Abstract
Chitosan produced by the deacetylation of chitin is a cationic polymer with antimicrobial properties. In this study, we demonstrate the improvement of chitosan properties by nanofibrillation. Nanofiber sheets were prepared from nanofibrillated chitosan under neutral conditions. The Young’s modulus and tensile strength of the chitosan NF sheets were higher than those of the chitosan sheets prepared from dissolving chitosan in acetic acid. The chitosan NF sheets showed strong mycelial growth inhibition against dermatophytes Microsporum and Trichophyton. Moreover, the chitosan NF sheets exhibited resistance to degradation by the fungi, suggesting potentials long-lasting usage. In addition, surface-deacetylated chitin nanofiber (SDCNF) sheets were prepared. The SDCNF sheet had a high Young’s modulus and tensile strength and showed antifungal activity to dermatophytes. These data indicate that nanofibrillation improved the properties of chitosan. Thus, chitosan NF and SDCNF sheets are useful candidates for antimicrobial materials.
Keywords: chitosan nanofibers, surface-deacetylated chitin nanofibers, antifungal activity, dermatophyte
1. Introduction
Chitosan, produced by the deacetylation of chitin, is a cationic linear polysaccharide [1,2]. Due to its biocompatibility and biodegradability, chitosan has great potential in various fields, and thus chemical and structural modifications have been made to widen its applicability [2,3,4,5,6,7]. We recently developed chitosan nanofiber (NF) and chitin NF (CNF) using the Star Burst system, which employs high-pressure water-jet technology [8,9,10]. NF has a highly uniform structure of 10–20 nm diameters and shows high dispersibility in water due to its submicron size and high surface-to-volume ratio [11]. Furthermore, we have reported surface-deacetylated chitin nanofibers (SDCNF) [12]. Surface-deacetylated chitin was prepared from chitin, whose surface had been transformed into chitosan by deacetylation, and the core part was maintained as chitin crystal. These techniques may help to widen the range of usability of chitosan: biosensor, filtration applications to water and air purification, drug delivery, wound dressing, tissue engineering [3,4,5,6,7].
Moreover, chitosan shows antimicrobial activity against a wide range of microorganisms, such as algae, bacteria, yeasts, and fungi, so it is of special interest for food, hygiene, and medical applications [6,13]. Chitosan showed antifungal activity against Alternaria brassicicola, A. solani, Aspergillus flavus, A. niger, A. parasiticus, Botrytis cinerea, Byssochlamys spp., Fusarium concentricum, F. oxysporum, Mucor piriformis, M. racemosus, Pythium debaryanum, Rhizoctonia solani, Rhizopus stolonifer, Saprolegnia parasitica, and Ustilago maydis [14,15,16,17,18,19,20,21,22,23,24,25,26]. These antifungal activity of chitosan in food and crop protection is well documented [27,28]. On the other hand, major effects of chitosan against gram-positive and negative bacteria are well reported in the area of cosmetic, hygiene and medical care, antifungal activity against Candida spp. and dermatophytes have been reported [29,30,31].
Dermatophytosis caused by Epidermophyton, Microsporum, or Trichophyton is the most frequent fungal infection in humans and is an important public health problem [32]. Microsporum and Trichophyton are human and animal pathogens that infect keratinized body surfaces, such as skin, hair, and nails [33,34,35]. Numerous fungicidal and fungistatic agents are used for the treatment of dermatophytosis. To avoid host damage, the targets of these drugs are fungal specific, such as the fungal cell wall and the enzyme involving its synthesis [35]. However, the emergence of antifungal resistance has been reported and attributed to the modification of the target enzyme by mutation and upregulation of the drug efflux [35]. Because dermatophytosis requires long-term treatment, the ideal antifungal drug should be nontoxic to the host, inexpensive, and eco-friendly. Moreover, the prevention of infection is important for disease control. Several reports suggested the availability of chitosan for biomedical application, and hence chitosan is one of the candidate antimicrobial agents used in antimicrobial textiles to prevent the transmission and expansion of infectious pathogen [4,5,6,36,37,38].
In this study, we demonstrate the antifungal activity to dermatophytes of chitosan NF and SDCNF, the nanofibrillation of which enhanced chitosan properties such as the water dispersibility and mechanical strength of a sheet. Chitosan is nontoxic to mammalian cells, hence chitosan NF and SDCNF should be useful materials in pharmaceuticals, cosmetics, household products, and textiles.
