Abstract
Periodontitis, an inflammatory disease, is caused by biofilms with a mixed microbial etiology and involves the progressive destruction of the tooth-supporting tissues. A rising number of studies investigate the clinical potential of photodynamic therapy (PDT) as an adjunct during active therapy. The aim of the present review was to evaluate the available literature for the in vitro antimicrobial efficacy of photodynamic therapy focusing on the periodontopathogenic bacteria Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Fusobacterium nucleatum. The focused question was: “Is it possible to decrease (at least 3 log steps or 99.9%) or even eliminate bacterial growth by photodynamic therapy in vitro when compared to untreated control groups or control groups treated by placebo?” In general, PDT resulted in a substantial reduction of surviving bacteria. However, not all studies showed the desired reduction or elimination. The ranges of log10-reduction were 0.38 (58%) to a complete eradication (100%) for P. gingivalis, 0.21 (39%) to 100% for A. actinomycetemcomitans and 0.3 (50%) to 100% for F. nucleatum. In conclusion, further and particularly more comparable studies are needed to evaluate if PDT can be clinically successful as an adjuvant in periodontal therapy.
Keywords: PDT, photodynamic therapy, in vitro, periodontitis, antimicrobial activity
1. Introduction
Periodontitis is an inflammatory disease caused by biofilms with a mixed microbial etiology and leads to a progressive destruction of the teeth-supporting tissues. The main objective of periodontal treatment is the removal of the supra- and subgingival biofilm from the root surface in order to eliminate the pathogenic bacteria, which initiate and cause the progression of periodontal disease [1]. At present, the most widely used treatment to achieve this goal is the mechanical instrumentation of the root surface, i.e., scaling and root planning (SRP). However, residual calculus is often observed after this treatment, especially in deep pockets and in posterior teeth [2]. Further, some pathogens are able to invade the surrounding soft tissues of the periodontal pocket, and re-colonization of treated sites may occur if intraoral niches remain untreated [3]. Therefore, new treatment approaches, such as the antimicrobial PDT (aPDT) have been proposed for a more efficient elimination of the pathogenic biofilm.
Antimicrobial PDT is considered a non-invasive therapeutic method able to selectively target periodontal pathogens, thus avoiding damage to the host tissues [4]. It involves the localization of a photoactivable agent—the photosensitizer—in a target region prior to activation by light of the appropriate wavelength. Singlet oxygen and free radicals are generated upon illumination, which are cytotoxic to microorganisms and their by-products [5]. Today, for curative applications, the photodynamic effects are used for cancers as an alternative to chemo- or radiotherapy; however, local infections such as those that occur within the oral cavity are also increasingly popular fields of application of the aPDT [6]. In the treatment of periodontitis, the additional benefits of the aPDT to the classical scaling and root planning are not completely clear [7]. Therefore, the aim of this review was to evaluate the available literature for the in vitro antimicrobial efficacy of photodynamic therapy against periodontopathogenic bacteria. It was analyzed if it is possible to decrease or even stop bacterial growth under laboratory conditions compared to non-treated controls or compared to control groups treated by placebo.
2. Results and Discussion
2.1. Search and Screening
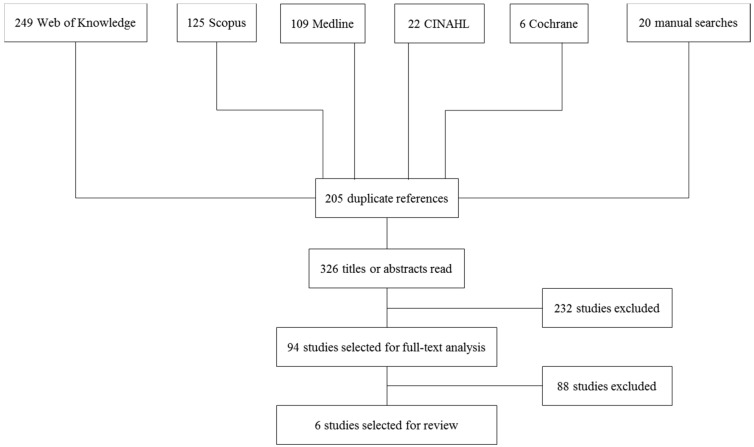
A total of 306 titles from the electronic databases and 20 titles from the hand search were identified (Figure 1). The full texts of 94 publications were further analyzed. Full text analysis led to exclusion of further 25 studies. Only 6 of the remaining 69 papers complied with the inclusion criteria.
Figure 1.
Flowchart of selection process of included studies.
2.2. Description of Studies
Five out of the six included studies used methylene blue as a photosensitizer [8,9,10,11,12], one applied toluidine blue [13]. The effect of PDT on Aggregatibacter actinomycetemcomitans only was investigated in two studies [11,12], Porphyromonas gingivalis only was studied in one trial [8]. Two studies investigated three pathogens, i.e., Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Fusobacterium nucleatum [9,10]. One study investigated Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis [13].
