Abstract
Factors affecting the blood-testis barrier function may be involved in testicular damage and male infertility. Two cytokines play an important role in the barrier regulation, namely transforming growth factor beta 3 (TGF-β3) and tumor necrosis factor (TNF-α). The aim of this study was to investigate the potential association between TGF-β3 (TGFB3) and TNF-α (TNF) gene polymorphisms and male infertility. A total of 846 subjects, 423 diagnosed with male infertility and 423 fertile men were enrolled. TGFB3 (rs2268626:T > C, rs3917158:C > T, rs2284792:A > G, rs2268625:T > C, rs3917187:C > T) and TNF (rs1800629:-308G > A) gene polymorphisms were genotyped. No association between TNF genotype and infertility was observed. As for TGFB3, the genotypes distribution was similar in infertile and fertile men. However, rs2284792 minor allele frequency was significantly higher among infertile subjects. Heterozygous rs2284792 AG genotype was associated with increased odds for infertility [OR = 1.40 (95% CI 1.05–1.86), p = 0.021] and similar results were observed for G allele carrier status [OR = 1.40 (95% CI 1.06–1.84), p = 0.017]. Heterozygosity in TGFB3 rs3917158 was also associated with the infertility [OR = 1.37 (95% CI 1.01–1.87), p = 0.041]. The TGFB3 variant genotypes were associated with lower spermatozoa motility parameters in fertile men. The results indicate that variants in TGFB3 gene may be associated with male infertility. However, the findings require further replication and validation.
Infertility is a common problem affecting one in six couples, and in 50% it is attributed to the male factor resulting from acquired and/or congenital abnormalities, in which 60–75% is found to be idiopathic1,2. The pattern of familial aggregation of male infertility implies also an important role of genetic factors, that influence a variety of physiological processes including hormonal homeostasis, spermatogenesis, and sperm quality3.
Most of male infertility genetic association studies explored factors involved in spermatogenesis. However, it seems reasonably to explore also an impact of genetic determinants of the blood-testis barrier integrity. Factors affecting the barrier function may be directly involved in the development of testicular damage and problems with fertility. The blood-testis barrier is a very restrictive blood-tissue barrier, and creates a specialized microenvironment for development and maturation of germ cells. It consists of three components: anatomical (tight junctions), physiological (transporters) and immunological. The barrier components restrict passage of molecules and cells (anatomical) and regulate movement of substances in or out of the lumen (physiological) as well as limit access by the immune system cells and sequester the majority of autoantigenic germ cells (immunological). The blood-testis barrier is not a static structure, as it undergoes extensive restructuring during spermatogenesis to meet the needs of germ cells and Sertoli cells. The anatomical barrier tight junctions undergo remodeling (opening, closing) to allow adaptation to Sertoli cell changes (but remain static for their function as integrity of the junctions remains intact) and transporter expression also adapts to various demands during spermatogenesis. Disruption of the barrier function and integrity (e.g. by environmental toxicants such as bisphenol A or cadmium) lead to testicular injury and infertility4,5.
Two crucial cytokines are involved in the regulation of the blood-testis barrier, namely transforming growth factor beta 3 (TGF-β3) and tumor necrosis factor (TNF-α). These cytokines determine the homeostasis of tight junctions and basal ectoplasmic specialization-structural proteins, proteases, protease inhibitors, and other extracellular proteins (e.g. collagen) in the seminiferous epithelium. Some of those molecules are known regulators of focal contacts between the extracellular matrix and other actively migrating cells, such as macrophages, fibroblasts or malignant cells. Experimental studies revealed that administration of IL-1β or TNF-α impair integrity of the blood-testis barrier6.
In the testis, TNF-α, a proinflammatory cytokine, is secreted by germ cells, predominantly pachytene spermatocytes and round spermatides as well as Sertoli cells, whereas its receptors are restricted to Sertoli cells7. During the assembly of Sertoli cell tight junction-barrier in vitro, the amount of TNF-α produced by Sertoli cells declines significantly, indicating that it can downregulate the Sertoli cell tight junction–barrier function. Co-incubation of Sertoli cells with recombinant TNF-α results in disruption of the integrity of tight junction barrier, as shown in experiments quantifying the transepithelial electrical resistance across the Sertoli cell monolayer7. The effect could be produced by direct effect of TNF-α on the tight junction-associated structural proteins’ complexes, as significant reduction of occluding level was observed after TNF-α exposure. Besides, it was shown that TNF-α was able to inhibit claudin-11 expression in cultured mouse Sertoli cells. TNF-α may also affect the blood-testis barrier function via activation of metalloproteinase 2 (MMP-2). MMP-2 is a defined factor regulating tight junction structure and function in the Sertoli cell-tight junction barrier8. Another possible route through which TNF-α affects tight junction-barrier function is its impact on follicle-stimulating hormone (FSH) level, since the hormone regulates assembly of Sertoli cell tight junction barrier9. Furthermore, subsequent studies have shown that TNF-α can mediate its effects via mitogen-activated protein kinase (MAPK) pathways and the integrin-linked kinase (ILK)/glycogen synthase kinase 3 β (GSK-3β)7. This in turn affects the homeostasis of proteases and protease inhibitors and subsequently the nature of the extracellular matrix adjacent to the blood-testis barrier6. Having in mind the aforementioned findings it is reasonable to undertake studies on associations of the genetic polymorphism of TNF gene (coding for TNF-α) with male infertility. It was documented that TNF-α activity depends on the polymorphic alleles of TNF. A single nucleotide polymorphism (SNP) in TNF gene promoter is a result of guanine for adenine transition (−308G > A). In vitro transfection studies revealed higher expression of −308A minor allele in comparison to −308G10, that may be responsible for variability of TNF-α concentration in patients with infectious diseases11.