2. Results and Discussion
2.1. Characterization of Nanofibers (NFs)
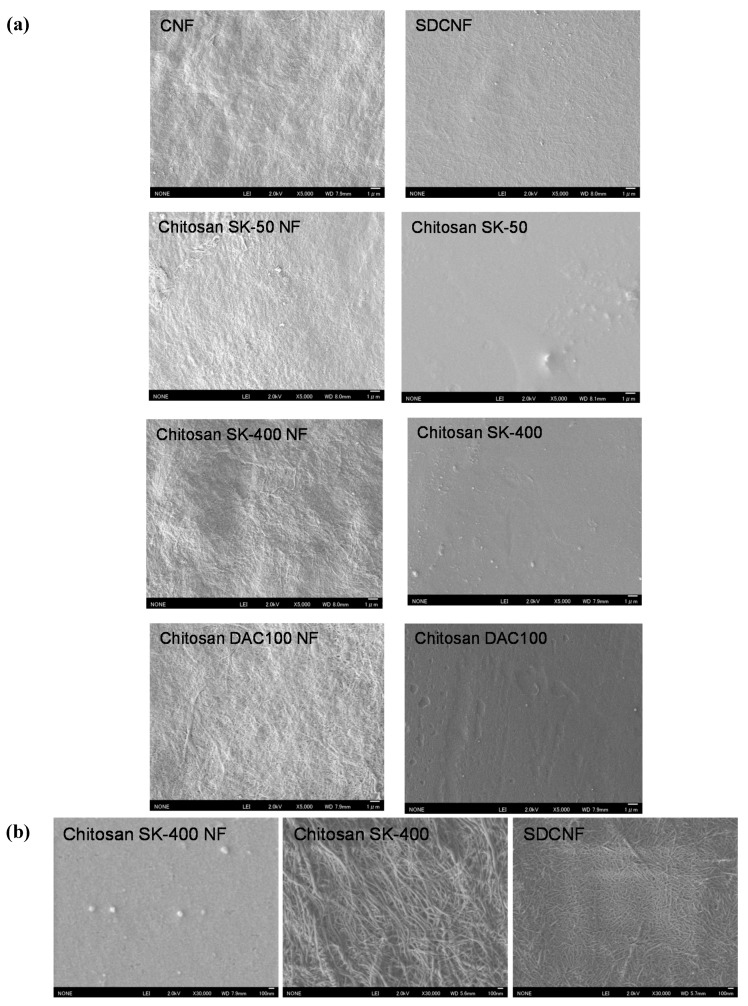
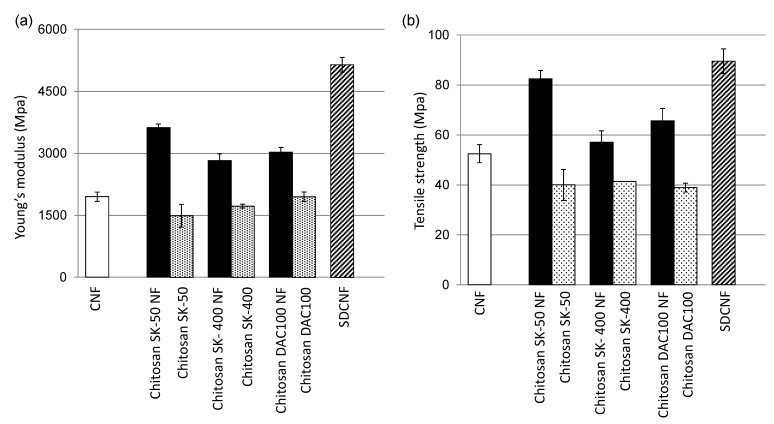
We prepared chitosan NF using the Star Burst system, which employs high-pressure water-jet technology [8,9,10]. Chitin or chitosan dispersed in water were passed through waterjet system equipped with a ball-collision chamber and then slurry was ejected from a small nozzle under high pressure. After mechanical treatments, the NFs became thinner as the number of treatments increased. Although chitosan is insoluble in water, chitosan NF shows high dispersibility in water due to its submicron size and high surface-to-volume ratio. Table 1 shows the properties of the chitosan and chitosan NF materials prepared in this work. It has been reported that antifungal activity depends on degrees of deacetylation (DDA) and molecular weight (Mw), and thus chitosan NF sheets were prepared from chitosan with different DDA and Mw [15,39,40]. Mw of commercial chitosan powder SK-50, SK-400, and DAC100 was 533 kDa, 4612 kDa, and 235 kDa respectively (Table 1). DDA of SK-50 and SK-400 were more than 80%, and DDA of DAC100 was 100%. Mechanical treatment did not affect DDA, but Mw was decreased (Table 1). It was reported that a large number of passes causes depolymerization [9,10]. Interestingly, the surfaces of the chitosan NFs and SDCNF were positively charged, even though these were prepared under neutral conditions. Figure 1 shows surface image of sheets. The chitosan SK-50, SK-400, and DAC100 sheets prepared from chitosan dissolved in aqueous acetic acid had a smooth surface. On the other hand, the surface of the chitosan NF, SDCNF, and CNF sheets which consists of lamination layers of NFs, showed a fibrous morphology (Figure 1A,B). Figure 2 show the tensile Young’s moduli and fracture strengths of the NF sheets. Both Young’s moduli and the fracture strengths of the chitosan NFs were higher than those of chitosan sheets. Moreover, SDCNF, whose surface was chitosan and whose core part was maintained as chitin crystal, showed the best mechanical properties. Chitosan NF and SDCNF showed low thermal expansion and high density [9,10,12]. These results indicate that the mechanical properties of chitosan were improved by nanofibrillation.
