]. It was done using PS sample size calculator software (ver. 3.0.43) with 80% power of the study, 0.05 type I error probability and 3 control subjects per case. Hence, 69 controls and around 23 cases in each PE group were included in this study.
The data was analyzed using the SPSS/PC + package (Version 20, Chicago, IL, USA). Values are reported as Mean ± Standard Deviation (S.D.). Skewed variables were normalized using the log10 transformation. Means were compared using one way ANOVA. Associations with birth weight and BP were performed using Pearson's partial correlation after adjusting for confounder i.e. gestational age, maternal age and BMI in NC and PE as separate groups. It was also done for whole cohort after adjusting for groups. Results were considered significant when p < 0.05.
3. Results
3.1. Maternal and neonatal characteristics
The systolic BP and diastolic BP of both PTPE and TPE women were higher (p < 0.01 for all) as compared with NC women. PTPE women had even higher systolic BP and diastolic BP (p < 0.01 for both) as compared with TPE women. BMIs of both the TPE (p < 0.01) and PTPE (p < 0.05) women were higher as compared with NC women.
In PTPE and TPE groups, birth weight and baby length were lower (p < 0.01 for all) as compared with NC group. These birth measures were even lower (p < 0.01 for both) in PTPE as compared with TPE group (Table 1).
Table 1.
Maternal and neonatal demographic characteristics.
| Groups | NC (n = 69) | TPE (n = 20) | PTPE (n = 24) |
|---|---|---|---|
| Maternal age (Yr) | 24.38 ± 3.47 | 25.24 ± 3.62 | 27.26 ± 4.87**,&& |
| Income (Rs) | 11,303.03 ± 7749.88 | 15,700 ± 12,260.76** | 10,673.91 ± 7612.20&& |
| BMI (kg/m2) | 24.91 ± 4.07 | 29.35 ± 4.68** | 27.18 ± 5.35* |
| Sys BP (mm Hg) | 119.19 ± 8.27 | 151.90 ± 18.06** | 155.74 ± 19.67**,&& |
| Dias BP (mm Hg) | 77.04 ± 5.19 | 94.86 ± 10.31** | 100.87 ± 11.31**,&& |
| Gestation (weeks) | 38.86 ± 1.14 | 38.49 ± 1.43 | 34.07 ± 2.13**,&& |
| Parity (%) | |||
| Nulliparous | 40.3 | 55 | 33.3 |
| Multiparous | 59.7 | 45 | 66.7 |
| Education (%) | |||
| Illiterate | 8.7 | 5 | 0 |
| Lower | 39.1 | 45 | 41.6 |
| Higher | 17.4 | 25 | 25 |
| Graduation | 31.9 | 25 | 29.2 |
| Post graduation | 2.9 | 0 | 4.2 |
| MOD (n) | |||
| Vaginal | 56 | 9 | 4 |
| C-section | 13 | 11 | 20 |
| Birth weight (kg) | 2.90 ± 0.31 | 2.62 ± 0.57** | 1.70 ± 0.44**,&& |
| Baby length (cm) | 49.30 ± 3.14 | 47.48 ± 2.87** | 42.21 ± 3.68**,&& |
| Baby sex (n) | |||
| Male | 40 | 9 | 19 |
| Female | 29 | 11 | 5 |
NC — normotensive control, TPE — term preeclampsia; PTPE — preterm preeclampsia; BMI — body mass index; Sys BP — systolic blood pressure; Dias BP — diastolic blood pressure; Education: Lower —grades 1 to 10, Higher —grades 11 to 12 or intermediate; MOD — mode of delivery; C-section — cesarean delivery; n — number; *p < 0.05; **p < 0.01 as compared with NC, &&p < 0.01 as compared with TPE. Data represented as Mean ± S.D.
3.2. Comparison of fatty acid levels across different placental regions within normotensive control, preterm-preeclampsia and term-preeclampsia
In the NC group, no differences were observed in the levels of LA within the placenta. However, AA levels were higher (p < 0.05 for both) in CF and PF regions as compared with the CM region. Omega 6 fatty acid levels were higher (p < 0.05 and p < 0.01 respectively) in the PF region as compared with the PM and CM regions. There were no significant regional differences in the levels of ALA, DHA, omega 3 fatty acid, omega 6:omega 3 and SFA. Further, MUFA levels were lower (p < 0.01 and p < 0.05 respectively) in the CF and PF regions as compared with the CM region.
