Abstract
Metastasis is the major cause of cancer mortality. A more thorough understanding of the mechanisms driving this complex multistep process will aid in the identification and characterization of therapeutically targetable genetic drivers of disease progression. We demonstrate that KLF6-SV1, an oncogenic splice variant of the KLF6 tumor suppressor gene, is associated with increased metastatic potential and poor survival in a cohort of 671 lymph node–negative breast cancer patients. KLF6-SV1 overexpression in mammary epithelial cell lines resulted in an epithelial-to-mesenchymal–like transition and drove aggressive multiorgan metastatic disease in multiple in vivo models. Additionally, KLF6-SV1 loss-of-function studies demonstrated reversion to an epithelial and less invasive phenotype. Combined, these findings implicate KLF6-SV1 as a key driver of breast cancer metastasis that distinguishes between indolent and lethal early-stage disease and provides a potential therapeutic target for invasive breast cancer.
INTRODUCTION
Breast cancer mortality is primarily a result of distant metastases (1). The entire metastatic cascade involves many distinct steps, which include (i) tumor cell invasion through the basement membrane and endothelial walls, (ii) intravasation and survival in the blood or lymphatic circulatory system, (iii) extravasation into distant sites, and (iv) colonization of a new organ site with a foreign microenvironment (2, 3). The initial pool of invasive cells, which may be transformed by either epigenetic or genetic alterations in conjunction with adverse microenvironment interactions, displays deregulated cell proliferation, angiogenesis, survival, invasion, and migration, thus, in aggregate, acquiring the ability to metastasize. Breast cancer cells metastasize to both regional lymph nodes and distant organs such as bone, lung, liver, and brain (1, 4). Unfortunately, the inability to identify the rate-limiting steps of the multistep metastatic cascade, along with heterogeneity of the primary tumor and the metastatic lesions, has left researchers unable to find a unifying molecular basis that drives this transition (5–8).
A combination of changes in the primary tumor cells and signals from the tumor microenvironment allows cancer cells to undergo epithelial-to-mesenchymal transition (EMT), a hallmark and key driver of many of the initial steps in the metastatic cascade (9–16). EMT occurs through a coordinated series of molecular changes acquired by protein modification and transcriptional events in response to both extracellular stimuli and activation of intracellular signal transduction pathways. This response leads to loss in apicobasal polarity, change in cell shape, and loss of cell-to-cell adhesion, resulting in increased cellular motility and invasion (16–20). With increased knowledge about the processes and genes regulating EMT in vitro and in vivo, more evidence is pointing toward EMT’s role in tumor progression and metastasis.
Several members of the Krüppel-like family (KLF) transcription factors contribute to EMT and metastasis. For example, KLF8 in breast cancer cells and KLF6 in renal proximal tubule cells are both EMT inducers (21–23), whereas KLF4 in breast cancer cells and KLF5 in non–small cell lung cancer cells are EMT repressors (24–26). Another KLF, KLF17, is a metastasis suppressor gene that acts as a negative regulator of EMT and breast cancer metastasis (27). Conversely, a splice variant of KLF6, KLF6-SV1, is a metastasis promoter gene in ovarian and prostate cancer models (28, 29). KLF6-SV1’s activity antagonizes the wild-type KLF6’s (wtKLF6) tumor-suppressive effects on cell proliferation, colony formation, invasion, and in vivo tumor growth through KLF6 degradation (28–32). Additionally, increased KLF6-SV1 expression has been associated with poor survival in patients with lung (33), prostate (28), ovarian (29), pancreatic (34), and head and neck squamous cell cancers (35), nasopharyngeal carcinoma (36), colorectal cancer (37), hepatocellular carcinoma (38), and glioblastoma (39, 40). Intriguingly, although KLF6-SV1 expression is present in both normal and cancerous tissues, the expression of this isoform has been found to be up-regulated in multiple cancers (41).
Here, we report that KLF6-SV1 is both a potent driver of breast cancer metastasis and a prognostic marker of poor outcome in human breast cancer. To evaluate the effects of KLF6-SV1 in breast cancer progression and metastasis, we performed gain-of-function and loss-of-function experiments on a panel of mammary epithelial cell lines and metastatic breast cancer cells. We demonstrated that KLF6-SV1 overexpression induced an EMT-like phenotype and resulted in marked cancer cell dissemination in vivo, independent of cellular proliferation. Consistent with its role as a metastasis promoter, loss-of-function studies using sequence-specific small interfering RNAs (siRNAs) in highly metastatic cells decreased the invasive and migratory potential of these cells. Furthermore, analysis of a large clinical cohort of lymph node–negative (LNN) breast cancer patients demonstrated that increased KLF6-SV1 expression was associated with both shorter metastasis-free survival (MFS) and increased EMT marker gene expression. Together, these findings suggest a role for KLF6-SV1 as a key initiator/driver of the metastatic cascade and a potential therapeutic target for invasive breast cancer.
RESULTS
Increased KLF6-SV1 expression in primary tumors correlates with increased metastatic potential
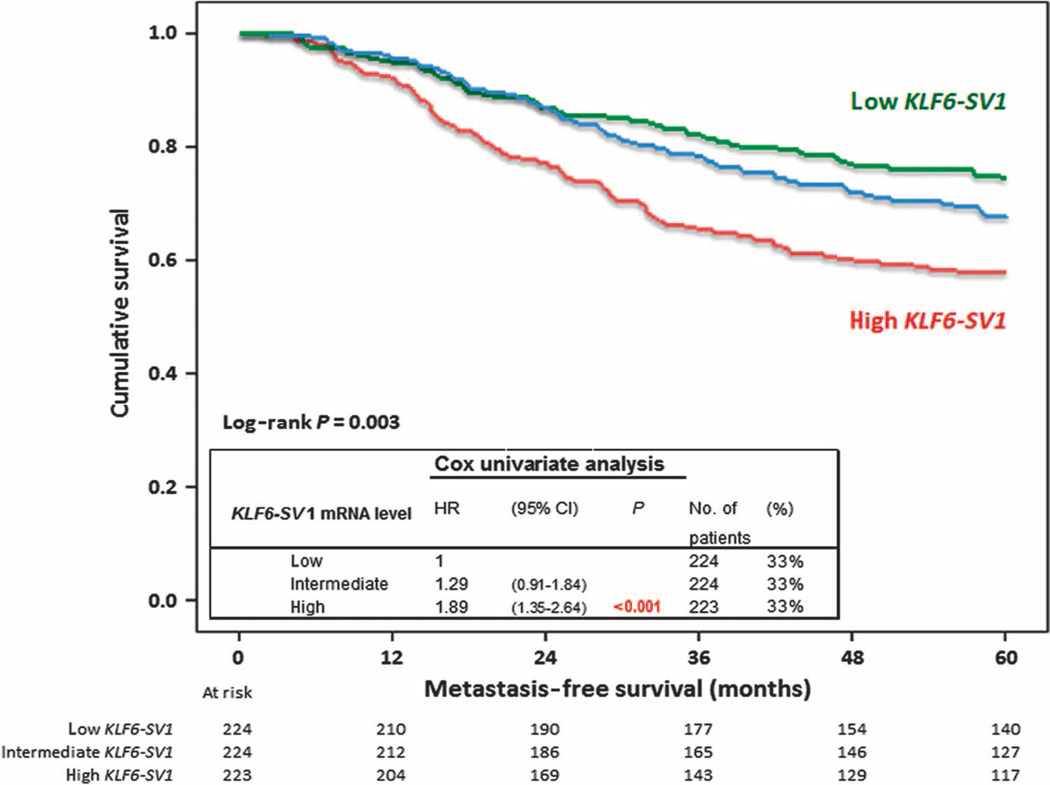
To assess the clinical relevance of KLF6-SV1 and patient outcome, we performed Cox univariate and multivariate proportional hazards analyses for MFS as a function of continuous and dichotomized KLF6-SV1 mRNA levels (Table 1). In these analyses, increasing levels of KLF6-SV1 were associated with a shorter MFS, independently of the traditional prognostic factors: age, menopausal status, tumor size, grade, and steroid hormone receptor levels [hazard ratio (HR) = 1.30, P = 0.02 and HR = 1.36, P = 0.02, respectively]. The prognostic value of KLF6-SV1 was visualized in Kaplan-Meier curves obtained after dividing KLF6-SV1 levels into three equal parts (low, intermediate, and high) (Fig. 1).
Table 1.
Cox univariate and multivariate analysis for MFS in 671 LNN primary breast cancer patients that received no adjuvant systemic treatment as a function of KLF6-SV1 mRNA levels measured in the primary tumor. CI, confidence interval.
| Factor | No. of patients (N = 671) |
(%) | Univariate analysis |
Multivariate analysis* |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age (years) | ||||||||
| ≤40 | 88 | 13 | 1 | 1 | ||||
| 41–55 | 241 | 36 | 0.85 | 0.60–1.21 | 0.89 | 0.62–1.29 | ||
| 56–70 | 214 | 32 | 0.72 | 0.50–1.04 | 0.65 | 0.36–1.17 | ||
| >70 | 128 | 19 | 0.5 | 0.32–0.79 | 0.01 | 0.45 | 0.23–0.87 | 0.09 |
| Menopausal status | ||||||||
| Premenopausal | 277 | 41 | 1 | 1 | ||||
| Postmenopausal | 394 | 59 | 0.76 | 0.59–0.97 | 0.03 | 0.99 | 0.62–1.57 | 0.95 |
| Tumor size | ||||||||
| pT1 (≤2 cm) | 274 | 41 | 1 | 1 | ||||
| pT2 (>2–5 cm) + unknown | 367 | 55 | 1.12 | 0.87–1.44 | 1.08 | 0.84–1.41 | ||
| pT3 (>5 cm) + pT4 | 30 | 4 | 1.54 | 0.87–2.74 | 0.32 | 1.93 | 1.07–3.47 | 0.13 |
| Grade† | ||||||||
| Poor | 351 | 52 | 1 | 1 | ||||
| Unknown | 208 | 31 | 1.03 | 0.79–1.35 | 1.12 | 0.86–1.47 | ||
| Moderate and good | 112 | 17 | 0.48 | 0.32–0.72 | <0.01 | 0.5 | 0.33–0.76 | <0.01 |
| ER-α mRNA level | ||||||||
| Continuous | 671 | 100 | 0.94 | 0.87–1.02 | 0.14 | 1.13 | 1.01–1.27 | 0.03 |
| PgR mRNA level | ||||||||
| Continuous | 671 | 100 | 0.92 | 0.87–0.97 | <0.01 | 0.89 | 0.83–0.95 | <0.01 |
| Additions to the base model | ||||||||
| KLF6-SV1 mRNA level | ||||||||
| Continuous | 671 | 100 | 1.3 | 1.06–1.60 | 0.01 | 1.3 | 1.05–1.62 | 0.02 |
| KLF6-SV1 mRNA level‡ | ||||||||
| ≤Median (≤0.0029) | 336 | 50 | 1 | 1 | ||||
| >Median (>0.0029) | 335 | 50 | 1.39 | 1.09–1.78 | <0.01 | 1.36 | 1.06–1.75 | 0.02 |
KLF6-SV1 was separately introduced to the base multivariate model that included the following factors: age, menopausal status, tumor size, grade, and ER-α and PgR mRNA levels as log-transformed continuous variables.
Grade was assessed by regional pathologists and reflects the current practice during the years the tumors were collected.
Dichotomized in high and low levels by median level.
Fig. 1.
Increased KLF6-SV1SV1 expression in primary breast tumors correlates with poor prognosis. Five-year MFS as a function of KLF6-SV1 in 671 LNN primary breast cancer patients after trichotomizing KLF6-SV1 mRNA levels in three equal parts (low, intermediate, and high). Patients at risk are indicated.