Table 1.
Properties of chitosan materials.
| Sheets | DDA (%) | Mw (kDa) | Surface Charge |
|---|---|---|---|
| CNF | <5 | 51 | ±0 |
| Chitosan SK-50 NF | >80 | 200 | + |
| Chitosan SK-400 NF | >80 | 368 | + |
| Chitosan DAC 100 NF | 100 | 10 | + |
| Chitosan SK-50 | >80 | 533 | NT |
| Chitosan SK-400 | >80 | 4612 | NT |
| Chitosan DAC 100 | 100 | 235 | NT |
| SDCNF | 18 | NT | + |
DDA, degree of deacetylation; NT, not tested. DDA and Mw were provided by manufacturer and surface charge was defined by zeta potential measurement.
Figure 1.
Field emission scanning electron microscopic (FE-SEM) micrographs of sheets. (a) SEM micrographs of chitin nanofiber (CNF), surface-deacetylated chitin nanofiber (SDCNF), chitosan NFs and chitosan sheets with low magnification (×5000); (b) Representative surface image of chitosan SK-400 NF, Chitosan SK400, and SDCNF sheets with high magnification (×30,000).
Figure 2.
Mechanical properties of nanofiber (NF) sheets. (a) Tensile Young’s modulus of NF sheets and (b) fracture strength of NF sheets. CNF, chitin nanofiber; SDCNF, surface-deacetylated CNF.
2.2. Antifungal Effects of Chitosan NFs and Surface-Deacetylated Chitin NF (SDCNF)
To assess antifungal effects, the mycelial growth of dermatophytes on mineral salt agar plates was measured (Table 2). CNF had no antifungal activity, whereas chitosan, chitosan NF, and SDCNF sheets inhibited fungal growth (Table 2). No antifungal effect was observed on any sheets to Trichophyton rubrum. The mycelial growth of Microsporum gypseum was highly inhibited on chitosan NF sheets, and the growth of M. canis, T. mentagrophytes, and T. tonsurans on chitosan sheets was comparable to that on chitosan NF sheets. It was reported that DDA and Mw affect antifungal activity against A. solani, F. concentricum, F. oxysporum, and R. solani [15,20,23,24,26]. According to these studies, there were no general trends in antifungal activity relating to high or low DDA or Mw, and efficacy of chitosan depended on the particular type of fungus. In our study, there were no significant differences among the sheets (Table 2). Moreover, the comparison of Mw of NF sheets was inappropriate due to its depolymerization during mechanical nanofibrillation processes. It was suggested that the antimicrobial activity of chitosan in solid state was influenced by surface morphology, size, hydrophilicity, electric charge, and DDA rather than Mw [13]. Because the surface of a chitosan NF sheet has a fibrous morphology with a high surface-to-volume ratio (Figure 1), it might increase contact between fungi and protonated amino group in chitosan NFs, thereby affecting antifungal activity. Particle size and shape reportedly affect the antibacterial activity of membranes prepared from chitosan particles [40]. Although the exact mechanism underlying the antimicrobial action of chitosan is not fully understood, the most accepted model is that interaction between positively charged chitosan molecules and negatively charged microbial cell membranes, leading to the leakage of intracellular constituents [13,41,42]. Several reports showed that chitosan induced morphological change in the fungal hyphae and spores including swellings and convolutions, and cellular disorganization [17,18,22,23,24,25,26]. On the other hand, another mode of action has indicated that chitosan acts as a chelating agent [16,19]. Therefore, it is conceivable that essential minerals and nutrients binding to chitosan NF and SDCNF might be unavailable for fungal growth. Further investigation will be needed to determine the mode of action of chitosan to dermatophytes.
