Abstract
Protein transduction using cell-penetrating peptides (CPPs) is useful for the delivery of large protein molecules, including some transcription factors. This method is safer than gene transfection methods with a viral vector because there is no risk of genomic integration of the exogenous DNA. Recently, this method was reported as a means for the induction of induced pluripotent stem (iPS) cells, directing the differentiation into specific cell types and supporting gene editing/correction. Furthermore, we developed a direct differentiation method to obtain a pancreatic lineage from mouse and human pluripotent stem cells via the protein transduction of three transcription factors, Pdx1, NeuroD, and MafA. Here, we discuss the possibility of using CPPs as a means of directing the differentiation of iPS cells and other stem cell technologies.
Keywords: cell-penetrating peptide, poly-arginine, protein transduction, induced pluripotent stem cell, pancreatic differentiation
1. Introduction
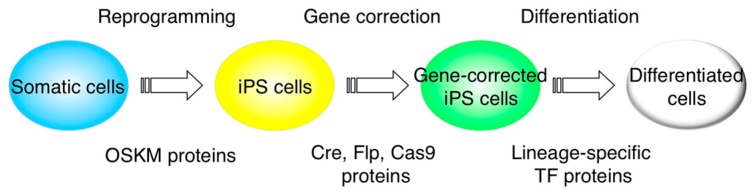
Induced pluripotent stem (iPS) cells are generated from somatic cells and they have a capacity to differentiate into multiple cell types [1]. The use of iPS cell technologies in regenerative medicine involves the key steps of reprogramming, gene editing/correction, and differentiation (Figure 1). Protein transduction via cell-penetrating peptides (CPPs) is a method for the delivery of peptides, recombinant proteins, and large molecules [2]. This method is safer than gene delivery via viral vectors because there is no risk of the genomic integration of exogenous genes. Therefore, this method has the possibility to substitute for virus-mediated gene delivery in the multi steps of reprogramming, gene editing/correction and differentiation (Figure 1). In this review, we summarize recent reports in this field and the future possibility of utilizing this method in iPS cell technologies.
Figure 1.
CPP-mediated protein transduction technologies in reprogramming, gene editing/correction, and differentiation of iPS cells. CPP-mediated protein transduction methods are used for key steps in iPS cell technologies. The reprogramming of somatic cells is induced with Yamanaka-4 factors fused to CPPs. Gene correction of disease-specific mutation is performed by the CRISPR-Cas9 system with CPP-fused Cas9 endonuclease. The differentiation of iPS cells is directed with CPP-fused transcription factors. OSKM, Oct4, Sox2, Klf4, c-Myc; TF, transcription factor.
2. CPP-Mediated Protein Transduction
It has been hypothesized that eukaryotic cells gained the function of endocytosis via evolution from a common origin of prokaryota [3]. Endocytosis was essential for biological diversity via the acquisition of mitochondria in animals and chloroplasts in plants [3]. Proteins fused with CPPs are internalized into cells via macropinocytosis [4,5], which is a form of fluid phase endocytosis [6]. Cell types with a macropinocytosis process can be transduced with recombinant proteins via CPPs. The CPP sequence was originally found in natural proteins as the HIV trans-activator of transcription (TAT) [7,8] and the Drosophila melanogastor homeodomain transcription factor Antennapedia [9]. That sequence in these proteins with the capacity of penetrating cells is called the protein transduction domain (PTD). Both TAT and Antennapedia contain arginine and lysine-rich residues in their PTDs [2]. Recombinant proteins fused to their PTD sequences or artificial CPPs like arginine-rich peptide (poly-arginine) can internalize into cells. In general, 6 to 12 arginines exhibit transduction activity as CPPs [10,11], while it has recently been reported that three arginines are sufficient for transduction capacity [12]. The first step of protein internalization into cells is mediated via binding to heparan sulfate proteoglycans, recruiting activated GTPase Rac1 to lipid rafts, followed by macropinocytosis [4,13,14,15,16]. However, there are some reports showing that heparan sulfate proteoglycans are not necessary for protein transduction [17,18,19]; therefore, detailed mechanisms are largely unknown. Several molecules including Rac1, p21-activated kinase 1 (Pak1), phosphatidylinositol 3-kinase, oncogene Ras, Src, histone deacetylase 6 (Hdac6), and heat shock protein 90 (Hsp90) have been implicated in macropinocytosis [20], suggesting that these molecules could influence the efficiency of protein transduction. Furthermore, it has been reported that protein entry into cells is also regulated by various molecules, such as coatomer subunit alpha and Na+/HCO3− cotransporter [21]. Recently, a unique method was reported, involving the intracellular delivery of naïve protein (not fused to any CPPs) via NaCl hypertonicity-induced macropinocytosis and a transduction compound, propanebetaine [22]. Surprisingly, the authors found these components in the buffer used on the purification of recombinant proteins. They also found that Na+/H+ exchanger 1 (Nhe1) plays an important role in this hypertonicity-induced protein transduction. Furthermore, another group also showed a transduction method without CPPs, involving the cationic lipid-mediated delivery of proteins with negatively supercharged proteins [23]. They used commonly available cationic lipid nucleic acid transfection reagents, lipofectamines.