In the PTPE group, no regional differences were observed in any of the omega 6 fatty acid levels. DHA, omega 3 fatty acid and MUFA levels were lower (p < 0.05 for all) in the CF region as compared with the PM region. Omega 6:omega 3 fatty acid ratio was higher (p < 0.01 and p < 0.05 respectively) in the CF region as compared with the PM and PF regions. SFA levels were higher (p < 0.05) in the CM as compared with the PM region.
In the TPE group, no regional differences were observed in any of the fatty acid levels within the placenta. All the results are represented in Table 2.
Table 2.
Regionwise fatty acid levels in normotensive control and preeclampsia placenta.
| Fatty acids (g/100 g fatty acids) | Groups | CM | CF | PM | PF |
|---|---|---|---|---|---|
| LA | NC | 10.22 ± 2.11 | 9.79 ± 2.47 | 9.90 ± 2.23 | 10.16 ± 2.59 |
| TPE | 9.39 ± 2.73 | 10.34 ± 2.18 | 9.69 ± 2.16 | 10.27 ± 2.64 | |
| PTPE | 9.92 ± 2.21 | 9.86 ± 2.15 | 10.40 ± 1.98 | 10.40 ± 1.86 | |
| AA | NC | 23.04 ± 4.76 | 24.80 ± 4.80@ | 23.52 ± 5.43 | 24.76 ± 5.03@ |
| TPE | 22.97 ± 3.65 | 22.37 ± 2.83* | 22.22 ± 3.79 | 22.82 ± 4.55 | |
| PTPE | 21.78 ± 5.19 | 24.17 ± 5.48 | 22.60 ± 4.53 | 22.33 ± 4.67* | |
| Omega 6 | NC | 37.42 ± 4.20 | 38.73 ± 3.44 | 37.48 ± 4.72 | 39.22 ± 3.68@@,% |
| TPE | 36.55 ± 3.05 | 37.33 ± 2.31 | 36.21 ± 3.91 | 37.63 ± 4.57 | |
| PTPE | 35.69 ± 4.72 | 37.99 ± 5.23 | 37.28 ± 3.66 | 36.93 ± 3.93* | |
| ALA | NC | 0.17 ± 0.15 | 0.15 ± 0.10 | 0.15 ± 0.12 | 0.18 ± 0.35 |
| TPE | 0.23 ± 0.23 | 0.18 ± 0.10 | 0.20 ± 0.16 | 0.17 ± 0.14 | |
| PTPE | 0.24 ± 0.16 | 0.21 ± 0.13* | 0.19 ± 0.12 | 0.22 ± 0.13* | |
| DHA | NC | 1.94 ± 0.73 | 1.80 ± 0.75 | 1.99 ± 0.87 | 1.85 ± 0.65 |
| TPE | 1.85 ± 0.74 | 2.01 ± 0.69 | 1.97 ± 0.90 | 2.00 ± 0.78 | |
| PTPE | 1.44 ± 0.75**,& | 1.43 ± 0.82*,& | 1.80 ± 0.77# | 1.60 ± 0.54& | |
| Omega 3 | NC | 2.28 ± 0.70 | 2.13 ± 0.74 | 2.31 ± 0.84 | 2.23 ± 0.78 |
| TPE | 2.27 ± 0.67 | 2.40 ± 0.65 | 2.31 ± 0.91 | 2.38 ± 0.79 | |
| PTPE | 1.88 ± 0.74*,& | 1.80 ± 0.84*,&& | 2.21 ± 0.72# | 2.08 ± 0.72 | |
| Omega 6:omega 3 | NC | 18.20 ± 6.71 | 20.54 ± 7.67 | 18.41 ± 7.18 | 19.76 ± 7.32 |
| TPE | 17.26 ± 4.38 | 16.93 ± 6.00 | 18.97 ± 11.07 | 17.84 ± 6.85 | |
| PTPE | 21.04 ± 6.30& | 25.01 ± 11.96*,&& | 18.72 ± 6.90## | 19.09 ± 4.61# | |
| SFA | NC | 41.97 ± 4.29 | 42.17 ± 4.04 | 42.09 ± 4.55 | 41.22 ± 3.14 |
| TPE | 43.84 ± 3.44 | 43.84 ± 3.43 | 44.53 ± 4.12* | 43.91 ± 4.77** | |
| PTPE | 44.84 ± 4.36** | 43.66 ± 3.77 | 42.50 ± 2.41@ | 43.47 ± 3.51** | |
| MUFA | NC | 7.60 ± 2.29 | 6.72 ± 1.54@@ | 7.29 ± 2.33 | 6.82 ± 1.63@ |
| TPE | 7.05 ± 1.33 | 6.79 ± 1.66 | 6.83 ± 1.49 | 6.78 ± 1.49 | |
| PTPE | 7.46 ± 1.82 | 6.73 ± 1.83 | 7.85 ± 1.53# | 7.26 ± 1.35 |
CM — central maternal region, CF — central fetal region, PM — peripheral maternal region, PF — peripheral fetal region; NC — normotensive control, TPE — term preeclampsia and PTPE — preterm preeclampsia; **p < 0.01, *p < 0.05 as compared with NC and &&p < 0.01, &p < 0.05 as compared with TPE in the corresponding region; @@p < 0.01, @p < 0.05 as compared with CM, ##p < 0.01, #p < 0.05 as compared with CF and %p < 0.05 as compared with PM of the same group; LA — linoleic acid, AA — arachidonic acid, omega 6 — total omega 6 fatty acid, ALA — alpha linolenic acid, DHA — docosahexaenoic acid, omega 3 — total omega 3 fatty acid, omega 6:omega 3 — total omega 6 fatty acids/total omega 3 fatty acid, SFA — saturated fatty acid, MUFA — monounsaturated fatty acid; data represented as Mean ± S.D.
