Abstract
All kinetoplastid parasites, including protozoa such as Leishmania species, Trypanosoma brucei, and Trypanosoma cruzi that cause devastating diseases in humans and animals, are flagellated throughout their life cycles. While flagella were originally thought of primarily as motility organelles, flagellar functions in other critical processes, especially in sensing and signal transduction, have become more fully appreciated in the recent past. The flagellar membrane is a highly specialized subdomain of the surface membrane, and flagellar membrane proteins are likely to be critical components for all the biologically important roles of flagella. In this review, we summarize recent discoveries relevant to flagellar membrane proteins in these parasites including the identification of such proteins, investigation of their biological functions, and mechanisms of selective trafficking to the flagellar membrane. Prospects for future investigations and current unsolved problems are highlighted.
Keywords: Flagellar membrane, kinetoplastid parasites, environmental sensing, transporters, channels, receptors, subcellular targeting
Introduction
Kinetoplastid parasites are flagellated protozoa that cause a variety of diseases of global importance that afflict millions of people. They include Trypanosoma brucei, the causative agent of African sleeping sickness, Trypanosoma cruzi, which causes Chagas' disease, and various species of Leishmania that cause a range of diseases collectively referred to as the leishmaniases (44). These parasites exist in multiple morphologically and biochemically distinct life cycle forms, some of which populate the insect vectors responsible for transmission between hosts and other of which infect the mammalian host. Molecular, cellular, and biochemical studies on these parasites have begun to reveal biological processes that mediate infection, persistence, and pathogenesis, whereas probing of the basic life processes in these eukaryotic microorganisms have unveiled a range of novel biological phenomena, including genetically controlled antigenic variation (24), massive RNA editing of mitochondrial transcripts (21), trans-splicing of mRNAs (54), tethering of membrane proteins via glycosylphosphatidyl inositol anchors (16), etc. One common feature of kinetoplastid protozoa is that they are all flagellated. Flagella may exist as extended, motile structures, as they do in insect stage promastigotes of Leishmania and all life cycle stages of T. brucei, or they may be present as short, non-motile structures that barely emerge from the cell body, as in the case of the intracellular amastigote stages of T. cruzi and Leishmania species (Fig. 1) that reside in the mammalian host. Considerable interest has emerged regarding flagella and cilia, similar subcellular structures that differ largely in length and are operationally considered to be the same class of ‘organelle’, with both terms being used interchangeably. In particular, the role of cilia and flagella as sensory organelles or antennae that monitor the extracellular environment and transfer information to the cell interior has been the focus of studies in many eukaryotes, from single cell algae such as Chlamydomonas reinhardtii to differentiated mammalian cells (2, 3). These sensory functions are typically mediated by flagellar or ciliary membrane proteins such as receptors, channels, or transporters (51). Thus ciliary membranes can be thought of as highly differentiated domains of the plasma membrane that are specialized for various biological functions, especially signal transduction. Hence, an emerging highly active area of cell biology focuses upon understanding such ciliary proteins, their modes of action, and the cellular trafficking processes that selectively target such proteins to the membranes of cilia or flagella (39).
Fig 1.
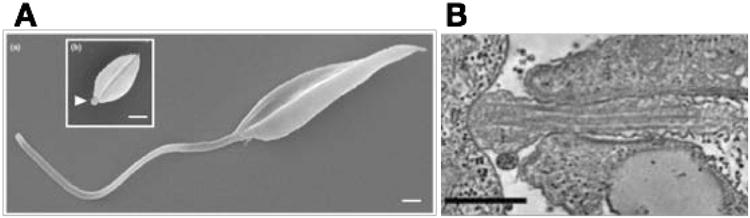
A) Scanning electron micrographs of a promastigote (a) and an amastigote (b) of Leishmania mexicana. The arrowhead in (b) shows the short amastigote flagellum just emerging from the cell body. Scale bars represent 1 μm. Image reproduced from reference (18) with permission. B) Transmission electron micrograph of the synapse between the flagellum of a L. mexicana amastigote (right side of image) and the phagolysomal vesicle of the host macrophage (left side of image). Scale bar represents 500 nm. Reproduced from reference (19) with permission.
Parasitic protozoa experience widely differing environments as they transit from invertebrate vectors into mammalian hosts. Successful colonization of vertebrate and invertebrate hosts depends upon the ability of the parasite to sense its environment and to respond accordingly, typically including differentiation into a distinctive life cycle form. Nonetheless, mechanisms for environmental sensing are generally poorly understood in eukaryotic parasites, especially among the kinetoplastid protozoa that lack many of the hallmarks of signal transduction found in other organisms, including heterotrimeric G proteins, G protein coupled receptors, and many classes of membrane bound kinases (29). Hence, the potential of flagella to serve sensory roles, in addition to other functions such as motility and tethering, has elicited increasing interest in these organelles, especially as studying these subcellular structures may help to deconvolute the currently obscure mechanisms whereby these parasites sense their environments.
In this review, we discuss recent studies on specific membrane proteins with demonstrated localization to flagellar membranes in the three kinetoplastid parasites mentioned above. In addition, we consider biological roles these flagellar membrane proteins may be playing, and we discuss potential mechanisms for selectively trafficking these proteins to the flagellar membrane.