Table 2.
Antifungal activity of chitosan nanofibers (NFs) on mycelial growth of dermatophytes.
| Sheets | Colony Diameter (mm) | ||||
|---|---|---|---|---|---|
| M. canis | M. gypseum | T. mentagrophytes | T. rubrum | T. tonsurans | |
| Blank | 2.4 ± 0.05 a | 2.4 ± 0.05 a | 1.3 ± 0.01 a | 1.8 ± 0.02 a | 1.7 ± 0.04 a |
| CNF | 1.6 ± 0.06 b | 3.0 ± 0.07 b | 1.5 ± 0.03 b | 1.6 ± 0.04 a | 1.7 ± 0.06 a |
| Chitosan SK-50 NF | 1.4 ± 0.02 b | 1.0 ± 0.0 c | 0.5 ± 0.05 cd | 1.9 ± 0.03 a | 0.4 ± 0.01 bc |
| Chitosan SK-400 NF | 1.1 ± 0.01 bc | 0.9 ± 0.04 c | 0.5 ± 0.02 c | 1.5 ± 0.2 a | 0.5 ± 0.01 bc |
| Chitosan DAC100 NF | 1.2 ± 0.08 bc | 0.8 ± 0.02 c | 0.5 ± 0.01 cd | 1.5 ± 0.04 a | 0.4 ± 0.02 bc |
| Chitosan SK-50 | 1.7 ± 0.2 b | 2.6 ± 0.2 ab | 0.4 ± 0.01 d | 2.0 ± 0.09 a | 0.4 ± 0.02 bc |
| Chitosan SK-400 | 1.1 ± 0.3 bc | 2.2 ± 0.2 a | 0.5 ± 0.01 cd | 1.8 ± 0.1 a | 0.4 ± 0.02 b |
| Chitosan DAC100 | 0.8 ± 0.08 c | 2.5 ± 0.2 ab | 1.3 ± 0.01 cd | 1.5 ± 0.1 a | 0.5 ± 0.03 c |
| SDCNF | 1.6 ± 0.1 b | 1.0 ± 0.06 ac | 0.7 ± 0.02 e | 1.8 ± 0.1 a | 0.7 ± 0.03 d |
Data represent the mean of three independent experiments and standard error. Means with the same letter are not significantly different according to Tukey’s test (p < 0.05). CNF, chitin NF; SDCNF, surface-deacetylated CNF.
2.3. Resistance of Chitosan NFs and SDCNF against Fungal Degradation
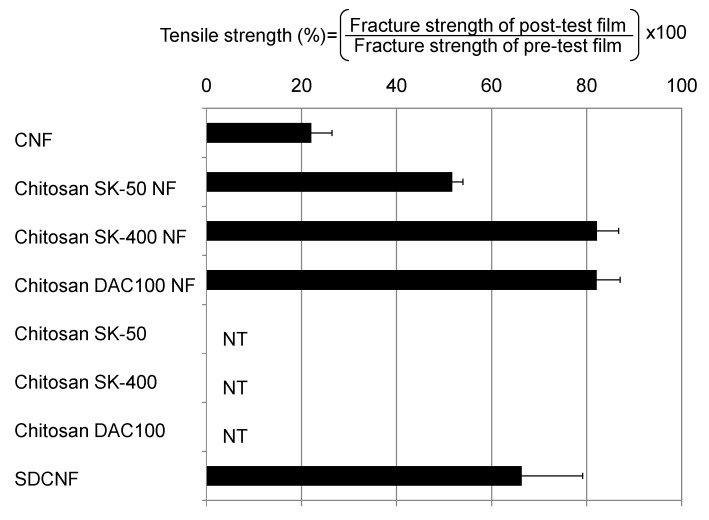
Since the mineral salt agar plate is a starvation medium, mycelial growth indicates the fungal assimilation of sheets. Dermatophytes secrete a great variety of enzymes in order to obtain nutrients such as carbon, nitrogen, phosphorus, and sulfur from host tissue [33]. Pathogenic fungi generally secrete more enzymes than nonpathogenic fungi, and this is associated with its virulence [33,35]. To assess the resistance of chitosan NF sheets against fungal degradation, the Young’s moduli and the tensile strengths of the sheets were compared from before to after the antifungal tests. Since the Chitosan sheets were fragile, none of chitosan sheets were recovered from the agar plates after the antifungal test (Figure 3). On the other hand, NF sheets were able to be harvested, and chitosan NF sheets and SDCNF showed high tensile strength than control CNF sheets (Figure 3). CNF had no antifungal effect and allowed marked fungal proliferation, which might be accompanied by active secretion of enzymes. Thus, CNF showed low tensile strength after the antifungal test. The chitosan, chitosan NF, and SDCNF sheets showed antifungal activity, indicating fungal biological activity may have been depressed. Yet, the chitosan sheets were degraded. These results indicated that the morphology of a sheet surface seems to affect the resistance against fungal degradation. The attachment of degrading enzymes to the chitosan molecule might be facile on the chitosan sheets than on the chitosan NF sheets due to the fiber-bundle structure of the NF. These results indicated that the chitosan NF and SDCNF sheets exhibited resistance to degradation by the fungi, suggesting their potential for long-lasting use.