3.3. Comparison of placental fatty acid levels between normotensive control, preterm-preeclampsia and term-preeclampsia groups
When PTPE placenta was compared with NC placenta, LA levels were similar in both the groups in all the four regions. AA and omega 6 fatty acid levels were lower (p < 0.05 for both) in the PF region. ALA levels were higher (p < 0.05 for both) in CF and PF regions. In contrast, DHA levels were lower (p < 0.01 and p < 0.05 respectively) in CM and CF regions. Similarly, omega 3 fatty acid levels were lower (p < 0.05 for both) in CM and CF regions. Omega 6:omega 3 fatty acid ratio was higher (p < 0.05) in CF region and similar trend (p = 0.05) was there in the CM region. SFA levels were higher in CM and PF (p < 0.01 for both) regions.
When TPE placenta was compared with the NC placenta, AA levels were lower (p < 0.05) in the CF region. SFA levels were higher (p < 0.05 and p < 0.01 respectively) in PM and PF regions. Other fatty acids did not show any difference between the two groups.
When PTPE placenta was compared with TPE placenta, DHA levels were lower (p < 0.05 for all) in CM, CF and PF regions. Omega 3 fatty acid levels were lower (p < 0.05 and p < 0.01 respectively) in CM and CF regions. ALA, LA, AA and omega 6 fatty acid did not show any difference between the two groups. Omega 6:omega 3 fatty acid ratio was higher (p < 0.05 and p < 0.01 respectively) in CM and CF regions. MUFA and SFA levels did not show any significant differences. All the results are represented in Table 2.
3.4. Regionwise association of LCPUFA with birth weight and blood pressure
DHA of PF region was positively (n = 39, r = 0.35, p = 0.035) associated with birth weight in the PE group (combining TPE and PTPE) and showed similar trend (n = 94, r = 0.2, p = 0.053) in the whole cohort.
Omega 6 fatty acid of CF region was positively (n = 54, r = 0.29, p = 0.037) associated with maternal systolic BP in the NC group and showed similar correlation (n = 93, r = 0.25, p = 0.019) in the whole cohort. Omega 6 fatty acid of PF region was positively (n = 39, r = 0.36, p = 0.032) associated with maternal systolic BP in the PE group and showed similar correlation (n = 93, r = 0.22, p = 0.042) in the whole cohort.
4. Discussion
To the best of our knowledge this study for the first time demonstrates regional fatty acid distribution in the normotensive and preeclampsia placenta. We have observed differential regional fatty acid distribution in both NC and PE placenta. Important findings of this study within groups are (1) AA levels were higher in CF region as compared to CM region of the placenta only in NC. (2) Omega 6:omega 3 fatty acid ratio was higher in the CF region as compared to PF region of the placenta only in PTPE.
Analysis of the data between groups indicates that (1) ALA levels were higher in both the fetal regions (CF & PF) of the placenta in the PTPE as compared with NC. However, DHA and omega 3 fatty acid levels were lower in both the central regions (CM & CF) of the placenta in PTPE as compared with NC and TPE. (2) AA and omega 6 fatty acid levels were lower in the PF region of the placenta in PTPE as compared with NC. (3) SFA levels were higher in PF region of the placenta in TPE and PTPE as compared with NC. (4) DHA of PF region was positively associated with baby birth weight in overall PE group. (5) Omega 6 fatty acid of the fetal regions (CF and PF) was positively associated with maternal systolic BP in whole cohort.