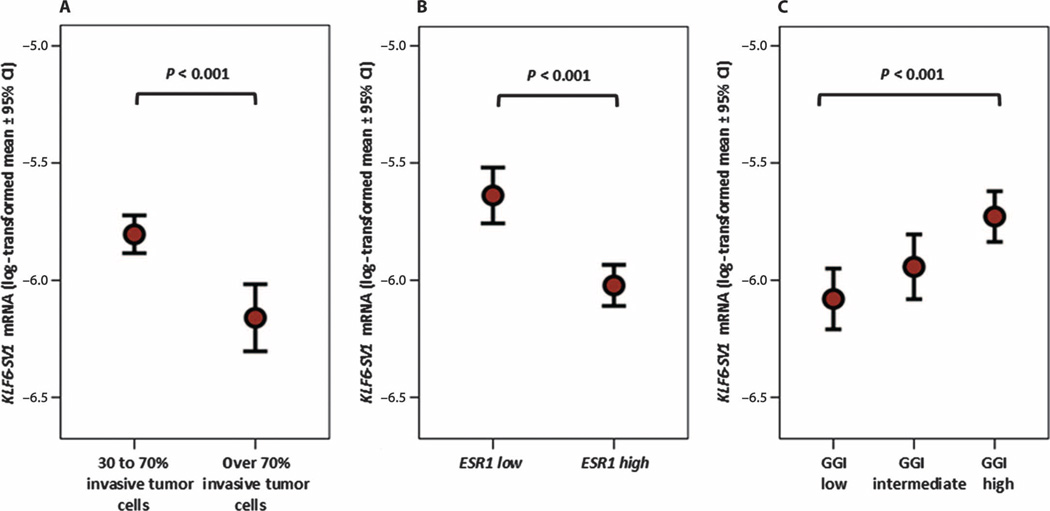
The associations of KLF6-SV1 mRNA expression levels with other patient and tumor characteristics are shown in Table 2. Although mucinous tumors expressed the lowest levels of KLF6-SV1, the highest median level of KLF6-SV1 was found in the histological subgroup of tumors with a ductal carcinoma in situ (DCIS) component present (P = 0.03). For the five main breast cancer subtypes as identified by gene expression profiling (42), the luminal B breast tumors expressed the lowest levels of KLF6-SV1 and the normal-like breast tumors expressed the highest levels (P < 0.001) (Table 2). Furthermore, primary tumors that contained a relatively large proportion of stromal cells expressed the highest levels of KLF6-SV1 (P < 0.001) (Fig. 2A). Finally, KLF6-SV1 mRNA levels were inversely related with age (P = 0.04) and steroid hormone receptors (P < 0.001) (Fig. 2B and Table 2) and did not associate with pathological grade, as has been assessed in those years by regional pathologists, but when correlated with genomic grade index (GGI), they were higher in tumors that were molecularly identified to be of high grade (43) (P < 0.001) (Fig. 2C).
Table 2.
Associations of KLF6-SV1 mRNA levels with clinicopathologic and biological factors. DCIS, ductal carcinoma in situ; GGI, genomic grade index; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; TC, tumor cells.
| Clinical characteristic |
No. of patients |
% | Median mRNA levels and interquartile range |
|
|---|---|---|---|---|
|
KLF6-SV1 (×10−3) |
Interquartile range |
|||
| All LNN patients | 671 | 100 | 2.97 | 2.86 |
| Age (years) | ||||
| ≤40 | 88 | 13 | 3.05 | 2.49 |
| 41–55 | 241 | 36 | 2.98 | 2.78 |
| 56–70 | 214 | 32 | 3.06 | 3.13 |
| >70 | 128 | 19 | 2.6 | 2.91 |
| P = 0.04* | ||||
| Menopausal status | ||||
| Premenopausal | 277 | 41 | 2.97 | 2.6 |
| Postmenopausal | 394 | 59 | 2.96 | 2.98 |
| P = 0.37† | ||||
| Tumor size | ||||
| pT1 (≤2 cm) | 274 | 41 | 3 | 3.04 |
| pT2 (>2–5 cm) + unknown | 367 | 55 | 2.94 | 2.78 |
| pT3 (>5 cm) + pT4 | 30 | 4 | 2.82 | 1.98 |
| P = 0.92‡ | ||||
| Pathological grade | ||||
| Poor | 351 | 52 | 2.95 | 2.65 |
| Unknown | 208 | 31 | 3.06 | 2.94 |
| Moderate and good | 112 | 17 | 2.9 | 3.4 |
| P = 0.95‡ | ||||
| TNM stage§ | ||||
| I | 274 | 41 | 3 | 3.04 |
| IIa | 361 | 54 | 2.94 | 2.76 |
| IIb | 22 | 3 | 2.82 | 1.98 |
| IIIb | 8 | 1 | 2.89 | 3.22 |
| Unknown | 6 | 1 | 3.45 | 2.94 |
| P = 0.99‡ | ||||
| ER protein status | ||||
| Negative (<10 fmol/mg protein) | 189 | 28 | 3.7 | 2.86 |
| Positive (≥10 fmol/mg protein) | 482 | 72 | 2.79 | 2.68 |
| P < 0.001* | ||||
| PgR protein status§ | ||||
| Negative (<10 fmol/mg protein) | 234 | 35 | 3.54 | 3 |
| Positive (≥10 fmol/mg protein) | 405 | 60 | 2.76 | 2.72 |
| P < 0.001* | ||||
| ESR1 mRNA status | ||||
| Negative (<0.2) | 179 | 27 | 3.6 | 3.44 |
| Positive (≥0.2) | 492 | 73 | 2.79 | 2.69 |
| P < 0.001* | ||||
| PGR mRNA status | ||||
| Negative (<0.1) | 273 | 41 | 3.39 | 2.81 |
| Positive (≥0.1) | 398 | 59 | 2.7 | 2.73 |
| P < 0.001* | ||||
| ERBB2 mRNA status§ | ||||
| Negative (<18) | 565 | 84 | 2.93 | 2.86 |
| Positive (≥18) | 101 | 15 | 3.21 | 2.97 |
| P = 0.30* | ||||
| GGI mRNA status§ | ||||
| Low | 218 | 32 | 2.5 | 2.32 |
| Intermediate | 220 | 33 | 3.05 | 2.93 |
| High | 221 | 33 | 3.46 | 2.92 |
| P < 0.001* | ||||
| Stromal content¶ | ||||
| Stroma-rich (≤70% TC) | 454 | 68 | 3.21 | 2.97 |
| Stroma-poor (>70% TC) | 217 | 32 | 2.4 | 2.58 |
| P < 0.001† | ||||
| Breast cancer subtype║ | ||||
| Luminal A | 70 | 24 | 3.93 | 3.44 |
| Luminal B | 64 | 22 | 2.45 | 2.3 |
| erbb2+ | 60 | 21 | 3.66 | 2.87 |
| Basal-like | 80 | 27 | 3.27 | 2.63 |
| Normal-like | 17 | 6 | 4.27 | 3.68 |
| P < 0.001‡ | ||||
| Tumor histology§ | ||||
| DCIS + IDC | 87 | 13 | 3.59 | 3.54 |
| IDC | 341 | 51 | 2.98 | 2.84 |
| ILC | 42 | 6 | 3.18 | 3.96 |
| Medullary | 17 | 3 | 3.38 | 2.1 |
| Mucinous | 27 | 4 | 2.15 | 2.57 |
| P = 0.03‡ | ||||
P for Spearman rank correlation test.
P for Mann-Whitney U test.
P for Kruskal-Wallis test.
Due to missing values, numbers do not add up to 671.
Dichotomized at the median level of 70% tumor cells.
Obtained after hybridizing n = 291 overlapping total RNA samples on the Affymetrix Oligonucleotide Human U133a GeneChips (present for 43% of the N = 671 samples).
Fig. 2.
High levels of KLF6-SV1 mRNA associated with primary tumor characteristics. (A) KLF6-SV1 mRNA levels were correlated with stromal content (n = 453 LNN primary breast tumors with 30 to 70% invasive tumor cells versus n = 218 with more than 70% invasive tumor cells). (B) ESR1 (n = 492 ESR1-positive LNN primary breast tumors versus n = 179 ESR1-negative LNN primary breast tumors). (C) GGI (n = 218 GGI low LNN primary breast tumors versus n = 220 GGI intermediate LNN primary tumors versus n = 221 GGI high LNN primary breast tumors). mRNA levels were measured by qRT-PCR. The strengths of the associations between continuous variables were tested with a two-tailed Mann-Whitney U test.
To investigate in more detail the relationship of KLF6-SV1 with the risk to develop a distant metastasis, we performed exploratory subgroup analyses for MFS (table S1). Overall, in these subgroups stratified by ESR1, PGR, ERBB2, GGI, stromal content, breast cancer subtype, and tumor histology, high levels of KLF6-SV1 were associated with an increased risk to develop a distant metastasis. In addition, the high level of KLF6-SV1 mRNA in primary tumors with a DCIS component was confirmed in 20 additional pure DCIS primary tumors (table S1).
EMT markers are expressed in primary breast tumors with high KLF6-SV1
Driven by the contribution of KLF6-SV1 in the high GGI, stromarich, and hormone receptor–negative primary tumors (Fig. 2), increasing levels of KLF6-SV1 were associated with poor prognosis in our MFS analyses. This result is particularly relevant because EMT harbors many characteristics associated with the above findings. We therefore next correlated KLF6-SV1 mRNA levels with the expression levels of 30 genes, as measured with Affymetrix U133A GeneChips for 294 of the 671 tumors that are known from the literature to be up- or down-regulated during EMT. These associations are shown in Table 3. Most notable in this analysis was the high number of EMT markers that correlated positively (17 of 30, P < 0.01) with KLF6-SV1 in the 294 primary tumors, and the finding that the correlation with genes up-regulated during EMT could be almost completely assigned to the n = 183 estrogen receptor (ER)–positive primary tumors. These data were validated by quantitative real-time polymerase chain reaction (qRT-PCR) in the whole cohort of N = 671 LNN patients for Twist homolog 1 (TWIST1) (rs = 0.30, P = 1.0 × 10−15) and vimentin (VIM) (rs = 0.34, P = 3.2 × 10−1 9) (fig. S1, A and B).
Table 3.
Spearman correlations (KLF6-SV1 versus EMT markers).
KLF6-SV1 enhances cell survival, migration, and invasion but not cell proliferation
Metastasis involves a complex series of steps where cancer cells leave the original tumor site and migrate to other parts of the body via either the bloodstream or the lymphatic system (10). Uncontrolled proliferation (resulting in tumor growth), migration, and invasion are key events that occur as initial and primary steps in the metastatic cascade (44–47). To investigate the biological and/or functional role of KLF6-SV1 in breast cancer biology, we generated KLF6-SV1 stable cell lines by retroviral transduction of three independent mammary epithelial cell lines with KLF6-SV1. To study the effects of KLF6-SV1 overexpression in vitro, we used two nontumorigenic mammary epithelial cell lines, MCF10A and MCF12A, as well as the tumorigenic BT474 cell line. Overexpression was confirmed by qRT-PCR analysis with KLF6-SV1–specific primers, and protein overexpression was confirmed with a polyclonal antibody to KLF6, which recognizes both wtKLF6 and its splice variants (fig. S2A) (28, 30, 31, 48). Additionally, using wtKLF6 and Total KLF6–specific primers (28, 31) (Total KLF6 primer recognizes both wtKLF6 and all alternatively spliced KLF6 transcripts) further demonstrated that Total KLF6 expression was up-regulated as expected (fig. S2B), whereas wtKLF6 mRNA expression did not change with stable overexpression of KLF6-SV1 (fig. S2C). Moreover, KLF6 did not seem to be affected by KLF6-SV1 overexpression at the protein level when examining the KLF6-SV1 to wtKLF6 ratio (fig. S2D).