Another cytokine which plays an important role in the regulation of the blood-testis barrier function is transforming growth factor-β3 (TGF-β3), which is the most abundant form of TGF-β in the testis. It is produced by Sertoli cells, and its level declines when Sertoli cell tight junction barrier is being assembled in vitro12. It is also a product of premeiotic germ cells, such as spermatogonia and early spermatocytes. In vitro studies documented that recombinant TGF-β3 led to disruption of Sertoli cell monolayer12. The disruption of Sertoli cell tight junctions is accompanied by a decline in the protein levels of several tight junction associated proteins, including occluding, ZO-1 and claudin-11 in Sertoli cells. Subsequent studies have shown that TGF-β3 mediates its effects on Sertoli cell tight junctions via the p38 MAPK pathway. More importantly, those observations have been validated in vivo using the CdCl2-treated rat model of infertility, i.e. an increase of TGF-β3 was paralleled by decreased content of occludin and ZO-113. Recent observations have also documented that TGF-β3 significantly downregulated junctional adhesion molecule-B expression via post-transcriptional and post-translational modulation, and resulted in the disruption of the blood-testis barrier and apical ectoplasmic specializations14. Current data indicate an implication of TGF-β3 polymorphism (TGFB3) in the pathology of connective tissue. A significant association was found between TGF-β3 gene (TGFB3) polymorphism and fibrosis in patients with sarcoidosis (SNP 4875A > G and 17369T > C)15 as well as heart fibrosis16. Based on the proved biological role of TGF-β3 a study on its polymorphism in male infertility seem to be well established.
Results
Standard sperm parameters of the study subjects are presented in Table 1. Comparison of sperm characteristics of infertile and fertile men revealed significant differences in all studied parameters. i.e. concentration, total number of spermatozoa, morphology and motility.
Table 1. Descriptive characteristics of standard sperm parameters of study subjects.
| Sperm parameters | unit | Infertile men (n = 344) | Fertile men (n = 124) | p value | ||
|---|---|---|---|---|---|---|
| Mean ± SD | % of normal values* | Mean ± SD | % of normal values | |||
| Concentration | ×106/mL | 39.9 ± 37.5 | 72.6 | 85.4 ± 72.8 | 94.4 | <10−5 |
| Total number of spermatozoa | ×106 | 167 ± 204 | 70.2 | 304 ± 299 | 97.2 | <10−5 |
| Morphologically normal spermatozoa | % | 4.5 ± 4.7 | 43.5 | 10.2 ± 7.1 | 90.2 | <10−5 |
| Progressive motility | % | 39.8 ± 21.8 | 62.2 | 47.5 ± 17.2 | 85.5 | 0.0007 |
| Non-progressive motility | % | 13.5 ± 10.4 | – | 18.8 ± 9.7 | – | <10−5 |
| Total motility | % | 53.0 ± 20.0 | 77.6 | 66.4 ± 14.8 | 96.0 | <10−5 |
| Immotile spermatozoa | % | 47.0 ± 20.0 | – | 33.6 ± 14.8 | – | <10−5 |
SD–standard deviation; p value calculated by means of Mann-Whitney U-test; *according to the WHO guidelines.
Distribution of all the genotypes was in concordance with Hardy-Weinberg equilibrium, both in fertile and infertile group (p > 0.1). No association was observed between TNF genotype and male infertility (Table 2). As for TGFB3, the studied genotypes’ distribution was similar in infertile and fertile men. However, minor allele frequency (MAF) was higher among infertile subjects in case of all the studied SNPs, reaching statistical significance level for TGFB3 rs2284792 (p = 0.025). Heterozygous rs2284792 AG genotype was associated with increased odds for infertility [OR = 1.40 (95% CI 1.05–1.86), p = 0.021] and similar results were observed for G allele carrier status [AG or GG genotype: OR = 1.40 (95% CI 1.06–1.84), p = 0.017]. As for TGFB3 rs3917158, only in the case of heterozygous subjects, an association with infertility was significant [OR = 1.37 (95% CI 1.01–1.87), p = 0.041]. For all other SNPs, only marginally significant associations were observed when dominant model was applied (p < 0.1, Table 2). As all the analyzed polymorphisms within the TGFB3 gene are non-coding SNPs in strong linkage disequilibrium (Fig. 1), they should be only considered as markers, not directly influencing gene expression nor protein function, with rs2284792:A > G SNP as the one potentially the most predictive.
Table 2. Frequency of the studied genotypes and alleles in infertile (n = 423) vs. fertile men (n = 423).