2.3. Antimicrobial Effects of Photodynamic Therapy (PDT) on Porphyromonas Gingivalis
The summarized data set regarding P. gingivalis is presented in Table 1.
Table 1.
Antimicrobial effects of photodynamic therapy (PDT) on P.g.
| Reference | Photo-Sensitizer | PDT | Control 1 | Control 2 | Control 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Light Source | Bacterial Strain | Mean | Conditions | Mean | Conditions | Mean | Conditions | Mean | ||
| Braham et al. [8] | MB 0.01% at dilution 0.03 | Diode laser λ: 670 nm P: 150 mW | ATCC 33277 | log10 reduction 3.8 ± 1.3 * | N/A | N/A | N/A | N/A | photosensitizer only | no effect |
| Chan and Lai [9] | MB 0.01% | He–Ne laser λ: 632.8 nm P: 30 mW He: 6.4 J/cm2 | ATCC 33277 | Viable count CFU 16 ± 5 | negative control no photosensitizer no light exposure | Viable count CFU 129 ± 7 | light only | Viable count CFU 132 ± 8 | photosensitizer only | Viable count CFU 108 ± 12 |
| Diode laser λ: 665 nm P: 100 mW He: 21.2 J/cm2 | Viable count CFU 1 ± 0.3 | Viable count CFU 117 ± 8 | Viable count CFU 67 ± 8 | Viable count CFU 111 ± 13 | ||||||
| Diode laser λ: 830 nm P: 100 mW He: 21.2 J/cm2 | Viable count CFU 58 ± 6 | Viable count CFU 138 ± 8 | Viable count CFU 81 ± 6 | Viable count CFU 126 ± 4 | ||||||
| Eick et al. [13] | TB 0.1 mg/mL | LED lamp λ: 625–635 nm E: 2 W/cm2 | ATCC 33277 | Viable count CFU (8.17 ± 1.01) log10 | negative control no photosensitizer no light exposure | Viable count CFU (9.73 ± 0.46) log10 | light only | Viable count CFU (9.59 ± 0.64) log10 | photosensitizer only | Viable count CFU (9.08 ± 0.25) log10 |
| M5-1-2 | Viable count CFU (2.73 ± 3.16) log10 | Viable count CFU (9.73 ± 0.65) log10 | Viable count CFU (9.71 ± 0.48) log10 | Viable count CFU (5.22 ± 4.03) log10 | ||||||
| Street et al. [10] | MB 0.01% | Diode laser λ: 670 nm He: 9.4 J/cm2 | planktonic ATCC 33277 | log10 reduction 6.8 ± 0.7 | N/A | N/A | N/A | N/A | N/A | N/A |
| MB 0.01% | Diode laser λ: 670 nm He: 6 J/cm2 | biofilm ATCC 33277 | log10 reduction 4.5 ± 1.2 | N/A | N/A | N/A | N/A | N/A | N/A | |
MB: Methylene Blue; TB: Toluidine Blue; λ: wavelength; P: Power Output; E: Irradiance; He: Radiant exposure; min: Minutes; CFU: colony forming units; *: value taken from diagram; P.g.: Porphyromonas gingivalis; Exposure time always 60 s unless otherwise specified.
Chan and Lai [9] performed experiments with methylene blue (MB) as a dye and three different light sources, i.e., a He–Ne laser (632.8 nm) with a 30 mW power output, a 100 mW diode laser at 665 nm, and a 100 mW diode laser at 830 nm. Incubation with MB alone without light irradiation resulted in a statistically significant decrease of P. gingivalis [9]. Light irradiation alone without previous incubation with MB showed statistically significant decrease for both diode lasers [9]. Using a MB and light application, all test arrangements led to a statistically significant decrease of P. gingivalis [9]. The most efficient combination was MB 0.01% with the diode laser at 665 nm.
Braham et al. [8] found no antimicrobial effect using MB only. MB in combination with light irradiation resulted in a decrease of P. gingivalis of (3.8 ± 1.3) log10. Another study using a diode laser with a wave length in the range of 650–675 nm and MB as a dye resulted in an eradication of >99.9% of planktonic P. gingivalis [10].
The reduction of P. gingivalis is summarized in Table 4, given as reduction in %, which ranged from 57.97% [9] to 100% [10], as well as log10 reduction, which ranged from 0.38 [9] to 6.8 [10].
Table 4.