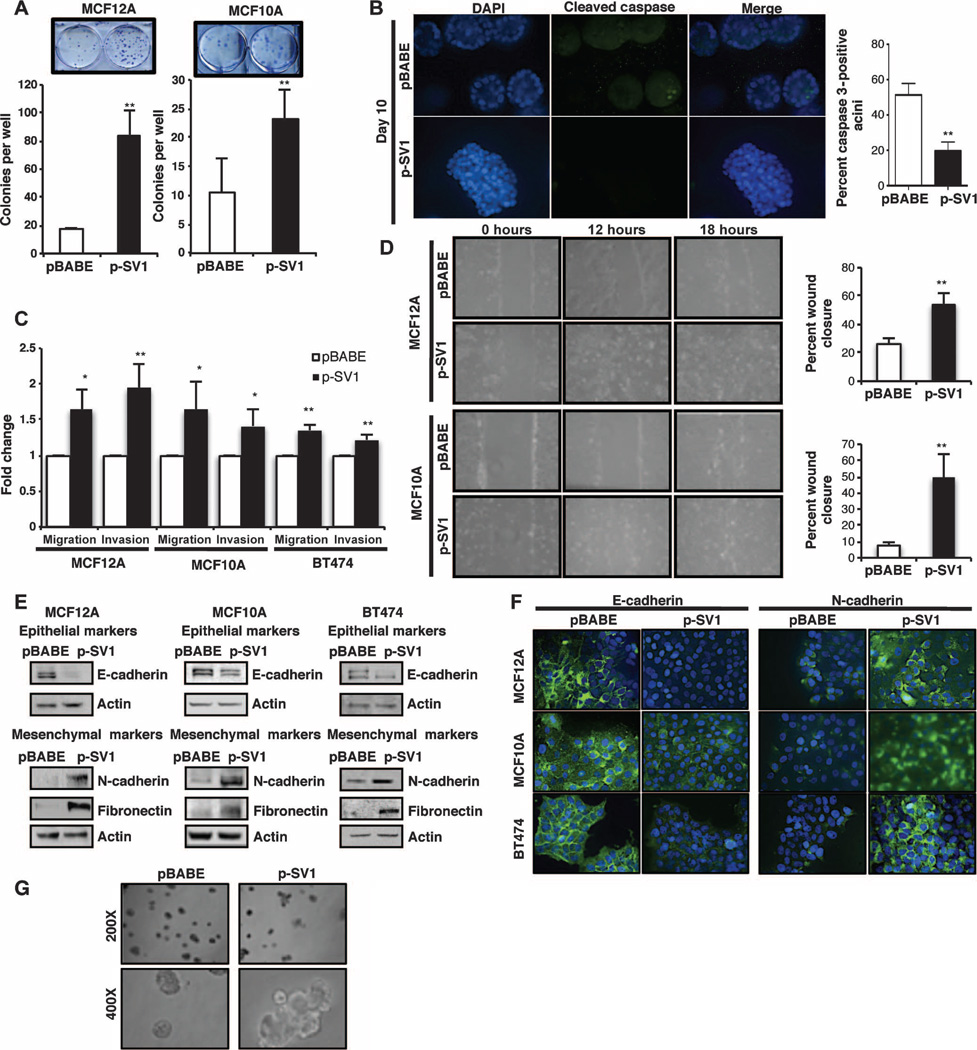
KLF6-SV1 overexpression resulted in a significant increase in colony formation in both nontumorigenic cell lines (Fig. 3A). In contrast, there were no notable differences in the rate of thymidine incorporation and cell number in the control pBABE- versus KLF6-SV1–expressing cells (fig. S3, A and B). Because cells grown two-dimensionally (2D) on plastic do not fully recapitulate the entire spectrum of the processes regulating proliferation, differentiation, and apoptosis, which are the major drivers of mammary morphogenesis and oncogenesis (49, 50), we decided to use a well-characterized 3D culture model to further investigate the role of KLF6-SV1 in breast cancer development and progression (51, 52). To determine whether KLF6-SV1 protected these cells from apoptosis, we immunostained acini with the apoptotic marker cleaved caspase 3 (53) and quantified cellular apoptosis in our pBABE-versus KLF6-SV1–overexpressing acini (Fig. 3B). Consistent with the overall appearance of the acini, the pBABE acini had a higher percentage of cleaved caspase 3–positive cells when compared to the KLF6-SV1-overexpressing acini. Acini derived from KLF6-SV1–overexpressing cells demonstrated decreased luminal clearance and complex multi-acinar structures as identified by the 4’,6-diamidino-2-phenylindole (DAPI) staining (Fig. 3B). These data were consistent with previous reports identifying KLF6-SV1 as an antiapoptotic protein (48) and with our clonogenic assay results, which both further support a role for KLF6-SV1 in sustaining breast cancer cell survival through the evasion of apoptosis. Together, these findings suggested that KLF6-SV1 expression primarily had an effect on cellular survival, but not proliferation, in mammary epithelial cell lines.
Fig. 3.
Overexpression of KLF6-SV1 promotes breast cancer development and progression in breast cell lines. (A) Colony assay of KLF6-SV1–expressing mammary epithelial cells stained at day 8; graphs represent the average number of colonies in pBABE control versus KLF6-SV1–expressing cells for MCF12A (P = 0.0027) and MCF10A (P = 0.0066). (B) Quantification of apoptosis in KLF6-SV1–expressing acini detected by cleaved caspase 3 staining by day 10 using indirect immunofluorescence (images taken at ×400 magnification). Acini containing two or more active caspase 3–positive cells were scored as positive, and 100 acini were counted per experiment (P = 0.0026). (C) Quantification of invasion and migration in Transwell chambers toward chemoattractant serum media over 24 hours in pBABE control versus KLF6-SV1–expressing cells in MCF12A migration (P = 0.034) and invasion (P = 0.002), MCF10A migration (P = 0.045) and invasion (P = 0.037), and BT474 migration (P = 0.011) and invasion (P = 0.0017). (D) Wound-healing assay over a period of 18 hours; graph represents percent wound closure over 12 hours for MCF12A (P = 0.006) and MCF10A (P = 0.007); images were taken at ×200 magnification. (E) Expression of epithelial marker E-cadherin and mesenchymal marker N-cadherin and fibronectin-expressing cells detected by Western blot. (F) Immunocytochemistry for N-cadherin and E-cadherin in pBABE versus p-SV1 cells taken at ×400 magnification. (G) KLF6-SV1–expressing MCF10A multiacinar structures during morphogenesis at day 20, taken at ×200 and ×400 magnifications. Representative images are demonstrated here and were repeated in triplicate; 1:100 of each antibody was used for staining. Data are represented as means ± SD of triplicate experiments.
Because cancer cell migration and invasion play central roles in the initial steps of the metastatic cascade, we next assessed KLF6-SV1’s potential to regulate cellular migration and invasion. In all cell lines tested, KLF6-SV1 overexpression consistently resulted in increased migration and invasion (Fig. 3C). These results were further extended and confirmed with a wound-healing assay, in which the ability of cells to migrate and induce wound closure was quantified as a function of time (Fig. 3D). Together, these results indicated that enhanced KLF6-SV1 expression was sufficient to promote invasion and migration in vitro. Because both evasion of apoptosis and increased motility and invasiveness are hallmarks of epithelial cells that have acquired mesenchymal characteristics (19), we next determined whether KLF6-SV1 induced EMT.
Overexpression of KLF6-SV1 induces an EMT-like phenotype
One hallmark of EMT is the expression switch from epithelial to mesenchymal markers. Specifically, this molecular program is characterized by the classic “cadherin switch” from E-cadherin to N-cadherin expression in cells. This switch confers loss of cell-cell adhesions because E-cadherin is an important regulator of these adherens junctions and cell polarity (11). Down-regulation of this cadherin has been shown to be one of the earliest indicators of the EMT process (20, 54). KLF6-SV1 overexpression resulted in increased expression of the mesenchymal fibroblastoid markers N-cadherin and fibronectin, with concomitant down-regulation of the expression of the epithelial marker E-cadherin. This result was consistent in all three cell lines tested at both the protein and mRNA levels (Fig. 3E and fig. S3C). There were no distinct or consistent morphological changes noted in these cell lines (fig. S3D). This cadherin switch was further corroborated through the use of indirect immunofluorescence staining for N-cadherin and E-cadherin (Fig. 3F). The pBABE control cells had intact membrane E-cadherin protein expression, as determined by the presence of strong cell-cell boundary staining, and low to no detectable membrane N-cadherin protein expression. Conversely, KLF6-SV1–expressing cells had lost this E-cadherin membrane protein expression and gained N-cadherin protein expression. Together, these data confirmed that enhanced KLF6-SV1 expression resulted in the loss of cell-cell adhesion and drove the acquisition of fibroblastoid properties in mammary epithelial cells. These findings were consistent with the statistically significant correlation between KLF6-SV1 expression and EMT marker gene expression in our cohort of early-stage breast cancer patient specimens (Table 3).
Next, we used our 3D model to study the effects of KLF6-SV1 on cell polarization, which is altered during the process of cancer development (51, 52). We observed that increased KLF6-SV1 expression disrupted the normal 3D architecture, resulting in complex multi-acinar structures (Fig. 3G). Specifically, the polarity of KLF6-SV1– overexpressing acini was determined by indirect immunostaining with α6 integrin, a marker of basolateral polarity, at day 20 (fig. S3E). Normal acini should have strong basal, but weaker lateral, α6 integrin staining, as was demonstrated in the pBABE acini. On the contrary, KLF6-SV1 acini had increased lateral staining, as represented by the arrows. Together, these results confirm that KLF6-SV1 disrupted the normal organization and architecture of the acinus by decreasing apoptosis and disrupting cell polarity. These disrupted acini during organoid development mimic features of invasive cells (51, 52). Therefore, these results supported a role for KLF6-SV1 in the induction of an EMT-like phenotype and in driving increased cellular invasiveness.
KLF6-SV1 knockdown in metastatic breast cancer cells reverts metastatic features
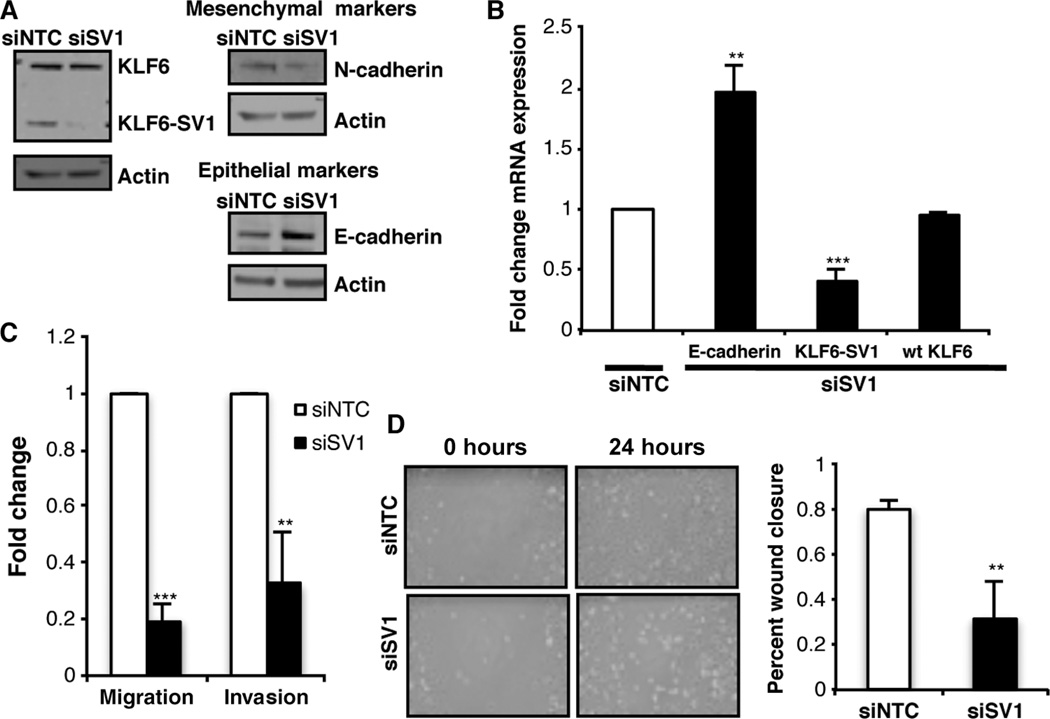
To further validate the biological role/function of KLF6-SV1 in breast cancer progression and metastasis, we performed loss-of-function studies using sequence-specific siRNA to KLF6-SV1. Using a previously established and validated siRNA to KLF6-SV1, whose sequence had the lowest degree of off-target effects (48, 55), we transiently transfected this siSV1 and a negative control siNTC in the highly aggressive and metastatic derivative of the breast carcinoma MDA-MB-231 cell line, MDA-MB-231-D3H2LN. These cells were derived from metastatic lymph node tissue, resulting spontaneously from an orthotopic MDA-MB-231-D3H1 tumor, after 12 weeks of in vivo growth (56). Knockdown of endogenous KLF6-SV1 was confirmed by Western blot analysis, with no effect seen on wtKLF6 protein expression (Fig. 4A). Additionally, qRT-PCR confirmed consistent and significant knockdown in these cells, with no change in wtKLF6 transcript expression (Fig. 4B). These studies validated the specificity of the KLF6-SV1 knockdown. Biologically, targeted reduction of KLF6-SV1 resulted in decreased expression of the mesenchymal marker N-cadherin, with a concomitant increase in expression of the epithelial E-cadherin marker, as demonstrated by Western blot analysis (Fig. 4A). These changes in E-cadherin expression were also noted at the transcriptional level as determined by qRT-PCR analysis (Fig. 4B).
Fig. 4.
Silencing KLF6-SV1 in metastatic cell line reverts cells back to an epithelial-like expression pattern. (A) Western blot analysis of wtKLF6, KLF6-SV1, N-cadherin, and E-cadherin expression after siRNA-mediated knockdown of KLF6-SV1 in MDA-MB-231-luc-D3H2LN cell line. (B) Gene expression analysis of KLF6-SV1 (P < 0.001) and E-cadherin (P = 0.002) analyzed by qRT-PCR. (C) Invasion (P < 0.001) and migration (P = 0.003) toward serum media over 24 hours. (D) Wound-healing assay from cells grown on a monolayer over 24 hours; graph represents percent wound closure over a period of 24 hours; images were taken at ×200 magnification (P = 0.007). Data are represented as means ± SD of triplicate experiments.
Targeted reduction of KLF6-SV1 resulted in a decrease in invasion compared to the nontargeting siRNA control in this highly metastatic cell line (Fig. 4C). Additionally, motility in the siSV1 cells decreased as assessed by directional migration using Transwell chambers over a 24-hour period. Moreover, this was confirmed with a wound-healing scratch assay, which demonstrated a decrease in wound closure ability over this same period in comparison to siNTC-transfected control cells (Fig. 4D). In conclusion, targeted reduction of endogenous KLF6-SV1 resulted in the reversion of the mesenchymal-like phenotype to a more epithelial one, similar to a process referred to as mesenchymal-to-epithelial transition. These loss-of-function results have fully corroborated the overexpression studies, demonstrating that KLF6-SV1 induces an EMT-like phenotype in breast epithelial cell lines. Combined, these data highlight a role for KLF6-SV1 in regulating several relevant cellular processes that play central roles in breast cancer progression and metastasis; nonetheless, KLF6-SV1’s role in breast cancer metastasis in vivo still remains to be elucidated.