| Infertile patients | Fertile controls | p1 | p2 | p3 | p4 | p5 | |||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | ||||||
| TNF rs1800629 | |||||||||
| GG | 312 | (73.8) | 301 | (71.2) | 0.507 | ||||
| GA | 105 | (24.8) | 118 | (27.9) | 0.568 | 0.330 | 0.397 | 0.525 | |
| AA | 6 | (1.4) | 4 | (0.9) | |||||
| MAF | (13.8) | (14.9) | 0.389 | ||||||
| TGFB3 rs2268626 | |||||||||
| TT | 275 | (65.0) | 298 | (70.5) | |||||
| CT | 134 | (31.7) | 111 | (26.2) | 0.214 | 0.836 | 0.079 | 0.090 | 1.000 |
| CC | 14 | (3.3) | 14 | (3.3) | |||||
| MAF | (19.1) | (16.4) | 0.143 | ||||||
| TGFB3 rs3917158 | |||||||||
| CC | 287 | (67.9) | 311 | (73.5) | |||||
| CT | 127 | (30.0) | 100 | (23.7) | 0.101 | 0.643 | 0.041 | 0.070 | 0.507 |
| TT | 9 | (2.1) | 12 | (2.9) | |||||
| MAF | (17.1) | (14.7) | 0.162 | ||||||
| TGFB3 rs2284792 | |||||||||
| AA | 240 | (56.8) | 274 | (64.8) | |||||
| AG | 163 | (38.5) | 133 | (31.4) | 0.056 | 0.303 | 0.021 | 0.017 | 0.496 |
| GG | 20 | (4.7) | 16 | (3.8) | |||||
| MAF | (24.0) | (19.5) | 0.025 | ||||||
| TGFB3 rs2268625 | |||||||||
| TT | 288 | (68.1) | 311 | (73.5) | 0.176 | 0.966 | 0.064 | 0.082 | 0.825 |
| TC | 125 | (29.6) | 101 | (23.9) | |||||
| CC | 10 | (2.3) | 11 | (2.6) | 0.143 | ||||
| MAF | (17.1) | (14.5) | |||||||
| TGFB3 rs3917187 | |||||||||
| CC | 257 | (60.8) | 282 | (66.7) | |||||
| CT | 149 | (35.2) | 125 | (29.6) | 0.192 | 0.669 | 0.071 | 0.074 | 0.859 |
| TT | 17 | (4.0) | 16 | (3.8) | |||||
| MAF | (21.6) | (18.6) | 0.115 | ||||||
MAF – minor allele frequency; p values calculated by means of χ2 test, with Yate’s corrections for n < 5. p1 – overall comparison; p2 – major homozygotes vs. minor homozygotes; p3 – major homozygotes vs. heterozygotes; p4 – dominant model (minor homozygotes and heterozygotes vs. major homozygotes); p5 – recessive model (minor homozygotes vs. other genotypes);
Figure 1. Pairwise LD between the studied TGFB3 SNPs.
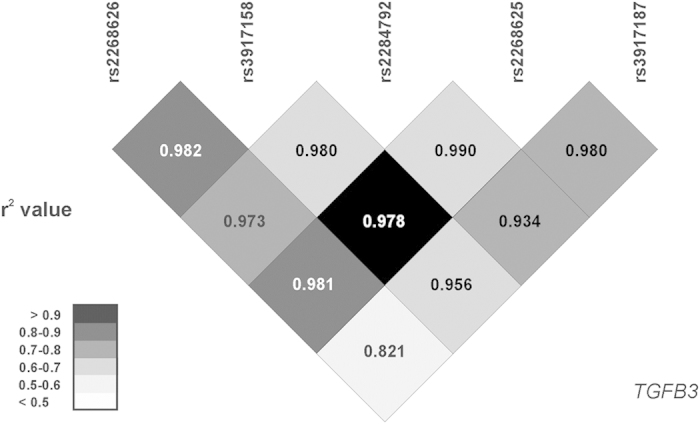
Analysis based on genotyping data from all study subjects (n = 846). Numbers represent D’, r2 values (square correlation coefficient) are color-coded.
Due to strong linkage between the TGFB3 SNPs, haplotype frequencies were assessed, in both infertile and control groups. It was revealed, that 5 SNPs form two most frequent haplotypes T_C_A_T_C (estimated frequency of 0.750 among infertile and 0.786 among fertile man), and C_T_G_C_T (0.165 and 0.139, respectively, Table 3), and only 3 more variants were present in the study population with frequency > 1% (from 32 potentially possible combinations for 5 loci). However, frequencies of the haplotypes did not differ significantly between infertile cases and controls (Table 3).
Table 3. Frequency of TGFB3 haplotypes in infertile (n = 423) and fertile men (n = 423).
| TGFB3 haplotype | Infertile patients | Fertile controls | p value |
|---|---|---|---|
| T_C_A_T_C | 0.750 | 0.786 | – |
| T_C_A_T_T | 0.006 | 0.012 | 0.301 |
| T_C_G_T_T | 0.041 | 0.029 | 0.186 |
| C_C_G_T_C | 0.020 | 0.017 | 0.587 |
| C_T_G_C_T | 0.165 | 0.139 | 0.117 |
| other | 0.018 | 0.017 | 0.851 |
Haplotypes formed by 5 SNPs within TGFB3 gene: rs2268626:T > C, rs3917158:C > T, rs2284792:A > G, rs2268625:T > C, rs3917187:C > T; p value calculated by means of Fisher exact test in relation to fertile controls, T_C_A_T_C as a referent haplotype. Haplotypes with estimated frequency > 1% in any study group are presented.
When the studied TGFB3/TNF genotypes were analyzed for the presence of potential association with sperm parameters, no significant genotype-dependent differences were observed among infertile men (Table 4). However, among fertile men, TGFB3 variant genotypes were associated with lower percentage of spermatozoa with progressive motility (reaching significance level for rs2268626), higher percentage of non-progressive motility (rs2268626, rs3917158, all the studied SNPs), as well as decreased total motility and higher percentage of immotile spermatozoa among variant carriers (rs2268626 and rs2284792). TNF rs1800629 variant was associated with lower percentage of spermatozoa with progressive motility. In infertile subjects the genetic associations and sperm parameters were mostly not observed.
