Abstract
Yersinia pseudotuberculosis is a foodborne enteric pathogen that causes a mild self-limiting diarrhea in humans. Yersinia pseudotuberculosis is able to persist in soil and water and in association with fresh produce, but the mechanism by which it persists is unknown. It has been shown that Y. pseudotuberculosis co-occurs with protozoans in these environments; therefore, this study investigates if bacterivorous free-living amoeba (FLA) are able to support persistence of Y. pseudotuberculosis. Coculture studies of Y. pseudotuberculosis and the prototype FLA, Acanthamoeba castellanii revealed that bacteria had an enhanced capacity to survive in association with amoeba and in the absence of any cytotoxic effects. Yersinia pseudotuberculosis is able to survive and replicate in trophozoites specifically localized within vacuoles, and persists within cysts over a period of at least a week. These data present the first evidence that Y. pseudotuberculosis is able to resist the bacterivorous nature of FLA and instead exhibits an enhanced ability to replicate and persist in coculture with amoeba. This study sheds light on the potential role of FLA in the ecology of Y. pseudotuberculosis which may have implications for food safety.
Keywords: Acanthamoeba, Yersinia, cyst, trophozoite
Yersinia pseudotuberculosis–amoeba interactions.
Graphical Abstract Figure.
Yersinia pseudotuberculosis–amoeba interactions.
INTRODUCTION
Yersinia pseudotuberculosis is a facultative anaerobic psychrotrophic bacterium that belongs to the family Enterobacteriaceae. Yersinia pseudotuberculosis causes a mild self-limiting enteric disease, called yersiniosis, which typically presents as a fever, abdominal pain and diarrhea. Yersinia pseudotuberculosis is frequently isolated from foods, e.g. raw seafood, fresh vegetables, unpasteurized milk and processed pork, and from animals, soil and water. The ability of Y. pseudotuberculosis to survive and proliferate at low temperatures of 0–4 °C within refrigerators, for example on raw vegetables or processed pork poses a challenge for proper food storage and preservation (Kangas et al. 2008; Rimhanen-Finne et al. 2009; Vaerewijck et al. 2010; Laukkanen-Ninios, Fredriksson-Ahomaa and Korkeala 2014). The ecology of Y. pseudotuberculosis with regard to its mode of transmission, survival in the environment and on food, and its source of contamination on farms, is not well known. The mechanisms of persistence of Y. pseudotuberculosis on food, farms and slaughterhouses (Laukkanen et al. 2008) need to be understood if new strategies are to be developed to minimize food contamination by these bacteria.
There is evidence for a role of free-living amoeba (FLA) as a reservoir or vehicle of dissemination of bacterial pathogens which addresses the ecology of these bacteria. FLA are ubiquitous, bacterivorous unicellular eukaryotic organisms that have two characteristic life stages (Khunkitti et al. 1998; Siddiqui and Khan 2012). One life stage is a metabolically active trophozoite form which phagocytozes bacteria (Khunkitti et al. 1998). These trophozoites are akin to macrophages and some other mammalian cell types because phagocytozed bacteria are trafficked through the canonical endocytic pathway to be digested in the phagolysosome. A second life stage is a dormant double-walled cyst form that is resistant to harsh environmental conditions (e.g. extreme temperatures, desiccation and nutrient deprivation) and supports prolonged survival under these conditions (Khunkitti et al. 1998). Exposure to favorable conditions activates excystment and return to the metabolically active trophozoite life stage (Khunkitti et al. 1998; El-Etr et al. 2009). Some pathogenic intracellular bacteria resist being killed by amoeba and instead exploit this association to enable their persistence in the environment. Such interactions have been demonstrated between the foodborne pathogens Campylobacter jejuni (Olofsson et al. 2013), Salmonella (Douesnard-Malo and Daigle 2011), Listeria monocytogenes (Pushkareva and Ermolaeva 2010) and Yersinia enterocolitica (Lambrecht et al. 2013) with soil- and water-borne FLA. These pathogens utilize conserved intracellular survival strategies to survive in unicellular amoeba. For instance, C. jejuni evades intracellular digestion by avoiding localization to the amoebal lysosomal vacuole (Olofsson et al. 2013), much like how it is trafficked within mammalian epithelial cells (Watson and Galan 2008). Other pathogens like Francisella tularensis and Mycobacterium spp. are able to survive for weeks within Acanthamoeba spp. cysts (El-Etr et al. 2009; Ben Salah and Drancourt 2010; Amissah et al. 2014).