Figure 3.
Tensile strength of sheets after the antifungal test. The sheets were harvested from agar plates after the antifungal test and their fracture strength was measured. Data represent the mean of three independent experiments with all five pathogens, and error bars indicate standard error. NT, not tested; CNF, chitin NF; SDCNF, surface-deacetylated CNF.
3. Experimental Section
3.1. Preparation of NF
Chitin powder (TC-L) from crab shells and chitosan powder (SK-50, SK-400, and DAC100) were purchased from Koyo Chemical (Tottori, Japan). CNF was prepared without acetic acid as described previously [8]. In brief, dry chitin powder was dispersed in water at 1 wt % and passed through a high-pressure water-jet system (Star Burst Mini, HJP-25001S, Sugino Machine, Toyama, Japan) equipped with a ball-collision chamber for mechanical disintegration. SDCNF was prepared as described previously [12] and modified as follows. Chitin powder was stirred in 20% (w/w) NaOH for 6 h at 150 °C under a nitrogen atmosphere. After deacetylation, the supernatant was removed and the precipitate was washed with distilled water. Surface-deacetylated chitin was dispersed in aqueous acetic acid (0.5% (w/w)) to remove any soluble products and then thoroughly washed with distilled water by centrifugation. The sample was passed through a grinder (MKCA6–3; Masuko Sangyo Co., Kawaguchi, Japan) once at 1500 rpm and then passed through a high-pressure water-jet system as described above. Chitosan NFs were prepared as described previously [9,10].
3.2. Preparation of Nanofiber Sheets
The above-prepared NFs dispersed in water were vacuum-filtered using a hydrophilic polytetrafluoroethylene membrane filter (Millipore, pore size: 0.2 μm) and washed with distilled water and ethanol. The obtained NF sheets were hot-pressed at 100 °C for 20 min to obtain a dried sheet consisting of 200 mg of chitin or chitosan.
3.3. Preparation of Chitosan Sheets
Chitosan was dissolved in aqueous acetic acid (1% (w/w)). The chitosan solution was poured into glass petri dishes coated with a release agent and dried under ambient conditions (40 °C, 3 days). The obtained chitosan sheets were neutralized by immersion in 0.5% (w/w) NaOH for 5 min and then were washed in distilled water. The sheets were hot-pressed at 100 °C for 20 min to obtain a dried sheet consisting of 200 mg of chitosan.
3.4. Characterization of Nanofibers
For field emission scanning electron microscopic (FE-SEM) observation, the sample was coated with an approximately 2 nm layer of Pt by an ion sputter coater and observed by a JSM-6700F scanning electron microscope (JEOL Ltd., Tokyo, Japan) operating at 2.0 kV. The Young’s modulus and tensile strength of each NF or chitosan sheet (3 cm in length and 1 cm in width) were measured using a universal testing instrument (AG-X, Shimadzu, Tokyo, Japan). The zeta potentials of the NF dispersants were measured with a Zeta-Potential & Particle Size Analyzer (ELSZ-1000ZS, Otsuka Electronics, Osaka, Japan).