Table 4. Association between the studied TGFB3/TNF genotypes and sperm parameters in infertile (n = 344) and fertile (n = 124) men.
| Sperm parameters | Unit | Group | TNF rs1800629 genotype | p value | ||||
|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | p1 | p2 | p3 | |||
| Concentration | ×106/mL | infertile | 39.7 ± 37.2 | 40.6 ± 38.9 | 33.7 ± 23.8 | 0.944 | 0.738 | 0.981 |
| fertile | 82.1 ± 74.3 | 94.5 ± 67.5 | ND | – | 0.198 | – | ||
| Morphologically normal spermatozoa | % | infertile | 4.5 ± 4.8 | 4.5 ± 4.9 | 4.5 ± 4.8 | 0.981 | 0.851 | 0.929 |
| fertile | 10.2 ± 7.3 | 10.7 ± 6.4 | ND | – | 0.513 | – | ||
| Progressive motility | % | infertile | 39.7 ± 21.9 | 40.1 ± 21.6 | 31.0 ± 16.7 | 0.611 | 0.959 | 0.338 |
| fertile | 49.2 ± 16.8 | 40.1 ± 16.3 | ND | – | 0.035 | – | ||
| Non-progressive motility | % | infertile | 13.8 ± 10.2 | 13.0 ± 11.2 | 11.6 ± 6.4 | 0.585 | 0.303 | 0.776 |
| fertile | 18.1 ± 9.2 | 22.1 ± 10.8 | ND | – | 0.084 | – | ||
| Total motility | % | infertile | 53.5 ± 19.9 | 52.2 ± 19.7 | 42.6 ± 12.0 | 0.357 | 0.516 | 0.166 |
| fertile | 67.5 ± 14.6 | 62.1 ± 14.9 | ND | – | 0.221 | – | ||
| Immotile spermatozoa | % | infertile | 45.7 ± 19.9 | 45.4 ± 19.7 | 57.4 ± 12.0 | 0.335 | 0.796 | 0.140 |
| fertile | 32.5 ± 14.6 | 37.8 ± 14.9 | ND | – | 0.230 | – | ||
| Sperm parameters | unit | TGFB3 rs2268626 genotype | p value | |||||
| TT | CT | CC | p1 | p2 | p3 | |||
| Concentration | ×106/mL | infertile | 40.8 ± 40.3 | 39.1 ± 32.0 | 27.4 ± 25.4 | 0.440 | 0.781 | 0.257 |
| fertile | 87.9 ± 76.3 | 80.5 ± 69.7 | 65.4 ± 20.4 | 0.876 | 0.618 | 0.974 | ||
| Morphologically normal spermatozoa | % | infertile | 4.5 ± 4.9 | 4.6 ± 4.6 | 3.7 ± 4.9 | 0.487 | 0.654 | 0.238 |
| fertile | 10.9 ± 7.3 | 9.5 ± 6.9 | 6.4 ± 2.1 | 0.326 | 0.226 | 0.239 | ||
| Progressive motility | % | infertile | 39.3 ± 21.8 | 41.5 ± 21.8 | 30.4 ± 19.0 | 0.208 | 0.673 | 0.120 |
| fertile | 50.7 ± 17.8 | 40.1 ± 16.7 | 41.0 ± 10.6 | 0.024 | 0.006 | 0.469 | ||
| Non-progressive motility | % | infertile | 14.1 ± 10.9 | 13.0 ± 9.6 | 9.2 ± 5.5 | 0.326 | 0.272 | 0.207 |
| fertile | 18.6 ± 10.3 | 18.3 ± 7.5 | 30.2 ± 8.2 | 0.034 | 0.289 | 0.010 | ||
| Total motility | % | infertile | 53.1 ± 19.5 | 54.4 ± 20.6 | 39.6 ± 18.8 | 0.414 | 0.924 | 0.016 |
| fertile | 69.3 ± 14.6 | 58.9 ± 13.3 | 70.8 ± 9.6 | 0.002 | 0.002 | 0.465 | ||
| Immotile spermatozoa | % | infertile | 46.5 ± 19.4 | 43.7 ± 20.1 | 52.1 ± 21.6 | 0.156 | 0.471 | 0.124 |
| fertile | 30.7 ± 14.6 | 41.1 ± 13.3 | 28.8 ± 9.8 | 0.002 | 0.002 | 0.427 | ||
| Sperm parameters | unit | TGFB3 rs3917158 genotype | p value | |||||
| CC | CT | TT | p1 | p2 | p3 | |||
| Concentration | ×106/mL | infertile | 41.4 ± 39.9 | 36.9 ± 32.1 | 32.1 ± 27.9 | 0.886 | 0.678 | 0.728 |
| fertile | 80.1 ± 77.5 | 78.6 ± 63.9 | 65.5 ± 20.4 | 0.950 | 0.758 | 0.974 | ||
| Morphologically normal spermatozoa | % | infertile | 4.6 ± 4.9 | 4.3 ± 4.4 | 4.8 ± 5.8 | 0.642 | 0.357 | 0.716 |
| fertile | 10.9 ± 7.6 | 9.1 ± 5.9 | 6.4 ± 2.1 | 0.326 | 0.214 | 0.238 | ||
| Progressive motility | % | infertile | 39.6 ± 21.6 | 40.2 ± 22.4 | 35.1 ± 19.7 | 0.785 | 0.961 | 0.507 |
| fertile | 49.0 ± 18.6 | 42.8 ± 11.6 | 41.0 ± 10.6 | 0.308 | 0.131 | 0.469 | ||
| Non-progressive motility | % | infertile | 14.0 ± 10.7 | 13.0 ± 9.8 | 9.0 ± 5.3 | 0.326 | 0.253 | 0.231 |
| fertile | 18.5 ± 10.1 | 18.4 ± 7.5 | 30.2 ± 8.2 | 0.034 | 0.245 | 0.010 | ||
| Total motility | % | infertile | 53.3 ± 19.4 | 53.2 ± 21.4 | 44.1 ± 16.9 | 0.331 | 0.827 | 0.139 |
| fertile | 67.6 ± 15.9 | 61.8 ± 10.8 | 70.8 ± 9.6 | 0.085 | 0.083 | 0.466 | ||
| Immotile spermatozoa | % | infertile | 46.3 ± 19.3 | 43.9 ± 20.8 | 55.9 ± 16.9 | 0.196 | 0.645 | 0.110 |
| fertile | 32.4 ± 15.9 | 38.2 ± 10.8 | 28.8 ± 9.8 | 0.085 | 0.085 | 0.427 | ||
| Sperm parameters | unit | TGFB3 rs2284792 genotype | p value | |||||
| AA | AG | GG | p1 | p2 | p3 | |||
| Concentration | ×106/mL | infertile | 40.6 ± 38.2 | 40.1 ± 37.5 | 27.8 ± 25.8 | 0.425 | 0.986 | 0.204 |