FLA and Y. pseudotuberculosis co-occur in natural soil and water environments, on fresh produce, including lettuce and carrots (Kangas et al. 2008; Rimhanen-Finne et al. 2009; Vaerewijck et al. 2011), and they are found in anthropogenic environments, like refrigerators associated with fresh produce (Vaerewijck et al. 2010). It has been shown that several different species of protozoa isolated from lettuce and spinach can harbor the food pathogens Escherichia coli O157:H7 and Salmonella enterica (Gourabathini et al. 2008) within vesicles that are expelled from protozoa. These bacteria-filled vesicles once expelled not only protect the bacteria from harsh conditions, but also support bacterial replication and subsequent escape back into the environment (Gourabathini et al. 2008). These factors led to the hypothesis that FLA may act as a reservoir or vehicle of dissemination of Y. pseudotuberculosis in the environment. To test if this was possible, we investigated the interaction of Y. pseudotuberculosis with the laboratory prototype FLA, A. castellanii using controlled coculture studies.
MATERIALS AND METHODS
Cultivation of Acanthamoeba castellanii
Acanthamoeba castellanii (Douglas) Page amoeba (ATCC® 30010; American Type Culture Collection) was maintained axenically in proteose peptone yeast extract glucose (PYG) medium (ATCC recipe; http://www.atcc.org/products/all/30010.aspx#culturemethod) at ambient room temperature (25°C) in 25-cm2 culture flasks. The adherent amoeba were harvested by tapping the flasks vigorously, followed by centrifugation (300 × g for 5 min) to collect dislodged amoeba. Amoeba were then washed with phosphate-buffered saline (PBS) and resuspended in nutrient-rich PYG medium. A Neubauer counting chamber was used to estimate amoeba numbers. For interaction studies with bacteria, the amoeba culture was diluted to approximately 1 × 105 living trophozoites/ml and seeded into 24-well plates and allowed to adhere to the wells overnight at 25°C ambient temperature; all plates were subsequently maintained at this temperature. The viability of A. castellanii trophozoites was confirmed using trypan blue exclusion.
Bacterial cultures
The Y. pseudotuberculosis IP32953 strain was grown for 12 h overnight in brain heart infusion (BHI) broth at 25 or 37°C to test if any adaptive influences at temperature conditions mimicking ambient environmental growth and a mammalian host, respectively, occurs. Escherichia coli DH5α cultured in Luria–Bertani (LB) medium at 37°C was also used. Bacteria were enumerated using a Petroff Hausser counting chamber. All bacteria are maintained at −80°C glycerol stocks in the laboratory. The Y. pseudotuberculosis IP32953 strain was maintained on Yersinia selective agar base (Oxoid) after culture from glycerol stocks.
Extracellular persistence assay
Amoeba were seeded on 24-well plates as described above and infected with Y. pseudotuberculosis cultured at 25°C and E. coli DH5α cultured at 37°C at a multiplicity of infection (MOI) of 100, i.e. 107 bacteria. Bacteria were also cultured singly. After 24 and 168 h co- and monoculture, serial dilutions were performed and plated without lysing of amoeba.
Bacterial entry and intracellular survival assays in PYG medium
Amoeba that were seeded overnight on 24-well plates were washed and incubated in fresh PYG medium for 1 h prior to infection. To determine if Y. pseudotuberculosis could enter or be phagocytozed by amoeba, bacteria were cocultured with amoeba at an MOI of 100. Yersinia pseudotuberculosis was cultured at either 25 or 37°C, and E. coli at 37°C prior to use in coculture experiments. After coculture of bacteria and amoeba for 1 h, cells were washed once with PBS and incubated with 200 μg ml−1 of gentamicin in PYG medium for a further 2 h to kill extracellular bacteria. The amoebas were then washed once with PBS to remove dead bacterial cells and debris, and then lysed with addition of 0.2% Triton X-100 for 10 min. Lysates were immediately serially diluted and plated on BHI agar. Following incubation for 48 h at 28°C, colony forming units (CFU) were enumerated. To assess if Y. pseudotuberculosis could survive intracellularly in amoeba trophozoites over a period of 1 week (168 h), assays in PYG were performed similarly to entry assays except that, following gentamicin treatment, the medium was replaced with fresh PYG medium and incubation of the coculture was allowed to proceed for a further 24 h or 1 week. Amoebas were lysed using 0.2% Triton X-100 immediately after incubation periods, serially diluted and plated. Monocultures of bacteria were included as controls and were treated with gentamicin and serially diluted and plated at the respective time points, to confirm that all bacteria succumbed to gentamicin treatment. All experiments were performed using three independent biological replicates, each consisting of two technical replicates.