3.5. Fungal Material
Dermatophytes including M. canis (NBRC9182), M. gypseum (NBRC5948), T. mentagrophytes (NBRC5466), T. rubrum (NBRC5807), and T. tonsurans (NBRC5946) were obtained from NBRC (NITE Biological Resource Center, Chiba, Japan). All fungi were maintained on Sabouraud agar medium.
3.6. Antifungal Activity Test
NF sheets or chitosan sheets (cut into 3 cm × 3 cm pieces) were placed on mineral salt agar plates (3 g NH4NO3, 1 g KH2PO4, 0.5 g MgSO4·7H2O, 0.25g KCl, 0.002 g FeSO4·7H2O, 20 g Agar per liter), and then a piece of fungal block (diameter 2 mm) was placed on each sheet. The plates were placed under 25 °C, and the colony diameter was measured at the appropriate time.
4. Conclusions
Chitosan NF and SDCNF sheets were prepared using a high-pressure waterjet system under neutral conditions. The NF sheets exhibited improved mechanical properties and inhibited mycelial growth against dermatophytes. Moreover, the chitosan NF and SDCNF sheets exhibited resistance to degradation by the fungi, suggesting their potential for long-lasting use. It is apparent that public concern for environmental and biological systems is growing, and thus ideal antimicrobial products should be safe for human and animals and eco-friendly. Because chitosan shows favorable biological properties such as non-toxicity, biocompatibility, biodegradability, and antimicrobial activity, chitosan is promising candidates as active natural material. Moreover there are many evidence of the beneficial effect of chitin and chitosan for biomedical area and these nano-fibrillation materials enhance their efficacy. Chitosan NF and SDCNF nanofibers are promising candidates for in pharmaceuticals, cosmetics, household products, and textiles.
Acknowledgments
This work was financially supported by the Creating STart-ups from the Advanced Research and Technology (START) project of JST.
Author Contributions
Mayumi Egusa and Shinsuke Ifuku planned the experiments; Mayumi Egusa and Ryo Iwamoto performed the experiments; Mayumi Egusa wrote the paper; Hironori Izawa, Minoru Morimoto, Hiroyuki Saimoto, Hironori Kaminaka, and Shinsuke Ifuku discussed the results and revised the manuscript; Shinsuke Ifuku supervised the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Younes I., Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, Properties and Applications. Mar. Drugs. 2015;13:1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillai C.K.S., Paul W., Sharma C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009;34:641–678. doi: 10.1016/j.progpolymsci.2009.04.001. [DOI] [Google Scholar]
- 3.Jayakumar R., Prabaharan M., Nair S.V., Tamura H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010;28:142–150. doi: 10.1016/j.biotechadv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Muzzarelli R.A.A. New techniques for optimization of surface area and porosity in nanochitins and nanochitosans. In: Jayakumar R., Prabaharan A., Muzzarelli R.A.A., editors. Advances in Polymer Science: Chitosan for Biomaterials. Volume 2. Springer-Verlag; Berlin, Germany: 2011. pp. 167–186. [Google Scholar]
- 5.Muzzarelli R.A.A. Nanochitins and nanochitosans, paving the way to eco-friendly and energy-saving exploitation of marine resources. Polym. Sci. Compr. Ref. 2012;10:153–164. [Google Scholar]
- 6.Muzzarelli R.A.A., El Mehtedi M., Mattioli-Belmonte M. Emerging biomedical applications of nano-chitins and nano-chitosans obtained via advanced eco-friendly technologies from marine resources. Mar. Drugs. 2014;12:5468–5502. doi: 10.3390/md12115468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalumon K.T., Sathish D., Nair S.V., Chennazhi K.P., Tamura H., Jayakumar R. Fabrication of aligned poly(lactic acid)-chitosan nanofibers by novel parallel blade collector method for skin tissue engineering. J. Biomed. Nanotechnol. 2012;8:405–416. doi: 10.1166/jbn.2012.1395. [DOI] [PubMed] [Google Scholar]
- 8.Ifuku S., Yamada K., Morimoto M., Saimoto H. Nanofibrillation of dry chitin powder by Star Burst system. J. Nanomater. 2012;2012:1–7. doi: 10.1155/2012/645624. [DOI] [Google Scholar]