| fertile | 93.0 ± 81.8 | 74.3 ± 58.5 | 60.7 ± 21.8 | 0.622 | 0.335 | 0.705 | ||
| Morphologically normal spermatozoa | % | infertile | 4.7 ± 5.1 | 4.2 ± 4.3 | 4.2 ± 5.0 | 0.467 | 0.251 | 0.475 |
| fertile | 10.8 ± 6.8 | 10.0 ± 7.9 | 6.3 ± 1.9 | 0.270 | 0.194 | 0.203 | ||
| Progressive motility | % | infertile | 39.9 ± 22.3 | 40.3 ± 21.0 | 32.3 ± 21.8 | 0.311 | 0.871 | 0.131 |
| fertile | 49.7 ± 16.5 | 44.5 ± 17.6 | 36.2 ± 15.1 | 0.114 | 0.060 | 0.171 | ||
| Non-progressive motility | % | infertile | 13.8 ± 10.9 | 13.5 ± 10.1 | 10.9 ± 6.5 | 0.797 | 0.748 | 0.506 |
| fertile | 19.0 ± 9.7 | 17.4 ± 9.2 | 30.0 ± 7.4 | 0.012 | 0.989 | 0.004 | ||
| Total motility | % | infertile | 53.3 ± 20.1 | 53.8 ± 19.5 | 43.2 ± 20.4 | 0.094 | 0.760 | 0.031 |
| fertile | 68.7 ± 14.0 | 62.3 ± 15.5 | 65.8 ± 14.9 | 0.086 | 0.034 | 0.985 | ||
| Immotile spermatozoa | % | infertile | 46.2 ± 20.1 | 44.7 ± 19.1 | 50.6 ± 21.5 | 0.304 | 0.868 | 0.149 |
| fertile | 31.3 ± 13.9 | 37.7 ± 15.5 | 33.8 ± 15.1 | 0.084 | 0.035 | 0.938 | ||
| Sperm parameters | unit | TGFB3 rs2268625 genotype | p value | |||||
| TT | TC | CC | p1 | p2 | p3 | |||
| Concentration | ×106/mL | infertile | 41.3 ± 39.8 | 37.4 ± 32.2 | 29.1 ± 27.6 | 0.746 | 0.731 | 0.453 |
| fertile | 86.3 ± 75.4 | 83.9 ± 71.8 | 65.5 ± 20.4 | 0.997 | 0.946 | 0.974 | ||
| Morphologically normal spermatozoa | % | infertile | 4.6 ± 4.9 | 4.4 ± 4.4 | 4.3 ± 5.6 | 0.656 | 0.456 | 0.488 |
| fertile | 11.0 ± 7.6 | 9.1 ± 5.8 | 6.4 ± 2.1 | 0.349 | 0.247 | 0.238 | ||
| Progressive motility | % | infertile | 39.5 ± 21.7 | 40.5 ± 22.5 | 34.5 ± 18.5 | 0.698 | 0.896 | 0.435 |
| fertile | 49.3 ± 18.7 | 42.4 ± 11.4 | 41.0 ± 10.6 | 0.183 | 0.067 | 0.469 | ||
| Non-progressive motility | % | infertile | 14.1 ± 10.7 | 12.8 ± 9.9 | 10.2 ± 6.2 | 0.358 | 0.178 | 0.426 |
| fertile | 18.3 ± 10.2 | 18.9 ± 7.6 | 30.2 ± 8.2 | 0.027 | 0.134 | 0.010 | ||
| Total motility | % | infertile | 53.3 ± 19.4 | 53.2 ± 21.6 | 44.7 ± 16.0 | 0.322 | 0.836 | 0.135 |
| fertile | 67.7 ± 16.1 | 61.8 ± 10.5 | 70.8 ± 9.6 | 0.070 | 0.065 | 0.465 | ||
| Immotile spermatozoa | % | infertile | 46.3 ± 19.3 | 43.8 ± 20.9 | 55.2 ± 15.9 | 0.180 | 0.632 | 0.105 |
| fertile | 32.3 ± 16.1 | 38.2 ± 10.5 | 28.8 ± 9.8 | 0.066 | 0.068 | 0.427 | ||
| Sperm parameters | unit | TGFB3 rs3917187 genotype | p value | |||||
| CC | CT | TT | p1 | p2 | p3 | |||
| Concentration | ×106/mL | infertile | 40.0 ± 36.6 | 41.1 ± 40.1 | 28.0 ± 24.3 | 0.549 | 0.927 | 0.282 |
| fertile | 88.1 ± 78.8 | 82.4 ± 66.1 | 60.7 ± 21.8 | 0.928 | 0.952 | 0.704 | ||
| Morphologically normal spermatozoa | % | infertile | 4.6 ± 4.7 | 4.4 ± 5.0 | 3.9 ± 4.7 | 0.522 | 0.293 | 0.503 |
| fertile | 10.9 ± 7.2 | 9.9 ± 7.3 | 6.3 ± 1.9 | 0.317 | 0.255 | 0.203 | ||
| Progressive motility | % | infertile | 39.8 ± 22.0 | 40.0 ± 21.6 | 36.3 ± 20.7 | 0.749 | 0.876 | 0.449 |
| fertile | 48.5 ± 17.7 | 46.5 ± 15.8 | 36.2 ± 15.1 | 0.274 | 0.232 | 0.171 | ||
| Non-progressive motility | % | infertile | 13.6 ± 10.6 | 13.6 ± 10.6 | 12.7 ± 5.8 | 0.853 | 0.802 | 0.676 |
| fertile | 18.3 ± 9.3 | 18.7 ± 9.9 | 30.0 ± 7.4 | 0.017 | 0.330 | 0.004 | ||
| Total motility | % | infertile | 53.0 ± 20.2 | 53.5 ± 19.7 | 49.0 ± 19.0 | 0.464 | 0.908 | 0.222 |
| fertile | 66.8 ± 15.7 | 65.5 ± 13.2 | 65.8 ± 14.9 | 0.727 | 0.450 | 0.986 | ||
| Immotile spermatozoa | % | infertile | 44.4 ± 20.1 | 44.0 ± 19.1 | 51.0 ± 19.0 | 0.276 | 0.676 | 0.167 |
| fertile | 33.2 ± 15.7 | 34.5 ± 13.2 | 33.8 ± 15.1 | 0.721 | 0.457 | 0.939 | ||
Mean values and standard deviation are presented; ND - genotypes were not detected in subjects with sperm parameters available; p1 – overall comparison – Kruskal-Wallis test; p2 – dominant model (minor homozygotes and heterozygotes vs. major homozygotes) – U-test; p3 –recessive model (minor homozygotes vs. other genotypes) -U-test.
Discussion