Bacterial entry and intracellular survival assays in high salt (HS) buffer
Incubation in nutritive-deficient HS buffer (95 mM NaCl, 5 mM KCl, 8 mM MgSO4, 0.4 mM CaCl2, 1 mM NaHCO3, 20 mM Tris–HCl, pH 9.0) results in encystment of amoeba (El-Etr et al. 2009) and allows testing of survival of Y. pseudotuberculosis in cysts. Entry and survival assays in HS buffer were performed similarly as above but with the following modifications: following 24 and 168 h of coculture, amoeba were washed once with PBS, and HS medium was replaced with fresh PYG medium and supplemented with IsoVitaleX (BD Biosciences) to allow excystment and bacterial recovery over 48 h. Amoeba were then lysed with 0.2% Triton X-100 immediately after incubation periods, serially diluted and plated. Monocultures of bacteria were included as controls and were treated with gentamicin and serially diluted and plated at the respective time points to confirm that all bacteria succumbed to gentamicin treatment. All experiments were performed using three independent biological replicates each consisting of two technical replicates.
Electron microscopy
For preparation of samples for electron microscopy (EM), bacteria that were cocultured with amoeba in PYG (for trophozoites) or HS medium (for cysts) for 168 h were pelleted and fixed with 2% paraformaldehyde/2.5% glutaraldehyde in 0.2 M cacodylate buffer (El-Etr et al. 2009). After fixing, samples were washed, dehydrated and embedded in an Epon resin. Ultrathin sections (∼90–100 nm) were prepared and placed on a grid for microscopic analysis.
Cytotoxicity assays
A Cytotox-96 non-radioactive kit (Promega) was used as per the manufacturer's instructions. Cocultures and monocultures were prepared as above but without gentamicin treatment and sampled after 24 h.
Statistical analyses
Graphpad Prism 5 was used for statistical evaluation of the data. One-way ANOVA with a post-hoc Tukey's Test was used to determine differences in viable bacteria or amoeba at each time point.
RESULTS
Persistence of Y. pseudotuberculosis in coculture with A. castellanii
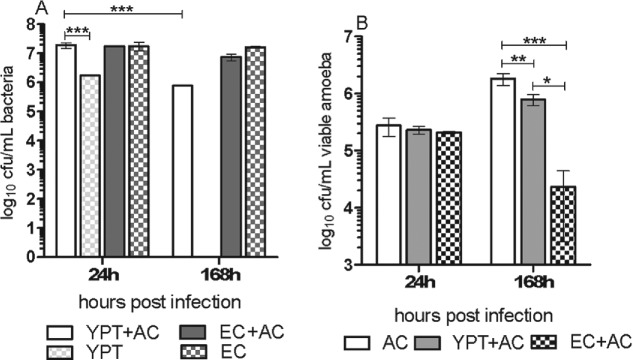
To establish if Y. pseudotuberculosis could survive in association with amoeba, coculture of bacteria and amoeba was allowed to proceed free of gentamicin treatment and amoeba were not lysed before plating. After 24 h of coculture, bacterial numbers remained the same in coculture and monoculture for both Y. pseudotuberculosis and E. coli (Fig. 1A). At 1 week of coculture, Y. pseudotuberculosis numbers were reduced by ∼1 log but viable bacteria were absent from the monoculture. In contrast, E. coli survived at equivalent numbers in both coculture and monoculture at 1 and 7 days.
Figure 1.
Yersinia pseudotuberculosis persists longer in association with A. castellanii than during monoculture. (A) Viable bacteria grown with A. castellanii, or grown singly. (B) Viable A. castellanii grown singly or in coculture with bacteria. Error bars indicate mean ± SD of at least three independent biological replicates. * = P < 0.01, ** = P < 0.001, *** = P < 0.0001. AC = A. castellanii, EC = E. coli DH5α, YPT = Y. pseudotuberculosis IP32953.
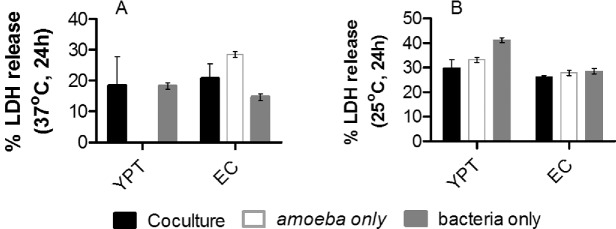
The amoeba in the Y. pseudotuberculosis amoeba coculture and amoeba monoculture were able to replicate ∼0.5–1 log greater over the 1 week period, but viable amoeba numbers decreased by 1 log in coculture containing E. coli (Fig. 1B). Many cysts were visible in cocultures of E. coli and amoeba at 1 week. Bacterial cytotoxicity assays were performed to determine if this accounted for the general decreases in viable amoeba present after 1 week of coculture. Cytotoxicity assays which measure release of lactate dehydrogenase (LDH) revealed that bacteria were not cytotoxic to amoeba because comparable levels of LDH were produced by single cultures of amoeba, or bacteria and the cocultures at both pre-culture temperatures (Fig. 2).
Figure 2.