Identification of Individual Flagellar Membrane Proteins
The first flagellar membrane protein to be identified among the kinetoplastid protozoa was a flagellar Ca2+ binding protein, FCaBP, from T. cruzi (15). Originally identified in cDNA expression libraries using sera from infected animals or humans, the protein showed homology to ‘EF hand’ Ca2+ binding proteins from other eukaryotes and exhibited Ca2+ binding activity when expressed in bacteria. FCaBP was dually labeled with myristate and palmitate at the second and fourth amino acids of its coding sequence (20), and this dual fatty acid modification was essential for trafficking to the flagellum. Furthermore, FCaBP associated with membranes in the presence of Ca2+ but was released into the cytosolic fraction when Ca2+ was removed from the preparations. This behavior suggested that FCaBP may act like the recoverin family of acylated proteins that participate in signal transduction in the vertebrate visual system, where Ca2+ bound recoverins associate with retinal rod cell membranes and inhibit rhodopsin kinase but dissociate from both the membrane and the kinase when Ca2+ levels drop. Furthermore, Ca2+ binds to multiple EF hand sites on FCaBP and induces conformational changes (6), suggesting that this protein functions as a calcium sensor. However, neither the upstream signals nor the downstream readout have been defined for FCaBP, so its specific role in signal transduction is still speculative.
An orthologous family of proteins, the calflagins, were identified in T. brucei as proteins that bound to a hydrophobic resin in a calcium-dependent manner (64, 63) and that trafficked to the flagellar membrane. RNAi mediated knockdowns of the entire calflagin family increased survival of mice infected with these parasites and resulted in a precipitous drop in parasitemia, compared to wild type T. brucei (12). Hence, although the specific biochemical functions of the calflagins are not known, they do play a role in supporting infection and pathogenesis.
Another dually acylated flagellar membrane protein, SMP-1, was identified in Leishmania major (59), initially as a protein that associated with detergent-resistant membranes. Immunofluorescence with polyclonal anti-SMP-1 antibodies exhibited flagellar localization and expression in insect stage promastigotes but not in intracellular amastigotes. The inner leaflet of the flagellar membrane is coated with SMP-1 homo-oligomers, an interaction which stabilizes this sterol- and sphingolipid-rich membrane and is required for flagellar membrane elongation during the amastigote to promastigote transition, as demonstrated using SMP-1 null mutants and complemented add-back lines (58).
Another flagellar membrane protein identified in early studies was ESAG-4 (41), a bloodstream stage specific protein with a functional adenylate cyclase domain located on the cytosolic side of the membrane and a large extracellular domain that may possess receptor activity. ESAG-4, and several related adenylate cyclases, were localized to the flagellar membrane by both light and electron microscopy. While no putative ligands for ESAG-4 have been identified and a specific role in sensing is thus obscure, these early results suggested a likely role for the trypanosome flagellum in sensing. Subsequent studies using a dominant negative mutant of ESAG-4 revealed an intriguing role for ESAG-4 in virulence (50). Adenylate cyclase (AC) activity, potentially arising form trypanosomes phagocytosed by liver myeloid cells, activates myeloid cell protein kinase A and downregulates the synthesis of TNF-α, a key innate immune response associated with the control of trypanosome infections. Hence, at least one role of ESAG-4 appears to control release of host TNF-α and thus to promote successful infection. In addition, a role for ESAG-4 and related ACs has been suggested in the control of cytokinesis in bloodstream trypanosomes (49), as RNAi mediated knockdown of these mRNAs leads to a cytokinesis defect.
Other members of the large family of receptor-like ACs of T. brucei have been studied by Hill and colleagues, focusing upon ACs that are upregulated in the insect stage procyclic trypanosomes (48). Intriguingly, different members of the family localized to distinct subdomains of the flagellar membrane. Whereas some AC isotypes were distributed along the entire length of the flagellum, others were restricted to the flagellar tip, emphasizing that the flagellar membrane consists of distinct subdomains. These results suggest that localized gradients of cAMP may be important in distinct processes governed by these ACs. Of further interest, the RNAi mediated knockdown of certain ACs resulted in hyperactive ‘social motility’ or the ability of procyclic trypanosomes to aggregate into multicellular groups and to coordinate movement over a solid surface (34). Recent evidence indicates that social motility is restricted to early but not late procyclic form trypanosomes and may be related to movement of trypanosomes to different sites within the Tsetse fly vector (26); hence, flagellar ACs may play important roles in vector colonization.
A third example of flagellar membrane proteins that emerged from early studies was a flagellar glucose transporter, initially identified in L. enriettii (43) and subsequently studied more extensively in L. mexicana (8), where it was designated GT1 to distinguish it from two related glucose transporters, GT2 and GT3, that were targeted to the plasma membrane surrounding the cell body but were excluded from the flagellar membrane (Fig. 2A,B). The three GT isoforms had similar substrate specificities and transport kinetics (46), and the largest region of sequence divergence was in the hydrophilic N-terminal domain, located in the cytosol, that was of extended length and unique sequence in GT1 (43, 8). GT1 is expressed and targeted to the extended flagella of promastigotes, but it was not detectable in intracellular amastigotes, which only possess a short, non-motile flagellum (45). Furthermore, expression of the GT1 protein is glucose sensitive, being more robustly expressed in glucose-starved parasites compared to promastigotes grown in glucose-replete medium. The lower levels of expression of GT1 in glucose-replete promastigotes and in amastigotes, compared to glucose-starved promastigotes, is due to relative destabilization of the protein (45). Of particular interest, Δgt1 null mutants exhibited a striking growth phenotype. Whereas wild type promastigotes transitioned from logarithmic to stationary phase, with a stable plateau in cell number at stationary phase, Δgt1 null mutants grew to a higher maximal density than wild type parasites but then underwent a catastrophic loss of viability when they depleted glucose from the medium.
Fig 2.