3. Protein Transduction into iPS Cells
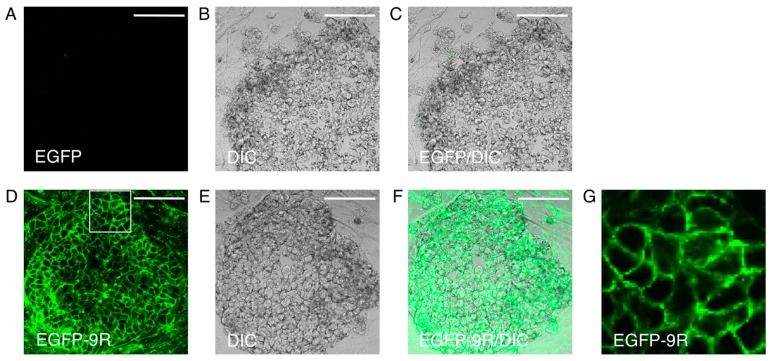
In general, lentivirus or retrovirus is used as a carrier for exogenous gene transduction to express protein, knockdown, or to edit endogenous genes in iPS cells and embryonic stem (ES) cells. These methods show high transduction efficiency; however, they lead to the integration of exogenous DNA into chromosomes of host cells, especially when viral vectors are used [24]. In the case of gene editing, the random occurrence of a deleterious mutation cannot be ruled out. Plasmid DNA transfection with cationic lipids can reduce the risk of integration into chromosomes; however, almost all pluripotent stem cells are generally difficult to transfect and the transfection efficiency is relatively low. Electroporation is a robust method to increase the efficiency of transfection, but it often leads to cell injury and death. The transduction methods combined with piggyBac transposon were developed for iPS cell generation as minimized genomic integration and the complete elimination of exogenous reprogramming factors, for application to regenerative medicine [25,26]. DNA transposons are genetic elements that can relocate between genomic sites by a “cut and paste” mechanism. Important features of the piggyBac transposon is that it transposes efficiently in many different species and that it nearly always excises itself precisely and leaves no footprint behind [27,28]. The piggyBac system has been shown to be applicable to human and mouse cell lines and this system becomes very attractive as a genetic tool. This piggyBac system has recently attracted attention, such as for the reprogramming of somatic cells and purification of differentiated cells [29]. A protein transduction method could also be useful for the transduction of exogenous proteins into iPS cells because of their high transduction efficiency and zero risk of genomic integration. In fact, proteins fused to poly-arginine were efficiently transduced into human iPS cells, whereas proteins without CPPs were not (Figure 2; unpublished data) [30]. In these cells, the signals of transduced EGFP-9R proteins were detected in the cytoplasm and cell membrane.
Figure 2.
Protein transduction into human iPS cells. Human iPS cells of 201B7 were treated with EGFP or 9R-EGFP for 6 h at a final concentration of 1 µM and GFP fluorescence was analyzed by confocal microscopy. (A–C) EGFP-treated cells. Images of EGFP fluorescence (A); DIC (B) and their merge (C) were shown. (D–F) EGFP-9R-treated cells. Images of EGFP fluorescence (D); DIC (E) and their merge (F) were shown; (G) Magnified image of indicated area by white box in (D). GFP fluorescence was detected in the cytoplasm and cell membrane. Scale bars are 100 µm. 9R, nine arginines. DIC, differential interference contrast.
Macropinocytosis occurs in most cell types, including pluripotent stem cells. Endocytosis processes are important in pluripotent stem cells for nutrient absorption [31], cellular signaling like Notch [32], Wnt [33,34], and gap junctional intercellular communication [35]. Under extracellular stimulation, GTPase, Rac1, and Cdc42 activate Pak1 [36] and these proteins trigger the active rearrangement of the actin cytoskelton and lead to macropinocytosis [20]. ES cells have been reported to express Rac1 and Cdc42, which regulate their migration [37]. In cancer cells, macropinocytosis is stimulated by the oncogene Ras, being important for macropinocytosis [38,39]. ES cells express embryonic stem cell-expressed Ras (E-Ras) [40], which have function in macropinocytosis in ES and iPS cells; however, its role is relatively unknown. Molecular mechanisms of macropinocytosis in iPS cells have also attracted research interest in this field.