Yersinia pseudotuberculosis is not cytotoxic to A. castellanii. Percentage of LDH released by bacteria, A. castellanii, or coculture of bacteria and A. castellanii at 37°C (A) or 25°C (B). Error bars indicate mean ± SD of at least three independent biological replicates. YPT = Y. pseudotuberculosis IP32953, EC = E. coli DH5α.
Yersinia pseudotuberculosis replicates and survives up to 1 week within trophozoites
To establish if Y. pseudotuberculosis is able to survive in intracellular association with A. castellanii, we determined if Y. pseudotuberculosis was phagocytozed by trophozoites using a gentamicin protection assay to kill extracellular bacteria that had not entered trophozoites. Monocultures of bacteria were treated with the same concentration of gentamicin but viable bacteria were not recovered. Irrespective of pre-culture temperature, Y. pseudotuberculosis entered amoeba at equal numbers (Fig. 3A), but E. coli was not similarly recovered from trophozoites.
Figure 3.
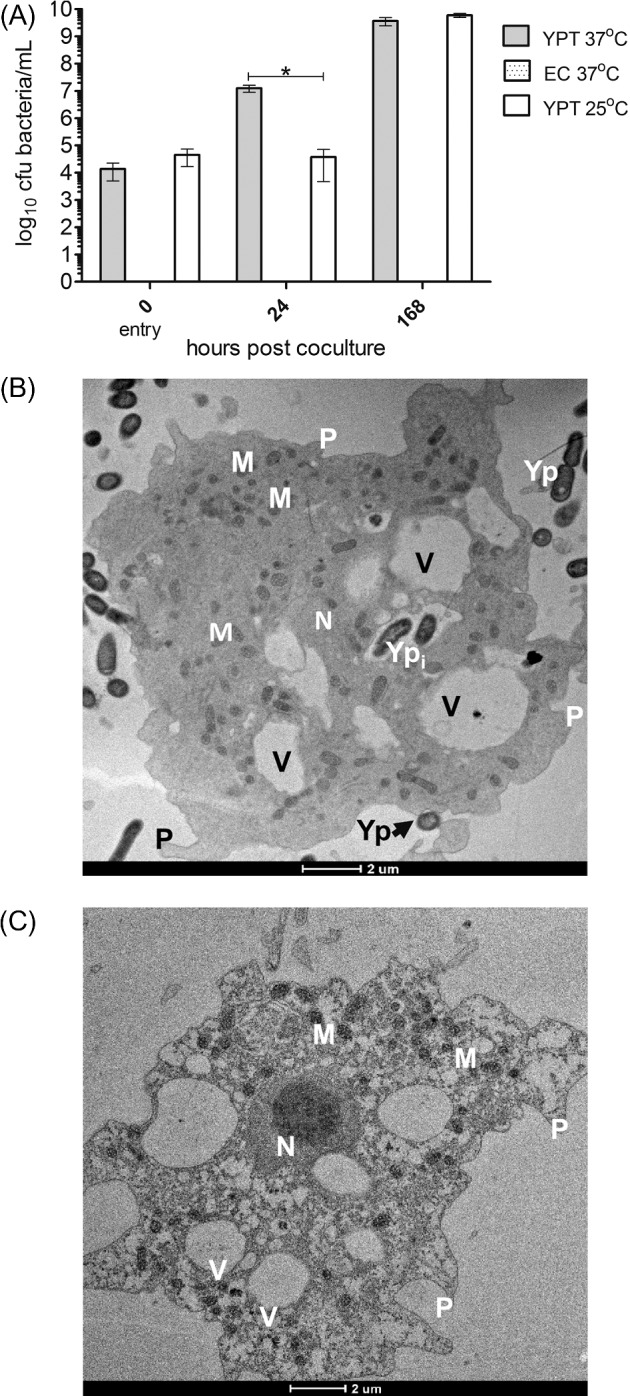
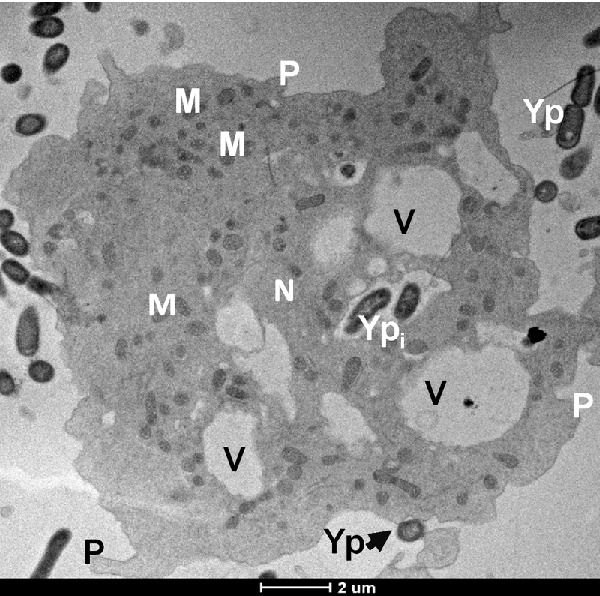
Yersinia pseudotuberculosis can enter, replicate and survive up to 1 week within A. castellanii trophozoites. (A) Entry and survival of bacterial strains in trophozoites after pre-culture at 37 or 25°C. Error bars indicate mean ± SD of at least three independent biological replicates. * = P < 0.0001. EC = E.coli DH5α, YPT = Y. pseudotuberculosis IP32953. (B) Transmission electron micrographs of Y. pseudotuberculosis IP32953 localized within A. castellanii trophozoite. (C) Uninfected trophozoite. M = mitochondria, Yp = extracellular Y. pseudotuberculosis, N = nucleus, V = vacuole, P = pseudopodia, Ypi = intracellular Y. pseudotuberculosis. Scale bar representing 2 μm is indicated.