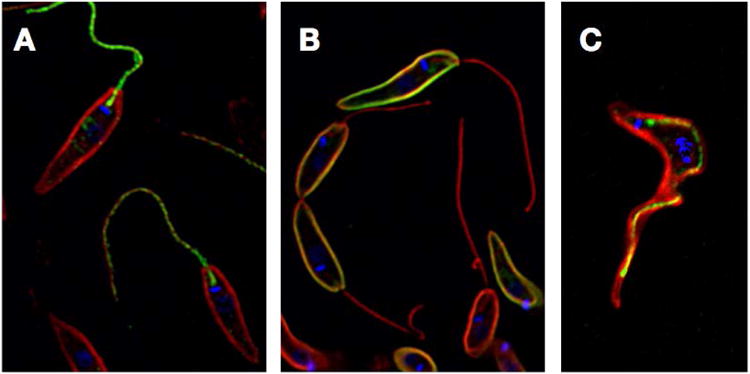
Immunofluorescence images of L. mexicana promastigotes expressing A) GT1 fused to GFP at its C-terminus, B) GT2 fused to GFP at its C-terminus. GFP (green) and α-tubulin (red, flagellar axoneme and subpellicular microtubules) were detected with antibodies directed against each protein. Blue represents DAPI staining of kinetoplast DNA. C) Immunofluorescence image of a bloodstream form T. brucei expressing the FS179 putative Ca2+ channel tagged at its C-terminus with a triple hemagglutinin epitope (green) and stained with antibody against α-tubulin (red) and DAPI (blue).
A number of these observations suggest that GT1 may be functioning as a glucose sensor in promastigotes. First, the established role of cilia and flagella as sensory organelles (51, 2, 3) elicits a suspicion that a flagellar membrane protein may be functioning in a sensory capacity. Second, various transporters or transporter-like proteins in a range of organisms have been demonstrated to function as sensors for their ligands, and this class of transporters/receptors has been named ‘transceptors’ (55). Transceptors are typically expressed at low levels, contain extended N- or C-terminal hydrophilic domains, and their levels of expression are often regulated by their ligands, all properties exhibited by GT1. The fact that the Δgt1 null mutants are unable to successfully negotiate the transition from logarithmic to stationary phase and that catastrophic loss of viability sets in when glucose is depleted suggests a natural biological function for GT1. This permease could sense glucose depletion and mediate a transition from active growth to the non-dividing state characteristic of stationary phase, a glucose-dependent switch that would be absent in the null mutants. Hence, GT1 may function as a transceptor that initiates a signal cascade to transition from growth to stationary phase when glucose concentrations become low. Alternatively, GT1 could play a sensory role by simply importing glucose into the flagellum where some internal flagellar protein senses the glucose level and sends a signal. Distinguishing between these two models will require further studies, particularly determining whether GT1 might interact physically with other proteins that mediate a signal transduction cascade, as has been established for the yeast glucose proteins Mth1 and Std1 (28) that bind respectively to the extended C-terminal tails of the Snf3 and Rgt2 glucose transceptors and initiate cascades that control transcription of bona fide glucose transporters.
Aquaporins and aquaglyceroporins are channels that mediate the flux of water, glycerol, and various other polar small molecules across the plasma membranes of cells. The aquaglyceroporin AQP1 from L. major attracted interest, since it is a major route for uptake into these parasites of Sb(III), the therapeutically active pharmacological agent derived from the antileishmanial antimonial drugs Pentostam and Glucantime (22). Immunofluorescence and immunoelectron microscopy using an antibody against AQP1 revealed that this channel is localized almost exclusively to the flagellar membrane in promastigotes and to the flagellar pocket and contractile vacuole in intracellular amastigotes (17). Overexpression of AQP1 in promastigotes promoted enhanced ability of the parasites to regulate cell volume in response to hypoosmotic stress and to migrate more rapidly towards an osmotic gradient. Hence, this flagellar permease is involved in volume regulation and osmotaxis in addition to solute transport. These results also support the role of the flagellum in interaction with, sensing of, and response to the extracellular environment. Notably, when AQP1 was phosphorylated on T197 by the parasite mitogen activated kinase 2, MPK2, it relocalized from the flagellum to the pellicular plasma membrane surrounding the promastigote cell body (35). Parasites in which this phosphorylation had occurred also had more rapid volume regulation in response to hypoosmotic stress, and AQP1 accumulated to a higher level due to stabilization against degradation. Hence, flagellar localization can be modulated by post-translational modification, at least in this case.
Some years ago, a T. cruzi epimastigote glycoprotein designated GP72 was shown by gene knockouts to be critical for attachment of the flagellum to the cell body (9). This protein was subsequently localized to the parasite surface, including the flagellum (23). Further work on the T. brucei ortholog of GP72, designated FLA1, showed that it is localized in the cell body membrane component of the flagellar attachment zone (FAZ) (32), an extended zone of adhesion between one side of the flagellar membrane and the plasma membrane of the cell body that has been characterized in bloodstream trypanosomes (31) (Fig. 3). FLA1 is also necessary for adhesion of the T. brucei flagellum to the cell body along most of its length. The FLA1 binding protein, FLA1BP, has recently been identified as a component of the flagellar membrane half of the FAZ that binds to FLA1 and maintains the flagellar-cell body attachment (53). Since the FAZ is a complex structure, there are likely multiple components in the flagellar membrane that are part of this adhesion zone, including the putative Ca2+ channel mentioned below (40).
Fig 3.
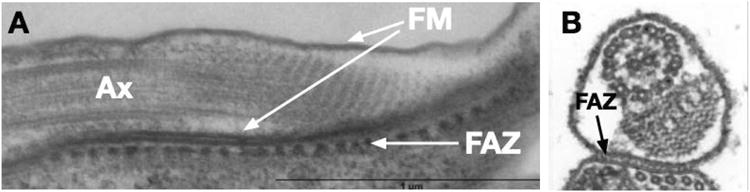
Transmission electron micrographs showing the Flagellar Attachment Zone (FAZ) in T. brucei bloodstream form parasites. A) Transverse section showing the flagellar axoneme (Ax), the flagellar membrane (FM) on both sides of the flagellum, and the FAZ that mediates attachment of the FM to the plasma membrane of the cell body. Repetitive electron dense structures that are not microtubules can be seen in the FAZ. Image reproduced from reference (7) with permission. B) A cross section of the flagellum showing its attachment to the cell body via the FAZ. Reproduced from reference (1) with permission.