4.1. Regional fatty acid levels within normotensive and preeclampsia placenta
In the current study, AA levels were higher in the CF region of the placenta as compared with CM and PM regions in the NC group. A combined analysis of AA and omega 6 fatty acid levels in maternal regions (Avg of CM & PM) with fetal regions (Avg of CF & PF) indicated that the levels of these fatty acids were higher in the fetal side as compared to the maternal side (data not shown) and may possibly indicate a preferential transfer of LCPUFA from the mother to the fetus. It has been reported that placental fatty acid binding and transport proteins (FABPs & FATPs) preferentially transfer LCPUFA which shifts the concentration gradient towards the fetal side of the placenta [28], [29]. AA is reported to dominate in the placental phospholipid membranes suggesting an important role in maintaining membrane structure, function, and integrity [30]. Further, its active metabolites like eicosanoids are also required by the fetus to regulate various metabolic processes [31].
In this study, we have reported lower levels of AA in TPE as well as PTPE placenta in the fetal regions (CF and PF respectively). Due to this the concentration gradient of AA from maternal to the fetal side which was observed in the NC placenta disappeared in PE placenta. The placental pathology may have altered this gradient in PE through dysregulation of fatty acid transport and binding proteins.
In this study, DHA and omega 3 fatty acid levels were lower in the CF region as compared to the PM region of the placenta in the PTPE group. A combined analysis of omega 3 fatty acid levels in the central regions (Avg of CM & CF) with the peripheral regions (Avg of PM & PF) (data not shown) also suggests lower levels in the central region (with respect to the cord insertion). Reports indicate that the onset of maternal blood flow starts earlier and is higher in the center in the abnormal placenta than the normal placenta [32]. This has been attributed to insufficient trophoblastic migration and vascular plugging which results in abnormally high maternal blood flow affecting the normal development of the villous tree in the center of the placenta [19]. Such conditions affect the placental functions by disrupting the nutrient transport system of the trophoblast cells [33]. Hence, lower omega 3 fatty acid may be due to an inefficient fatty acid metabolism and transfer in the center of the placenta in PTPE group.
The present study showed highest omega 6:omega 3 fatty acid ratio in CF region of the placenta as compared to both the peripheral regions (PM & PF) in PTPE group. Various studies have associated elevated ratio of omega 6 to omega 3 fatty acids with increased risk of PE [34], [35], [36]. Higher proportions of omega 6 fatty acids are known to increase inflammation due to excessive formation of inflammatory eicosanoids [37]. In contrast omega 3 fatty acid EPA and DHA form protectins and resolvins those are known to have anti-inflammatory properties [38]. Hence, the increased ratio of these LCPUFA may contribute to increased inflammation in the center of the placenta.
The pathology of PTPE is known to be more severe than TPE [39]. It has also been reported that TPE placenta has minimal histopathological features than the control placenta [40]. However, PTPE histopathology reports chronic inflammatory and placental vasoocclusive lesions [41]. This may explain why regional differences in LCPUFA levels are more prominent in PTPE placenta than in the TPE placenta in this study.
LCPUFAs are susceptible to peroxidation and its byproduct MDA forms adduct with proteins and DNA, injuring the cell [42]. Recently we have reported lower MDA levels in the CF as compared with the CM region of the PTPE placenta [23]. However, in the current study no such differences were observed in any of the LCPUFA levels between these two regions in PTPE placenta.
Our results indicate differential regional fatty acid distribution in the placenta. Reports suggest that differential placental trophoblast cell progression and onset of maternal blood are the two main phenomena which regionally affect the trophoblast differentiation as it is controlled by the interplay of oxygen tension, transcription factors, hormones, growth factors, and other signaling molecules [12], [43].
4.2. Regional fatty acid levels between normotensive control and preeclampsia placenta
In the current study, ALA levels were higher in both the fetal regions (CF & PF) of the placenta of PTPE as compared to NC group. However, DHA levels were lower in the CF region of PTPE placenta as compared to control. ALA is an essential omega 3 fatty acid and the conversion of ALA to DHA depends upon the availability of several desaturase and elongase enzymes [44]. We have reported the presence of desaturase enzymes in the placenta and its lower expression in PE [7]. The observed lower DHA levels despite higher precursor ALA levels may indicate altered biosynthesis in the PTPE placenta.
DHA levels were lower in both the central regions (CM & CF) of the placenta in PTPE as compared to NC and TPE groups. Similar observations were seen in the case of omega 3 fatty acid. Essential role of omega 3 fatty acids especially DHA in fetal brain and retinal development is well documented in the literature [45], [46]. Omega 3 fatty acids are not only required for fetal development but also to maintain several metabolic processes in the placenta [21]. Maximum accretion of DHA to the fetus has been reported to occur in the third trimester of the pregnancy [47]. We have extensively demonstrated the importance of DHA during pregnancy and effects of its reduced levels in pregnancy disorders [8], [48], [49].