- 9.Dutta A.K., Kawamoto N., Sugino G., Izawa H., Morimoto M., Saimoto H., Ifuku S. Simple preparation of chitosan nanofibers from dry chitosan powder by the Star Burst system. Carbohydr. Polym. 2013;97:363–367. doi: 10.1016/j.carbpol.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Aklog Y.F., Dutta A.K., Izawa H., Morimoto M., Saimoto H., Ifuku S. Preparation of chitosan nanofibers from completely deacetylated chitosan powder by a downsizing process. Int. J. Biol. Macromol. 2015;72:1191–1195. doi: 10.1016/j.ijbiomac.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Ifuku S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules. 2014;19:18367–18380. doi: 10.3390/molecules191118367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ifuku S., Ikuta A., Egusa M., Kaminaka H., Izawa H., Morimoto M., Saimoto H. Preparation of high-strength transparent chitosan sheet reinforced with surface-deacetylated chitin nanofibers. Carbohydr. Polym. 2013;98:1198–1202. doi: 10.1016/j.carbpol.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Rahman M.H., Shovan L.R., Hjeljord L.G., Aam B.B., Eijsink V.G.H., Sørlie M., Tronsmo A. Inhibition of fungal plant pathogens by synergistic action of chito-oligosaccharides and commercially available fungicides. PLoS ONE. 2014;9:e93192. doi: 10.1371/journal.pone.0093192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younes I., Sellimi S., Rinaudo M., Jellouli K., Nasri M. Influence of acetylation degree and molecular weight of homogenous chitosan on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014;185:57–63. doi: 10.1016/j.ijfoodmicro.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Cuero R.G., Osuji G., Washington A. N-carboxymethylchitosan inhibition of aflatoxin production: Role of zinc. Biotechnol. Lett. 1991;13:441–444. doi: 10.1007/BF01030998. [DOI] [Google Scholar]
- 17.Martinez-Camacho A.P., Cortez-Rocha M.O., Ezquerra-Brauer J.M., Graciano-Verdugo A.Z., Rodriguez-Felix F., Castillo-Ortega M.M., Yepiz-Gomez M.S., Plascencia-Jatomea M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010;82:305–315. doi: 10.1016/j.carbpol.2010.04.069. [DOI] [Google Scholar]
- 18.El Ghaouth A., Arul J., Grenier J., Asselin A. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology. 1992;82:398–402. doi: 10.1094/Phyto-82-398. [DOI] [Google Scholar]
- 19.Roller S., Covill N. The antifungal properties of chitosan in laboratory media and apple. Int. J. Food Microbiol. 1999;47:64–77. doi: 10.1016/S0168-1605(99)00006-9. [DOI] [PubMed] [Google Scholar]
- 20.Qiu M., Wu C., Ren G., Liang X., Wang X., Huang J. Effect of chitosan and its derivatives as antifungal and preservative agents on postharvest green asparagus. Food Chem. 2014;155:105–111. doi: 10.1016/j.foodchem.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Rabea E.I., Badawy M.E.I., Steurbaut W., Stevens C.V. In vitro assessment of N-(benzyl)chitosan derivatives against some plant pathogenic bacteria and fungi. Eur. Polym. J. 2009;45:237–245. doi: 10.1016/j.eurpolymj.2008.10.021. [DOI] [Google Scholar]
- 22.Benhamou N. Ultrastructural and cytochemical aspects of chitosan on Fusarium oxysporum f. sp. radicis-lycopersici, agent of tomato crown and root rot. Phytopathology. 1992;10:1185–1193. [Google Scholar]
- 23.Liu H., Tian W., Li B., Wu G., Ibrahim M., Tao Z., Wang Y., Xie G., Li H., Sun G. Antifungal effect and mechanism of chitosan against the rice sheath blight pathogen, Rhizoctonia solani. Biotechnol. Lett. 2012;34:2291–2298. doi: 10.1007/s10529-012-1035-z. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Lauzardo A.N., Bautista-Banos S., Velazquez-del Valle M.G., Mendez-Montealvo M.G., Sanchez-Rivera M.M., Bello-Perez L.A. Antifungal effects of chitosan with different molecular weights on in vitro development of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Carbohydr. Polym. 2008;73:541–547. doi: 10.1016/j.carbpol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Muzzarelli R.A.A., Muzzarelli C., Tarsi R., Miliani M., Gabbanelli F., Cartolari M. Fungistatic activity of modified chitosans against Saprolegnia parasitica. Biomacromolecules. 2001;2:165–169. doi: 10.1021/bm000091s. [DOI] [PubMed] [Google Scholar]