Overview of the results (percentage reduction and log reduction) of all included studies.
| Reference | Bacteria Investigated | PDT | P.g. | A.a. | F.n. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Photosensitizer | Light Source | Reduction in % | Log10 Reduction | Reduction in % | Log10 Reduction | Reduction in % | Log10 Reduction | |||
| Braham et al. [8] | P.g. | MB 0.01% at dilution 0.03 | Diode laser λ: 670 nm; P: 150 mW | 99.98 | 3.8 * | N/A | N/A | N/A | N/A | |
| Chan and Lai [9] | P.g. A.a. F.n. | MB 0.01% | He–Ne laser λ: 632.8 nm; P: 30 mW; He: 6.4 J/cm2 | 90.7 | 1.03 | 87.5 | 0.9 | 83.76 | 0.79 | |
| Diode laser λ: 665 nm; P: 100 mW; He: 21.2 J/cm2 | 99.15 | 2.07 | 95.45 | 1.3 | 96.23 | 1.42 | ||||
| Diode laser λ: 830 nm; P: 100 mW; He: 21.2 J/cm2 | 57.97 | 0.38 | 39.2 | 0.21 | 49.59 | 0.3 | ||||
| Eick et al. [13] | P.g. A.a. | ATCC 33277 Y4 | TB 0.1 mg/mL | LED lamp λ: 625-635 nm; E: 2 W/cm2 | 97.25 | 1.56 | 98.42 | 1.8 | N/A | N/A |
| M5-1-2 J7 | LED lamp λ: 625-635 nm; E: 2 W/cm2 | 99.99 | 7 | 99.78 | 2.66 | N/A | N/A | |||
| Street et al. [10] | P.g. A.a. F.n. | planktonic | MB 0.01% | Diode laser λ: 670 nm; He: 9.4 J/cm2 | 100 | 6.8 | 98.74 | 1.9 | 99.99 | 5.2 |
| P.g. A.a. F.n. | biofilm | Diode laser λ: 670 nm; He: 6 J/cm2 | 100 | 4.5 | 100 | 4.9 | 99.96 | 3.4 | ||
| Alvarenga et al. [12] | A.a. | MB 100 µM | L: Diode laser λ: 660 nm; P: 100 mW; He: 15 J/cm2 | N/A | N/A | 50 | 0.3 | N/A | N/A | |
| Goulart et al. [11] | A.a. | MB 0.5 µmol/L | Dental photopolymerizer λ: 400–500 nm; E: 350–500 mW/cm2 He: 0.65 J/cm2 | N/A | N/A | 15 | 0.07 | N/A | N/A | |
| MB 1 µmol/L | Dental photopolymerizer λ: 400–500 nm; E: 350–500 mW/cm2 He: 0.65 J/cm2 | N/A | N/A | 25 | 0.12 | N/A | N/A | |||
MB: Methylene Blue; TB: Toluidine Blue; λ: wavelength; P: Power Output; E: Irradiance; He: Radiant exposure; *: value taken from diagram; Exposure time always 60 s.
2.4. Antimicrobial Effects of Photodynamic Therapy (PDT) on Aggregatibacter Actinomycetemcomitans
A summary of the data is shown in Table 2.
Table 2.
Antimicrobial effects of photodynamic therapy (PDT) on A.a.
| Reference | Photo-Sensitizer | Light Source | Bacterial Strain | PDT | Control 1 | Control 2 | Control 3 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Conditions | Mean | Conditions | Mean | Conditions | Mean | ||||
| Chan and Lai [9] | MB 0.01% | He-Ne laser λ: 632.8 nm P: 30 mW He: 6.4 J/cm2 | ATCC 29522 | Viable count CFU 17 ± 6 | negative control no photosensitizer no light exposure | Viable count CFU 136 ± 12 | light only | Viable count CFU 116 ± 5 | photosensitizer only | Viable count CFU 141 ± 10 |
| Diode laser λ: 665nm P: 100 mW He: 21.2 J/cm2 | Viable count CFU 6 ± 3 | Viable count CFU 132 ± 12 | Viable count CFU 88 ± 5 | Viable count CFU 137 ± 11 | ||||||
| Diode laser λ: 830 nm P: 100 mW He: 21.2 J/cm2 | Viable count CFU 76 ± 5 | Viable count CFU 125 ± 11 | Viable count CFU 65 ± 10 | Viable count CFU 128 ± 8 | ||||||
| Eick et al. [13] | TB 0.1 g/mL | LED lamp λ: 625–635 nm E: 2 W/cm2 | Y4 | Viable count CFU 7.47 ± 1.24 log10 | negative control no photosensitizer no light exposure | Viable count CFU 9.27 ± 0.14 log10 | light only | Viable count CFU 9.36 ± 0.08 log10 | photosensitizer only | Viable count CFU 8.7 ± 0.41 log10 |
| J7 | Viable count CFU 4.11 ± 2.77 log10 | Viable count CFU 6.77 ± 0.78 log10 | Viable count CFU 6.86 ± 0.8 log10 | Viable count CFU 5.73 ± 0.21 log10 | ||||||
| Goulart et al. [11] | MB 0.5 µmol/L | Dental photo-polymerizer λ: 400–500 nm E: 350–500 mW/cm2 He: 0.65 J/cm2 | planktonic JP2 | 30 min incubation 15% cell death | N/A | N/A | N/A | N/A | photosensitizer only ≤0.1 µmol/L 10 min incubation 30 min incubation | 0% reduction |