Several membrane proteins have been localized to the distal tip of the flagellum, including a putative cation/proton antiporter on T. brucei (25) and a cation channel, TcCat, from T. cruzi that localizes to an intracellular spot in trypomastigotes but migrates to the flagellar tip when these life cycle forms are treated with acid pH to induce incipient differentiation into amastigotes (27). Along with the observation of an AC at the flagellar tip cited above, these results suggest that this domain of the flagellar membrane is highly differentiated and may play a role in sensing and signaling.
Global Analysis of Flagellar Membrane Proteomes in African Trypanosomes
A significant advance in identification of flagellar membrane proteins in African trypanosomes was achieved by the Hill laboratory by applying mass spectrometry to large scale identification of such components (40). While flagella had previously been isolated from bloodstream trypanosomes, the procedure required removal of the surface membrane. In this study, the authors developed a procedure for physical isolation of trypanosome flagella with intact membranes by first inducing RNAi knockdown of Fla1 mRNA. The consequent reduction in FLA1 protein produced parasites with detached flagella that could be readily removed by shearing and then purified by sucrose density gradient centrifugation. Prior to flagellar isolation, parasites were surface labeled with biotin so that the flagellar surface proteins could subsequently be purified by streptavidin affinity chromatography and identified by multidimensional protein identification technology. This process led to identification of 158 potential flagellar surface proteins representing a wide variety of species, most of which were predicted to be integral membrane proteins. Notable among the surface components were many proteins likely to play roles in signaling and sensing, such as ACs with extracellular domains that exhibited similarity to bacterial periplasmic binding proteins, probable receptor kinases, a phosphoglycerate kinase, a protein with an EGF-like binding domain, a putative Ca2+ channel named FS179, and a Ca2+-ATPase. Localization of epitope tagged versions of several candidate proteins identified different patterns of flagellar membrane distribution. The Ca2+ channel was located on the flagellar side of the FAZ ((40) and Fig. 2C), whereas several other proteins like the Ca2+- ATPase exhibited punctate staining over the flagellum.
An extensive proteomic study of isolated flagella from procyclic form T. brucei (52) exhibited very little overlap with the surface proteome of bloodstream trypanosomes. However, this study did identify an adenosine kinase as being localized to the procyclic form flagellar membrane.
Trafficking of Proteins to the Flagellar Membrane
One fundamental problem of great interest is to understand how flagellar and ciliary membrane proteins are selectively trafficked to the relevant membrane system, and much work has been devoted to this problem using mammalian cells and several model eukaryotes (39). A number of protein complexes, such as the BBSome and Intraflagellar Transport Proteins, have been implicated in transport of ciliary membrane proteins. Paralogs of many of these proteins exist among the Kinetoplastids, but for the most part, their potential roles in trafficking of flagellar membrane proteins remain to be assessed. However, some insights into flagellar trafficking of several kinetoplastid membrane proteins have emerged from studies with these proteins. An earlier review by Engman and colleagues (13) discussed flagellar targeting of kinetoplastid membrane proteins as of 2010.
Studies on the T. cruzi FCaBP demonstrating that dual palmitoylation and myristoylation near the N-terminus are necessary for membrane association of this protein (20) suggested these fatty acids might also play a role in flagellar targeting. Flagellar membranes are enriched in sterol- and sphingolipid-rich rafts, and dually acylated FCaBP isolated from T. cruzi epimastigotes is found in detergent-resistant membranes and floats to low-density regions of discontinuous density gradients (60), confirming that it is associated with rafts. Hence, one initial idea was that FCaBP might spontaneously partition into the flagellar membrane due to the raft-rich properties of that organelle. However, several observations now suggest that flagellar trafficking is more complicated. Fusion proteins containing either the first 12 or first 24 amino acids of FCaBP fused to GFP were both dually acylated. However, only the 24 amino acid fusion mediated flagellar localization of GFP, indicating that sequences with the 13-24 amino acid segment were required for flagellar targeting. Three lysine residues at positions 13, 19, and 22 were conserved between T. cruzi FCaBP and the T. brucei calflagins and hence were interrogated by site-directed mutagenesis for a potential role in flagellar targeting. A mutant in which all three lysines were replaced with alanines did not target to the flagellar membrane, confirming the importance of these residues for flagellar targeting. The crystal structure of FCaBP (61) shows that these lysines form a cluster of positive charges on a face of FCaBP, and it was suggested that these residues could promote electrostatic interactions with negatively charged head groups on the inner leaflet of the flagellar membrane, thus dictating flagellar association. However, an alternative model could be that these residues mediate interaction with another protein or proteins that actively traffic FCaBP from the adjacent flagellar pocket into the flagellar membrane. A second indication that dual acylation is insufficient to explain flagellar membrane targeting is the observation that other dually acylated proteins do not traffic to this organelle. In particular, the dually acylated HASPB protein of L. major is transported to the entire cell surface (11) using a non-classical pathway for trafficking across the membrane during biosynthesis, another dually acylated protein phosphatase of L. major (38) is localized in the endomembrane system of the parasite, and a myristoylated and palmitoylated phosphatidylinositol phospholipase C from T. cruzi is expressed on the extracellular surface of amastigotes (10, 36).