4. iPS Cell Differentiation with Protein Transduction of Specific Transcription Factor
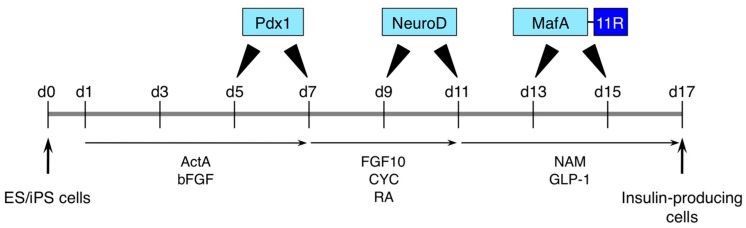
In the general method, some cytokines and growth factors are used to mimic organ development in pluripotent stem cells and direct the differentiation into specific cell types. Small molecules are also used to inhibit selective molecular signaling and guide to specific molecular activation. There have been several reports of efficient methods promoting differentiation from human iPS cells into neurons [41], retinal cells [42], lung cells [43], and pancreatic β cells [44], using some cytokines, growth factors, and small molecules. In addition to these biological factors and chemicals, the protein transduction of specific transcription factors is a useful method for directing the differentiation. We previously developed a differentiation method by the step-wise transduction of recombinant Pdx1, NeuroD, and MafA-11R proteins [45]. Pdx1 and NeuroD have their own PTDs [46,47], while MafA is fused with 11 arginines (11R) as CPPs. In mouse ES cells, these three proteins improved the efficiency of differentiation into insulin-producing cells (Figure 3) [45]. In human iPS cells, culture in differentiation medium with recombinant Pdx1 facilitates differentiation into pancreatic endocrine progenitors [45]. In this method, the order of transduced proteins and timing of protein transduction are important components for efficient differentiation. The protocol of Pdx1 transduced on days 5 and 7, NeuroD on days 9 and 11, and MafA-11R on days 13 and 15, was the most efficient by determining the number of insulin-positive cells, while a different order and timing reduced this efficiency (Figure 3) [45]. The order of transduced proteins is similar to developmental expression in vivo. Thus, the order of Pdx1, NeuroD, and MafA-11R is crucial for differentiation into insulin-producing cells. However, the yield of insulin-producing cells was relatively low (~1%). One reason is that full activity of protein transduced into cells was not achieved with this simple method using CPP-containing proteins. A well-optimized protocol for protein transduction will be required. Recently, well-established protocols for the pancreatic differentiation of human iPS cells have been reported [44,48,49]. These protocols use many cytokines, growth factors, hormones, and chemicals. Molecules originating from endogenous factors are thought to be safer and suited for usage in regenerative medicine, although some chemicals have a risk of mutagenicity. In these protocols, toxic chemicals are used, such as phorbol dibutyrate [44]. The possibility that differentiated cells cause tumorigenesis cannot be ruled out. It will be necessary to replace mutagenic chemicals with safe materials as recombinant proteins.
Figure 3.
Scheme of the protocol for pancreatic differentiation with Pdx1, NeuroD and MafA-11R protein transduction. Dissociated mouse ES or iPS cells were plated at day 0 and directed to pancreatic differentiation in medium supplemented with Activin A (ActA) and basic fibroblast growth factor (bFGF) from days 1 to 7, followed by medium supplemented with fibroblast growth factor 10 (FGF10), KAAD-cyclopamine (CYC), and retinoic acid (RA) from day 7 to 11, and medium supplemented with nicotinamide (NAM) and glucagon-like peptide-1 (GLP-1) from day 11 to 17. At day 17, a part of differentiated cells express insulin and mature pancreatic β-cell markers. Blue boxes show recombinant proteins of Pdx1, NeuroD, and MafA-11R and these proteins were added at the indicated time-points. d: day; 11R: 11 arginine.
Specific transcription factors are used for directing differentiation into other cell types. For neural differentiation, the forced expression of Ngn2 by lentiviral vectors is used for the efficient induction of functional neurons [50]. Transient overexpression of Nkx2-1 and Pax8 directs the differentiation of mouse ES cells into thyroid follicular cells [51]. Mesp1 expression in the doxycycline-inducible Mesp1 ES cell line promotes skeletal myogenic derivates in the absence of serum-derived factors [52] and the inducible expression of MyoD by piggyBac vector leads to efficient differentiation into mature myocytes [53]. In pancreatic differentiation, the combined expression of Pdx1 and MafA with either Ngn3 or NeuroD by adenoviral vectors facilitates the differentiation of mouse ES cells into insulin-producing cells [54]. Each method uses exogenous genes for the induction of transcription factors with lentiviral, adenoviral, and piggyBac vectors. These transduction methods cannot exclude the risk of the genomic integration of exogenous DNA and such methods are not desirable for clinical application. The protein transduction method is safer than viral vectors because there is no risk of genomic integration. Therefore, this method has the capacity to substitute for such transcription factors. Recently, there have been several reports of a differentiation protocol with protein transduction, as neural induction by Nkx2.2, Olig2, or Pax6 [55,56,57], myogenic induction by MyoD [58,59]. It is hoped that this method will become widely used for directing the differentiation.