| MB 1 µmol/L | Dental photo-polymerizer λ: 400–500 nm E: 350–500 mW/cm2 He: 0.65 J/cm2 | planktonic JP2 | 30 min incubation 25% cell death | N/A | N/A | N/A | N/A | photosensitizer only 10 min incubation 30 min incubation | 19% reduction * 25% reduction * | |
| MB 10 µmol/L | N/A | planktonic JP2 | N/A | N/A | N/A | N/A | N/A | photosensitizer only 10 min incubation 30 min incubation | 23% reduction * 31% reduction * | |
| MB 20 µmol/L | N/A | N/A | N/A | N/A | N/A | N/A | photosensitizer only incubation time unclear | 50% reduction | ||
| MB 0.5 µmol/L | Dental photo-polymerizer λ: 400–500 nm E: 350–500 mW/cm2 He: 0.65 J/cm2 | biofilm JP2 | 30 min incubation 73% reduction of absorbance * | N/A | N/A | N/A | N/A | photosensitizer only 30 min incubation | 73% reduction of absorbance * | |
| MB 1 µmol/L | biofilm JP2 | 30 min incubation 58% reduction of absorbance * | N/A | N/A | N/A | N/A | photosensitizer only 30 min incubation | 60% reduction of absorbance * | ||
| Street et al. [10] | MB 0.01% | Diode laser λ: 670 nm He: 9.4 J/cm2 | planktonic ATCC 33384 | log10 reduction 1.9 ± 0.6 | N/A | N/A | N/A | N/A | N/A | N/A |
| Diode laser λ: 670 nm He: 6 J/cm2 | biofilm ATCC 43717 | log10 reduction 4.9 ± 1.4 | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Alvarenga et al. [12] | MB 100 µM | L: Diode laser λ: 660 nm P: 100 mW He: 15 J/cm2 | ATCC 29523 | log10 reduction 0.3 | negative control no photosensitizer no light exposure | (cfu/mL) 8.87 ± 0.34 log10 | light only | (cfu/mL) 8.13 ± 0.67 | photosensitizer only | (cfu/mL) 8.47 ± 0.06 |
MB: Methylene Blue; TB: Toluidine Blue; λ: wavelength; P: Power Output; E: Irradiance; He: Radiant exposure; min: Minutes; CFU: colony forming units; *: value taken from diagram; A.a.: Aggregatibacter actinomycetemcomitans; Exposure time always 60 s unless otherwise specified.
Chan and Lai [9] found no effect against A. actinomycetemcomitans using MB alone in a concentration of 0.01% without light application. MB in combination with light application resulted in a statistically significant decrease of A. actinomycetemcomitans with the most efficient combination being MB 0.01% with a diode laser (665 nm). Light application alone without previous incubation with MB, however, led to a statistically significant decrease of A. actinomycetemcomitans regardless of the light source applied (He–Ne laser at 632.8 nm, two different diode laser at 665 nm or at 830 nm) as well.
When studying A. actinomycetemcomitans organized in a biofilm, Alvarenga et al. [12] could not find a statistically significant effect on the viable count of the bacteria, irrespective of the use of dye only (MB), light activation only (diode laser at 660 nm), or dye in combination with a 60 s light application.
The test arrangement for planktonic growth of Street et al. [10] using a diode laser with a wavelength of 670 nm and the photosensitizer MB resulted in eradication of >99.9% of planktonic A. actinomycetemcomitans.
Eick et al. [13] found that toluidine blue and a diode laser (625–635 nm) were effective in reducing the viability of biofilms of two different strains of A. actinomycetemcomitans. Similarly, Goulart et al. [11] found that exposure of an A. actinomycetemcomitans bacterial lineage to a dental photopolymerizer light source (400–500 nm) led to a decrease in bacterial viability both in planktonic culture and as a cellular aggregate in the presence of MB.
The reduction of A. actinomycetemcomitans is again summarized in Table 4, given as reduction in %, which ranged from 15% [11] to 100% [10], as well as log10 reduction which ranged from 0.07 [11] to 4.9 [10].
2.5. Antimicrobial Effects of Photodynamic Therapy (PDT) on Fusobacterium Nucleatum
Table 3 shows the summarized data set regarding F. nucleatum.
Table 3.