KLF6-SV1 promotes tumor metastasis in a subcutaneous model
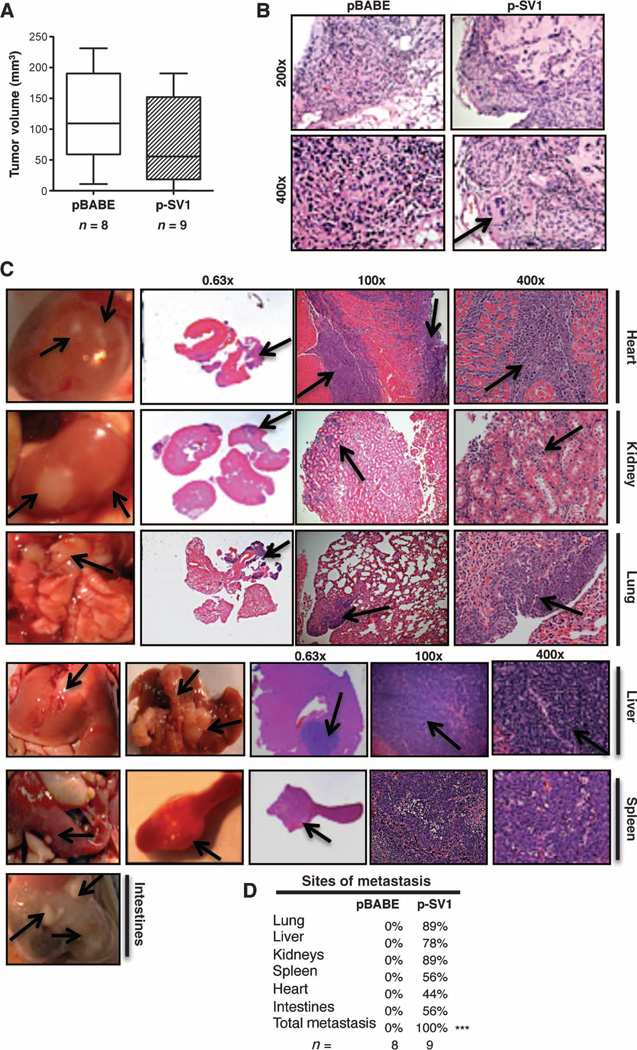
To identify whether KLF6-SV1–induced EMT could drive the phenotypic switch from indolent, localized breast cancer to highly metastatic lethal disease in vivo, we subcutaneously injected the tumorigenic, nonmetastatic BT474 cells overexpressing stably transduced KLF6-SV1 into the flank of immunodeficient mice. Consistent with both our in vitro and clinical data, tumor size was not correlated with KLF6-SV1 expression (Fig. 5A). Histological studies using hematoxylin and eosin (H&E) staining of pBABE-derived tumors showed no signs of local invasion compared to the KLF6-SV1 tumors, which had features consistent with a locally invasive phenotype (Fig. 5B). Remarkably, we found that KLF6-SV1 overexpression (fig. S4A) alone drove the entire metastatic cascade resulting in multiorgan dissemination, including distant metastasis to the liver, kidney, heart, lung, intestinal tract, and spleen (Fig. 5, C and D). Visible metastatic nodules and histopathologic examination confirmed the presence of undifferentiated tumor cells infiltrating into the heart and invasion into the kidneys, lung, liver, and spleen (Fig. 5, C and D). These results supported a role for KLF6-SV1 endowing tumor cells with metastatic potential/capacity, which resulted in metastatic dissemination in this disease-relevant in vivo model.
Fig. 5.
KLF6-SV1 induces metastasis in a subcutaneous mouse model. (A) Tumor volume of BT474 pBABE-expressing (n = 8) compared to p-SV1–expressing (n = 9) tumors. (B) Representative H&E staining of pBABE and p-SV1 tumors, with arrow pointing to local invasion in the p-SV1 tumor. (C) Representative images and H&E-stained sections of cardiac myocytes of the heart, kidney, lung, liver, spleen, and intestinal tract from a BT474 KLF6-SV1 mouse. Arrows point to metastatic lesions. (D) Sites of metastasis in BT474 pBABE- versus p-SV1–injected mice in the KLF6-SV1 mice (P < 0.001) analyzed by χ2 analysis. All data are represented as means ± SD.
KLF6-SV1 enhances metastatic potential in an orthotopic mouse model of the disease
To further extend our cell culture and in vivo findings, we used an orthotopic in vivo model to assay metastatic dissemination by bioluminescence imaging (BLI) of luciferase-transduced MDA-MB-231-luc-D3H2LN cells (30, 57, 58). We generated KLF6-SV1 MDA-MB-231-luc-D3H2LN luciferase-positive stable cells (fig. S5A). This BLI model system allowed for in vivo monitoring of local tumor growth and dissemination in real time.
Similar to our previous in vitro results, when characterizing these stable cells, KLF6-SV1 overexpression had no effects on the rate of thymidine incorporation (fig. S5B) but drove a significant increase in cellular migration, invasion, and wound healing (fig. S5,C and D). Similar to the other breast epithelial and cancer cell lines, overexpression of KLF6-SV1 in this mesenchymal cell line had no clear morphological changes (fig. S5E) and enhanced the EMT-like phenotype (fig. S5, F and G), further supporting a role for KLF6-SV1 in promoting this EMT-like process.
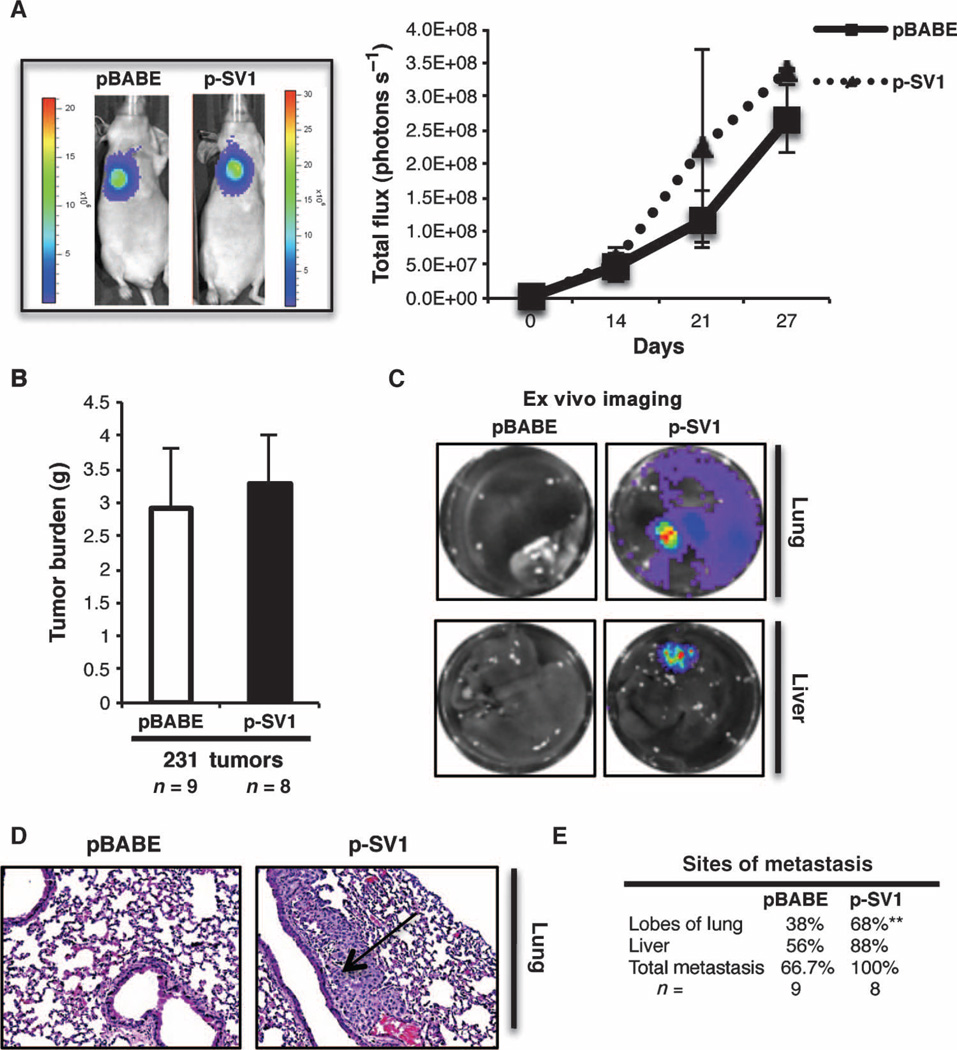
We next injected these metastatic cells into the intramammary fat pad of immunodeficient mice to recapitulate the entire spectrum of disease progression. We quantified the light emitted from the pBABE versus KLF6-SV1 tumors (Fig. 6A) and validated KLF6-SV1 overexpression by qRT-PCR (fig. S4B), which demonstrated no significant difference in local tumor growth consistent with our previous data (Fig. 6B). To identify distant sites of metastasis, we performed ex vivo imaging to assess whether increased KLF6-SV1 expression promoted metastasis (Fig. 6C). Additionally, histopathologic examination confirmed the presence of metastasis (Fig. 6D) and identified enhanced liver and lung metastases (Fig. 6E). Consistent with our BT474 xenograft model, we were able to validate that KLF6-SV1 overexpression increased the metastatic propensity of these cells in this complementary, orthotopic, in vivo model of breast cancer.
Fig. 6.
Overexpression of KLF6-SV1 in an orthotopic model of breast cancer progression enhances metastasis. (A) Female nu/nu mice injected orthotopically with MDA-MB-231-luc-D3H2LN pBABE (n = 9) or p-SV1 (n = 8) cells into mammary fat pad; representative luminescent images from each group at day 21 and bioluminescence graph of local tumor growth for 27 days. (B) Tumor burden of pBABE (n = 9) and p-SV1 (n = 8) mammary tumors from mice sacrificed at day 27. (C) Representative ex vivo imaging of lung and liver from each group. (D) Representative H&E-stained lung section, with arrow pointing at a metastatic lung lesion. (E) Percentage of mice with metastasis observed using ex vivo analysis, lung metastasis (P = 0.006), and total metastasis (P = 0.072). Data are represented as means ± SD. P value was obtained by χ2 analysis.
KLF6-SV1 affects TWIST1 expression in multiple model systems
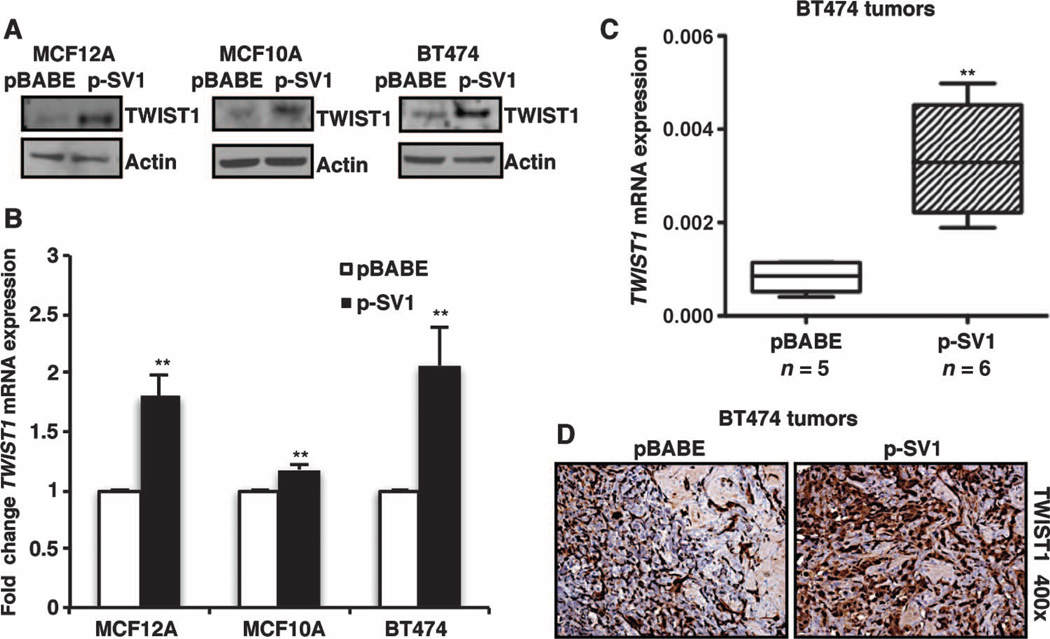
In an effort to understand the potential mechanisms by which KLF6-SV1 regulates breast cancer metastasis, we analyzed our patient cohort to identify which of the EMT-associated genes previously identified to be master regulators of this process were most highly correlated with KLF6-SV1 expression. On the basis of this analysis, KLF6-SV1 mRNA levels correlated significantly with TWIST1 expression in patient tumors in our cohort of breast cancer patients (fig. S1A). TWIST1 is an E-box transcription factor belonging to the family of basic helix-loop-helix transcription factors and a key regulator of EMT in embryogenesis and carcinogenesis. In addition, TWIST1 expression has been strongly correlated with high-grade invasive breast carcinoma (59–62). KLF6-SV1 overexpression resulted in TWIST1 up-regulation at both the protein (Fig. 7A and fig. S5H) and mRNA levels in the KLF6-SV1–overexpressing mammary cell lines generated (Fig. 7B). Consistent with the cell culture data, KLF6-SV1 overexpression in the BT474 xenograft tumors also resulted in TWIST1 up-regulation (Fig. 7, C and D). Conversely, silencing KLF6-SV1 in the mesenchymal metastatic 231 cell line resulted in down-regulated TWIST1 protein expression (fig. S5I). Furthermore, when TWIST1 was silenced in the KLF6-SV1 and pBABE stable cells in culture (fig. S6A), a reversal of EMT marker expression to a less mesenchymal and more epithelial expression pattern occurred (fig. S6B). Together, these data suggest that KLF6-SV1 cells are primed to undergo EMT through a TWIST-induced mechanism, thus becoming more susceptible to metastasis (16, 17, 61, 63–66). Combined, these data highlight a dynamic role for the KLF6-SV1 splice variant as a key functional driver of the entire metastatic cascade through TWIST1 in human breast cancer, and provide a mechanistic link between the association of increased KLF6-SV1 expression and poor survival in human breast cancer.