5. Gene Editing with CPP-Mediated Protein Transduction
The protein transduction method via CPPs is also useful for introducing Cre recombinase and FLP recombinase proteins into cells to excise target genes [60,61,62] and for introducing Cas9 endonuclease and guide RNA to edit or correct genes [63]. Recently, D’Astolfo’s group and Zuris’s group reported native protein transduction via the hypertonicity- or cationic lipid-mediated delivery of Cre and Cas9, respectively [22,23] and D’Astolfo’s group also succeeded in Cas9 protein transduction into H1 human ES cells by this method [22]. Furthermore, protein transduction via CPPs can be used for siRNA delivery into pluripotent stem cells by fusing siRNA to the RNA-binding domain with CPPs [64]. These technologies are now being used in human pluripotent stem cells as a research element, especially TAT-Cre-mediated gene excision [65,66,67]. Gene-editing/correction technologies in iPS cells are desired for generating disease models carrying specific mutations or the transplantation of gene-corrected autologous tissues [68,69]. Thus the protein transduction method is also attractive in this gene-editing technology as a method without exogenous genes.
6. Usage of Protein Transduction in iPS Cell Generation or Direct Conversion
In contrast to directing the differentiation of stem cells, there is some difficulty in reprogramming somatic cells to iPS cells and the direct conversion of somatic cells to other cell types with protein transduction. Some groups reported the generation of mouse or human iPS cells by protein transduction via CPPs [70,71,72]. We have also attempted the reprogramming of fibroblasts with protein transduction of Oct4, Sox2, Klf4, and c-Myc proteins. However we failed to generate iPS cell colonies. In previous reports, the efficiency of iPS cell generation by proteins was significantly lower (about 0.001%) [71] compared to transduction via retroviral vectors (0.02%) [1]. Furthermore, it was reported that cells transduced with these four proteins via CPPs resembled the ES cell morphology but failed to expand like iPS cells; therefore, only partial reprogramming occurred using this method [73]. For complete reprogramming, the robust expression of the four factors might be needed equally to retroviral vector-mediated transduction. To use this for clinical utilization, more efficient protocols with robust expression are needed for this protein-mediated reprogramming.
Direct conversion occurs by the robust expression of specific transcription factors. Ascl1, Brn2, and Myt1l convert fibroblasts into neurons [74], Gata4, Mef2c, and Tbx5 convert fibroblasts into cardiomyocytes [75], Gata4, Hnf1α, and Foxa3 and the inactivation of p19Arf convert fibroblasts into hepatocytes [76], Hnf4α plus Foxa1, Foxa2, and Foxa3 convert fibroblasts into hepatocytes [77] and Sox10, Olig2, and Zfp536 convert fibroblasts into oligodendrocyte precursor cells [78]. They used retroviral or lentiviral vectors for gene transduction and the robust expression of these transcription factors. The protein transduction method has the capacity to replace these viral vector-mediated transductions; however, there is no report at present. Practical protocols are desired regarding protein-mediated direct conversion.
7. Conclusions
As stated above, it has been shown by many reports that some steps in iPS cell technologies can be done by protein transduction methods (Table 1). The transduction of exogenous genes via plasmids, viral vectors, and nucleic acids cannot completely exclude the risk of genomic integration. Proteins transduced via CPPs function transiently, but not stably in the cell. This kinetics could be suitable to mimic a differentiation process, because the expression of key transcription factors rapidly and dynamically fluctuates in defined periods in in vivo development and stable expression is rare. This method is useful as a means for directing the differentiation of iPS cells and for clinical application.
Table 1.