The normal function of the blood–testis barrier protects developing germ cells against harmful agents and immunological influences. Protection is necessary because many agents or immune system cells can disturb the delicate process of meiotic cell division, thus leading to infertility. The barrier include structural component build of tight junctions as well as functional elements being mainly membrane transporters, e.g. glycoprotein P. TNF-α and TGF-β3 are two crucial cytokines, that are involved in the regulation of the blood-testis barrier, affecting both its structure as well as activity of functional components6. Therefore, the present study evaluated associations between polymorphisms of genes coding for TNF-α and TGF-β3, i.e. TNF and TGFB3, and male infertility.
Our study did not reveal significant association between TNF rs1800629 polymorphism and male infertility in a Polish population. This observation is contrary to the report of Tronchon et al. from French population, who revealed significantly increased frequency of the TNFα -308 A allele in infertile male patients with testicular failure or with altered sperm motility compared with patients with normal sperm parameters17. Indian population data published by Shukla et al. are in keeping with French observations. i.e. substitution level from G to A in the TNF-α gene was significantly higher in the infertile subjects as compared to fertile controls18. Likewise, Egyptian population report by Zalata et al. documented significant overrepresentation of TNF -308AA carriers among patients diagnosed with asthenozoospermia, asthenoteratozoospermia and oligoasthenoteratozoospermia19. The latter study revealed also that TNF-α -308AA genotype was significantly associated with decreased sperm count, sperm motility, normal sperm morphology in the above mentioned groups of infertile patients. Contrary, our data revealed that in the group of infertile patients any of the studied sperm parameters was not significantly associated the studied TNF-α gene polymorphism. In the control group a marked association was seen with lower percentage of spermatozoa with progressive motility.
To our knowledge, this is the first communication on the association between male infertility and TGFB3 polymorphism. The results of the study demonstrated similar (borderline for rs2284792, p = 0.056) distribution of the studied TGFB3 genotypes in infertile and fertile men. However, higher frequency of the minor alleles (in all the studied SNPs) in infertile men, being significant for TGFB3 rs2284792. It was also found that heterozygous rs2284792 AG genotype was associated with significantly increased odds for infertility and similar results were observed for G allele carrier status. In the case of TGFB3 rs3917158 a significant association with infertility in heterozygous subjects was found, with odd ratio of 1.37. Analysis in the subgroup of infertile men with semen abnormalities according to the WHO criteria (65.1% of infertile subjects) revealed a significant association of TGFB3 rs2284792 variant with infertility; differences were significant for overall comparison, dominant model and allele frequency. Significant association for TGFB3 rs3917158 SNP could also be observed for that subgroup analysis (p < 0.05 for dominant model and increased frequency of heterozygotes among infertile men with semen abnormalities, p = 0.056 for differences in minor allele frequency) (Supplementary Table 1). Those data may suggest that TGFB3 polymorphism could be considered as a factor predisposing to male infertility. TGF-β3 is an important factor determining the blood-testis barrier integrity and its protective functions. Therefore, altered status for TGF-β3 generation produced by polymorphic variants in TGFB3 gene may affect function and integrity of the blood-testis barrier, thus leading to infertility. However, confirmation from studies involving higher number of subjects as well as recruited in different populations is required.