The levels of SFA were higher in PM and PF regions of the of TPE and CM and PF regions of the PTPE placenta as compared to NC. Animal studies have shown high saturated fat diet during pregnancy increases placental oxidative stress and loss of trophoblast cells [50], [51]. Our recent departmental study has shown increased oxidative stress in all the regions of the TPE as well as PTPE placenta [23].
4.3. Association of LCPUFA with birth weight and blood pressure
In this study, DHA levels of the PF region were positively associated with birth weight in the PE group. Similar trend was also observed in the whole cohort although not significant. Reports indicate that maternal DHA levels are positively associated with birth weight [52], [53], [54]. However, future studies need to examine the possible mechanisms behind the role of PF region in determining the fetal outcome.
The present study reports positive association between omega 6 fatty acid of the fetal regions (CF and PF) with systolic BP in whole cohort. Omega 6 fatty acids are known to increase inflammation [55]. Inflammation may play a significant role in the incidence of high blood pressure [56]. Further, the fetal region of the placenta has been reported to have higher endothelin-1 receptors which are potent vasoconstrictors affecting the renal function and blood flow associated with the pregnancy disorders [57], [58].
4.4. Limitations
Consistent with our earlier and other studies, in this study, women with PE had a higher BMI [7], [59]. It is known that obesity increases the risk of PE [60]. However, in this study it was not possible to compare obese women with or without PE which is a limitation of the study. Secondly, the PE women delivering preterm were not compared with controls of similar gestational age which is another limitation of the study.
5. Conclusion
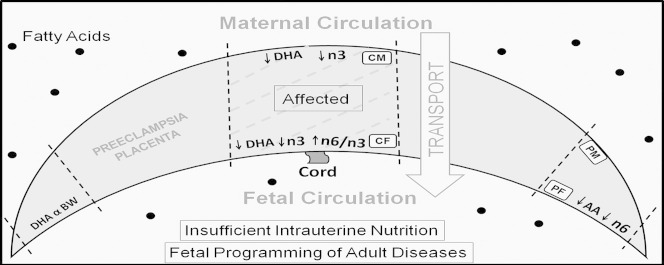
This study confirms regional differences in fatty acid levels in normal placenta which is further altered in preeclampsia. Further, it was observed that placenta is more affected in PTPE with greater alterations in the regional LCPUFA levels as compared with control (Fig. 2). A disturbed fatty acid distribution in the PE placenta in a region specific manner may have an implication for altered regional placental growth thereby influencing fetal development leading to programming for adult diseases. Further investigations on regional differences in fatty acid transport proteins, binding proteins and desaturase enzymes in the placenta are needed to explain the differential distribution of fatty acids observed in this study.
Fig. 2.
Regionwise alterations in fatty acid levels in the preterm preeclampsia placenta as compared with control; DHA — docosahexaenoic acid, AA — arachidonic acid, n3 — omega 3 fatty acids, n6 — omega 6 fatty acids; BW — birth weight, CM — central maternal region, PM — peripheral maternal region, CF — central fetal region, PF — peripheral fetal region.
Acknowledgements
The authors are grateful to the staff members of the Department of Ob&Gy, Bharati Hospital, Pune, India for their assistance in obtaining placenta samples and to the participants of this study. Author AR was the recipient of an ‘INSPIRE fellowship’ from the Department of Science and Technology, Government of India.