Summary of pluripotent stem cell technologies via protein transduction methods.
| CPPs | Proteins | Supplements | Technologies | Cell Types | References |
|---|---|---|---|---|---|
| Poly-arginine | OSKM | NA | Reprogramming | MEFs | [70] |
| Poly-arginine | OSKM | NA | Reprogramming | HNFs | [71] |
| NA | ES cell-derived extract proteins | Streptolysin O | Reprogramming | Mouse cardiac fibroblasts | [72] |
| Hydrophobic MTDs | OSKMN or OSKML | NA | Partial reprogramming | HDFs | [73] |
| TAT | Cre | NA | Recombination | Mouse ES cells | [60] |
| TAT | Cre | NA | Recombination | Human ES cells | [61] |
| TAT | FLP | dTAT-HA2 peptide | Recombination | Mouse or human ES cells | [62] |
| Poly-arginine | Cas9 and sgRNA | NA | Gene disruption | Human ES cells | [63] |
| NA | Cre or Cas9 | Hypertonic solution and NDSB-201 | Gene editing | Mouse or human ES cells | [22] |
| NA | Cre, TALE or Cas9 | Anionic proteins and cationic lipids | Gene editing | Mouse ES cells | [23] |
| PTDs or Poly-arginine | Pdx1, NeuroD and MafA | NA | Pancreatic differentiation | Mouse ES cells or human iPS cells | [45] |
| TAT | Nkx2.2 | NA | Neural differentiation | Mouse NSCs | [55] |
| TAT | Pax6 | NA | Neural differentiation | Rat NSCs | [57] |
CPP, cell-penetrating peptide; ES, embryonic stem; HDF, human dermal fibroblasts; HNF, human newborn fibroblast; iPS, induced pluripotent stem; MEF, mouse embryonic fibroblast; MTD, macromolecule transduction domain; NA, not applicable; NSC, neural stem cells; OSKM, Oct-4, Sox2, Klf4, c-Myc; OSKMN, Oct-4, Sox2, Klf4, c-Myc, Nanog; OSKML, Oct-4, Sox2, Klf4, c-Myc, Lin28; PTD, protein transduction domain; sgRNA, single-guide RNA; TAT, trans-activator of transcription.
Acknowledgments
This work was supported by a Grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; by the Japan Society for the Promotion of Science (JSPS) through its “Funding Program for Next Generation World-Leading Researchers”; by the Uehara Memorial Foundation; and by the Takeda Science Foundation.
Author Contributions
Taku Kaitsuka performed experiments; Taku Kaitsuka and Kazuhito Tomizawa wrote the manuscript; Kazuhito Tomizawa supervised the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berg A., Dowdy S.F. Protein transduction domain delivery of therapeutic macromolecules. Curr. Opin. Biotechnol. 2011;22:888–893. doi: 10.1016/j.copbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 3.De Duve C. The origin of eukaryotes: A reappraisal. Nat. Rev. Genet. 2007;8:395–403. doi: 10.1038/nrg2071. [DOI] [PubMed] [Google Scholar]
- 4.Nakase I., Niwa M., Takeuchi T., Sonomura K., Kawabata N., Koike Y., Takehashi M., Tanaka S., Ueda K., Simpson J.C., et al. Cellular uptake of arginine-rich peptides: Roles for macropinocytosis and actin rearrangement. Mol. Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan I.M., Wadia J.S., Dowdy S.F. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J. Control. Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Swanson J.A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frankel A.D., Pabo C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 8.Green M., Loewenstein P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus TAT trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 9.Joliot A., Pernelle C., Deagostini-Bazin H., Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA. 1991;88:1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Futaki S., Ohashi W., Suzuki T., Niwa M., Tanaka S., Ueda K., Harashima H., Sugiura Y. Stearylated arginine-rich peptides: A new class of transfection systems. Bioconjug. Chem. 2001;12:1005–1011. doi: 10.1021/bc015508l. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita M., Tomizawa K., Moriwaki A., Li S.T., Terada H., Matsui H. A high-efficiency protein transduction system demonstrating the role of PKA in long-lasting long-term potentiation. J. Neurosci. 2001;21:6000–6007. doi: 10.1523/JNEUROSCI.21-16-06000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hitsuda T., Michiue H., Kitamatsu M., Fujimura A., Wang F., Yamamoto T., Han X.J., Tazawa H., Uneda A., Ohmori I., et al. A protein transduction method using oligo-arginine (3R) for the delivery of transcription factors into cell nuclei. Biomaterials. 2012;33:4665–4672. doi: 10.1016/j.biomaterials.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi M., Rusnati M., Presta M., Giacca M. Internalization of HIV-1 TAT requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T., Futaki S., Niwa M., Tanaka S., Ueda K., Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J. Biol. Chem. 2002;277:2437–2443. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- 15.Wadia J.S., Stan R.V., Dowdy S.F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 16.Imamura J., Suzuki Y., Gonda K., Roy C.N., Gatanaga H., Ohuchi N., Higuchi H. Single particle tracking confirms that multivalent Tat protein transduction domain-induced heparan sulfate proteoglycan cross-linkage activates Rac1 for internalization. J. Biol. Chem. 2011;286:10581–10592. doi: 10.1074/jbc.M110.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gump J.M., June R.K., Dowdy S.F. Revised role of glycosaminoglycans in TAT protein transduction domain-mediated cellular transduction. J. Biol. Chem. 2010;285:1500–1507. doi: 10.1074/jbc.M109.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose H., Takeuchi T., Osakada H., Pujals S., Katayama S., Nakase I., Kobayashi S., Haraguchi T., Futaki S. Transient focal membrane deformation induced by arginine-rich peptides leads to their direct penetration into cells. Mol. Ther. 2012;20:984–993. doi: 10.1038/mt.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katayama S., Nakase I., Yano Y., Murayama T., Nakata Y., Matsuzaki K., Futaki S. Effects of pyrenebutyrate on the translocation of arginine-rich cell-penetrating peptides through artificial membranes: Recruiting peptides to the membranes, dissipating liquid-ordered phases, and inducing curvature. Biochim. Biophys. Acta. 2013;1828:2134–2142. doi: 10.1016/j.bbamem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 21.Tsumuraya T., Matsushita M. COPA and SLC4A4 are required for cellular entry of arginine-rich peptides. PLoS ONE. 2014;9:e86639. doi: 10.1371/journal.pone.0086639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Astolfo D.S., Pagliero R.J., Pras A., Karthaus W.R., Clevers H., Prasad V., Lebbink R.J., Rehmann H., Geijsen N. Efficient intracellular delivery of native proteins. Cell. 2015;161:674–690. doi: 10.1016/j.cell.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Zuris J.A., Thompson D.B., Shu Y., Guilinger J.P., Bessen J.L., Hu J.H., Maeder M.L., Joung J.K., Chen Z.Y., Liu D.R. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2015;33:73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waehler R., Russell S.J., Curiel D.T. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaji K., Norrby K., Paca A., Mileikovsky M., Mohseni P., Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woltjen K., Michael I.P., Mohseni P., Desai R., Mileikovsky M., Hämäläinen R., Cowling R., Wang W., Liu P., Gertsenstein M., et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding S., Wu X., Li G., Han M., Zhuang Y., Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Feschotte C. The piggyBac transposon holds promise for human gene therapy. Proc. Natl. Acad. Sci. USA. 2006;103:14981–14982. doi: 10.1073/pnas.0607282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruyama M., Yamashita Y., Kase M., Trifonov S., Sugimoto T. Lineage-specific purification of neural stem/progenitor cells from differentiated mouse induced pluripotent stem cells. Stem Cells Transl. Med. 2013;2:420–433. doi: 10.5966/sctm.2012-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaitsuka T., Tomizawa K. Generation of functional insulin-producing cells from human pluripotent stem cells via protein transduction methods. 2015. Manuscript in preparation. [DOI] [PubMed]
- 31.Sun C., Velazquez M.A., Marfy-Smith S., Sheth B., Cox A., Johnston D.A., Smyth N., Fleming T.P. Mouse early extra-embryonic lineages activate compensatory endocytosis in response to poor maternal nutrition. Development. 2014;141:1140–1150. doi: 10.1242/dev.103952. [DOI] [PubMed] [Google Scholar]
- 32.Bray S.J. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 33.Dobrowolski R., de Robertis E.M. Endocytic control of growth factor signalling: Multivesicular bodies as signalling organelles. Nat. Rev. Mol. Cell Biol. 2011;13:53–60. doi: 10.1038/nrm3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niehrs C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 35.Wörsdörfer P., Maxeiner S., Markopoulos C., Kirfel G., Wulf V., Auth T., Urschel S., von Maltzahn J., Willecke K. Connexin expression and functional analysis of gap junctional communication in mouse embryonic stem cells. Stem Cells. 2008;26:431–439. doi: 10.1634/stemcells.2007-0482. [DOI] [PubMed] [Google Scholar]
- 36.Knaus U.G., Wang Y., Reilly A.M., Warnock D., Jackson J.H. Structural requirements for PAK activation by Rac GTPases. J. Biol. Chem. 1998;273:21512–21518. doi: 10.1074/jbc.273.34.21512. [DOI] [PubMed] [Google Scholar]
- 37.Suh H.N., Han H.J. Laminin regulates mouse embryonic stem cell migration: Involvement of Epac1/Rap1 and Rac1/cdc42. Am. J. Physiol. Cell Physiol. 2010;298:C1159–C1169. doi: 10.1152/ajpcell.00496.2009. [DOI] [PubMed] [Google Scholar]