Fig. 7.
Increased KLF6-SV1 up-regulates TWIST1 overexpression. (A) Expression of TWIST1 analyzed by Western blot in MCF12A, MCF10A, and BT474 cell lines. (B and C) Gene expression analysis of TWIST1 by qRT-PCR in (B) MCF12A (P = 0.0018), MCF10A (P = 0.0011), and BT474 (P = 0.0048) cell lines and (C) BT474 tumors (P = 0.002). (D) Immunohistochemical analysis of TWIST1 in pBABE and p-SV1 BT474 tumors demonstrating increased TWIST1 staining at ×400 magnification. Data are represented as means ± SD. Whiskers represent minimum and maximum values. Experiments were repeated in triplicate. Tumors were analyzed with Mann-Whitney statistical analysis.
DISCUSSION
The goal of this study was to elucidate and characterize the functional role and potential clinical relevance of the oncogenic splice variant KLF6-SV1 in breast cancer development and progression. We demonstrate that aberrant expression of KLF6-SV1 plays a central role in promoting an EMT-like phenotype and metastasis, as well as in validating KLF6-SV1 expression as an independent prognostic risk factor for poor MFS in a large cohort of early-stage breast cancer patients. We report a role for this splice variant in breast cancer, implicating KLF6-SV1’s overexpression as an early driver of disease progression. In addition, biologically, we demonstrate that KLF6-SV1 drives an EMT-like transition and promotes aggressive multiorgan metastatic disease in multiple in vivo models independent of the effects on cellular proliferation. Combined, these findings implicate KLF6-SV1 as a key driver of breast cancer metastasis, discriminating between indolent and lethal early-stage disease, and a potential therapeutic target for invasive breast cancer.
Previous studies have demonstrated that KLF6-SV1 over-expression in prostate and ovarian cancer cell lines results in increased proliferation, contrary to our current findings in breast epithelial and cancer lines (28–31). Here, in the context of mammary cells, KLF6-SV1 overexpression was identified to have a cell proliferation–independent effect in both in vitro and in vivo models of the disease. These data support the notion that KLF6-SV1–enhanced cell growth is cell context–dependent. Remarkably, these results corroborate our clinical data, which showed no correlation between KLF6-SV1 expression and primary tumor size. Expression profiling has allowed researchers to identify a different signature in primary breast tumors with increased propensities to metastasize compared to tumors that remained localized (67–70). This further supports a model in which KLF6-SV1–expressing breast tumors acquire metastatic potential independent of tumor size.
The early steps of the metastatic cascade resemble developmental programs transitioning epithelial cells to a less polarized and mesenchymal state. This complex and dynamic process is referred to as EMT and is implicated as a key driver in the initiation of the metastatic cascade (17, 71, 72). In accordance with our observed EMT-like phenotype in culture, our clinical data show increased expression of KLF6-SV1 that correlates with the expression of EMT markers in primary breast cancer. We demonstrate that KLF6-SV1 is highly expressed in breast tumors with high GGI and hormone receptor–negative tumors, which has also been associated with EMT in breast carcinomas (73). Our data support the role of KLF6-SV1 as an early molecular determinant of invasive breast cancer. Therefore, KLF6-SV1 overexpression in breast cancer patients could potentially identify a distinct patient population that might need to be treated more aggressively because of their increased propensity to metastasize. Our observation that primary tumors that contained a DCIS component expressed relatively high levels of KLF6-SV1 is especially intriguing in this respect. Whether and how these DCIS-derived KLF6-SV1 levels contribute to prognosis remain to be determined because no clinical follow-up data are available for these patients.
Metastasis is a complex and multistep process that results from both molecular alterations occurring at the primary site as well as in the tumor microenvironment (10, 74). We herein demonstrate that high KLF6-SV1 mRNA levels in primary tumors were associated with shorter MFS in 671 primary breast cancer patient specimens. Additionally, through the use of the BT474 xenograft model, we uncovered that enhanced expression of KLF6-SV1 alone was sufficient to convert a nonmetastatic cell line to a highly aggressive metastatic variant. Furthermore, our orthotopic MDA-MB-231-D3H2LN model, which is highly metastatic, demonstrated an increased metastatic propensity with KLF6-SV1 over-expression. TWIST1 has been identified to promote an EMT-like transition and aggressive phenotype driving metastasis (61, 62, 65, 66, 75–77). Here, we demonstrate that TWIST1 positively associates with KLF6-SV1 expression both in culture, in vivo, and in patient samples, which is in accordance with TWIST1 associating with high-grade invasive breast carcinomas (62). Furthermore, loss-of-function studies demonstrate that the increased TWIST1 expression noted in KLF6-SV1–overexpressing cells was required for the KLF6-SV1–driven EMT-like phenotype.
Our data and the published literature seem to suggest cell context–specific relationships between wtKLF6 and KLF6-SV1 (23, 28–33, 35, 38, 39, 48, 55, 78–80). For example, recently, Vetter et al. demonstrated that KLF6-SV1 directly binds to KLF6, targeting the wtKLF6 for acceleration degradation through a proteasome-mediated pathway, a potential mechanism for the antagonistic function of KLF6-SV1 (32). In the paper by Holian et al., the authors suggest that wtKLF6 is an important regulator of EMT in proximal tubular cells of the kidney (23). In this particular context, the expression and role of KLF6-SV1 were not studied. In the mammary cell context in our current study, KLF6 does not seem to be affected by KLF6-SV1 overexpression at either the mRNA or protein level. Specifically, KLF6-SV1 expression was consistently higher than wtKLF6 in both our pBABE- and p-SV1–overexpressing stable cell lines. The ratio of KLF6-SV1 to wtKLF6 at both the mRNA and protein levels was greater, making the likelihood of a wtKLF6-dependent effect for the biology observed with KLF6-SV1 overexpression minimal. Therefore, the effects demonstrated in this report are an example of a de novo function of KLF6-SV1 in driving EMT through a TWIST1-dependent mechanism. Overall, these data support a role for KLF6-SV1 as an early driver/initiator of breast cancer progression and metastasis through a TWIST1-dependent mechanism that is independent of effects on wtKLF6 expression and function.
There are several key limitations to this study, including the retrospective nature of the analysis performed in identifying a strong correlation between KLF6-SV1 and poor outcome in human breast cancer. Further prospective studies in independent large well-annotated clinical cohorts are necessary to validate these initial observations. Furthermore, although xenograft models can faithfully recapitulate many aspects of tumorigenesis, ultimately, the true potential of KLF6-SV1 to drive tumor metastasis needs to be tested in disease-relevant transgenic mouse models of the disease. In addition, this study did not validate the therapeutic potential of targeting KLF6-SV1 in disease-relevant in vivo models. Further insight into the molecular mechanisms regulating the KLF6-SV1–driven EMT-like and invasive phenotype is still needed to determine the full spectrum of pathway perturbations associated with the reported biological effects of this splice variant.
The issues of tumor recurrence, drug resistance, enhanced invasion, and metastasis remain a challenge in the treatment of breast cancer. Moreover, molecular studies have revealed the inherently heterogeneous and genetically complex nature of this cancer, and more recently, this has been complemented with imaging analysis (6, 81–86). Despite a better understanding of genetic drivers and cancer biology, our ultimate ability to translate these findings into better, more effective, and safer treatments for patients has been limited. One reason for these failures is that most of the cancer drugs target drivers of local disease and not the events/alterations driving and sustaining the metastatic cascade. Our findings highlight a role for the oncogenic splice variant KLF6-SV1 as a potent initiator and driver of breast cancer metastasis as well as implicate KLF6-SV1’s overexpression as a key prognostic factor in distinguishing between indolent and lethal early-stage human breast cancer. This study paves the way for future studies aimed at the pathways perturbed by KLF6-SV1 overexpression and lays the molecular and conceptual framework for the development of targeted therapies directed against this metastasis-promoting splice variant for the treatment of breast cancer. Previous work published by our group (48) showed that siRNAs directed against KLF6-SV1 injected intraperitoneally in an ovarian mouse model demonstrated antitumorigenic effects. Moreover, Kaplan-Meier survival curves showed increased survival in the siSV1-treated mice in comparison to the siNTC-treated mice. This study and others highlight the potential role for therapeutic targeting of KLF6-SV1 in a variety of advanced cancers (30, 32, 33, 41, 48, 55, 87). Ultimately, we hypothesize that these approaches or small molecules designed to target this splice variant for degradation could have therapeutic use in a broad range of human cancers.
MATERIALS AND METHODS
Patients, breast cancer samples, tissue processing, and RNA analysis
The study was approved by the institutional medical ethics committee (MEC 02·953). Freshly frozen tumor tissues were originally submitted to our reference laboratory from 25 regional hospitals for measurements of steroid hormone receptors. Guidelines for primary treatment were similar for all hospitals. To avoid bias, selection of tumors from our tumor bank at the Erasmus University Medical Center (Rotterdam, The Netherlands) was done by processing all available frozen tumor samples from female patients with LNN breast cancer, who entered the clinic during 1979 to 1996 and from whom detailed clinical follow-up was available. Nodal status and tumor size were based on pathological examination by regional pathologists. Information on pathological grade was extracted from the pathology records and reflects clinical practice during those years. ER and progesterone receptor (PgR) were assessed both by enzyme-linked immunosorbent assay and by qRT-PCR as described before (88). Exclusion criteria were as follows: residual disease or distant spread diagnosed at or within 1 month after primary surgery, noninvasive breast cancer, neoadjuvant or adjuvant systemic therapy, a previous other cancer (except basal cell skin cancer or early-stage cervical cancer stage Ia/Ib), less than 100 mg of frozen tissue available, evaluation of tumor content not reliable (2%), less than 30% tumor cell nuclei in the sample (15%), and poor RNA quality (8%). Furthermore, to study the natural course of the disease (pure prognosis) without possible confounding effects of treatment or multiple tumors, we excluded patients who had received adjuvant systemic therapy or had both breasts simultaneously affected. The remaining 671 eligible LNN patients were treated either with breast-conserving surgery (55%) or with modified mastectomy (45%). Forty-one percent of the patients had T1 tumors. The median age of the patients at surgery was 56 years (range, 25 to 88 years). Sixty-two percent of the patients received adjuvant radiotherapy. Routine postsurgical follow-up and definition of the length of MFS were as described (70, 89). The median follow-up time of patients alive was 96 months (range, 12 to 202 months). For MFS, 261 events were observed; for overall survival, 242 events were observed. Other relevant clinicopathological and biological characteristics are listed in Tables 1 and 2. Tissue processing was done as described in detail before (90). In brief, 20 to 60 cryostat sections of 30-µm thickness, corresponding to 30 to 100 mg, were cut from frozen tissues. Before, in between, and after cutting the sections for RNA isolation, 5-µm sections were cut for H&E staining to assess the amount of tumor cells relative to the amount of surrounding stromal cells. The amount of nuclei evidently of epithelial tumor cell origin relative to the amount of surrounding stromal cells was estimated with a 100-fold magnification in 10 different areas covering the area of each of the three H&E sections. Only specimens with at least 30% of the nuclei of epithelial tumor cell origin and distributed uniformly over at least 70% of the section area were included. As done before (90), these estimates were used to dichotomize our tumor cohort at the median level of 70% tumor cell nuclei as stroma-rich (primary tumors containing 30 to 70% invasive epithelial tumor cells) and epithelial-rich (primary tumors containing more than 70% invasive epithelial tumor cells). RNA isolation, complementary DNA (cDNA) synthesis, quantification of specific mRNA species, and quality control checks were done as described in detail before (90). qRT-PCR was performed in a Mx3000P Real-Time PCR System (Agilent) with the Taqman-based Hs00361186_m1 gene expression assay from Applied Biosystems/Life Technologies for TWIST1- and SYBR-based intron-spanning forward and reverse primer combinations for the other genes. Primer sequences for KLF6-SV1 and VIM are listed in the Supplementary Materials and Methods. Primer sequences for ERS1, PGR, ERBB2, the GGI, and reference genes, as well as how PCRs and validations were performed to ensure PCR specificity, have all been previously described (43, 90, 91). Levels of the target genes, expressed relative to our reference gene set HMBS, HPRT1, and B2M, were quantified as follows: mRNA target = 2(mean Ct ref genes – mean Ct target). In addition, KLF6-SV1 levels were compared with expression data we had available of 294 LNN breast tumors that were analyzed before on the Affymetrix Oligonucleotide Human U133a GeneChips (70, 92, 93).