References
- 1.Craici I.M., Wagner S.J., Weissgerber T.L., Grande J.P., Garovic V.D. Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int. 2014;86:275–285. doi: 10.1038/ki.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regnault T.R., Galan H.L., Parker T.A., Anthony R.V. Placental development in normal and compromised pregnancies — a review. Placenta. 2002;23(Suppl. A):S119–S129. doi: 10.1053/plac.2002.0792. [DOI] [PubMed] [Google Scholar]
- 3.Roberts J.M., Escudero C. The placenta in preeclampsia. Pregnancy Hypertens. 2012;2:72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shekhawat P., Bennett M.J., Sadovsky Y., Nelson D.M., Rakheja D., Strauss A.W. Human placenta metabolizes fatty acids: implications for fetal fatty acid oxidation disorders and maternal liver diseases. Am. J. Physiol. 2003;284:E1098–E1105. doi: 10.1152/ajpendo.00481.2002. [DOI] [PubMed] [Google Scholar]
- 5.Calder P.C. Mechanisms of action of (n − 3) fatty acids. J. Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 6.Duttaroy A.K., Basak S. Docosahexaenoic acid and angiogenesis: a role in early placentation. Clin. Lipidol. 2012;7:303–312. [Google Scholar]
- 7.Wadhwani N., Patil V., Pisal H., Joshi A., Mehendale S., Gupte S., Wagh G., Joshi S. 2014. Altered Maternal Proportions of Long Chain Polyunsaturated Fatty Acids and Their Transport Leads to Disturbed Fetal Stores in Preeclampsia, Prostaglandins, Leukotrienes, and Essential Fatty Acids. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni A.V., Mehendale S.S., Yadav H.R., Joshi S.R. Reduced placental docosahexaenoic acid levels associated with increased levels of sFlt− 1 in preeclampsia. Prostaglandins Leukot. Essent. Fatty Acids. 2011;84:51–55. doi: 10.1016/j.plefa.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Matheus M., Sala M.A. Measurement of the villus surface area and its regional variation in the human full-term placenta. Gegenbaurs Morphol. Jahrb. 1989;135:851–854. [PubMed] [Google Scholar]
- 10.Dempsey E.W., Luse S.A. Regional specializations in the syncytial trophoblast of early human placentas. J. Anat. 1971;108:545–561. [PMC free article] [PubMed] [Google Scholar]
- 11.Benirschke K., Kaufmann P. Springer; 2000. Pathology of the Human Placenta. (Place Published) [Google Scholar]
- 12.Matijevic R., Meekins J.W., Walkinshaw S.A., Neilson J.P., McFadyen I.R. Spiral artery blood flow in the central and peripheral areas of the placental bed in the second trimester. Obstet. Gynecol. 1995;86:289–292. doi: 10.1016/0029-7844(95)00129-f. [DOI] [PubMed] [Google Scholar]
- 13.Hempstock J., Bao Y.P., Bar-Issac M., Segaren N., Watson A.L., Charnock-Jones D.S., Jauniaux E., Burton G.J. Intralobular differences in antioxidant enzyme expression and activity reflect the pattern of maternal arterial bloodflow within the human placenta. Placenta. 2003;24:517–523. doi: 10.1053/plac.2002.0955. [DOI] [PubMed] [Google Scholar]
- 14.Saleh S.A., Maraqa A.D., Ali M.E., Mustafa Z.S., Qadoumi O.F. 2010. Regional Distribution of Superoxide Dismutase Activity in Human Placenta and its Correlation with Lipid Peroxidation. [Google Scholar]
- 15.Roland-Zejly L., Moisan V., St-Pierre I., Bilodeau J.F. Altered placental glutathione peroxidase mRNA expression in preeclampsia according to the presence or absence of labor. Placenta. 2011;32:161–167. doi: 10.1016/j.placenta.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Buttery L.D., McCarthy A., Springall D.R., Sullivan M.H., Elder M.G., Michel T., Polak J.M. Endothelial nitric oxide synthase in the human placenta: regional distribution and proposed regulatory role at the feto-maternal interface. Placenta. 1994;15:257–265. doi: 10.1016/0143-4004(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 17.Manci E.A., Blackburn W.R. Regional variations in the levels of zinc, iron, copper, and calcium in the term human placenta. Placenta. 1987;8:497–502. doi: 10.1016/0143-4004(87)90078-6. [DOI] [PubMed] [Google Scholar]
- 18.Gratton R.J., Gluszynski M., Mazzuca D.M., Nygard K., Han V.K. Adrenomedullin messenger ribonucleic acid expression in the placentae of normal and preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2003;88:6048–6055. doi: 10.1210/jc.2003-030323. [DOI] [PubMed] [Google Scholar]