- 26.Olicón-Hernández D.R., Hernández-Lauzardo A.N., Pardo J.P., Pena A., Velázquez-del Valle M.G., Guerra-Sánchez G. Influence of chitosan and its derivatives on cell development andphysiology of Ustilago maydis. Int. J. Biol. Macromol. 2015;79:654–660. doi: 10.1016/j.ijbiomac.2015.05.057. [DOI] [PubMed] [Google Scholar]
- 27.El Hadrami A., Adam L.R., El Hadrami I., Daayf F. Chitosan in plant protection. Mar. Drugs. 2010;8:968–987. doi: 10.3390/md8040968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elsabee M.Z., Abdou E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C. 2013;33:1819–1841. doi: 10.1016/j.msec.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Jung B.-O., Kim C.-H., Choi K.-S., Lee Y.M., Kim J.-J. Preparation of amphiphilic chitosan and their antimicrobial activities. J. Appl. Polym. Sci. 1999;72:1713–1719. doi: 10.1002/(SICI)1097-4628(19990624)72:13<1713::AID-APP7>3.0.CO;2-T. [DOI] [Google Scholar]
- 30.Alburquenque C., Bucarey S.A., Neira-Carrillo A., Urzua B., Hermosilla G., Tapia C.V. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med. Mycol. 2010;48:1018–1023. doi: 10.3109/13693786.2010.486412. [DOI] [PubMed] [Google Scholar]
- 31.Sajomsang W., Gonil P., Saesoo S., Ovatlarnporn C. Antifungal property of quaternized chitosan and its derivatives. Int. J. Biol. Macromol. 2012;50:263–269. doi: 10.1016/j.ijbiomac.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Vena G.A., Chieco P., Posa F., Garofalo A., Bosco A., Cassano N. Epidemiology of dermatophytoses: Retrospective analysis from 2005 to 2010 and comparison with previous data from 1975. New Microbiol. 2012;35:207–213. [PubMed] [Google Scholar]
- 33.Monod M., Capoccia S., Lechenne B., Zaugg C., Holdom M., Jousson O. Secreted proteases from pathogenic fungi. Int. J. Med. Microbiol. 2002;292:405–419. doi: 10.1078/1438-4221-00223. [DOI] [PubMed] [Google Scholar]
- 34.Mendez-Tovar L.J. Pathogenesis of dermatophytosis and tinea versicolor. Clin. Dermatol. 2010;28:185–189. doi: 10.1016/j.clindermatol.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Peres N.T.A., Maranhao F.C.A., Rossi A., Martinez-Rossi N.M. Dermatophytes: Host-pathogen interaction and antifungal resistance. An. Bras. Dermatol. 2010;85:657–667. doi: 10.1590/S0365-05962010000500009. [DOI] [PubMed] [Google Scholar]
- 36.Azuma K., Izumi R., Osaki T., Ifuku S., Morimoto M., Saimoto H., Minami S., Okamoto Y. Chitin, chitosan, and its derivatives for wound healing: Old and new materials. J. Funct. Biomater. 2015;6:104–142. doi: 10.3390/jfb6010104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Shahid M., Mohammad F. Green chemistry approaches to develop antimicrobial textiles based on sustainable biopolymers—A review. Ind. Eng. Chem. Res. 2013;52:5245–5260. doi: 10.1021/ie303627x. [DOI] [Google Scholar]
- 38.Mitchell A., Spencer M., Edmiston C., Jr. Role of healthcare apparel and other healthcare textiles in the transmission of pathogens: A review of the literature. J. Hosp. Infect. 2015;90:285–292. doi: 10.1016/j.jhin.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.No H.K., Park N.Y., Lee S.H., Samuel P., Meyers S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002;74:65–72. doi: 10.1016/S0168-1605(01)00717-6. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi T., Imai M., Suzuki I., Sawai J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem. Eng. J. 2008;40:485–491. doi: 10.1016/j.bej.2008.02.009. [DOI] [Google Scholar]
- 41.Helander I.M., Nurmiaho-Lassila E.-L., Ahvenainen R., Rhoades J., Roller S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001;71:235–244. doi: 10.1016/S0168-1605(01)00609-2. [DOI] [PubMed] [Google Scholar]
- 42.Liu H., Du Y., Wang X., Sun L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004;95:147–155. doi: 10.1016/j.ijfoodmicro.2004.01.022. [DOI] [PubMed] [Google Scholar]