Next the survival of bacteria was assessed over a period of 1 week. Although pre-culture at 37 and 25°C resulted in equal replication of Y. pseudotuberculosis by 1 week, pre-culture at 37°C enabled faster replication as demonstrated by the significant difference (P < 0.01) in bacteria between the two pre-culture temperatures after 24 h of coculture.
To determine the localization of Y. pseudotuberculosis and E. coli in trophozoites of A. castellanii, EM was performed after coculture at 1 week. Intact Y. pseudotuberculosis cells were observed to reside intracellularly within vacuoles (Fig. 3B) of A. castellanii but this was not observed for E. coli (data not shown).
Yersinia pseudotuberculosis can survive for up to 1 week within cysts
During amoeba encystment, which lasts several hours, digestive vacuoles and other components (e.g. golgi, nucleus) are broken down and extruded leaving only lipid droplets to remain in the double-walled mature cyst (Bowers and Korn 1969). Hence, any intracellular bacteria that were not digested or expelled during encystment would represent bacteria that remained in cysts upon initial entry into trophozoites in nutritive-deficient HS buffer. Upon coculture with bacteria, encystment occurred within 24 h, which is typical for Acanthamoeba growing in HS medium (El-Etr et al. 2009). Yersinia pseudotuberculosis entered into trophozoites and was recovered from cysts at 24 and 168 h at both 25 and 37°C (Fig. 4A). Interestingly, the pre-culture of Y. pseudotuberculosis at 37°C, which is representative of mammalian physiological conditions, favors entry into trophozoites cultured in HS medium and faster growth rate in PYG medium. Escherichia coli was never recovered from cysts, which correlates with its inability to survive in trophozoites. Consistent with these data, EM confirmed that Y. pseudotuberculosis was localized within mature double-walled cysts at 1 week of coculture (Fig. 4B), but E. coli was not observed within cysts.
Figure 4.
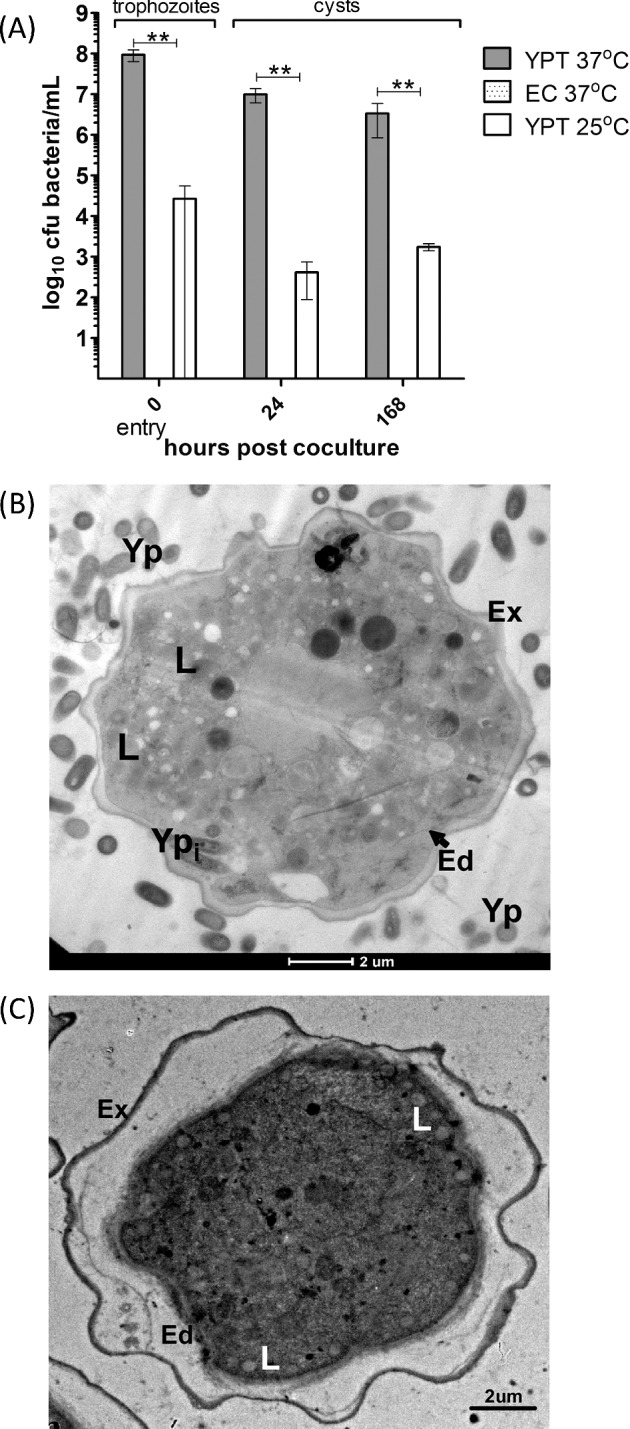
Yersinia pseudotuberculosis can survive up to 1 week within A. castellanii cysts. (A) Entry into trophozoites and survival of bacteria in cysts after pre-culture at 37 or 25°C. Error bars indicate mean ± SD of at least three independent biological replicates. ** = P < 0.001. EC = E. coli DH5α, YPT = Y. pseudotuberculosis IP32953. (B) Transmission electron micrographs of Y. pseudotuberculosis IP32953 localized within an A. castellanii cyst. (C) An uninfected A. castellanii cyst. L = lipid droplet, Yp = extracellular Y. pseudotuberculosis, Ypi = intracellular Y. pseudotuberculosis, Ed = endocyst wall, Ex = exocyst wall. Scale bar representing 2 μm is indicated.
DISCUSSION