Antimicrobial effects of photodynamic therapy (PDT) on F.n.
| Reference | Photosensitizer | Light Source | Bacterial Strain | PDT | Control 1 | Control 2 | Control 3 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Conditions | Mean | Conditions | Mean | Conditions | Mean | ||||
| Chan and Lai [9] | MB 0.01% | He-Ne laser λ: 632.8 nm; P: 30 mW; He: 6.4 J/cm2 | ATCC 23726 | Viable count CFU 19 ± 3 | negative control no photosensitizer no light exposure | Viable count CFU 117 ± 9 | light only no photosensitizer | Viable count CFU 113 ± 6 | photosensitizer only no light | Viable count CFU 112 ± 9 |
| Diode laser λ: 665 nm; P: 100 mW He: 21.2 J/cm2 | Viable count CFU 4 ± 2 | Viable count CFU 106 ± 14 | Viable count CFU 65 ± 9 | Viable count CFU 96 ± 6 | ||||||
| Diode laser λ: 830 nm; P: 100 mW He: 21.2 J/cm2 | Viable count CFU 61 ± 4 | Viable count CFU 121 ± 9 | Viable count CFU 67 ± 11 | Viable count CFU 117 ± 9 | ||||||
| Street et al. [10] | MB 0.01% | Diode laser λ: 670 nm He: 9.4 J/cm2 | planktonic ATCC 25586 | log10 reduction 5.2 ± 0.6 | N/A | N/A | N/A | N/A | N/A | N/A |
| Diode laser λ: 670 nm He: 6 J/cm2 | biofilm ATCC 25586 | log10 reduction 3.4 ± 1.1 | N/A | N/A | N/A | N/A | N/A | N/A | ||
MB: Methylene Blue; TB: Toluidine Blue; λ: wavelength; P: Power Output; E: Irradiance; He: Radiant exposure; F.n.: Fusobacterium nucleatum; Exposure time always 60 s.
Chan and Lai [9] found that treatment with MB alone in the absence of laser irradiation did not cause a significant reduction in the viability of the bacteria. A statistically significant decrease of colony forming units was found if a diode laser at a wavelength of either 665 nm or 830 nm illuminated the probes without previous incubation with MB. If MB and a light source (either a He–Ne laser at 632.8 nm, or a diode laser at 665 nm or at 830 nm) were applied, all of the three combinations showed a statistically significant decrease of CFU regarding F. nucleatum, with the most efficient set being MB 0.01% in combination with the diode laser (665 nm). Only 4% of the amount of CFU (F.n.) compared to the negative control (no dye, no light) grew on the platelets using this combination.
Street et al. [10] showed that it is possible to kill nearly 100% of F. nucleatum using MB in combination with a diode laser with a wave length of 650–675 nm. The degree of reduction depended on the growing model used (planktonic growth or biofilm), where PDT proved to be more effective in planktonic growing bacteria.
The reduction of F. nucleatum is also presented in Table 4, given as reduction in %, which ranged from 49.6% [9] to 100% [10], as well as log10 reduction which ranged from 0.3 [9] to 5.2 [10].
2.6. Discussion
The purpose of the present review was to evaluate the available literature for the in vitro antimicrobial efficacy of photodynamic therapy regarding P. gingivalis, A. actinomycetemcomitans, F. nucleatum. In the initial search, many studies with different light sources, photosensitizers and application protocols were found. Only six papers could be found that complied with these. Originally, also studies assessing C. albicans were planned to be included in this review. Eight studies, which investigated the effect of PDT against C. albicans, were included in the fulltext analysis. Finally, however, none of these studies matched the inclusion criteria. Therefore, the studies concerning C. albicans had to be excluded and no results regarding antifungal efficacy could be presented.
Only studies were included, which applied either methylene blue or toluidine blue as photosensitizer. This criterion was chosen because these two dyes rank among the most commonly used photosensitizers for oral antimicrobial PDT [14,15]. Both dyes have been shown to have no toxic effect in the commonly used concentration of 0.01%; furthermore, they present with a high degree of selectivity for damage for gram-positive and gram-negative bacteria, as well as viruses and yeasts [16,17,18].