Cell culture and retroviral stable transduction
All cell lines were obtained from the American Type Culture Collection, with the exception of MDA-MB-231-luc-D3H2LN (obtained from Xenogen), and were maintained at 37°C with 5% CO2. BT474 cells were cultured in RPMI 1640 medium (Cellgro, Mediatech Inc.) supplemented with 10% (50 ml/500 ml) fetal bovine serum (FBS) (HyClone) and 0.5% (2.5 ml/500 ml) penicillin-streptomycin (Invitrogen). MDA-MB-231-luc-D3H2LN cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Cellgro, Mediatech Inc.) also supplemented with 10% FBS and 0.5% penicillin-streptomycin. MCF10A and MCF12A were cultured in DMEM/F12 (50:50) (Invitrogen), epidermal growth factor (EGF) (20 ng/ml) (BD Biosciences), cholera toxin (CT) (100 ng/ml) (Sigma), insulin (10 µg/ml) (Sigma), hydrocortisone (500 µg/ml) (Sigma), and 5% horse serum (Invitrogen) as previously described (51). Stable cell lines were generated by retroviral infection with either control pBABE or pBABE-KLF6-SV1 (p-SV1) virus. Retrovirus was generated by cotransfection of the pBABE or p-SV1 constructs with packaging plasmids into Phoenix cells with Lipofectamine 2000 (Invitrogen). For standard infection, about 1 × 106 viral particles were incubated with cells (at 60 to 70% confluence) in a final volume of 2 ml in the presence of polybrene (4 mg/ml) for 24 hours. Infected cells were then selected in puromycin (2 µg/ml) as described previously (29). Polyclonal pools of the pBABE- and p-SV1–infected cell lines were collected, and KLF6-SV1 overexpression was determined by qRT-PCR and Western blot analysis.
RNA extraction and quantitative real-time PCR
RNA from cell lines and xenografts was isolated with the RNeasy Mini Kit according to the manufacturer’s protocol (Qiagen). A total of 1 µg of RNA was reverse-transcribed with the Fermentas cDNA Synthesis kit. qRT-PCR was subsequently performed in triplicate with a 1:15 dilution of cDNA with SYBR Green PCR System qRT-PCR on an ABI PRISM 7900HT Fast Real-Time PCR machine (Applied Biosystems) with the primers listed in Table 4 below. The data were collected and analyzed with SDS 2.3 software accompanying the PCR machine, and relative expression levels were determined with the comparative quantification feature of the SDS 2.3 software. All mRNA quantification data were normalized to β-Actin and 18S expression (Table 4).
Table 4.
qRT-PCR primers.
| Gene | Forward | Reverse |
|---|---|---|
| wtKLF6 | 5′-CGGACGCACACAGGAGAAAA-3′ | 5′-CGGTGTGCTTTCGGAAGTG-3′ |
| Total KLF6 | 5′-CTGCCGTCTCTGGAGGAGT-3′ | 5′-TCCACAGATCTTCCTGGCTGTC-3′ |
| KLF6-SV1 endogenous | 5′-CCTCGCCAGGGAAGGAGAA-3′ | 5′-CGGTGTGCTTTCGGAAGTG-3′ |
| KLF6-SV1 exogenous | 5′-CCTCGCCAGGGAAGGAGAA-3′ | 5′-AAAACGCCACTCACACC-3′ |
| E-cadherin (CDH1) | 5′-CAAAGTGGGCACAGATGGTGTG-3′ | 5′-CTGCTTGGATTCCAGAAACGG-3′ |
| N-cadherin (CDH2) | 5′-GACGGTTCGCCATCCAGAC-3′ | 5′-TCGATTGGTTTGACCACGG-3′ |
| Fibronectin (FN1) | 5′-CAGTGGGAGACCTCGAGAAG-3′ | 5′-TCCCTCGGAACATCAGAAAC-3′ |
| Twist homolog 1 (TWIST1) | 5′-GGAGTCCGCAGTCTTACGAG-3′ | 5′-TCTGGAGGACCTGGTAGAGG-3′ |
| Vimentin (VIM) | 5′-CAGATTCAGGAACAGCATGTC-3′ | 5′-TCAGAGAGGTCAGCAAACTTG-3′ |
Western blot analysis
Whole-cell protein extracts were obtained with radioimmunoprecipitation assay buffer following standard protocols (Santa Cruz Biotechnology). The protein extracts were denatured, and 40 to 50 µg, as determined by the Bio-Rad DC Protein quantification assay, was separated on 12% SDS–polyacrylamide gel electrophoresis gels and transferred onto nitrocellulose membranes. After being blocked with 5% nonfat milk (LabScientific Inc.) in tris-buffered saline–Tween buffer, the membranes were probed with the following antibodies: KLF6 (sc-7158, Santa Cruz Biotechnology Inc.), N-cadherin (sc-59987, Santa Cruz Biotechnology Inc.), E-cadherin (sc-7870, Santa Cruz Biotechnology Inc.), fibronectin (610077, BD Biosciences), TWIST1 (sc-15393, Santa Cruz Biotechnology Inc.), actin (sc-1616, Santa Cruz Biotechnology Inc.), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sc-32233, Santa Cruz Biotechnology Inc.). Membranes were exposed to ECL (Roche) following the manufacturer’s instructions.
Colony formation
MCF10A (500 cells per well) and MCF12A (100 cells per well) cells were plated at low densities in six-well plates and incubated for 8 days with the medium replaced every 3 days. Cells were fixed and stained with 1% crystal violet staining solution and quantified. Each experiment was plated and repeated in triplicate.
Invasion migration and wound-healing assays
With the BD BioCoat Tumor Invasion System (BD Biosciences), cells were grown to 70 to 80% confluency and serum-starved for 24 hours. The BD FluoroBlok 24-well plates coated with BD Matrigel Matrix were rehydrated with warm phosphate-buffered saline (PBS) for 2 hours before plating. Twenty-four–well migration plates were not coated with BD Matrigel Matrix. Cells were then resuspended in serum-free medium, and 0.5 ml (1 × 105 cells/ml) was plated in triplicate onto the upper chamber. Then, 0.75 ml of medium with 10% FBS was added in the lower Boyden chamber as a chemoattractant. Migration plates were plated the same. After 20 hours of incubation at 37°C, the medium was removed from the upper chamber. The entire insert plate was transferred to a second 24-well plate containing 0.5 ml per well of calcein AM (4 µg/ml) (BD Biosciences) in Hanks’ balanced salt solution (Cellgro, Mediatech Inc.). These plates were incubated for 1 hour at 37°C, and invasive cells were quantified with a fluorescence plate reader. Cell migration was also determined by means of wound-healing assay. Cells were seeded into six-well plates and grown to confluence. At confluence, cells were serum-starved for 24 hours, and then a sterile 200-µl pipette tip was used to scratch the cells to form a wound. The cells were washed multiple times with PBS and kept in complete medium. Migration of the cells to the wound was visualized with an inverted Nikon Eclipse TS100 phase-contrast microscope and measured with Axioplan image analysis software. All experiments were performed in triplicate.
Immunocytochemistry and immunohistochemistry
Cell lines were plated on glass coverslips (Fisher Scientific) in 24-well plates. Cells were then fixed and washed twice with PBS, fixed with 4% paraformaldehyde (PFA) (USB Corporation), and incubated at room temperature for 20 min, followed by an overnight incubation with primary antibodies to N-cadherin (1:100) (12221, Abcam) and E-cadherin (1:500) (sc-7870, Santa Cruz Biotechnology Inc.). Then, cells were incubated with secondary goat anti-rabbit immunoglobulin G (IgG) (H+L)/fluorescein isothiocyanate (FITC) conjugate (AP307F, Millipore) for 1 hour. Vectashield mounting medium with DAPI (Vector Laboratories) was used as counterstain. Multiple independent fields were captured for each condition at high-power fields (×400) by fluorescence microscopy. Images were obtained with NIS-Elements for Basic Sciences (Nikon) and a Nikon Eclipse E-800 microscopy system. For immunohistochemistry, tumors were fixed in 10% formalin (Sigma) and prepared for histological analysis. Tissue sections were stained with H&E and TWIST1 (1:100) (49254, Abcam). Paraffin-embedded tumor sections were deparaffinized with xylene (Fisher) and rehydrated through graded alcohol washes (100 to 70%) followed by antigen retrieval in sodium citrate buffer (10 mM, pH 6.0) with a pressure cooker (Dako). Slides were then blocked in normal goat serum (1:20) (S-1000, Vector Laboratories) incubated in primary antibody overnight at 4°C, and biotinylated anti-rabbit IgG (Vector Laboratories) was used as secondary antibody (1:200). Staining was then visualized with 3,3′-diaminobenzidine (Zymed Laboratories), and sections were counterstained with hematoxylin, dehydrated, and mounted with Permount (Fisher). Bright-field images were all captured with either a Stereoscope or an Axioplan 2 IE microscope (Zeiss).
3D Matrigel culture
This assay was performed as described previously (51). Cells were briefly treated with trypsin, resuspended in DMEM/F-12 medium supplemented with 20% horse serum, and then centrifuged and resuspended in assay medium [DMEM/F-12 supplemented with 2% horse serum, insulin (10 µg/ml), CT (1 ng/ml), hydrocortisone (100 µg/ml), streptomycin (50 µg/ml), and penicillin/streptomycin]. Eight-chambered glass slides were coated with 50 µl of Matrigel (BD Biosciences) per well and then left to solidify for 30 min. Cells (5000 per well) were plated in medium containing 2% Matrigel and EGF (5 ng/ml), and assay medium containing EGF (5 ng/ml) and 2% Matrigel was replaced every 4 days. Immunostaining of these acinar structures was performed as previously described (51). Acini were fixed with 4% PFA for 20 min at room temperature. Cells were permeabilized with 0.5% Triton X-100 for 10 min at 4°C and rinsed with PBS-glycine three times for 10 min before blocking. Cells were incubated with primary cleaved caspase 3 (9661, Cell Signaling Technology) and α6 integrin (1378, Millipore) antibodies overnight at 4°C (1:100) and then with anti-rabbit FITC (AP307F, Millipore) or anti-rat Cy3-conjugated (The Jackson Laboratory) secondary antibodies (1:200) in the dark for 1 hour. Cells were mounted with DAPI (Vector Laboratories). All microscopy was performed with a Nikon Eclipse E-800 microscopy system, and independent multiple high-power fields were taken (×400).
Small interfering RNA
Transient transfection of sequence-specific siRNA to KLF6-SV1 (5′-CAGGGAAGGAGAAAAGCCUUU, Dharmacon) or nontargeting scrambled control (siNTC) (inverted β-galactosidase sequence; 5′-UAGCGACUAAACACAUCAAUU, Dharmacon) was performed with Lipofectamine 2000 (Invitrogen). Targeted knockdown of KLF6-SV1 was conducted with 100 nM KLF6-SV1–specific siRNA as previously described (29, 48).
Subcutaneous xenograft model
A pellet of 17β-estradiol (0.72 mg/ml) from Innovative Research of America was surgically implanted subcutaneously into the upper back of each 6- to 8-week-old BALB/c nude mouse. To do this, mice were anesthetized with ketamine and xylazine at a dose of 0.1 mg/kg and a small incision was made. Using a precision trochar, we inserted the pellet and sealed the incision with a clip. Cell pellets (5 × 106 cells) were mixed with an equal volume of Matrigel (Basement Membrane Matrix, BD Biosciences). The mixture was injected subcutaneously in the flank of each animal 1 week after the insertion of the estrogen pellet. Tumor size was measured biweekly with a digital caliper, and independent measurements (length and width) were taken for each tumor weekly. All in vivo experiments were performed according to approved protocols from the Animal Research Committee at the Mount Sinai School of Medicine.
BLI mouse studies
In vivo BLI was performed with an IVIV-200 Imaging System (Xenogen). Fifteen minutes before imaging, animals received d-luciferin substrate (Biosynth) at 150 mg/kg in PBS by intraperitoneal injection and were anesthetized with 3% isoflurane (Abbott Laboratories). Imaging time ranged from 1 to 5 min, depending on the signal emitted from each mouse, and five mice were imaged at a time. The light emitted from the tumors and organs from each mouse was detected and quantified as photons per second with Living Image software (Xenogen) (30).