Our findings reveal that the foodborne pathogen, Y. pseudotuberculosis is capable of resisting grazing by bacterivorous protozoa in a non-cytotoxic manner. Instead, these bacteria show enhanced persistence in extracellular association with amoeba. The enhanced ability to persist in association with A. castellanii but not in monoculture has also been demonstrated for Salmonella enterica serovar Typhi and Y. enterocolitica (Douesnard-Malo and Daigle 2011; Lambrecht et al. 2013). Simultaneously, we demonstrated that Y. pseudotuberculosis is able to survive and replicate within trophozoites, especially at 37°C, similar to its phenotype in macrophages (Moreau et al. 2010; Ligeon et al. 2014). Bacterial factors expressed at 37°C might trigger improved uptake and replication of Y. pseudotuberculosis within amoeba. As such, because HS buffer is nutritionally deficient, turnover of the expression factors is slower than during PYG growth, thus sustaining higher entry. The intravacuolar residence of Y. pseudotuberculosis was reminiscent of its infection in macrophages within which the bacteria can survive by inhibiting acidification of the vacuole (Wang and Ahearn 1997). Other bacterial pathogens, such as C. jejuni (Axelsson-Olsson et al. 2005), F. tularensis (El-Etr et al. 2009) and Legionella pneumophila (Greub and Raoult 2004) survive within Acanthamoeba vacuoles and prevent acidification of the intravacuolar niche as an intracellular survival strategy. Interestingly, this is in contrast to Y. enterocolitica which resides in the cytosol of A. castellanii (Lambrecht et al. 2013) although both species of Yersinia are intracellular foodborne pathogens that cause yersiniosis.
Our data demonstrating the maintenance of viable Y. pseudotuberculosis in cysts is a relatively rare observation because not many other human pathogens have been observed in cysts. Several Mycobacterium spp. (Ben Salah and Drancourt 2010; Amissah et al. 2014) are able to survive in the exocyst while F. tularensis was shown to survive within cysts for a period of at least 3 weeks (El-Etr et al. 2009). Legionella pneumophila survival in cysts has been proposed (Skinner et al. 1983; Kilvington and Price 1990) but is contentious because viable L. pneumophila was demonstrated in vesicles expelled from encysting amoeba (Bouyer et al. 2007; Hoffmann, Harrison and Hilbi 2014). Cysts are supposedly dormant bodies; however, it is not known whether bacteria are metabolically active while inside. Our data suggest that Y. pseudotuberculosis is not replicating within cysts. This observation is based on the experimental design in which amoeba excystment and bacterial recovery is allowed to ensue for 48 h prior to plating, but the number of Y. pseudotuberculosis in trophozoites remains similar to those recovered from cysts. During long-term persistence in a soil microcosm, Y. pseudotuberculosis has been observed to adopt physiological characteristics that are indicative of lower metabolic activity, which is reversed following several passages in a nutritive medium (Buzoleva and Somov 2003).