Dissection of flagellar trafficking of GT1 from L. mexicana has led to discovery of novel flagellar trafficking machinery that is likely to be operational among other kinetoplastid parasites. GT1 is closely related in sequence to two other glucose transporter isoforms, GT2 and GT3, which do not traffic to the flagellum (8). The principal sequence differences between GT1 and the non-flagellar isoforms is the unique 130 amino acid N-terminal hydrophilic domain of GT1 that is cytosolically oriented. Deletion and site-directed mutagenesis of this N-terminal domain (56) localized a segment from amino acids 84-100 that was critical for flagellar targeting, and alanine scanning mutagenesis identified three adjacent amino acids within this segment, N95-P96-M97, whose mutation strongly impaired flagellar targeting and caused the transporter to arrest in the flagellar pocket. Computational modeling of the N-terminal domain suggested that this NPM motif was localized on the solvent accessible surface of the domain where it could interact with other partner proteins. Subsequently, formaldehyde crosslinking followed by tandem affinity purification/mass spectrometry identified a novel kinetoplastid-specific protein, designated KHARON1, which interacted with the NPM-containing segment of the GT1 N-terminus (57). Genetic deletion of the Kharon1 gene in promastigotes produced a Δkharon1 null mutant in which the GT1-GFP fusion protein arrested in the flagellar pocket. Epitope tagged KHARON1 localized to the base of the flagellar axoneme, as determined by immunoelectron microscopy, where it could potentially mediate trafficking of GT1 from the adjacent flagellar pocket membrane into the flagellar membrane. KHARON1 also associated with the subpellicular microtubules underneath the pellicular plasma membrane and is thus a cytoskeletally tethered protein in both locations.
The Δkharon1 promastigotes were not impaired in growth and were able to enter macrophages and transform into amastigotes, but these intracellular parasites died over the course of seven days, implying an essential role for KHARON1 in infectious amastigotes. These null mutants were also completely avirulent in the BALB/c murine model of leishmaniasis, and they failed to undergo cytokinesis, forming multinucleate amastigotes (Tran, K.D. et al., manuscript submitted). It is not yet clear whether this lethal phenotype in amastigotes is due to the absence of KHARON1 at the base of the flagellum, on the subpellicular microtubules, or both. Preliminary data employing blue native gel electrophoresis (4) and biotin proximity labeling (47) also suggest that KHARON1 associates with multiple other subunits and thus probably functions as part of a complex. To date GT1 is the only membrane protein in L. mexicana known to depend upon KHARON1 for flagellar targeting, and neither AQP1 nor SMP-1 was mistargeted in the Δkharon1 null mutant. However, it seems likely that other cargo proteins exist for this flagellar targeting machinery.
Subsequent studies in African trypanosomes (unpublished data of M.A. Sanchez, K.D. Tran, and S.M. Landfear) have established that the T. brucei ortholog of KHARON1 is required for flagellar targeting of the putative Ca2+ channel, FS179, that targets to the flagellar side of the FAZ ((40) and Fig. 2C). RNAi-mediated knockdown of Kharon1 mRNA was also lethal to both bloodstream and procyclic form trypanosomes, resulting in multinucleate ‘monster cells’ that could not undergo cytokinesis. Hence, KHARON1 plays roles in flagellar targeting of distinct membrane proteins in both L. mexicana and T. brucei and is essential for the infectious stages of both life cycles. The well-established association of the lethal monster cell phenotype with defects in flagellar structure (5) suggests that KHARON1's role in targeting of flagellar membrane proteins is likely to explain at least part of this protein's critical function in trypanosomes.
Another component of flagellar membrane trafficking that is poorly understood is how different proteins localize to distinct subdomains of the flagellar membrane, but studies on differentially localized ACPs (adenylate cyclase proteins) from procyclic form T. brucei have begun to uncover some determinants (48). ACP1 that traffics to the distal tip of the flagellum and ACP2 that is evenly distributed along this organelle differ almost exclusively in the C-terminal domain, suggesting that this region might control flagellar targeting. Deletion mutants removing the C-terminal 45 and 46 amino acids respectively disrupted flagellar trafficking of both ACs, confirming that this sequence was important for routing these proteins to the flagellum. Furthermore among the 31 C-terminal residues that differed between ACP1 and ACP2 and therefore could control targeting to the flagellar tip, only five C-terminal residues were conserved between ACP1 and ACP4, another flagellar tip AC. Hence, these five residues are of particular interest for specifying suborganellar location.
Biological Functions of Flagella in Kinetoplastid Parasites
Flagella have long been appreciated to play important roles in motility and attachment to insect epithelia among the Kinetoplastida (33). However, other likely or potential roles have only been taken more seriously in the recent past. For instance, the short non-motile flagella of amastigotes perplexed many parasitologists and were often referred to pejoratively or dismissively as ‘residual’ organelles thought to exist only to allow outgrowth of a motile flagellum when the parasite transformed into a genuine ‘flagellated’ life cycle stage. A closer examination of these shorter relatives of motile flagella establishes that they have morphologies reminiscent of sensory cilia (19), such as the primary cilia present on many mammalian cells (2), and that they may thus be important in sensing the environment within the phagolysosomal vesicle in Leishmania amastigotes or the cytosol in T. cruzi. Furthermore, the striking attachment of the flagellar tip of L. mexicana amastigotes (Fig. 1B and reference (19)) to the phagolysomal membrane of the host macrophage in 65% of the amastigotes examined suggests that the flagellar tip may be especially important in sensing the state of the host cell.
Infectious microbes often secrete ‘effector’ proteins into their host cells to modulate their physiology and generate a response favorable to the pathogen, with some remarkable examples coming to light originally in bacteria (37) and subsequently in the parasitic protozoan Toxoplasma gondii (14). The flagellum has been suggested to be a secretory organelle, and recent studies on the green alga Chlamydomonas reinhardtii (62) have confirmed that vesicles harboring specific proteins are indeed delivered from the tip of the flagellum to the extracellular space. Hence, another potential role for the flagellum in kinetoplastid parasites could be to deliver parasite proteins to either the lumen of the phagolysome or the host cell cytosol, as articulated previously (18).