- 38.Bar-Sagi D., Feramisco J.R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 39.Commisso C., Davidson S.M., Soydaner-Azeloglu R.G., Parker S.J., Kamphorst J.J., Hackett S., Grabocka E., Nofal M., Drebin J.A., Thompson C.B., et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi K., Mitsui K., Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541–545. doi: 10.1038/nature01646. [DOI] [PubMed] [Google Scholar]
- 41.Sasai Y. Next-generation regenerative medicine: Organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12:520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Kamao H., Mandai M., Okamoto S., Sakai N., Suga A., Sugita S., Kiryu J., Takahashi M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014;2:205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gotoh S., Ito I., Nagasaki T., Yamamoto Y., Konishi S., Korogi Y., Matsumoto H., Muro S., Hirai T., Funato M., et al. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Rep. 2014;3:394–403. doi: 10.1016/j.stemcr.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagliuca F.W., Millman J.R., Gürtler M., Segel M., van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaitsuka T., Noguchi H., Shiraki N., Kubo T., Wei F.Y., Hakim F., Kume S., Tomizawa K. Generation of functional insulin-producing cells from mouse embryonic stem cells through 804G cell-derived extracellular matrix and protein transduction of transcription factors. Stem Cells Transl. Med. 2014;3:114–127. doi: 10.5966/sctm.2013-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noguchi H., Kaneto H., Weir G.C., Bonner-Weir S. PDX-1 protein containing its own antennapedia-like protein transduction domain can transduce pancreatic duct and islet cells. Diabetes. 2003;52:1732–1737. doi: 10.2337/diabetes.52.7.1732. [DOI] [PubMed] [Google Scholar]
- 47.Noguchi H., Bonner-Weir S., Wei F.Y., Matsushita M., Matsumoto S. BETA2/NeuroD protein can be transduced into cells due to an arginine- and lysine-rich sequence. Diabetes. 2005;54:2859–2866. doi: 10.2337/diabetes.54.10.2859. [DOI] [PubMed] [Google Scholar]
- 48.Shahjalal H.M., Shiraki N., Sakano D., Kikawa K., Ogaki S., Baba H., Kume K., Kume S. Generation of insulin-producing beta-like cells from human iPS cells in a defined and completely xeno-free culture system. J. Mol. Cell Biol. 2014;6:394–408. doi: 10.1093/jmcb/mju029. [DOI] [PubMed] [Google Scholar]
- 49.Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A., O’Dwyer S., Quiskamp N., Mojibian M., Albrecht T., et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Pak C., Han Y., Ahlenius H., Zhang Z., Chanda S., Marro S., Patzke C., Acuna C., Covy J., et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antonica F., Kasprzyk D.F., Opitz R., Iacovino M., Liao X.H., Dumitrescu A.M., Refetoff S., Peremans K., Manto M., Kyba M., et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71. doi: 10.1038/nature11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan S.S., Shi X., Toyama A., Arpke R.W., Dandapat A., Iacovino M., Kang J., Le G., Hagen H.R., Garry D.J., et al. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell. 2013;12:587–601. doi: 10.1016/j.stem.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka A., Woltjen K., Miyake K., Hotta A., Ikeya M., Yamamoto T., Nishino T., Shoji E., Sehara-Fujisawa A., Manabe Y., et al. Efficient and reproducible myogenic differentiation from human iPS cells: Prospects for modeling Miyoshi Myopathy in vitro. PLoS ONE. 2013;8:e61540. doi: 10.1371/journal.pone.0061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H., Tsang K.S., Chan J.C., Yuan P., Fan R., Kaneto H., Xu G. The combined expression of Pdx1 and MafA with either Ngn3 or NeuroD improves the differentiation efficiency of mouse embryonic stem cells into insulin-producing cells. Cell Transplant. 2013;22:147–158. doi: 10.3727/096368912X653057. [DOI] [PubMed] [Google Scholar]
- 55.Stock K., Nolden L., Edenhofer F., Quandel T., Brüstle O. Transcription factor-based modulation of neural stem cell differentiation using direct protein transduction. Cell. Mol. Life Sci. 2010;67:2439–2449. doi: 10.1007/s00018-010-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mie M., Kaneko M., Henmi F., Kobatake E. Induction of motor neuron differentiation by transduction of Olig2 protein. Biochem. Biophys. Res. Commun. 2012;427:531–536. doi: 10.1016/j.bbrc.2012.09.090. [DOI] [PubMed] [Google Scholar]