All papers included showed that the respective dye used in combination with a light source resulted in a substantial reduction of the number of surviving bacteria or of the growing bacteria compared to the control groups. In the result section of this review, the individual outcomes of the PDT are presented, and a conversion from percentage reduction to log reduction and vice versa was performed for a better overview and comparison of the results. In general, PDT resulted in a substantial reduction of surviving bacteria. However, not all studies showed the desired reduction or elimination. The reduction of P. gingivalis, A. actinomycetemcomitans and F. nucleatum ranged from 57.8% [9], 15% [11] and 49.6% [9] to 100% [10], respectively. The wide range between the publications can be explained by various factors. One important factor is that PDT is more efficient in killing bacteria in their planktonic phase than in biofilms derived from the same plaque samples [19]. This trend was confirmed by studies included in this review, where PDT was more efficient if bacteria were in a planktonic phase than when they were organized in a biofilm [9,10,11]. Another publication, which was not included in this review, could not prove any effect of PDT in a multispecies biofilm because the matrix-embedded microbial populations in a biofilm were well protected against antimicrobial agents [7]. Similar effects are observed using antibiotics in periodontal treatment. These difficulties depend on more than one factor [20]. Two approaches are considered to explain the reduced antibiotic susceptibility. First, the medicament is not able to penetrate the biofilm. Furthermore, antibiotics are more effective in killing rapidly growing cells. This is the second approach. Because of nutrient limitation, microorganisms are growing slowly and their antimicrobial susceptibility is reduced [20]. There are antibiotics not able to kill non-growing cells. Probable reasons that PDT is less effective in biofilms compared to planktonic condition are the distinct and protected phenotypes expressed by dental plaque microorganisms once they attached to the tooth [19]. Furthermore, MB has reduced penetration which may result from presence of proteins derived from gingival crevicular fluid and saliva [19]. Other possibilities are that MB and toluidine blue are substrates of multidrug resistance pumps existing in bacteria [21] or that microorganisms organized in a biofilm are able to exist in a slow-growing or starved state [22]. It was shown in some publications, that the degree of photo-inactivation was dependent upon the time of the irradiation [9,12]. One test arrangement showed a statistically significant decrease of bacteria (P.g.) incubated by methylene blue only with no light application [9]. Furthermore, the kind of light activation, concentration of photosensitizer and pathogen treated will also influence the results.
In vitro test arrangements have led to significant advances in the study of dental biofilm, but their results cannot be imported into clinical situations [23]. In vitro models often involve a small number of species of microorganisms, and are performed under laboratory conditions that cannot reproduce the physiologic situation [24]. Several factors such as salivary flow, the capacity of antimicrobial substances to adhere to the teeth or soft tissues, and the interactions of non-cultivable bacteria cannot be reflected in an in vitro set-up [25]. Anyhow it is important to test the validity of any medical procedure before applying it in clinic. This review focused on in vitro studies investigating the antimicrobial efficacy of photodynamic therapy. Nevertheless, a rising number of clinical studies are available which focus on PDT as an adjunctive therapy modality to periodontal treatment in vivo. A systematic review by Sgolastra and co-workers [26] investigated the potential of antimicrobial photodynamic therapy adjunctive to scaling and root planning in patients with chronic periodontitis. A meta-analysis was conducted to evaluate any clinical adjunctive effect of PDT to SRP when compared to SRP alone or in combination with placebo. Fourteen randomized clinical trials, which were published between 2007 and 2012, were included. The results of the latter review indicated, that the adjunctive use of PDT to SRP could provide additional benefits when compared to SRP alone, in terms of probing depth reduction and clinical attachment level gain. However, these clinical improvements, although statistically significant, proved not to be relevant in terms of clinical applicability. Further, they were only observed at the 3-month-follow up, whereas no significant differences were found after 6 months [26]. Another systematic review corroborated these findings and also showed an additional benefit regarding the treatment of chronic periodontitis with regard to probing depth and clinical attachment level gain [27]. However, only four studies were included in this meta-analysis.
In summary, well-controlled in vitro studies as well as randomized clinical trials are necessary to determine whether this adjunctive treatment provides significant additional benefits to periodontal therapy.
3. Experimental Section
3.1. Search Strategy
The electronic databases CINAHL, Cochrane, Medline, Scopus and WoK were searched for studies published up to and including Mai 2015. The search was limited to laboratory (in vitro) studies on photodynamic therapy (PDT) that tested the antimicrobial effect to strains of periodontopathogenic bacteria. No language or time restrictions were applied.
The following strategy was applied: ((photodynam* or photocem*) NEAR/3 (therapy or treatment or intervention or effect)) OR (photochemotherapy or phototherapy) OR (photodynam* or photochem), (dent* or oral or periodont*), (periodont* NEAR 3 (disease or pocket)) OR ((attachment or “alveolar bone”) NEAR/3 (loss or level)) OR (periodontitis or (pocket NEAR/3 depth)), (therapy or treatment or intervention), (root NEAR/3 planing) OR ((dental or periodontal) NEAR/3 (scaling or debridement)) OR (calculus NEAR/3 (remov* or debridement)), (disinfection or antimicrobial or anti-microbial or “anti microbial” or bactericidal or bacteriostatic or anti-infective or antiinfective or “anti infective”) OR ((kill* or inactivat* or inhibit* or block* or viability) NEAR/15 bacteria*), and (“in vitro” or “ex vivo” or experimental or laboratory) OR ((cell* or strain or bacteria or colony) NEAR/10 (grow* or culture* or count* or plate*)). After title and abstract screening, an additional hand search was performed in the reference lists of all reviews and full texts of interest.