Orthotopic metastasis model
Female BALB/c nude mice, 6 to 8 weeks of age, were injected with 1 × 106 MDA-MB-231-luc-D3H2LN pBABE or MDA-MB-231-luc-D3H2LN p-SV1 stable cells into their mammary fat pad. Mice were then imaged after 1 hour to confirm successful orthotopic injection, showing systemic bioluminescence distributed throughout the mouse. Whole-body bioluminescence was measured biweekly until mice were euthanized at day 27. Upon necropsy, all organs were excised and prepared for ex vivo imaging as well as subsequent histopathology analysis.
Statistics
Computations were done with the SPSS Statistics version 20.0 and the STATA statistical package, release 12.0 (STATA Corp.). Differences in levels were assessed with the Mann-Whitney U test or Kruskal-Wallis test. In these tests, patient and tumor characteristics were used as grouping variables. The strengths of the associations between continuous variables were tested with the Spearman rank correlation (rs). Variables were log-transformed to reduce the skewness. The prognostic values of the clinical and biological variables, with MFS as the endpoint in the univariate and multivariate analyses, were investigated with the Cox proportional hazards model. The HR and its 95% confidence interval were derived from these results. Because we were interested in the effect added by KLF6-SV1, for which the proportional hazards assumptions were not violated in multivariate analysis, we analyzed the MFS using the full period of follow-up. Univariate analysis of KLF6-SV1 showed, however, a violation of the proportional hazards assumption (P = 0.005). For the univariate exploration of the relationship of KLF6-SV1 with MFS (Fig. 1 and table S1), we therefore restricted the analysis to the first 5 years of follow-up. In this analysis with 216 events, the proportional hazards assumption was no longer violated (P = 0.39). Estimates were similar when compared to those of the full follow-up but showed stronger relationships with MFS. So, the use of the complete follow-up data might underestimate the effect of KLF6-SV1. For that reason, we also chose to use the 5-year follow-up for the explorative analysis as shown in table S1. Kaplan-Meier survival plots and log-rank tests were used to assess the differences in time to distant metastasis of the predicted high- and low-risk groups. Student’s t test was used to compare two groups for the in vitro experiments. All P values were two-sided, and P < 0.05 was considered statistically significant. Unless otherwise noted, all error bars represented the means ± SD.
Supplementary Material
Acknowledgments
We thank S. Yao, F. Huang, A. Avivar-Valderas, H. Fernandez, T. Bellone, C. Lee, S. Friedman, and J. A. Martignetti for helpful discussions and technical guidance/assistance with this manuscript. Y. Zhou and the In-Vivo Molecular Imaging Shared Resource Facility and the Microscopy Shared Resource Facility at Mount Sinai School of Medicine. Funding: S.I. is supported by NIH grant KL2TR000069. This work was supported by the Howard Hughes Medical Institute (HHMI). G.N. is a recipient of the HHMI Physician-Scientist Early Career Award, a Harrington Distinguished Scholar (Early Career Award) (University Hospitals/Case Western Reserver University), and an Irma T. Hirschl Award. G.N. is supported by the Harrington Discovery Institute (HDI).
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/5/169/169ra12/DC1
Materials and Methods
Fig. S1. KLF6-SV1 mRNA levels associate with mRNA levels of TWIST1 and VIM.
Fig. S2. Stable KLF6-SV1 overexpression does not affect wtKLF6 in cell lines.
Fig. S3. KLF6-SV1 overexpression does not effect cell proliferation but induces mammary epithelial cells to revert to a mesenchymal phenotype.
Fig. S4. BT474 and 231 mouse tumors stably overexpress KLF6-SV1.
Fig. S5. KLF6-SV1 expression in a mesenchymal metastatic breast cancer cell line further enhances EMT-like properties.
Fig. S6. Knockdown of TWIST1 in KLF6-SV1 stable cells reverses EMT-like phenotype.
Table S1. Cox univariate subgroup analysis for 5-year MFS in 671 LNN primary breast cancer patients that received no adjuvant systemic treatment as a function of KLF6-SV1 mRNA levels measured in the primary tumor. (Microsoft Excel format)
Author contributions: R.H. performed all the nonclinical experiments, analyzed all the nonclinical data, and wrote the manuscript. A.M.S. performed the clinical breast cancer experiments, analyzed the clinical breast cancer data, and wrote the clinical part of the manuscript. S.I. helped with the BT474 in vivo experiment (Fig. 5), siTWIST experiments (fig. S6), and the manuscript. Z.Y. performed the sectioning and H&E for Fig. 5. R.F.Q. performed the sectioning and H&E for Fig. 6. L.P. performed the surgeries and helped with the design for the BT474 in vivo experiment. M.P.L. performed the statistics on the clinical breast data. M.S. analyzed the clinical breast cancer array data. J.O. assisted with Western blots in Fig. 3. D.G. guided and assisted with the BT474 in vivo experiment. A.C.L. helped with the manuscript. D.B. and A.K. analyzed the pathology and histology of the tumors and A.K. helped with the manuscript. A.D. helped supervise the study and helped with manuscript. J.A.F. supplied the clinical breast cancer specimen and reviewed the interpretation of the clinical breast cancer data. G.N., A.C.L., and A.D. supervised the study, analyzed the data, and aided in the writing of the manuscript.
Competing interests: G.N. is an author on patent 20090325150 related to this work. The other authors declare that they have no competing interests.
REFERENCES
- 1.Weigelt B, Peterse JL, van ‘t Veer LJ. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, Foster R, Gardner B, Lerner H, Margolese R, Poisson R, Shibata H, Volk H. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52:1551–1557. doi: 10.1002/1097-0142(19831101)52:9<1551::aid-cncr2820520902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Friedl P, Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Polyak K. Heterogeneity in breast cancer. J. Clin. Invest. 2011;121:3786–3788. doi: 10.1172/JCI60534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the micro-environment in restraining cancer progression. Nat. Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 11.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y, Pertsemlidis A, Kurie JM. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 2009;23:2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Kalluri R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen DX, Bos PD, Massagué J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 16.Thiery JP. Metastasis: Alone or together? Curr. Biol. 2009;19:R1121–R1123. doi: 10.1016/j.cub.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 18.Thiery JP. Cell adhesion in development: A complex signaling network. Curr. Opin. Genet. Dev. 2003;13:365–371. doi: 10.1016/s0959-437x(03)00088-1. [DOI] [PubMed] [Google Scholar]
- 19.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Zhao J. KLF8 transcription factor participates in oncogenic transformation. Oncogene. 2007;26:456–461. doi: 10.1038/sj.onc.1209796. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Zheng M, Liu G, Xia W, McKeown-Longo PJ, Hung MC, Zhao J. Krüppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res. 2007;67:7184–7193. doi: 10.1158/0008-5472.CAN-06-4729. [DOI] [PubMed] [Google Scholar]
- 23.Holian J, Qi W, Kelly DJ, Zhang Y, Mreich E, Pollock CA, Chen XM. Role of Kruppel-like factor 6 in transforming growth factor-β1-induced epithelial-mesenchymal transition of proximal tubule cells. Am. J. Physiol. Renal Physiol. 2008;295:F1388–F1396. doi: 10.1152/ajprenal.00055.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yori JL, Johnson E, Zhou G, Jain MK, Keri RA. Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J. Biol. Chem. 2010;285:16854–16863. doi: 10.1074/jbc.M110.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yori JL, Seachrist DD, Johnson E, Lozada KL, Abdul-Karim FW, Chodosh LA, Schiemann WP, Keri RA. Krüppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer. Neoplasia. 2011;13:601–610. doi: 10.1593/neo.11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimamura T, Imoto S, Shimada Y, Hosono Y, Niida A, Nagasaki M, Yamaguchi R, Takahashi T, Miyano S. A novel network profiling analysis reveals system changes in epithelial-mesenchymal transition. PLoS One. 2011;6:e20804. doi: 10.1371/journal.pone.0020804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gumireddy K, Li A, Gimotty PA, Klein-Szanto AJ, Showe LC, Katsaros D, Coukos G, Zhang L, Huang Q. KLF17 is a negative regulator of epithelial-mesenchymal transition and metastasis in breast cancer. Nat. Cell Biol. 2009;11:1297–1304. doi: 10.1038/ncb1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narla G, DiFeo A, Yao S, Banno A, Hod E, Reeves HL, Qiao RF, Camacho-Vanegas O, Levine A, Kirschenbaum A, Chan AM, Friedman SL, Martignetti JA. Targeted inhibition of the KLF6 splice variant, KLF6 SV1, suppresses prostate cancer cell growth and spread. Cancer Res. 2005;65:5761–5768. doi: 10.1158/0008-5472.CAN-05-0217. [DOI] [PubMed] [Google Scholar]
- 29.DiFeo A, Narla G, Hirshfeld J, Camacho-Vanegas O, Narla J, Rose SL, Kalir T, Yao S, Levine A, Birrer MJ, Bonome T, Friedman SL, Buller RE, Martignetti JA. Roles of KLF6 and KLF6-SV1 in ovarian cancer progression and intraperitoneal dissemination. Clin. Cancer Res. 2006;12:3730–3739. doi: 10.1158/1078-0432.CCR-06-0054. [DOI] [PubMed] [Google Scholar]
- 30.Narla G, DiFeo A, Fernandez Y, Dhanasekaran S, Huang F, Sangodkar J, Hod E, Leake D, Friedman SL, Hall SJ, Chinnaiyan AM, Gerald WL, Rubin MA, Martignetti JA. KLF6-SV1 overexpression accelerates human and mouse prostate cancer progression and metastasis. J. Clin. Invest. 2008;118:2711–2721. doi: 10.1172/JCI34780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narla G, Difeo A, Reeves HL, Schaid DJ, Hirshfeld J, Hod E, Katz A, Isaacs WB, Hebbring S, Komiya A, McDonnell SK, Wiley KE, Jacobsen SJ, Isaacs SD, Walsh PC, Zheng SL, Chang BL, Friedrichsen DM, Stanford JL, Ostrander EA, Chinnaiyan AM, Rubin MA, Xu J, Thibodeau SN, Friedman SL, Martignetti JA. A germline DNA polymorphism enhances alternative splicing of the KLF6 tumor suppressor gene and is associated with increased prostate cancer risk. Cancer Res. 2005;65:1213–1222. doi: 10.1158/0008-5472.CAN-04-4249. [DOI] [PubMed] [Google Scholar]
- 32.Vetter D, Cohen-Naftaly M, Villaneuva A, Lee YA, Kocabayoglu P, Hannivoort R, Narla G, Llovet JM, Thung SN, Friedman SL. Enhanced hepatocarcinogenesis in mouse models and human hepatocellular carcinoma by coordinate KLF6 depletion and increased messenger RNA splicing. Hepatology. 2012;56:1361–1370. doi: 10.1002/hep.25810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiFeo A, Feld L, Rodriguez E, Wang C, Beer DG, Martignetti JA, Narla G. A functional role for KLF6-SV1 in lung adenocarcinoma prognosis and chemotherapy response. Cancer Res. 2008;68:965–970. doi: 10.1158/0008-5472.CAN-07-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartel M, Narla G, Wente MN, Giese NA, Martignoni ME, Martignetti JA, Friess H, Friedman SL. Increased alternative splicing of the KLF6 tumour suppressor gene correlates with prognosis and tumour grade in patients with pancreatic cancer. Eur. J. Cancer. 2008;44:1895–1903. doi: 10.1016/j.ejca.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teixeira MS, Camacho-Vanegas O, Fernandez Y, Narla G, DiFeo A, Lee B, Kalir T, Friedman SL, Schlecht NF, Genden EM, Urken M, Brandwein-Gensler M, Martignetti JA. KLF6 allelic loss is associated with tumor recurrence and markedly decreased survival in head and neck squamous cell carcinoma. Int. J. Cancer. 2007;121:1976–1983. doi: 10.1002/ijc.22926. [DOI] [PubMed] [Google Scholar]
- 36.Chen HK, Liu XQ, Lin J, Chen TY, Feng QS, Zeng YX. Mutation analysis of KLF6 gene in human nasopharyngeal carcinomas. Ai Zheng. 2002;21:1047–1050. [PubMed] [Google Scholar]