- 19.Burton G.J., Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Benyo D.F., Smarason A., Redman C.W., Sims C., Conrad K.P. Expression of inflammatory cytokines in placentas from women with preeclampsia. J. Clin. Endocrinol. Metab. 2001;86:2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 21.Jones M.L., Mark P.J., Waddell B.J. Maternal dietary omega-3 fatty acids and placental function. Reproduction. 2014;147:R143–R152. doi: 10.1530/REP-13-0376. [DOI] [PubMed] [Google Scholar]
- 22.Cheeseman K.H. Mechanisms and effects of lipid peroxidation. Mol. Asp. Med. 1993;14:191–197. doi: 10.1016/0098-2997(93)90005-x. [DOI] [PubMed] [Google Scholar]
- 23.Sahay A.S., Sundrani D.P., Wagh G.N., Mehendale S.S., Joshi S.R. Regional differences in the placental levels of oxidative stress markers in pre-eclampsia. Int. J. Gynecol. Obstet. 2015 doi: 10.1016/j.ijgo.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Ghabour M.S., Eis A.L.W., Brockman D.E., Pollock J.S., Myatt L. Immunohistochemical characterization of placental nitric oxide synthase expression in preeclampsia. Am. J. Obstet. Gynecol. 1995;173:687–694. doi: 10.1016/0002-9378(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 25.Abdulsid A., Hanretty K., Lyall F. Heat shock protein 70 expression is spatially distributed in human placenta and selectively upregulated during labor and preeclampsia. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054540. e54540. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Abdulsid A., Lyall F. Heat shock protein 27 expression is spatially distributed in human placenta and selectively regulated during preeclampsia. J. Reprod. Immunol. 2014;101–102:89–95. doi: 10.1016/j.jri.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Dangat K.D., Mehendale S.S., Yadav H.R., Kilari A.S., Kulkarni A.V., Taralekar V.S., Joshi S.R. Long-chain polyunsaturated fatty acid composition of breast milk in pre-eclamptic mothers. Neonatology. 2010;97:190–194. doi: 10.1159/000252971. [DOI] [PubMed] [Google Scholar]
- 28.Campbell F.M., Gordon M.J., Dutta-Roy A.K. Placental membrane fatty acid-binding protein preferentially binds arachidonic and docosahexaenoic acids. Life Sci. 1998;63:235–240. doi: 10.1016/s0024-3205(98)00267-7. [DOI] [PubMed] [Google Scholar]
- 29.Dutta-Roy A.K. Cellular uptake of long-chain fatty acids: role of membrane-associated fatty-acid-binding/transport proteins. Cell. Mol. Life Sci. 2000;57:1360–1372. doi: 10.1007/PL00000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bitsanis D., Crawford M.A., Moodley T., Holmsen H., Ghebremeskel K., Djahanbakhch O. Arachidonic acid predominates in the membrane phosphoglycerides of the early and term human placenta. J. Nutr. 2005;135:2566–2571. doi: 10.1093/jn/135.11.2566. [DOI] [PubMed] [Google Scholar]
- 31.Min Y., Crawford M.A. John Wiley & Sons, Ltd; 2004. Essential Fatty Acids, The Eicosanoids; pp. 257–265. (Place Published) [Google Scholar]
- 32.Jauniaux E., Hempstock J., Greenwold N., Burton G.J. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am. J. Pathol. 2003;162:115–125. doi: 10.1016/S0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kingdom J., Huppertz B., Seaward G., Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 34.Erskine K.J., Iversen S.A., Davies R. An altered ratio of 18:2 (9,11) to 18:2 (9,12) linoleic acid in plasma phospholipids as a possible predictor of pre-eclampsia. Lancet. 1985;1:554–555. doi: 10.1016/s0140-6736(85)91210-3. [DOI] [PubMed] [Google Scholar]
- 35.Mehendale S., Kilari A., Dangat K., Taralekar V., Mahadik S., Joshi S. Fatty acids, antioxidants, and oxidative stress in pre-eclampsia. Int. J. Gynaecol. Obstet. 2008;100:234–238. doi: 10.1016/j.ijgo.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., Walsh S.W., Kay H.H. Placental tissue levels of nonesterified polyunsaturated fatty acids in normal and preeclamptic pregnancies. Hypertens. Pregnancy. 2005;24:235–245. doi: 10.1080/10641950500281118. [DOI] [PubMed] [Google Scholar]
- 37.Simopoulos A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomedecine PharmacotherapieBiomed. Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 38.Serhan C.N., Petasis N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krielessi V., Papantoniou N., Papageorgiou I., Chatzipapas I., Manios E., Zakopoulos N., Antsaklis A. Placental pathology and blood pressure's level in women with hypertensive disorders in pregnancy. Obstet. Gynecol. Int. 2012;2012:684083. doi: 10.1155/2012/684083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sebire N., Goldin R., Regan L. Term preeclampsia is associated with minimal histopathological placental features regardless of clinical severity. J. Obstet. Gynaecol. 2005;25:117–118. doi: 10.1080/014436105400041396. [DOI] [PubMed] [Google Scholar]