3.2. Eligibility Criteria for Studies
Studies were only included if they had an in vitro-design, if they used methylene blue or toluidine blue as a dye. Furthermore, we decided to include only studies with an irradiation time of 60 seconds, because most of the studies, which were included in the fulltext analysis, used only this irradiation time. Thereby, a better comparability of the results in this review should to be achieved. With regard to the selected microorganisms, only studies were included that investigated the effect of PDT on Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Fusobacterium nucleatum.
3.3. Outcome Measures
The main focus of this study was to critically assess the in vitro antimicrobial efficacy of photodynamic therapy focusing on the periodontopathogenic bacteria Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Fusobacterium nucleatum. The focused question was: “Is it possible to decrease (at least 3 log steps or 99.9%) or even eliminate bacterial growth by photodynamic therapy in vitro when compared to untreated control groups or control groups treated by placebo?”
3.4. Study Selection
First, three reviewers (Philippe A. Haag, Valerie Steiger-Ronay, Patrick R. Schmidlin) independently screened all titles and abstracts of the electronic search and assessed them for possible inclusion in the review. Thereafter, all full texts of potentially eligible studies were assessed. Any disagreement concerning inclusion was resolved by discussion.
3.5. Analysis of the Results
Because it was not possible to extract all data out of the original papers, we tried to contact the corresponding authors per E-Mail asking for raw data. Where this was not possible, we took the values from the diagrams. So there may be a certain inaccuracy in the correspondent values. To present the results we created different tables. To analyze the results we converted the outcomes into different values. For each outcome we converted the percentage value to log reduction value or vice versa, if possible. The formulas for these conversions are the following:
The formula to convert percent reduction to log reduction is where P is the percent reduction and L is the log reduction. The formula to convert log reduction to percent reduction is where P is the percent reduction and L is the log reduction. In certain cases, we also needed to calculate the log reduction on the basis of existing values for viable microorganisms. The formula for this calculation is where A is the number of viable microorganisms before treatment and B is the number of viable microorganisms after treatment.
4. Conclusions
This review of the literature does not allow drawing any concrete conclusions regarding the efficacy of PDT due to the small number of included studies and the very different test arrangements. Although PDT seems to be a promising option for reducing the quantity of periodontopathogenic bacteria in combination with conventional therapy modalities. It would be desirable to develop methods they are able to get along with thicker, well organized biofilm. Further, particularly more comparable, studies are needed to evaluate if PDT can be clinically successful as an adjuvant in periodontal therapy.
Author Contributions
All authors contributed to varying degrees to the production of the review. Philippe A. Haag: Literature search and screening, writing the paper, references. Valerie Steiger-Ronay: Literature search and screening. Patrick R. Schmidlin: Literature search and screening, study conception and supervision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Teles R.P., Haffajee A.D., Socransky S.S. Microbiological goals of periodontal therapy. Periodontology. 2006;42:180–218. doi: 10.1111/j.1600-0757.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan S.A., Robertson P.B. Calculus removal by scaling/root planing with and without surgical access. J. Periodontol. 1987;58:159–163. doi: 10.1902/jop.1987.58.3.159. [DOI] [PubMed] [Google Scholar]
- 3.Quirynen M., Mongardini C., Pauwels M., Bollen C.M., van Eldere J., van Steenberghe D. One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. Ii. Long-term impact on microbial load. J. Periodontol. 1999;70:646–656. doi: 10.1902/jop.1999.70.6.646. [DOI] [PubMed] [Google Scholar]
- 4.Soukos N.S., Ximenez-Fyvie L.A., Hamblin M.R., Socransky S.S., Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob. Agents Chemother. 1998;42:2595–2601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharman W.M., Allen C.M., van Lier J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today. 1999;4:507–517. doi: 10.1016/S1359-6446(99)01412-9. [DOI] [PubMed] [Google Scholar]