An additional potential role for the flagellar membrane is to mediate mating between parasites. Recent evidence indicates that African trypanosomes have haploid gamete-like forms that emerge in the tsetse fly salivary gland (42) and mate to form hybrid diploids. These gametes interact initially by tangling together their flagella prior to cell fusion. This arrangement is reminiscent of a similar well-characterized process in C. reinhardtii gametes, where binding of receptor proteins on the flagella of different mating types initiates mating and the remodeling of gene expression required for zygote formation (30).
Prospects for the Future
Significant progress has been made over the past decade or so regarding understanding functions of flagella in kinetoplastid parasites and in identifying specific membrane proteins that populate the surface of this organelle. Challenges for the future involve connecting individual flagellar membrane proteins with specific biochemical or biological functions. For receptors or transceptors, what are the ligands that initiate sensation, what are the pathways that mediate signal transduction, and what are the biological outputs of signaling? What other proteins interact with the primary sensors and constitute a cascade that elicits a particular biological response? Why are there so many ACs in T. brucei, ∼80 isoforms (49) only some of which are flagellar in location, and how do they differ in their functions? Finally, our understanding of selective subcellular targeting of flagellar membrane proteins is still in its infancy. There are likely to be multiple pathways for flagellar targeting that engage different subsets of flagellar membrane proteins. What are these pathways, what are the proteins and protein complexes that constitute such machinery, and how do these molecular machines achieve selective targeting to this organelle? These latter questions are important, because proper formation of the flagellum and flagellar membrane appears to be essential for viability of infectious life cycle stages of multiple kinetoplastid parasites. Overall, understanding how and to what extent the flagellar membrane mediates interactions with the extracellular environment is an important objective in the study of these parasites, especially since mechanisms of sensation and signaling are still so poorly understood and yet so central to the successful execution of the parasite life cycles.
Acknowledgments
This work was supported by NIH grants R01AI25920 and R21AI114822 to SML and NIH NRSA F32 fellowship (1F32AI096854) to KDT. We thank Darin Halkides with help in preparation of figures.
References
- 1.Bastin P, Sherwin T, Gull K. Paraflagellar rod is vital for trypanosome motility. Nature. 1998;391:548. doi: 10.1038/35300. [DOI] [PubMed] [Google Scholar]
- 2.Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci. 2010;123:505–509. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- 4.Braz VA, Howard KJ. Separation of protein oligomers by blue native gel electrophoresis. Anal Biochem. 2009;388:170–172. doi: 10.1016/j.ab.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, Portman N, Shaw MK, Ginger ML, Gaskell SJ, McKean PG, Gull K. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–227. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan KT, Ames JB, Asfaw SH, Wingard JN, Olson CL, Campana PT, Araujo AP, Engman DM. A flagellum-specific calcium sensor. J Biol Chem. 2005;280:40104–40111. doi: 10.1074/jbc.M505777200. [DOI] [PubMed] [Google Scholar]
- 7.Buisson J, Bastin P. Flagellum structure and function. Microbiology Monographs. 2010;17:63–86. [Google Scholar]
- 8.Burchmore RJS, Landfear SM. Differential regulation of multiple glucose transporter genes in the parasitic protozoan Leishmania mexicana. J Biol Chem. 1998;273:29118–29126. doi: 10.1074/jbc.273.44.29118. [DOI] [PubMed] [Google Scholar]
- 9.Cooper R, Ribeiro de Jesus A, Cross GAM. Deletion of an immunodominant Trypanosoma cruzi surface glycoprotein disrupts flagellum-cell adhesion. J Cell Biol. 1993;122:149–156. doi: 10.1083/jcb.122.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Paulo Martins V, Okura M, Maric D, Engman DM, Vieira M, Docampo R, Moreno SN. Acylation-dependent export of Trypanosoma cruzi phosphoinositide-specific phospholipase C to the outer surface of amastigotes. J Biol Chem. 2010;285:30906–30917. doi: 10.1074/jbc.M110.142190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denny PW, Gokool S, Russell DG, Field MC, Smith DF. Acylation-dependent protein export in Leishmania. J Biol Chem. 2000;275:11017–11025. doi: 10.1074/jbc.275.15.11017. [DOI] [PubMed] [Google Scholar]
- 12.Emmer BT, Daniels MD, Taylor JM, Epting CL, Engman DM. Calflagin inhibition prolongs host survival and suppresses parasitemia in Trypanosoma brucei infection. Eukaryot Cell. 2010;9:934–942. doi: 10.1128/EC.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J Cell Sci. 2010;123:529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.English ED, Adomako-Ankomah Y, Boyle JP. Secreted effectors in Toxoplasma gondii and related species: determinants of host range and pathogenesis? Parasite Immunol. 2015;37:127–140. doi: 10.1111/pim.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engman DM, Krause KH, Blumin JH, Kim KS, Kirchhoff LV, Donelson JE. A novel flagellar Ca+2-binding protein in trypanosomes. J Biol Chem. 1989;264:18627–18631. [PubMed] [Google Scholar]