The association of TGFB3 polymorphism with male infertility can be also explained by other findings of the present study. It was found that the TGFB3 polymorphism may affect sperm parameters. The study revealed that TGFB3 polymorphism was associated with sperm motility parameters, i.e. TGFB3 variant genotypes were associated with lower percentage of spermatozoa with progressive motility (reaching significance level for rs2268626), higher percentage of non-progressive motility (rs2268626, rs3917158, all the studied SNPs), as well as decreased total motility and higher percentage of immotile spermatozoa among fertile men variant allele carriers (rs2268626 and rs2284792). No associations were found in the whole infertile men group, possibly due to more pronounced dysfunction of spermatozoa. However, separate analysis of sperm parameters in normozoospermic infertile men and infertile men with semen abnormalities revealed no significant genotype-related differences, but lower percentage of motile spermatozoa was observed in TGFB3 rs2268626 and rs2284792 minor homozygotes (Supplementary Table 2). That observation is in concordance with the results obtained for the control group of fertile men. There is no direct evidence from human studies on relationship between blood-testis barrier damage and spermatozoa-motility defects. However, it has been presented, that mice with disruption of blood-testis barrier integrity, induced by a high fat diet, were characterized by significantly decreased total and progressive motility of spermatozoa20. Similarly, administration of lipopolysaccharide with resultant disruption of blood-testis barrier, was associated with significant reduction in sperm count and spermatozoa motility in rats21. Other known disruptors of blood-testis barrier, i.e. TNF-α and IL-6, were also documented to significantly reduce spermatozoa progressive motility22,23. Those data may contribute to explanation of the observed associations between polymorphic variants in TGF-β3 and spermatozoa motility parameters. However, the results of the present study do not provide direct evidence that TGF-β3 may impact spermatozoa motility, only associations of TGFB3 polymorphism and spermatozoa motility parameters were evidenced. As it was stated above, TGF-β3 may also via effects on the barrier function affect testicular microenvironment, and thus indirectly the motility.
In conclusion, the results of the present study may suggest an association between TGFB3 polymorphism and male infertility. However, the observations should be verified in other studies, as the observed associations may result from a limited sample size (ethnic and environmental variables should also be addressed).
Methods
Subjects and study protocol
The study was carried out on 423 consecutive, otherwise healthy male patients, aged 32.9 ± 4.5 years (range: 19–52 years) without any chromosomal abnormalities, undergoing semen analysis due to infertility workup. The inclusion criteria were as following: age 18 to 56 years; no children from current or previous relations with at least a year history of at least a year of regular (2–3 weekly), unprotected sexual activity without conception; female partners aged up to 35 years with regular menstrual bleedings and/or progesterone levels in the luteal phase of the cycle >10 ng/ml, normal transvaginal ultrasound examination, negative testing for Chlamydia trachomatis infection, without history of pelvic inflammatory disease or abdominal operations. Male factor exclusion criteria included: clinical picture suggestive of obturatory azoospermia; history of testicular, epididymis or accessory gland infection; testicular torsio, maldescence or injury; varicocele; co-existing systemic disease; history of mumps.
The control group consisted of 423 healthy males, aged 34.3 ± 8.3 years, (range: 21–62) recruited among consecutive men accompanying their female partners at term labor at the Department of Reproductive Medicine and Gynecology, Siedlecka str., Police, Poland. Paternity was confirmed by women; however the possible paternal discrepancy was additionally checked based on blood group verification.
Both the men undergoing infertility workup as well as the fertile controls were of Polish origin, recruited within the same geographical region.
Semen samples were collected by masturbation after two to seven days of abstinence from sexual activity. Among infertile men, full seminograms, allowing for detailed data analysis were available for 344 patients. As for the fertile controls, that group was recruited among men accompanying their female partners at term labor, and only 124 of men from this group agreed to provide sperm sample for analysis (29.3%). However, that subgroup is representative for the whole study control group (no significant differences for age nor ethnicity) and provides necessary data on sperm parameters in healthy Polish males.
Sperm parameters were evaluated manually within one hour after the sample collection. Concentration, motility and morphology of spermatozoa were assessed according to the 2010 World Health Organization guidelines24. Sperm concentration was determined in an improved Neubauer haemocytometer (Heinz Hernez Medizinalbedarf GmbH, Hamburg, Germany), whereas sperm motility was analyzed under a phase-contrast microscope (Eclipse 200, Nikon, Japan), equipped with a heated stage. Sperm morphology was assessed, following the Papanicolaou staining of semen smears, under a bright light microscope (BX 41 Olympus Optical Co., Ltd., Tokyo, Japan) at 1000 × magnification. Study participants were categorized according to sperm concentration, motility and morphology as normozoospermic (with normal morphology) and subjects with abnormal standard sperm parameters (below the lower reference limits).
The study was approved by the Ethics Committee of Pomeranian Medical University (KB-0012/116/10) and conducted according to the Declaration of Helsinki. All participants provided written informed consent prior to participating in the study.
Genotyping
All the subjects were genotyped for rs1800629 (−308G > A, MAF: 0.173) SNP in the promoter region of the TNF, previously associated with higher gene expression (A allele). Five tag SNPs with frequencies >0.1, mapping the TGFB3 gene (rs2268626:T > C, MAF: 0.221; rs3917158:C > T, MAF: 0.199; rs2284792:A > G, MAF: 0.261; rs2268625:T > C, MAF: 0.190; rs3917187:C > T, MAF: 0.226) were selected with tag SNP picker using data for Caucasian population from the International HapMap Project (http://www.hapmap.org). MAFs are given for CEU population (Utah residents with Northern and Western European ancestry) from HapMap database. All the SNPs are located in gene introns. Genomic DNA was extracted from 200 mL of whole blood samples using Gene-MATRIX Quick Blood DNA Purification Kit (EURx, Poland). Commercially available, pre-validated allelic discrimination TaqMan real-time PCR assays (Life Technologies, USA) were used for genotyping. Fluorescence data was captured using ViiA 7 Real-Time PCR System (Life Technologies, USA) after 40 cycles of PCR.