In contrast to the interaction of Y. pseudotuberculosis with A. castellanii, E. coli DH5α was able to survive in extracellular association with A. castellanii but resisted being phagocytozed as indicated by our inability to quantify viable bacteria from intracellular assays, or observe E. coli within trophozoites or cysts. This may indicate an alternate mechanism of grazing resistance for E. coli DH5α, since the pathogenic E. coli O157:H7 is able to survive within amoeba vacuoles (Gourabathini et al. 2008). However, E. coli survives at high numbers extracellularly with a concomitant decrease in trophozoites over 1 week. The faster growth of E. coli may inhibit the growth and promote encystment of the amoeba due to competition for media nutrients and/or by pH decreases caused by metabolic by-products. This has been shown previously in amoeba coculture studies using MOIs of 100 or above (Avery, Harwood and Lloyd 1995; Wang and Ahearn 1997).
Collectively, it appears that Y. pseudotuberculosis is able to survive both intracellularly and in extracellular association with A. castellanii, and our data imply that this interaction is favourable and enhance persistence and survival of Y. pseudotuberculosis in the environment. Interestingly, some intracellular bacteria are able to cycle in and out of the trophozoite (Greub and Raoult 2004) within vesicles that are passively expelled from amoeba. These bacteria containing vesicles have been demonstrated to allow bacterial persistence in the environment by providing protection from harsh conditions, and support both replication and escape of bacteria (Gourabathini et al. 2008). Bacteria that have escaped are expected to be phagocytozed by the same or adjacent amoeba. Cycling of L. pneumophila (Bouyer et al. 2007), S. enterica (Brandl et al. 2005) and F. tularensis within vesicles has been implicated in the dissemination of these pathogens in the environment. Similarly the ability of Y. pseudotuberculosis to be sustained at high numbers both intracellularly and extracellularly may imply that these bacteria are cycling in and out of trophozoites.
In conclusion, our study demonstrates that the Y. pseudotuberculosis–FLA association favours persistence and replication of this foodborne pathogen. Taken together with data that Y. pseudotuberculosis and FLA co-occur, our findings indicate that the protozoa may play a significantly role in the ecology of Y. pseudotuberculosis.
Acknowledgments
We thank Jim Bliska for the Y. pseudotuberculosis IP32953 strain, and together with Doug Call and Anders Omsland, for comments to improve this manuscript.
FUNDING
This work was supported by intramural grant funds from Washington State University College of Veterinary Medicine
Conflict of interest. None declared. ()
REFERENCES
- Amissah NA, Gryseels S, Tobias NJ, et al. Investigating the role of free-living amoeba as a reservoir for Mycobacterium ulcerans. PLoS Neglect Trop D. 2014;8:e3148. doi: 10.1371/journal.pntd.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SV, Harwood JL, Lloyd D. Quantification and characterization of phagocytosis in the soil amoeba Acanthamoeba castellanii by flow cytometry. Appl Environ Microb. 1995;61:1124–32. doi: 10.1128/aem.61.3.1124-1132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson-Olsson D, Waldenstrom J, Broman T, et al. Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni. Appl Environ Microb. 2005;71:987–92. doi: 10.1128/AEM.71.2.987-992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Salah I, Drancourt M. Surviving within the amoebal exocyst: the Mycobacterium avium complex paradigm. BMC Microbiol. 2010;10:99. doi: 10.1186/1471-2180-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer S, Imbert C, Rodier MH, et al. Long-term survival of Legionella pneumophila associated with Acanthamoeba castellanii vesicles. Environ Microbiol. 2007;9:1341–4. doi: 10.1111/j.1462-2920.2006.01229.x. [DOI] [PubMed] [Google Scholar]
- Bowers B, Korn ED. The fine structure of Acanthamoeba castellanii (Neff strain). II. Encystment. J Cell Biol. 1969;41:786–805. doi: 10.1083/jcb.41.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl MT, Rosenthal BM, Haxo AF, et al. Enhanced survival of Salmonella enterica in vesicles released by a soilborne Tetrahymena species. Appl Environ Microb. 2005;71:1562–9. doi: 10.1128/AEM.71.3.1562-1569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzoleva LS, Somov GP. Adaptation variability of Yersinia pseudotuberculosis during long-term persistence in soil. Bull Exp Biol Med. 2003;135:456–9. doi: 10.1023/a:1024915409187. [DOI] [PubMed] [Google Scholar]