- 16.Ferguson MAJ. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112:2799–2809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- 17.Figarella K, Uzcategui NL, Zhou Y, LeFurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol. 2007;65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- 18.Gluenz E, Ginger ML, McKean PG. Flagellum assembly and function during the Leishmania life cycle. Curr Opin Microbiol. 2010;13:473–479. doi: 10.1016/j.mib.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Gluenz E, Hoog JL, Smith AE, Dawe HR, Shaw MK, Gull K. Beyond 9+0: noncanonical axoneme structures characterize sensory cilia from protists to humans. Faseb J. 2010;24:3117–3121. doi: 10.1096/fj.09-151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godsel LM, Engman DM. Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J. 1999;18:2057–2065. doi: 10.1093/emboj/18.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Göringer HU. RNA editing in African trypanosomes: a u-ser's G-U-ide. Nucleic Acids Mol Biol. 2012;28:149–165. [Google Scholar]
- 22.Gourbal B, Sonuc N, Bhattacharjee H, Legare D, Sundar S, Ouellette M, Rosen BP, Mukhopadhyay R. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem. 2004;279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- 23.Haynes PA, Russell DG, Cross GA. Subcellular localization of Trypanosoma cruzi glycoprotein Gp72. J Cell Sci. 1996;109(Pt 13):2979–2988. doi: 10.1242/jcs.109.13.2979. [DOI] [PubMed] [Google Scholar]
- 24.Horn D. Antigenic variation in African trypanosomes. Mol Biochem Parasitol. 2014;195:123–129. doi: 10.1016/j.molbiopara.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang G, Ulrich PN, Storey M, Johnson D, Tischer J, Tovar JA, Moreno SN, Orlando R, Docampo R. Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells. PLoS Pathog. 2014;10:e1004555. doi: 10.1371/journal.ppat.1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imhof S, Knusel S, Gunasekera K, Vu XL, Roditi I. Social motility of African trypanosomes is a property of a distinct life-cycle stage that occurs early in tsetse fly transmission. PLoS Pathog. 2014;10:e1004493. doi: 10.1371/journal.ppat.1004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jimenez V, Docampo R. Molecular and electrophysiological characterization of a novel cation channel of Trypanosoma cruzi. PLoS Pathog. 2012;8:e1002750. doi: 10.1371/journal.ppat.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston M, Kim JH. Glucose as a hormone: receptor-mediated glucose sensing in the yeast Saccharomyces cerevisiae. Biochem Soc Trans. 2005;33:247–252. doi: 10.1042/BST0330247. [DOI] [PubMed] [Google Scholar]
- 29.Kramer S. Developmental regulation of gene expression in the absence of transcriptional control: the case of kinetoplastids. Mol Biochem Parasitol. 2012;181:61–72. doi: 10.1016/j.molbiopara.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Kurvari V, Grishin NV, Snell WJ. A gamete-specific, sex-limited homeodomain protein in Chlamydomonas. J Cell Biol. 1998;143:1971–1980. doi: 10.1083/jcb.143.7.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacomble S, Vaughan S, Deghelt M, Moreira-Leite FF, Gull K. A Trypanosoma brucei protein required for maintenance of the flagellum attachment zone and flagellar pocket ER domains. Protist. 2012;163:602–615. doi: 10.1016/j.protis.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaCount DJ, Barrett B, Donelson JE. Trypanosoma brucei FLA1 is required for flagellum attachment and cytokinesis. J Biol Chem. 2002;277:17580–17588. doi: 10.1074/jbc.M200873200. [DOI] [PubMed] [Google Scholar]
- 33.Langousis G, Hill KL. Motility and more: the flagellum of Trypanosoma brucei. Nat Rev Microbiol. 2014;12:505–518. doi: 10.1038/nrmicro3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez MA, Saada EA, Hill KL. Insect stage-specific adenylate cyclases regulate social motility in African trypanosomes. Eukaryot Cell. 2015;14:104–112. doi: 10.1128/EC.00217-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandal G, Sharma M, Kruse M, Sander-Juelch C, Munro LA, Wang Y, Vilg JV, Tamas MJ, Bhattacharjee H, Wiese M, Mukhopadhyay R. Modulation of Leishmania major aquaglyceroporin activity by a mitogen-activated protein kinase. Mol Microbiol. 2012;85:1204–1218. doi: 10.1111/j.1365-2958.2012.08169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martins Vde P, Galizzi M, Salto ML, Docampo R, Moreno SN. Developmental expression of a Trypanosoma cruzi phosphoinositide-specific phospholipase C in amastigotes and stimulation of host phosphoinositide hydrolysis. Infect Immun. 2010;78:4206–4212. doi: 10.1128/IAI.00473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattoo S, Lee YM, Dixon JE. Interactions of bacterial effector proteins with host proteins. Curr Opin Immunol. 2007;19:392–401. doi: 10.1016/j.coi.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Mills E, Price HP, Johner A, Emerson JE, Smith DF. Kinetoplastid PPEF phosphatases: dual acylated proteins expressed in the endomembrane system of Leishmania. Mol Biochem Parasitol. 2007;152:22–34. doi: 10.1016/j.molbiopara.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oberholzer M, Langousis G, Nguyen HT, Saada EA, Shimogawa MM, Jonsson ZO, Nguyen SM, Wohlschlegel JA, Hill KL. Independent analysis of the flagellum surface and matrix proteomes provides insight into flagellum signaling in mammalian-infectious Trypanosoma brucei. Mol Cell Proteomics. 2011;10:M111 010538. doi: 10.1074/mcp.M111.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paindavoine P, Rolin S, Van Assel S, Geuskens M, Jauniaux J, Dinsart C, Huet G, Pays E. A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of Trypanosoma brucei. Mol Cell Biol. 1992;12:1218–1225. doi: 10.1128/mcb.12.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peacock L, Bailey M, Carrington M, Gibson W. Meiosis and haploid gametes in the pathogen Trypanosoma brucei. Curr Biol. 2014;24:181–186. doi: 10.1016/j.cub.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piper RC, Xu X, Russell DG, Little BM, Landfear SM. Differential targeting of two glucose transporters from Leishmania enriettii is mediated by an NH2-terminal domain. J Cell Biol. 1995;128:499–508. doi: 10.1083/jcb.128.