- 37.Reeves HL, Narla G, Ogunbiyi O, Haq AI, Katz A, Benzeno S, Hod E, Harpaz N, Goldberg S, Tal-Kremer S, Eng FJ, Arthur MJ, Martignetti JA, Friedman SL. Kruppel-like factor 6 (KLF6) is a tumor-suppressor gene frequently inactivated in colorectal cancer. Gastroenterology. 2004;126:1090–1103. doi: 10.1053/j.gastro.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Kremer-Tal S, Narla G, Chen Y, Hod E, DiFeo A, Yea S, Lee JS, Schwartz M, Thung SN, Fiel IM, Banck M, Zimran E, Thorgeirsson SS, Mazzaferro V, Bruix J, Martignetti JA, Llovet JM, Friedman SL, Downregulation of KLF6 is an early event in hepatocarcinogenesis. and stimulates proliferation while reducing differentiation. J. Hepatol. 2007;46:645–654. doi: 10.1016/j.jhep.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camacho-Vanegas O, Narla G, Teixeira MS, DiFeo A, Misra A, Singh G, Chan AM, Friedman SL, Feuerstein BG, Martignetti JA. Functional inactivation of the KLF6 tumor suppressor gene by loss of heterozygosity and increased alternative splicing in glioblastoma. Int. J. Cancer. 2007;121:1390–1395. doi: 10.1002/ijc.22809. [DOI] [PubMed] [Google Scholar]
- 40.Tchirkov A, Sapin V, Marceau G, Chautard E, Narla G, Veronese L, Friedman S, Khalil T, Vago P, Kemeny JL, Verrelle P. Increased expression of the oncogenic KLF6-SV1 transcript in human glioblastoma. Clin. Chem. Lab. Med. 2010;48:1167–1170. doi: 10.1515/CCLM.2010.219. [DOI] [PubMed] [Google Scholar]
- 41.DiFeo A, Martignetti JA, Narla G. The role of KLF6 and its splice variants in cancer therapy. Drug Resist. Updat. 2009;12:1–7. doi: 10.1016/j.drup.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toussaint J, Sieuwerts AM, Haibe-Kains B, Desmedt C, Rouas G, Harris AL, Larsimont D, Piccart M, Foekens JA, Durbecq V, Sotiriou C. Improvement of the clinical applicability of the Genomic Grade Index through a qRT-PCR test performed on frozen and formalin-fixed paraffin-embedded tissues. BMC Genomics. 2009;10:424. doi: 10.1186/1471-2164-10-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleinman HK, Jacob K. Invasion assays. Curr. Protoc. Cell Biol. Chapter. 2001;12 doi: 10.1002/0471143030.cb1202s00. Unit 12.2. [DOI] [PubMed] [Google Scholar]
- 45.Shaw LM. Tumor cell invasion assays. Methods Mol. Biol. 2005;294:97–105. doi: 10.1385/1-59259-860-9:097. [DOI] [PubMed] [Google Scholar]
- 46.Valster A, Tran NL, Nakada M, Berens ME, Chan AY, Symons M. Cell migration and invasion assays. Methods. 2005;37:208–215. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 47.van de Loosdrecht AA, Ossenkoppele GJ, Beelen RH, Broekhoven MG, Schweitzer KM, Langenhuijsen MM. In vitro purging of clonogenic leukaemic cells from human bone marrow by interferon-γ-activated monocytes. Cancer Immunol. Immunother. 1994;38:346–352. doi: 10.1007/BF01525514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Difeo A, Huang F, Sangodkar J, Terzo EA, Leake D, Narla G, Martignetti JA. KLF6-SV1 is a novel antiapoptotic protein that targets the BH3-only protein NOXA for degradation and whose inhibition extends survival in an ovarian cancer model. Cancer Res. 2009;69:4733–4741. doi: 10.1158/0008-5472.CAN-08-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bissell MJ, Radisky D. Putting tumours in context. Nat. Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- 51.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 52.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 53.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 54.Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int. J. Dev. Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- 55.Sangodkar J, DiFeo A, Feld L, Bromberg R, Schwartz R, Huang F, Terzo EA, Choudhri A, Narla G. Targeted reduction of KLF6-SV1 restores chemotherapy sensitivity in resistant lung adenocarcinoma. Lung Cancer. 2009;66:292–297. doi: 10.1016/j.lungcan.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenkins DE, Hornig YS, Oei Y, Dusich J, Purchio T. Bioluminescent human breast cancer cell lines that permit rapid and sensitive in vivo detection of mammary tumors and multiple metastases in immune deficient mice. Breast Cancer Res. 2005;7:R444–R454. doi: 10.1186/bcr1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guise TA, Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 59.Thisse B, el Messal M, Perrin-Schmitt F. The twist gene: Isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987;15:3439–3453. doi: 10.1093/nar/15.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, Maestro R, Voeltzel T, Selmi A, Valsesia-Wittmann S, Caron de Fromentel C, Puisieux A. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Mironchik Y, Winnard PI, Jr, Vesuna F, Kato Y, Wildes F, Pathak AP, Kominsky S, Artemov D, Bhujwalla Z, Van Diest P, Burger H, Glackin C, Raman V. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res. 2005;65:10801–10809. doi: 10.1158/0008-5472.CAN-05-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang Y, Massagué J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 64.Thiery JP. Epithelial-mesenchymal transitions in cancer onset and progression. Bull. Acad. Natl. Med. 2009;193:1969–1978. [PubMed] [Google Scholar]
- 65.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ansieau S, Morel AP, Hinkal G, Bastid J, Puisieux A. TWISTing an embryonic transcription factor into an oncoprotein. Oncogene. 2010;29:3173–3184. doi: 10.1038/onc.2010.92. [DOI] [PubMed] [Google Scholar]
- 67.van‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 68.van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 69.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 71.Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 72.Micalizzi DS, Ford HL. Epithelial-mesenchymal transition in development and cancer. Future Oncol. 2009;5:1129–1143. doi: 10.2217/fon.09.94. [DOI] [PubMed] [Google Scholar]
- 73.Jeong H, Ryu YJ, An J, Lee Y, Kim A. Epithelial-mesenchymal transition in breast cancer correlates with high histological grade and triple-negative phenotype. Histopathology. 2012;60:E87–E95. doi: 10.1111/j.1365-2559.2012.04195.x. [DOI] [PubMed] [Google Scholar]
- 74.Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smit MA, Geiger TR, Song JY, Gitelman I, Peeper DS. A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol. Cell. Biol. 2009;29:3722–3737. doi: 10.1128/MCB.01164-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xue G, Hemmings BA. Phosphorylation of basic helix-loop-helix transcription factor Twist in development and disease. Biochem. Soc. Trans. 2012;40:90–93. doi: 10.1042/BST20110678. [DOI] [PubMed] [Google Scholar]
- 77.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1a promotes metastasis. Nat. Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 78.Kremer-Tal S, Reeves HL, Narla G, Thung SN, Schwartz M, Difeo A, Katz A, Bruix J, Bioulac-Sage P, Martignetti JA, Friedman SL. Frequent inactivation of the tumor suppressor Kruppel-like factor 6 (KLF6) in hepatocellular carcinoma. Hepatology. 2004;40:1047–1052. doi: 10.1002/hep.20460. [DOI] [PubMed] [Google Scholar]
- 79.Sangodkar J, Shi J, DiFeo A, Schwartz R, Bromberg R, Choudhri A, McClinch K, Hatami R, Scheer E, Kremer-Tal S, Martignetti JA, Hui A, Leung WK, Friedman SL, Narla G. Functional role of the KLF6 tumour suppressor gene in gastric cancer. Eur. J. Cancer. 2009;45:666–676. doi: 10.1016/j.ejca.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yea S, Narla G, Zhao X, Garg R, Tal-Kremer S, Hod E, Villanueva A, Loke J, Tarocchi M, Akita K, Shirasawa S, Sasazuki T, Martignetti JA, Llovet JM, Friedman SL. Ras promotes growth by alternative splicing-mediated inactivation of the KLF6 tumor suppressor in hepatocellular carcinoma. Gastroenterology. 2008;134:1521–1531. doi: 10.1053/j.gastro.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 82.Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ, Kim JH, Kang E, Kim SW, Park SY. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: Its clinicopathological significance. Mod. Pathol. 2012;25:938–948. doi: 10.1038/modpathol.2012.36. [DOI] [PubMed] [Google Scholar]
- 83.Nik-Zainal S, Van Loo P, Wedge DC, Alexandrov LB, Greenman CD, Lau KW, Raine K, Jones D, Marshall J, Ramakrishna M, Shlien A, Cooke SL, Hinton J, Menzies A, Stebbings LA, Leroy C, Jia M, Rance R, Mudie LJ, Gamble SJ, Stephens PJ, McLaren S, Tarpey PS, Papaemmanuil E, Davies HR, Varela I, McBride DJ, Bignell GR, Leung K, Butler AP, Teague JW, Martin S, Jönsson G, Mariani O, Boyault S, Miron P, Fatima A, Langerød A, Aparicio SA, Tutt A, Sieuwerts AM, Borg Å, Thomas G, Salomon AV, Richardson AL, Børresen-Dale AL, Futreal PA, Stratton MR, Campbell PJ. Breast Cancer Working Group of the International Cancer Genome Consortium, The life history of 21 breast cancers. Cell. 2012;149:994–1007. doi: 10.1016/j.cell.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Gräf S, Ha G, Haffari G, Bashashati A, Russell R, McKinney S, Group METABRIC, Langerød A, Green A, Provenzano E, Wishart G, Pinder S, Watson P, Markowetz F, Murphy L, Ellis I, Purushotham A, Børresen-Dale AL, Brenton JD, Tavaré S, Caldas C, Aparicio S. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, Bashashati A, Prentice LM, Khattra J, Burleigh A, Yap D, Bernard V, McPherson A, Shumansky K, Crisan A, Giuliany R, Heravi-Moussavi A, Rosner J, Lai D, Birol I, Varhol R, Tam A, Dhalla N, Zeng T, Ma K, Chan SK, Griffith M, Moradian A, Cheng SW, Morin GB, Watson P, Gelmon K, Chia S, Chin SF, Curtis C, Rueda OM, Pharoah PD, Damaraju S, Mackey J, Hoon K, Harkins T, Tadigotla V, Sigaroudinia M, Gascard P, Tlsty T, Costello JF, Meyer IM, Eaves CJ, Wasserman WW, Jones S, Huntsman D, Hirst M, Caldas C, Marra MA, Aparicio S. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan Y, Failmezger H, Rueda OM, Ali HR, Gräf S, Chin SF, Schwarz RF, Curtis C, Dunning MJ, Bardwell H, Johnson N, Doyle S, Turashvili G, Provenzano E, Aparicio S, Caldas C, Markowetz F. Quantitative image analysis of cellular heterogeneity in breast tumors complements genomic profiling. Sci. Transl. Med. 2012;4:157ra143. doi: 10.1126/scitranslmed.3004330. [DOI] [PubMed] [Google Scholar]
- 87.Chen H, Chen L, Sun L, Zhen H, Li X, Zhang Q. A small interfering RNA targeting the KLF6 splice variant, KLF6-SV1, as gene therapy for gastric cancer. Gastric Cancer. 2011;14:339–352. doi: 10.1007/s10120-011-0049-x. [DOI] [PubMed] [Google Scholar]
- 88.Sieuwerts AM, Usher PA, Meijer-van Gelder ME, Timmermans M, Martens JW, Brünner N, Klijn JG, Offenberg H, Foekens JA. Concentrations of TIMP1 mRNA splice variants and TIMP-1 protein are differentially associated with prognosis in primary breast cancer. Clin. Chem. 2007;53:1280–1288. doi: 10.1373/clinchem.2006.082800. [DOI] [PubMed] [Google Scholar]
- 89.Foekens JA, Atkins D, Zhang Y, Sweep FC, Harbeck N, Paradiso A, Cufer T, Sieuwerts AM, Talantov D, Span PN, Tjan-Heijnen VC, Zito AF, Specht K, Hoefler H, Golouh R, Schittulli F, Schmitt M, Beex LV, Klijn JG, Wang Y. Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J. Clin. Oncol. 2006;24:1665–1671. doi: 10.1200/JCO.2005.03.9115. [DOI] [PubMed] [Google Scholar]
- 90.Sieuwerts AM, Meijer-van Gelder ME, Timmermans M, Trapman AM, Garcia RR, Arnold M, Goedheer AJ, Portengen H, Klijn JG, Foekens JA. How ADAM-9 and ADAM-11 differentially from estrogen receptor predict response to tamoxifen treatment in patients with recurrent breast cancer: A retrospective study. Clin. Cancer Res. 2005;11:7311–7321. doi: 10.1158/1078-0432.CCR-05-0560. [DOI] [PubMed] [Google Scholar]
- 91.van Agthoven T, Sieuwerts AM, Meijer-van Gelder ME, Look MP, Smid M, Veldscholte J, Sleijfer S, Foekens JA, Dorssers LC. Relevance of breast cancer antiestrogen resistance genes in human breast cancer progression and tamoxifen resistance. J. Clin. Oncol. 2009;27:542–549. doi: 10.1200/JCO.2008.17.1462. [DOI] [PubMed] [Google Scholar]
- 92.Smid M, Wang Y, Klijn JG, Sieuwerts AM, Zhang Y, Atkins D, Martens JW, Foekens JA. Genes associated with breast cancer metastatic to bone. J. Clin. Oncol. 2006;24:2261–2267. doi: 10.1200/JCO.2005.03.8802. [DOI] [PubMed] [Google Scholar]
- 93.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.