- Douesnard-Malo F, Daigle F. Increased persistence of Salmonella enterica serovar Typhi in the presence of Acanthamoeba castellanii. Appl Environ Microb. 2011;77:7640–6. doi: 10.1128/AEM.00699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Etr SH, Margolis JJ, Monack D, et al. Francisella tularensis type A strains cause the rapid encystment of Acanthamoeba castellanii and survive in amoebal cysts for three weeks postinfection. Appl Environ Microb. 2009;75:7488–500. doi: 10.1128/AEM.01829-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourabathini P, Brandl MT, Redding KS, et al. Interactions between food-borne pathogens and protozoa isolated from lettuce and spinach. Appl Environ Microb. 2008;74:2518–25. doi: 10.1128/AEM.02709-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greub G, Raoult D. Microorganisms resistant to free-living amoeba. Clin Microbiol Rev. 2004;17:413–33. doi: 10.1128/CMR.17.2.413-433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Harrison CF, Hilbi H. The natural alternative: protozoa as cellular models for Legionella infection. Cell Microbiol. 2014;16:15–26. doi: 10.1111/cmi.12235. [DOI] [PubMed] [Google Scholar]
- Kangas S, Takkinen J, Hakkinen M, et al. Yersinia pseudotuberculosis O:1 traced to raw carrots, Finland. Emerg Infect Dis. 2008;14:1959–61. doi: 10.3201/eid1412.080284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khunkitti W, Lloyd D, Furr JR, et al. Acanthamoeba castellanii: growth, encystment, excystment and biocide susceptibility. J Infect. 1998;36:43–8. doi: 10.1016/s0163-4453(98)93054-7. [DOI] [PubMed] [Google Scholar]
- Kilvington S, Price J. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J Appl Bacteriol. 1990;68:519–25. doi: 10.1111/j.1365-2672.1990.tb02904.x. [DOI] [PubMed] [Google Scholar]
- Lambrecht E, Bare J, Van Damme I, et al. Behavior of Yersinia enterocolitica in the presence of the bacterivorous Acanthamoeba castellanii. Appl Environ Microb. 2013;79:6407–13. doi: 10.1128/AEM.01915-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen R, Martinez PO, Siekkinen KM, et al. Transmission of Yersinia pseudotuberculosis in the pork production chain from farm to slaughterhouse. Appl Environ Microb. 2008;74:5444–50. doi: 10.1128/AEM.02664-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen-Ninios R, Fredriksson-Ahomaa M, Korkeala H. Enteropathogenic Yersinia in the pork production chain: challenges for control. Compr Rev Food Sci F. 2014;13:1165–91. [Google Scholar]
- Ligeon LA, Moreau K, Barois N, et al. Role of VAMP3 and VAMP7 in the commitment of Yersinia pseudotuberculosis to LC3-associated pathways involving single- or double-membrane vacuoles. Autophagy. 2014;10:1588–602. doi: 10.4161/auto.29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau K, Lacas-Gervais S, Fujita N, et al. Autophagosomes can support Yersinia pseudotuberculosis replication in macrophages. Cell Microbiol. 2010;12:1108–23. doi: 10.1111/j.1462-5822.2010.01456.x. [DOI] [PubMed] [Google Scholar]
- Olofsson J, Axelsson-Olsson D, Brudin L, et al. Campylobacter jejuni actively invades the amoeba Acanthamoeba polyphaga and survives within non digestive vacuoles. PLoS One. 2013;8:e78873. doi: 10.1371/journal.pone.0078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkareva VI, Ermolaeva SA. Listeria monocytogenes virulence factor Listeriolysin O favors bacterial growth in co-culture with the ciliate Tetrahymena pyriformis, causes protozoan encystment and promotes bacterial survival inside cysts. BMC Microbiol. 2010;10:26. doi: 10.1186/1471-2180-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimhanen-Finne R, Niskanen T, Hallanvuo S, et al. Yersinia pseudotuberculosis causing a large outbreak associated with carrots in Finland, 2006. Epidemiol Infect. 2009;137:342–7. doi: 10.1017/S0950268807000155. [DOI] [PubMed] [Google Scholar]
- Siddiqui R, Khan NA. Biology and pathogenesis of Acanthamoeba. Parasit Vectors. 2012;5:6. doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner AR, Anand CM, Malic A, et al. Acanthamoeba and environmental spread of Legionella pneumophila. Lancet. 1983;2:289–90. doi: 10.1016/s0140-6736(83)90277-5. [DOI] [PubMed] [Google Scholar]
- Vaerewijck MJ, Sabbe K, Bare J, et al. Occurrence and diversity of free-living protozoa on butterhead lettuce. Int J Food Microbiol. 2011;147:105–11. doi: 10.1016/j.ijfoodmicro.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Vaerewijck MJ, Sabbe K, Van Hende J, et al. Sampling strategy, occurrence and diversity of free-living protozoa in domestic refrigerators. J Appl Microbiol. 2010;109:1566–78. doi: 10.1111/j.1365-2672.2010.04783.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Ahearn DG. Effect of bacteria on survival and growth of Acanthamoeba castellanii. Curr Microbiol. 1997;34:212–5. doi: 10.1007/s002849900170. [DOI] [PubMed] [Google Scholar]
- Watson RO, Galan JE. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 2008;4:e14. doi: 10.1371/journal.ppat.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]