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues JC, Godinho JL, de Souza W. Biology of human pathogenic trypanosomatids: epidemiology, lifecycle and ultrastructure. Subcell Biochem. 2014;74:1–42. doi: 10.1007/978-94-007-7305-9_1. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Contreras D, Aslan H, Feng X, Tran K, Yates PA, Kamhawi S, Landfear S. Regulation and biological function of a flagellar glucose transporter in Leishmania mexicana: a potential glucose sensor. FASEB J. 2015;29:11–24. doi: 10.1096/fj.14-251991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Contreras D, Feng X, Keeney KM, Bouwer HG, Landfear SM. Phenotypic characterization of a glucose transporter null mutant in Leishmania mexicana. Mol Biochem Parasitol. 2007;153:9–18. doi: 10.1016/j.molbiopara.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196:801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saada EA, Kabututu ZP, Lopez M, Shimogawa MM, Langousis G, Oberholzer M, Riestra A, Jonsson ZO, Wohlschlegel JA, Hill KL. Insect stage-specific receptor adenylate cyclases are localized to distinct subdomains of the Trypanosoma brucei flagellar membrane. Eukaryot Cell. 2014;13:1064–1076. doi: 10.1128/EC.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salmon D, Bachmaier S, Krumbholz C, Kador M, Gossmann JA, Uzureau P, Pays E, Boshart M. Cytokinesis of Trypanosoma brucei bloodstream forms depends on expression of adenylyl cyclases of the ESAG4 or ESAG4-like subfamily. Mol Microbiol. 2012;84:225–242. doi: 10.1111/j.1365-2958.2012.08013.x. [DOI] [PubMed] [Google Scholar]
- 50.Salmon D, Vanwalleghem G, Morias Y, Denoeud J, Krumbholz C, Lhomme F, Bachmaier S, Kador M, Gossmann J, Dias FB, De Muylder G, Uzureau P, Magez S, Moser M, De Baetselier P, Van Den Abbeele J, Beschin A, Boshart M, Pays E. Adenylate cyclases of Trypanosoma brucei inhibit the innate immune response of the host. Science. 2012;337:463–466. doi: 10.1126/science.1222753. [DOI] [PubMed] [Google Scholar]
- 51.Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 52.Subota I, Julkowska D, Vincensini L, Reeg N, Buisson J, Blisnick T, Huet D, Perrot S, Santi-Rocca J, Duchateau M, Hourdel V, Rousselle JC, Cayet N, Namane A, Chamot-Rooke J, Bastin P. Proteomic analysis of intact flagella of procyclic Trypanosoma brucei cells identifies novel flagellar proteins with unique sub-localization and dynamics. Mol Cell Proteomics. 2014;13:1769–1786. doi: 10.1074/mcp.M113.033357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun SY, Wang C, Yuan YA, He CY. An intracellular membrane junction consisting of flagellum adhesion glycoproteins links flagellum biogenesis to cell morphogenesis in Trypanosoma brucei. J Cell Sci. 2013;126:520–531. doi: 10.1242/jcs.113621. [DOI] [PubMed] [Google Scholar]
- 54.Sutton RE, Boothroyd JC. Trypanosome trans-splicing utilizes 2′-5′ branches and a corresponding debranching activity. EMBO J. 1988;7:1431–1437. doi: 10.1002/j.1460-2075.1988.tb02960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thevelein JM, Voordeckers K. Functioning and evolutionary significance of nutrient transceptors. Mol Biol Evol. 2009;26:2407–2414. doi: 10.1093/molbev/msp168. [DOI] [PubMed] [Google Scholar]
- 56.Tran KD, Rodriguez-Contreras D, Shinde U, Landfear SM. Both sequence and context are important for flagellar targeting of a glucose transporter. J Cell Sci. 2012;125:3293–3298. doi: 10.1242/jcs.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran KD, Rodriguez-Contreras D, Vieira DP, Yates PA, David L, Beatty W, Elferich J, Landfear SM. KHARON1 mediates flagellar targeting of a glucose transporter in Leishmania mexicana and is critical for viability of infectious intracellular amastigotes. J Biol Chem. 2013;288:22721–22733. doi: 10.1074/jbc.M113.483461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tull D, Naderer T, Spurck T, Mertens HD, Heng J, McFadden GI, Gooley PR, McConville MJ. Membrane protein SMP-1 is required for normal flagellum function in Leishmania. J Cell Sci. 2010;123:544–554. doi: 10.1242/jcs.059097. [DOI] [PubMed] [Google Scholar]
- 59.Tull D, Vince JE, Callaghan JM, Naderer T, Spurck T, McFadden GI, Currie G, Ferguson K, Bacic A, McConville MJ. SMP-1, a member of a new family of small myristoylated proteins in kinetoplastid parasites, is targeted to the flagellum membrane in Leishmania. Mol Biol Cell. 2004;15:4775–4786. doi: 10.1091/mbc.E04-06-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, Hazlett TL, Engman DM. Flagellar membrane localization via association with lipid rafts. J Cell Sci. 2009;122:859–866. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wingard JN, Ladner J, Vanarotti M, Fisher AJ, Robinson H, Buchanan KT, Engman DM, Ames JB. Structural insights into membrane targeting by the flagellar calcium-binding protein (FCaBP), a myristoylated and palmitoylated calcium sensor in Trypanosoma cruzi. J Biol Chem. 2008;283:23388–23396. doi: 10.1074/jbc.M803178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wood CR, Huang K, Diener DR, Rosenbaum JL. The cilium secretes bioactive ectosomes. Curr Biol. 2013;23:906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Y, Deford J, Benjamin R, Lee MGS, Ruben L. The gene family of EF-hand calcium-binding proteins from the flagellum of Trypanosoma brucei. Biochem J. 1994;304:833–841. doi: 10.1042/bj3040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y, Haghighat NG, Ruben L. The predominant calcimedins from Trypanosoma brucei comprise a family of flagellar EF-hand calcium-binding proteins. Biochem J. 1992;287:187–193. doi: 10.1042/bj2870187. [DOI] [PMC free article] [PubMed] [Google Scholar]