Statistical analysis
Genotype distribution was tested for Hardy-Weinberg equilibrium using Chi2 test. Continuous variables were compared between groups using Mann-Whitney U-test (comparison between two groups) or Kruskal-Wallis test (more than 2 groups). Associations between categorical variables were assessed by the Chi2 test, with Yate’s corrections for n < 5 (Statistica 10.0, Statsoft Software, Poland). The EH program (Jurg Ott, Rockefeller University, New York) was used to estimate haplotype frequencies. Linkage disequilibrium (LD) was measured: the D’ was calculated using 2LD software and squared correlation coefficient (r2) was evaluated. A p level of less than 0.05 was considered statistically significant.
Additional Information
How to cite this article: Droździk, M. et al. TGFβ3 (TGFB3) polymorphism is associated with male infertility. Sci. Rep. 5, 17151; doi: 10.1038/srep17151 (2015).
Supplementary Material
Acknowledgments
The study was supported by the National Science Centre, Poland grant no. UMO-2011/01/B/NZ5/03811.
Footnotes
Author Contributions M.D. and M.K. designed study, analyzed data and wrote the paper. M.K. and D.M. performed genotyping. U. B. analyzed data, managed subjects files. A.K. and R.K. patient recruitment, diagnosis and sperm analysis.
References
- Irvine D. S. Epidemiology and aetiology of male infertility. Hum. Reprod. 13 Suppl 1, 33–44 (1998). [DOI] [PubMed] [Google Scholar]
- Abu-Halima M. et al. Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments. Fertil. Steril. 99, 1249–1255. e16 (2013). [DOI] [PubMed] [Google Scholar]
- Meschede D. et al. Clustering of male infertility in the families of couples treated with intracytoplasmic sperm injection. Hum. Reprod. 15, 1604–1608 (2000). [DOI] [PubMed] [Google Scholar]
- Cheng C. Y. & Mruk D. D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 64, 16–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital P., Hinton B. T. & Dufour J. M. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol. Reprod. 84, 851–858 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. et al. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology 146, 1893–1908 (2005). [DOI] [PubMed] [Google Scholar]
- Siu M. K. et al. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology 144, 371–378 (2003). [DOI] [PubMed] [Google Scholar]
- Yao P. L. et al. TNF alpha-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol. Reprod. 80, 581–589 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudiuit C. et al. Tumor necrosis factor-alpha antagonizes follicle-stimulating hormone action in cultured Sertoli cells. Endocrinology 133, 69–76 (1993). [DOI] [PubMed] [Google Scholar]
- Kaluza W. et al. Different transcriptional activity and in vitro TNF-α production in psoriasis patients carrying the TNF-α 238A promoter polymorphism. J. Invest. Dermatol. 114, 1180–1183 (2000). [DOI] [PubMed] [Google Scholar]
- Wilson A. G. et al. Effects of a polymorphism in the human tumor necrosis factor α promoter on transcriptional activation. Proc. Natl. Acad. Sci. USA 94, 3195–3199 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui W. Y. et al. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier In vitro possibly mediated via its effects on occludin, zonula occludens-1 and claudin-11. Endocrinology 142, 1865–1877 (2001). [DOI] [PubMed] [Google Scholar]
- Lui W. Y. et al. TGF-β3 regulates the blood testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology 144, 1139–1142 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang X. & Lui W. Y. Transforming growth factor-β3 regulates cell junction restructuring via MAPK-mediated mRNA destabilization and Smad-dependent protein degradation of junctional adhesion molecule B (JAM-B). Biochim. Biophys. Acta 1849, 601–611 (2015). [DOI] [PubMed] [Google Scholar]
- Kruit A. et al. Transforming growth factor-β gene polymorphisms in sarcoidosis patients with and without fibrosis. Chest 129, 1584–1591 (2006). [DOI] [PubMed] [Google Scholar]
- Hu B. C. The association between transforming growth factor beta3 polymorphisms and left ventricular structure in hypertensive subjects. Clin. Chim. Acta 411, 558–562 (2010). [DOI] [PubMed] [Google Scholar]
- Tronchon V. et al. Tumor necrosis factor-alpha -308 polymorphism in infertile men with altered sperm production or motility. Hum. Reprod. 23, 2858–2866 (2008). [DOI] [PubMed] [Google Scholar]
- Shukla K. K. et al. Significant association of TNFα and IL-6 gene with male infertility - an explorative study in Indian populations of Uttar Pradesh. Immunol. Lett. 156, 30–37 (2013). [DOI] [PubMed] [Google Scholar]
- Zalata A. et al. Tumor necrosis factor-α gene polymorphism relationship to seminal variables in infertile men. Urology 81, 962–966 (2013). [DOI] [PubMed] [Google Scholar]
- Fan Y. et al. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS One 10, e0120775 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-Allah A. R. et al. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxid. Med. Cell. Longev. 2, 73–81 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampiao F. & du Plessis S. S. TNF-alpha and IL-6 affect human sperm function by elevating nitric oxide production. Reprod. Biomed. Online 17, 628–631 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells. Sci. Rep. 4, 4260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO laboratory manual for the examination and processing of human semen (World Health Organization Press, 2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