- 41.Salafia C.M., Pezzullo J.C., López-Zeno J., Simmens S., Minior V.K., Vintzileos A.M. Placental pathologic features of preterm preeclampsia. Am. J. Obstet. Gynecol. 1995;173:1097–1105. doi: 10.1016/0002-9378(95)91333-5. [DOI] [PubMed] [Google Scholar]
- 42.Ayala A., Munoz M.F., Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji L., Brkić J., Liu M., Fu G., Peng C., Wang Y.-L. Placental trophoblast cell differentiation: physiological regulation and pathological relevance to preeclampsia. Mol. Asp. Med. 2013;34:981–1023. doi: 10.1016/j.mam.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Burdge G.C., Calder P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005;45:581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 45.Uauy R., Hoffman D.R., Peirano P., Birch D.G., Birch E.E. Essential fatty acids in visual and brain development. Lipids. 2001;36:885–895. doi: 10.1007/s11745-001-0798-1. [DOI] [PubMed] [Google Scholar]
- 46.Haast R.A., Kiliaan A.J. 2014. Impact of Fatty Acids on Brain Circulation, Structure and Function, Prostaglandins, Leukotrienes, and Essential Fatty Acids. [DOI] [PubMed] [Google Scholar]
- 47.Haggarty P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010;30:237–255. doi: 10.1146/annurev.nutr.012809.104742. [DOI] [PubMed] [Google Scholar]
- 48.Kulkarni A., Mehendale S., Pisal H., Kilari A., Dangat K., Salunkhe S., Taralekar V., Joshi S. Association of omega-3 fatty acids and homocysteine concentrations in pre-eclampsia. Clin. Nutr. 2011;30:60–64. doi: 10.1016/j.clnu.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Dhobale M.V., Wadhwani N., Mehendale S.S., Pisal H.R., Joshi S.R. Reduced levels of placental long chain polyunsaturated fatty acids in preterm deliveries. Prostaglandins Leukot. Essent. Fatty Acids. 2011;85:149–153. doi: 10.1016/j.plefa.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Liang C., DeCourcy K., Prater M.R. High-saturated-fat diet induces gestational diabetes and placental vasculopathy in C57BL/6 mice. Metab. Clin. Exp. 2009;59:943–950. doi: 10.1016/j.metabol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 51.Liang C., Oest M.E., Prater M.R. Intrauterine exposure to high saturated fat diet elevates risk of adult-onset chronic diseases in C57BL/6 mice. Birth Defects Res. B Dev. Reprod. Toxicol. 2009;86:377–384. doi: 10.1002/bdrb.20206. [DOI] [PubMed] [Google Scholar]
- 52.Lucia Bergmann R., Bergmann K.E., Haschke-Becher E., Richter R., Dudenhausen J.W., Barclay D., Haschke F. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? J. Perinat. Med. 2007;35:295–300. doi: 10.1515/JPM.2007.085. [DOI] [PubMed] [Google Scholar]
- 53.Olsen S.F., Dalby J. So̸rensen, N.J. Secher, M. Hedegaard, T. Brink Henriksen, H.S. Hansen, A. Grant, Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet. 1992;339:1003–1007. doi: 10.1016/0140-6736(92)90533-9. [DOI] [PubMed] [Google Scholar]
- 54.Helland I.B., Smith L., Saarem K., Saugstad O.D., Drevon C.A. Maternal supplementation with very-long-chain n − 3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 55.Calder P., Grimble R. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002;56:S14–S19. doi: 10.1038/sj.ejcn.1601478. [DOI] [PubMed] [Google Scholar]
- 56.Bautista L.E. Inflammation, endothelial dysfunction, and the risk of high blood pressure: epidemiologic and biological evidence. J. Hum. Hypertens. 2003;17:223–230. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- 57.Robaut C., Mondon F., Bandet J., Ferre F., Cavero I. Regional distribution and pharmacological characterization of [125I]endothelin-1 binding sites in human fetal placental vessels. Placenta. 1991;12:55–67. doi: 10.1016/0143-4004(91)90510-m. [DOI] [PubMed] [Google Scholar]
- 58.Singh H.J., Rahman A., Larmie E.T., Nila A. Endothelin-1 in feto-placental tissues from normotensive pregnant women and women with pre-eclampsia. Acta Obstet. Gynecol. Scand. 2001;80:99–103. doi: 10.1034/j.1600-0412.2001.080002099.x. [DOI] [PubMed] [Google Scholar]
- 59.Sohlberg S., Stephansson O., Cnattingius S., Wikstrom A.K. Maternal body mass index, height, and risks of preeclampsia. Am. J. Hypertens. 2012;25:120–125. doi: 10.1038/ajh.2011.175. [DOI] [PubMed] [Google Scholar]
- 60.Zavalza-Gomez A.B. Obesity and oxidative stress: a direct link to preeclampsia? Arch. Gynecol. Obstet. 2011;283:415–422. doi: 10.1007/s00404-010-1753-1. [DOI] [PubMed] [Google Scholar]