- 6.Meisel P., Kocher T. Photodynamic therapy for periodontal diseases: State of the art. J. Photochem. Photobiol. B. 2005;79:159–170. doi: 10.1016/j.jphotobiol.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Muller P., Guggenheim B., Schmidlin P.R. Efficacy of gasiform ozone and photodynamic therapy on a multispecies oral biofilm in vitro. Eur. J. Oral Sci. 2007;115:77–80. doi: 10.1111/j.1600-0722.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- 8.Braham P., Herron C., Street C., Darveau R. Antimicrobial photodynamic therapy may promote periodontal healing through multiple mechanisms. J. Periodontol. 2009;80:1790–1798. doi: 10.1902/jop.2009.090214. [DOI] [PubMed] [Google Scholar]
- 9.Chan Y., Lai C.H. Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med. Sci. 2003;18:51–55. doi: 10.1007/s10103-002-0243-5. [DOI] [PubMed] [Google Scholar]
- 10.Street C.N., Pedigo L.A., Loebel N.G. Energy dose parameters affect antimicrobial photodynamic therapy-mediated eradication of periopathogenic biofilm and planktonic cultures. Photomed. Laser Surg. 2010;28(Suppl. S1):S61–S66. doi: 10.1089/pho.2009.2622. [DOI] [PubMed] [Google Scholar]
- 11.Goulart Rde C., Thedei G., Jr., Souza S.L., Tedesco A.C., Ciancaglini P. Comparative study of methylene blue and erythrosine dyes employed in photodynamic therapy for inactivation of planktonic and biofilm-cultivated aggregatibacter actinomycetemcomitans. Photomed. Laser Surg. 2010;28(Suppl. S1):S85–S90. doi: 10.1089/pho.2009.2698. [DOI] [PubMed] [Google Scholar]
- 12.Alvarenga L.H., Prates R.A., Yoshimura T.M., Kato I.T., Suzuki L.C., Ribeiro M.S., Ferreira L.R., Pereira S.A., Martinez E.F., Saba-Chujfi E. Aggregatibacter actinomycetemcomitans biofilm can be inactivated by methylene blue-mediated photodynamic therapy. Photodiagn. Photodyn. Ther. 2015;12:131–135. doi: 10.1016/j.pdpdt.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Eick S., Markauskaite G., Nietzsche S., Laugisch O., Salvi G.E., Sculean A. Effect of photoactivated disinfection with a light-emitting diode on bacterial species and biofilms associated with periodontitis and peri-implantitis. Photodiagn. Photodyn. Ther. 2013;10:156–167. doi: 10.1016/j.pdpdt.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Rajesh S., Koshi E., Philip K., Mohan A. Antimicrobial photodynamic therapy: An overview. J. Indian Soc. Periodontol. 2011;15:323–327. doi: 10.4103/0972-124X.92563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soukos N.S., Goodson J.M. Photodynamic therapy in the control of oral biofilms. Periodontology. 2011;55:143–166. doi: 10.1111/j.1600-0757.2010.00346.x. [DOI] [PubMed] [Google Scholar]
- 16.Wilson M., Dobson J., Harvey W. Sensitization of oral bacteria to killing by low-power laser radiation. Curr. Microbiol. 1992;25:77–81. doi: 10.1007/BF01570963. [DOI] [PubMed] [Google Scholar]
- 17.Wilson M., Dobson J. Lethal photosensitization of oral anaerobic bacteria. Clin. Infect. Dis. 1993;16(Suppl. S4):S414–S415. doi: 10.1093/clinids/16.Supplement_4.S414. [DOI] [PubMed] [Google Scholar]
- 18.Usacheva M.N., Teichert M.C., Biel M.A. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg. Med. 2001;29:165–173. doi: 10.1002/lsm.1105. [DOI] [PubMed] [Google Scholar]
- 19.Fontana C.R., Abernethy A.D., Som S., Ruggiero K., Doucette S., Marcantonio R.C., Boussios C.I., Kent R., Goodson J.M., Tanner A.C., et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J. Periodontal Res. 2009;44:751–759. doi: 10.1111/j.1600-0765.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tegos G.P., Hamblin M.R. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob. Agents Chemother. 2006;50:196–203. doi: 10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown M.R., Allison D.G., Gilbert P. Resistance of bacterial biofilms to antibiotics: A growth-rate related effect? J. Antimicrob. Chemother. 1988;22:777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- 23.Palmer R.J., Jr. Supragingival and subgingival plaque: Paradigm of biofilms. Compend. Contin. Educ. Dent. 2010;31:104–106. [PubMed] [Google Scholar]
- 24.Auschill T.M., Hellwig E., Sculean A., Hein N., Arweiler N.B. Impact of the intraoral location on the rate of biofilm growth. Clin. Oral Investig. 2004;8:97–101. doi: 10.1007/s00784-004-0255-6. [DOI] [PubMed] [Google Scholar]
- 25.Al-Ahmad A., Wunder A., Auschill T.M., Follo M., Braun G., Hellwig E., Arweiler N.B. The in vivo dynamics of streptococcus spp., actinomyces naeslundii, fusobacterium nucleatum and veillonella spp. in dental plaque biofilm as analysed by five-colour multiplex fluorescence in situ hybridization. J. Med. Microbiol. 2007;56:681–687. doi: 10.1099/jmm.0.47094-0. [DOI] [PubMed] [Google Scholar]
- 26.Sgolastra F., Petrucci A., Severino M., Graziani F., Gatto R., Monaco A. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2013;40:514–526. doi: 10.1111/jcpe.12094. [DOI] [PubMed] [Google Scholar]
- 27.Atieh M.A. Photodynamic therapy as an adjunctive treatment for chronic periodontitis: A meta-analysis. Lasers Med. Sci. 2010;25:605–613. doi: 10.1007/s10103-009-0744-6. [DOI] [PubMed] [Google Scholar]