- 57.Spitere K., Toulouse A., O’Sullivan D.B., Sullivan A.M. TAT-PAX6 protein transduction in neural progenitor cells: A novel approach for generation of dopaminergic neurones in vitro. Brain Res. 2008;1208:25–34. doi: 10.1016/j.brainres.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 58.Hidema S., Tonomura Y., Date S., Nishimori K. Effects of protein transduction with intact myogenic transcription factors tagged with HIV-1 Tat-PTD (T-PTD) on myogenic differentiation of mouse primary cells. J. Biosci. Bioeng. 2012;113:5–11. doi: 10.1016/j.jbiosc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 59.Noda T., Fujino T., Mie M., Kobatake E. Transduction of MyoD protein into myoblasts induces myogenic differentiation without addition of protein transduction domain. Biochem. Biophys. Res. Commun. 2009;382:473–477. doi: 10.1016/j.bbrc.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 60.Peitz M., Pfannkuche K., Rajewsky K., Edenhofer F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: A tool for efficient genetic engineering of mammalian genomes. Proc. Natl. Acad. Sci. USA. 2002;99:4489–4494. doi: 10.1073/pnas.032068699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nolden L., Edenhofer F., Haupt S., Koch P., Wunderlich F.T., Siemen H., Brüstle O. Site-specific recombination in human embryonic stem cells induced by cell-permeant Cre recombinase. Nat. Methods. 2006;3:461–467. doi: 10.1038/nmeth884. [DOI] [PubMed] [Google Scholar]
- 62.Patsch C., Peitz M., Otte D.M., Kesseler D., Jungverdorben J., Wunderlich F.T., Brüstle O., Zimmer A., Edenhofer F. Engineering cell-permeant FLP recombinase for tightly controlled inducible and reversible overexpression in embryonic stem cells. Stem Cells. 2010;28:894–902. doi: 10.1002/stem.417. [DOI] [PubMed] [Google Scholar]
- 63.Ramakrishna S., Kwaku Dad A.B., Beloor J., Gopalappa R., Lee S.K., Kim H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 2014;24:1020–1027. doi: 10.1101/gr.171264.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eguchi A., Meade B.R., Chang Y.C., Fredrickson C.T., Willert K., Puri N., Dowdy S.F. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat. Biotechnol. 2009;27:567–571. doi: 10.1038/nbt.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wernig M., Meissner A., Foreman R., Brambrink T., Ku M., Hochedlinger K., Bernstein B.E., Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 66.Kadari A., Lu M., Li M., Sekaran T., Thummer R.P., Guyette N., Chu V., Edenhofer F. Excision of viral reprogramming cassettes by Cre protein transduction enables rapid, robust and efficient derivation of transgene-free human induced pluripotent stem cells. Stem Cell Res. Ther. 2014;5:47. doi: 10.1186/scrt435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Redmer T., Diecke S., Grigoryan T., Quiroga-Negreira A., Birchmeier W., Besser D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011;12:720–726. doi: 10.1038/embor.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin Y., Gao W.Q. Concise Reviews: Patient-Derived Stem Cell Research for Monogenic Disorders. Stem Cells. 2015 doi: 10.1002/stem.2112. [DOI] [PubMed] [Google Scholar]
- 69.Li H.L., Nakano T., Hotta A. Genetic correction using engineered nucleases for gene therapy applications. Dev. Growth Differ. 2014;56:63–77. doi: 10.1111/dgd.12107. [DOI] [PubMed] [Google Scholar]
- 70.Zhou H., Wu S., Joo J.Y., Zhu S., Han D.W., Lin T., Trauger S., Bien G., Yao S., Zhu Y., et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim D., Kim C.H., Moon J.I., Chung Y.G., Chang M.Y., Han B.S., Ko S., Yang E., Cha K.Y., Lanza R., et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho H.J., Lee C.S., Kwon Y.W., Paek J.S., Lee S.H., Hur J., Lee E.J., Roh T.Y., Chu I.S., Leem S.H., et al. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood. 2010;116:386–395. doi: 10.1182/blood-2010-02-269589. [DOI] [PubMed] [Google Scholar]
- 73.Lim J., Kim J., Kang J., Jo D. Partial somatic to stem cell transformations induced by cell-permeable reprogramming factors. Sci. Rep. 2014;4:4361. doi: 10.1038/srep04361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Südhof T.C., Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ieda M., Fu J.D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B.G., Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang P., He Z., Ji S., Sun H., Xiang D., Liu C., Hu Y., Wang X., Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 77.Sekiya S., Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 78.Yang N., Zuchero J.B., Ahlenius H., Marro S., Ng Y.H., Vierbuchen T., Hawkins J.S., Geissler R., Barres B.A., Wernig M. Generation of oligodendroglial cells by direct lineage conversion. Nat. Biotechnol. 2013;31:434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]